Abstract
Introduction:
Several renal denervation (RDN) systems are currently under investigation for treatment of hypertension by ablation of renal sympathetic nerves. The procedural efficacy of devices, however, is variable and incompletely understood. This study aimed at investigating procedural and anatomical predictors of RDN efficacy by comparing two radiofrequency catheter systems in a porcine model.
Methods:
Domestic swine were assigned into two treatment groups (n = 10) and one sham group (n = 3). Bilateral RDN in main and in branch segments of renal arteries was performed using two different multielectrode catheter systems [Symplicity Spyral (SPY) and IberisBloom (IBB)]. After 7 days, measurement of norepinephrine (NEPI) tissue concentrations and histological analyses have been performed.
Results:
Renal NEPI tissue concentration following RDN was significantly reduced when compared with Sham (SPY: −95 ± 3% vs. Sham, P < 0.001; IBB: −88 ± 11% vs. Sham, P < 0.001). Histological evaluation showed comparable lesion depth and lesion area (lesion depth: SPY-main 6.26 ± 1.62 mm vs. SPY-branch 3.49 ± 1.11 mm; IBB-main 5.93 ± 1.88 mm vs. IBB-branch: 3.26 ± 1.26 mm, P < 0.001; lesion area: SPY-main 43.5 ± 29.5 mm2 vs. SYP-branch 45.0 ± 38.0 mm2; IBB-main 52.3 ± 34.8 mm2 vs. IBB-branch 44.0 ± 42.6 mm2, P = 0.77; intergroup SPY vs. IBB, P = 0.73). Histological investigations documented a significant correlation between number of ablations per millimeter length of renal artery and reduction in NEPI tissue concentration.
Conclusion:
The two devices under investigation demonstrated similar histopathological lesion characteristics and similar reduction of renal NEPI levels. An increase in number of ablations per millmeter length of renal artery resulted in improved efficacy and reduced variability in treatment effects.
Keywords: hypertension, renal denervation devices, sympathetic nervous system
INTRODUCTION
Hypertension is the most prevalent cardiovascular disease worldwide and is associated with poor cardiovascular outcome [1,2]. Despite the availability of multiple effective antihypertensive drugs, control rates remain unacceptably low [3]. Catheter-based renal denervation (RDN) is under investigation to disrupt renal sympathetic nerve activity and produce a reduction of blood pressure in patients with uncontrolled hypertension [4–7]. The recently published Spyral HTN-OFF MED trial has proven the biological proof of principle for the blood pressure-lowering efficacy of RDN in the absence of antihypertensive medication [8]. The treatment effects, however, were variable and, in a relevant proportion of patients undergoing the procedure, blood pressure was not reduced sufficiently [9]. The neutral outcome of the SYMPLICITY HTN-3 study underscores the considerable heterogeneity in individual responses and the potential limitations of a poorly conceived ablation protocol. In the SYMPLICITY HTN-3 trial a mono-electrode catheter was utilized [10], whereas in the Spyral HTN-OFF-MED trial, a revised procedural approach using a multielectrode catheter was executed [8]. Previous studies in the porcine model have shown that radiofrequency energy delivered to the branches and the main renal artery resulted in less variability and greater effect on norepinephrine (NEPI) reduction and axonal degeneration when compared with conventional ablation in the main artery alone [11,12]. A detailed understanding of the effect of procedural parameters and different ablative strategies and their outcomes is mandatory for further procedural and catheter refinements. The present porcine study aimed at investigating different procedural parameters and their value in predicting treatment efficacy following RDN with two different radiofrequency devices.
METHODS AND MATERIAL
Study design
A cohort of 13 female Yorkshire cross swine was approved for use by the Institutional Animal Care and Use Committee (IACUC) of the test facility, which is accredited by the Association for Assessment and Accreditation of Laboratory Animal Care (AAALAC). All procedures for this study were performed at SynchronyLabs Inc (Durham, North Carolina, USA) in compliance with USDA Regulations and the Animal Welfare Act (9 CFR Parts 1–3). The Guide for the Care and Use of Laboratory Animals was followed. Study animals were acclimated for 10 days prior to intervention. The animals were assembled in three groups. Group SPY (n = 5, SP1–SP5) and Group IBB (n = 5, IB1–IB5) underwent RDN procedures. Pigs were treated with radiofrequency energy in the proximal, mid, and/or distal regions of both main renal arteries as well as branch arteries measuring at least 3.0 mm in diameter and at least 10 mm in length. The number of ablations was determined based on the length and diameters of the arterial segments. Sham group (n = 3, Sham1–Sham3) served as sham controls for the purposes of obtaining NEPI in animals, which did not undergo radiofrequency catheter placement or radiofrequency energy delivery. Table 1 displays the treatment matrix and procedural details.
TABLE 1.
Treatment matrix and procedural details
| Number of ablations | Ratio of numbers of ablations (branch/main) | |||||||||||
| Group | Animal ID | Left | Right | Left | Right | Mean ± SD | ||||||
| Branch | Main | Total | Branch | Main | Total | Total/animal | Mean ± SD | |||||
| Group SPY | SP1 | 14 | 5 | 19 | 11 | 9 | 20 | 39 | 33.2 ± 3.3 | 2.8 | 1.2 | 1.4 ± 0.7 |
| SP2 | 7 | 6 | 13 | 8 | 10 | 18 | 31 | 1.2 | 0.8 | |||
| SP3 | 8 | 4 | 12 | 10 | 9 | 19 | 31 | 2.0 | 1.1 | |||
| SP4 | 10 | 5 | 15 | 8 | 9 | 17 | 32 | 2.0 | 0.9 | |||
| SP5 | 8 | 9 | 17 | 6 | 10 | 16 | 33 | 0.9 | 0.6 | |||
| Group IBB | IB1 | 7 | 6 | 13 | 7 | 10 | 17 | 30 | 26.2 ± 3.3 | 1.2 | 0.7 | 0.9 ± 0.5 |
| IB2 | 8 | 6 | 14 | 5 | 8 | 13 | 27 | 1.3 | 0.6 | |||
| IB3 | 5 | 4 | 9 | 6 | 6 | 12 | 21 | 1.3 | 1.0 | |||
| IB4 | 2 | 10 | 12 | 4 | 11 | 15 | 27 | 0.2 | 0.4 | |||
| IB5 | 8 | 5 | 13 | 4 | 9 | 13 | 26 | 1.6 | 0.4 | |||
| Sham group | Sham1 | |||||||||||
| Sham2 | No ablations performed | |||||||||||
| Sham3 | ||||||||||||
Top: five animals were treated with the Symplicity Spyral system (group SPY). All animals of this group received 33.2 ± 3.3 ablations in main and branch arteries. The ratio of number of ablations (branch/main) was 1.4 ± 0.7. Middle: five animals were treated with the IberisBloom system (group IBB). All animals of this group received 26.2 ± 3.3 ablations in main and branch arteries. The ratio of number of ablations (branch/main) was 0.9 ± 0.5. Bottom: three animals received sham procedure (Sham Group) with zero ablations. IBB, IberisBloom; SPY, Symplicity Spyral.
Device overview
Two different RDN devices were used. The IberisBloom (Terumo, Tokyo, Japan) RDN system consists of a multielectrode catheter, with four electrodes arranged helically and its multichannel generator [13]. The Symplicity Spyral (Medtronic, Santa Rosa, California, USA) RDN system consists of a multielectrode catheter with four electrodes mounted approximately 5 mm apart at 90° of separation from each other in a helical pattern, thus providing automated four-quadrant ablation treatments. Figure 1 visualizes the two systems.
FIGURE 1.
(a) Symplicity Spyral (SPY) RDN catheter. (b) IberisBloom (IBB) RDN catheter. [Pictures: (a) www.medtronicrdn.com; (b) http://www.terumomedical.com.]
Catheter-based renal sympathetic denervation
All animals were treated with acetylsalicylic acid (325 mg per os) and clopidogrel (75 mg per os) 24 h prior to the procedure and administered daily until the day of euthanasia. Animals were anesthetized with tiletamine (4 mg/kg intramuscularly) followed by isoflurane anaesthesia by mask to facilitate endotracheal intubation. Animals were maintained on isoflurane anaesthesia for the duration of the procedure and butorphanol (0.3 mg/kg i.m.) was administered for analgesia. Animals were prepared for aseptic surgery and vascular access was obtained percutaneously via the femoral artery. A 6 French introducer sheath was placed, and intravenous heparin was administered to achieve an activated clotting time of at least 250 s. The right and left renal arteries of the pig were engaged using a 6 French guide catheter (Medtronic PLC, Dublin, Ireland) and quantitative vascular analysis measurements were performed. Thereafter, a 6 French renal denervation catheter was delivered over an 0.014′′ guidewire (Abbott Vascular, Santa Clara, California, USA) introduced and positioned in the right and left renal artery via fluoroscopic guidance. Bilateral RDN was initiated within each renal artery starting in a distal position then repositioning more proximally for sequential treatments, overlap of treatments was avoided. All procedures were performed by the same interventionist. Meticulous adherence to the protocol with respect to four quadrant ablation and spacing between ablation sites was followed. All animals were survived for 7 days.
Angiography
Angiography was performed before and after RDN. Baseline angiography was done after placement of the guide catheter into the ostium of the intended artery. Arteriographic images of the vessel were obtained and target locations for denervation treatment were measured using quantitative vascular analysis (QVA). Terminal angiography of the treated renal arteries was performed using the same projection as during the initial procedure and QVA measurements were performed.
Gross pathology and tissue collections
Prior to sacrifice, renal angiography was performed to assess renal artery changes (e.g. stenosis, aneurysm) then a ventral midline laparotomy was performed to access both kidneys. Samples of renal cortical tissue were obtained and frozen in 1 g aliquots for NEPI measurements. The animals were then euthanized, and a gross pathology was performed in order to evaluate macroscopic changes to the treated kidneys, heart, lungs, liver, spleen, bowel, ovaries, adrenal glands prior to en-block resection of the treated kidneys and surrounding musculature.
Histopathology
Renal arteries, including distal branches were trimmed sequentially with adjoining tissues, processed in paraffin, sectioned at 5 μm twice serially and stained with hematoxylin and eosin and Elastin Trichrome. The microscopic slides were assessed for healing and safety endpoints to include endothelialization, mural thrombosis, neointima formation and maturity, procedural and mural injury, media hypocellularity and fibrosis, inflammation, necrosis, adventitial fibrosis, and other changes. Quantitative and semi-quantitative assessments including measurements of the depth of radiofrequency changes, circumferential extension in the media of radiofrequency changes, and circumferential extension of radiofrequency lesion in the vessel wall and perivascular tissue combined were employed. For branched levels, some parameters were scored individually for each branch (e.g. presence and extent of radiofrequency changes), whereas other parameters were given a single score, which considered both branches (namely perivascular qualitative changes). Kidneys were assessed for presence of thrombi, emboli, granulomas, necrosis, renal infarcts, and any other pathological changes. All assessments were performed by a single ACVP board certified veterinary pathologist who was blinded to treatment groups. Metric measurements were obtained using an ocular micrometre.
Kidney tissue norepinephrine concentration
All tissue specimens harvested for NEPI analysis in this study were obtained from the renal cortex. A total of six sections per kidney of renal cortical tissue were harvested. Three samples from cranial, mid, and caudal were obtained from the dorsal aspect of the kidney and three samples from cranial, mid, and caudal from the ventral aspect of the kidney. Each tissue sample was approximately 2–3 mm thick and weighed 0.5–1.0 g. Samples were flash frozen before being sorted at −80 °C. For evaluation a HPLC-MS ASSAY was used.
Statistical analyses
Data are presented as mean ± SD. Quantitative vessel analysis data were compared by unpaired t-test between Group SPY and Group IBB. NEPI renal tissue concentration of each group was compared by Tukey's multiple comparison test. To assess main effects of arterial segments and devices and interactive effects, histomorphometric data were analysed by two-way analysis of variance (ANOVA). Pearson's correlation coefficients were calculated to investigate the correlation among NEPI reduction and histopathological parameters. To investigate effects of number of ablations normalized by arterial length on variation of renal tissue NEPI reduction, means and SDs of NEPI reduction were calculated in kidneys whose number of ablations per millimeter length renal artery was more and less than median, respectively, and compared by unpaired t-test. All statistical analyses were performed with GraphPad Prism 7 (GraphPad Software, San Diego, California, USA). A P value greater than 0.05 was considered to indicate statistical significance.
RESULTS
Angiography
Late lumen loss and diameter stenosis are depicted in Table 2 and exhibited no significant difference between either of the treatment groups (baseline mean lumen of main and branch arteries: SPY: 4.63 ± 1.2 mm, IBB: 4.48 ± 1.1 mm, P = 0.60; late lumen loss of main and branch arteries: SPY: 0.29 ± 0.4 mm, IBB: 0.30 ± 0.4 mm, P = 0.90; diameter stenosis of main and branch arteries: SPY: 6.6 ± 10.9%, IBB: 7.7 ± 9.1%, P = 0.67).
TABLE 2.
Angiographic changes following catheter-based renal denervation
| N | Baseline mean lumen diameter (mm) | Late lumen loss (mm) | Diameter stenosis (%) | |
| Group SPY | ||||
| All | 32 | 4.63 ± 1.21 | 0.29 ± 0.42 | 6.6 ± 10.9 |
| Main | 10 | 6.26 ± 0.43 | 0.37 ± 0.42 | 6.2 ± 7.2 |
| Branch | 22 | 3.90 ± 0.51 | 0.25 ± 0.42 | 6.7 ± 12.4 |
| Group IBB | ||||
| All | 28 | 4.48 ± 1.08 | 0.30 ± 0.39 | 7.7 ± 9.1 |
| Main | 10 | 5.74 ± 0.27 | 0.18 ± 0.46 | 3.3 ± 8.5 |
| Branch | 18 | 3.77 ± 0.58 | 0.37 ± 0.34 | 10.2 ± 8.7 |
No significant diameter stenosis between both RDN-devices (Group SPY and Group IBB). Data are presented as mean ± SD. IBB, IberisBloom; SPY, Symplicity Spyral.
Gross pathology and histopathology
There were no gross pathology changes noted in any of the animals treated with RDN. Lesion depth and lesion area were comparable in both treatment arms (Table 3). Lesion depth was the greatest in main arteries compared with branch artery in both treatment groups (SPY: 6.26 ± 1.62 mm in main and 3.49 ± 1.06 mm in branch; IBB: 5.93 ± 1.77 mm in main and 3.26 ± 1.15 mm in branch; intergroup main vs. branch, P < 0.001; intergroup SPY vs. IBB, P = 0.54). Lesion area between main or branch ablations was not different (SPY: 43.5 ± 29.5 mm2 in main and 45.0 ± 38.0 mm2 in branch; IBB: 52.3 ± 34.8 mm2 in main and 44.0 ± 42.6 mm2 in branch; intergroup main vs. branch, P = 0.77; intergroup SPY vs. IBB, P = 0.73). The luminal surface of the renal artery was almost completely endothelialized by day 7, regardless of the device used or the number of ablations. Effects on adjacent structures, including focal psoas muscle necrosis, injury to the ureter, and focal necrosis of lymph node were observed with both devices. Ablation of anterior and posterior branch arteries showed more necrosis of kidney tissue. Localized necrosis of ureter was prevalent in both segments. Overall collateral changes did not pose a safety concern because of their low severity and limited extension. The renal veins were not affected (Fig. 2). Increasing the number of treatments along the length of the artery positively correlated with an increase in the number of nerves directly affected (Pearson's correlation coefficient = 0.67, P = 0.001).
TABLE 3.
Histomorphometric data
| Group SPY | Group IBB | P values of two-way ANOVA | |||||
| Main segment | Branch segment | Main segment | Branch segment | Main vs. branch renal arteries | Group SP vs. Group IB | Intergroup effect | |
| Lesion depth (mm) | 6.26 ± 1.62 | 3.49 ± 1.06 | 5.93 ± 1.77 | 3.26 ± 1.15 | <0.001 | 0.54 | 0.92 |
| Lesion area (mm2) | 43.5 ± 29.5 | 45.0 ± 38.0 | 52.3 ± 34.8 | 44.0 ± 42.6 | 0.77 | 0.73 | 0.67 |
| Lesion area (%) | 11.8 ± 8.0 | 11.3 ± 8.6 | 12.7 ± 7.3 | 12.4 ± 8.8 | 0.96 | 0.71 | 0.96 |
| Lesion circumferential extension (%) | 41.9 ± 14.9 | 38.5 ± 9.8 | 47.9 ± 18.8 | 40.7 ± 14.2 | 0.26 | 0.39 | 0.68 |
| Lesion circumferential extension at media (%) | 28.3 ± 12.3 | 31.7 ± 11.0 | 33.8 ± 14.6 | 36.6 ± 14.7 | 0.46 | 0.22 | 0.95 |
Comparable lesion depth in lesion area in both arms. Lesion depth is significantly greater in main than in a branch. Data are presented as mean ± SD. IBB, IberisBloom; SPY, Symplicity Spyral.
FIGURE 2.
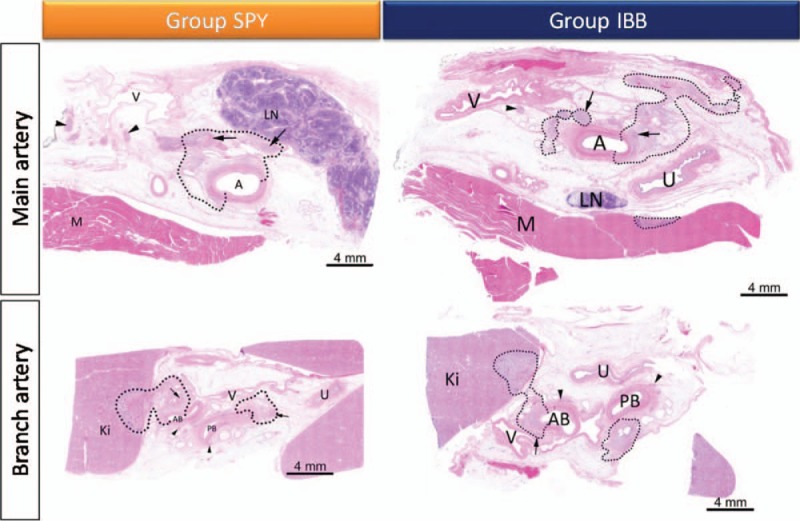
Focal changes following radiofrequency renal denervation shown within dotted lines. Focal changes were documented in both treatment groups. Ablation of main arteries (A) led to focal affection of lymph node (LN) and of psoas muscle (M). Ablation of anterior and posterior branch arteries (AB, PB) shows more irritations of kidney tissue (KI). Inflammation of ureter (U) was prevalent in both segments. The renal veins (V) have not been affected.
Kidney tissue norepinephrine concentration
Treatment with RDN (SPY and IBB) resulted in a significant reduction in kidney tissue NEPI concentration compared with the Sham group, whereas there was no significant difference between the treatment groups (SPY vs. Sham, −95 ± 3%, P < 0.001; IBB vs. Sham, −88 ± 11%, P < 0.001; SPY vs. IBB, P = 0.33; Fig. 3). Interestingly, a more intensive ablation strategy led to a higher NEPI reduction with less variation. In pigs treated with less or more than the median of 0.2 ablations per 1 mm artery length, NEPI reduction was reduced by 88.5 ± 10.9 and 94.9 ± 3.3%, respectively. When assessing the effectiveness of the procedure in the main compared with the branch arteries separately, less ablations were needed per 1 mm artery length in the branches. The median number in the main renal arteries was 0.26 ablations per 1 mm (NEPI reduction in <0.26 ablations per 1 mm: 88.4 ± 10.8% vs. >0.26 ablations per 1 mm: 95.0 ± 3.3%, P = 0.08) compared with a median number of 0.16 ablations per 1 mm in branch renal arteries (NEPI reduction in <0.16 ablations per 1 mm: 89.8 ± 10.4% vs. >0.16 ablations per 1 mm: 93.6 ± 6.0%, P = 0.33; Fig. 4), indicating that ablations in branch arteries were associated with higher efficacy and less variability compared with main arteries.
FIGURE 3.
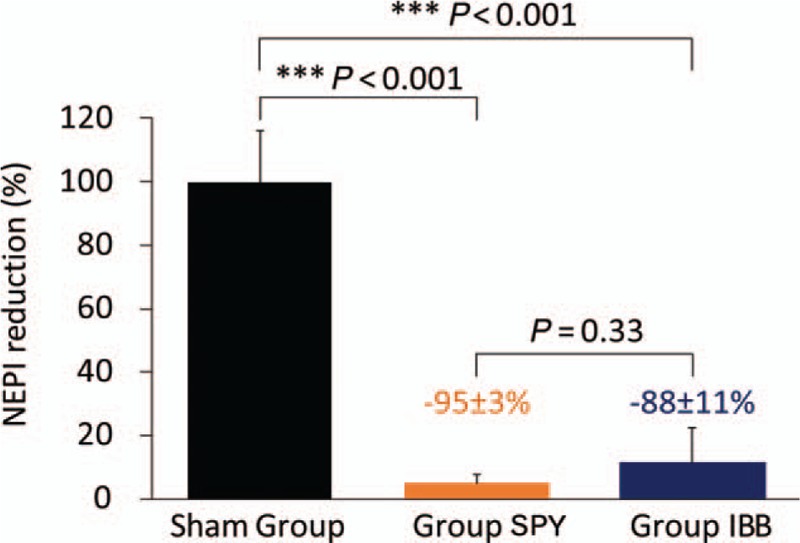
Relative change in kidney norepinephrine concentration. Data are presented as mean ± SD.
FIGURE 4.
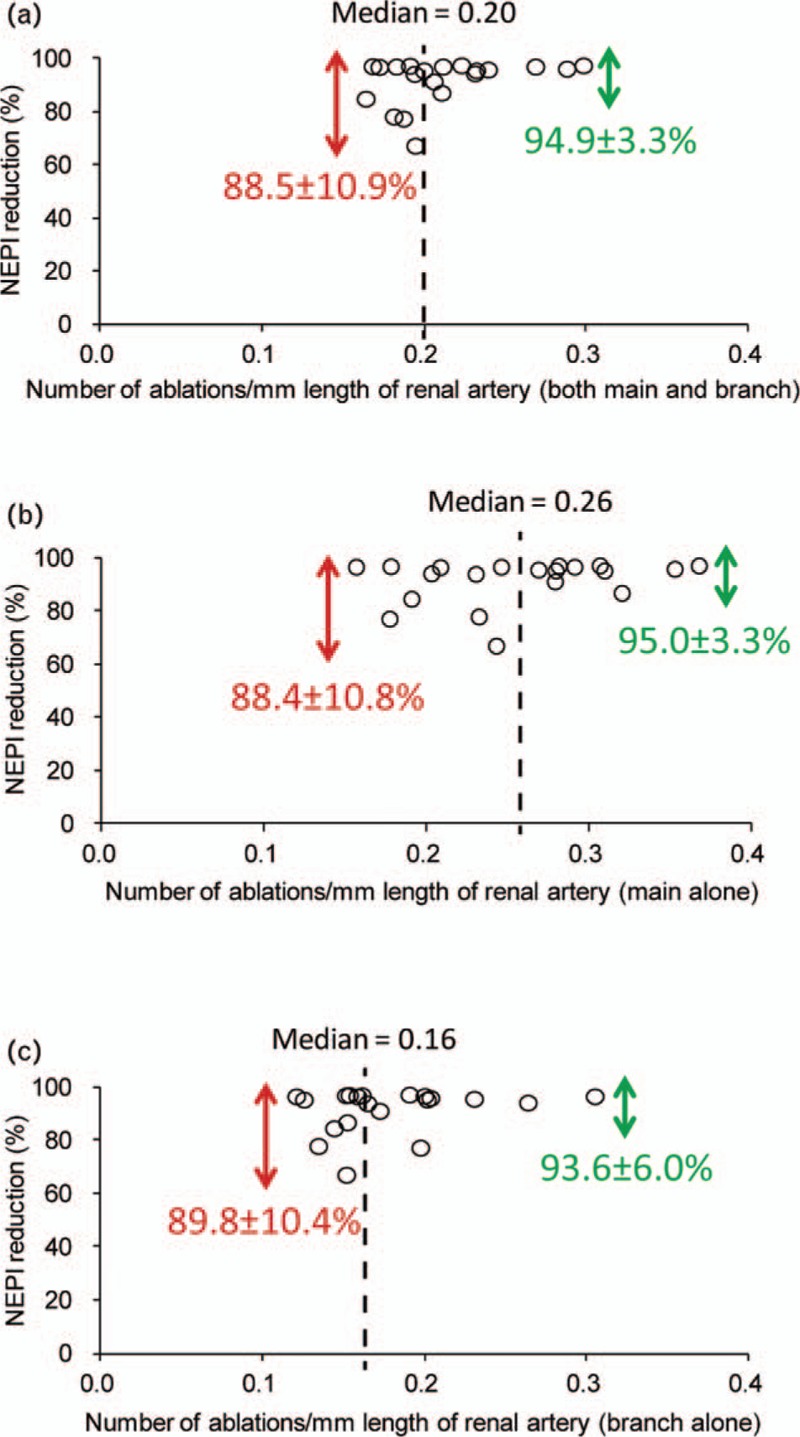
Dependence of ablations per millimeter length of artery to reduction of kidney norepinephrine concentration. (a) 0.2 ablations per 1 mm length of renal artery are needed to reduce the variation of norepinephrine reduction in main and branch arteries. (b) 0.26 ablations per 1 mm length of renal artery are needed to reduce the variation of norepinephrine reduction in main arteries. (c) 0.16 ablations per 1 mm length of renal artery are needed to reduce the variation of norepinephrine reduction in branch arteries. Data are presented as mean ± SD.
DISCUSSION
The present study explored procedural and anatomical predictors of RDN efficacy using two different radiofrequency catheters in a porcine model. Both systems were highly effective in reducing NEPI kidney tissue content with no significant difference between them. Further, lesion depth and lesion areas were comparable in both groups. Lesion depth was highest in main arteries compared with branch arteries, which is in line with previous reports [12]. There was no clear dose–response relationship with number of ablations and NEPI reduction established. However, histological evaluation demonstrated that ablations in the branches were associated with higher efficacy based on nerve necrosis and distal nerve atrophy and less variability compared with main arteries.
Catheter-based RDN is currently under investigation for treatment of hypertension [4,14,15]. Early studies in patients with resistant hypertension have shown mixed results [10,16], whereas the recently published Spyral HTN-OFF MED study elegantly provided the biological proof of principle for the blood pressure-lowering efficacy of RDN in the absence of antihypertensive medication [8]. Although several confounding factors have been identified and addressed in ongoing studies, such as the exclusion of isolated systolic hypertensive patients and revised procedural protocols with ablations in the main and branch arteries, the blood pressure-lowering effectiveness was variable [16–19]. Identification of patients not responding to the RDN therapy remains a major objective of future studies in the field.
One reason for nonresponse to the procedure may, in part, relate to an ineffective targeting of renal afferent and efferent sympathetic nerves, which are located in the adventitia of renal arteries. Disruption of these structures reduces the sympathetic nerve innervation of the kidney and through alteration of afferent nerve signalling whole body sympathetic activity [20]. The distribution pattern and density of sympathetic nerves along the renal arteries have been identified to impact the success of RDN [21]. Human histological studies as well as preclinical animal studies indicate that the maximal number of nerves are located around the proximal and middle segments of the renal artery, whereas lower number of nerves are located around distal structures. However, the mean distance of the sympathetic afferent and efferent nerves to the lumen of the renal arteries is shorter in the distal arterial segments [21]. Even if the mean lesion depth, which is influenced by surrounding structures [12], is less pronounced in branch arteries, the effectiveness of radiofrequency RDN is enhanced in this area likely because of the proximity of the nerves to the treated artery. One of the major unresolved issue of the procedure is how to monitor treatment success intraprocedurally. As no feedback is provided to the interventionist during the procedure, targeting the wrong arterial segments and performing too few ablations per artery could result in a high variability of blood pressure reduction or even in a complete negative outcome. On the other hand, ablations that exceed the required threshold needed to reduce local sympathetic activity are unnecessarily exposing patients to prolonged procedures and potential long-term vascular safety risk. Intraprocedural guidance on the number of ablations that need to be performed is currently lacking, though urgently desired. Herein, numerically more ablations per length were needed in the main when compared with branches to increase effectiveness and reduce variability in NEPI changes. The breaking point for ablations per 10 mm lengths of the main renal artery was 2.6 compared with 1.6 in the branches.
Clinical and preclinical trials indicate that catheter-based RDN, using radiofrequency energy is well tolerated [12,22]. Injury and inflammation of adjacent structures were minimal. Furthermore, no significant endothelial damage or stenosis of treated renal arteries were observed. Nevertheless, as the animals were sacrificed after 5 months, it remains unclear if injury of the ureter, renal cortex or psoas muscle by radiofrequency energy delivery may cause deleterious long-term effects. Of note, the recently published studies that utilized a revised approach of treating the main and branch renal arteries with radiofrequency energy, documented a favourable safety profile [8,23]. However, long-term follow-up in a larger set of patients is required before statements on the safety profile can be made.
Limitations
The study was performed in normotensive juvenile swine. Their arteries may not reflect the characteristics of human arteries with hypertension, which may alter anatomy. In addition, the microanatomy of the swine artery may not accurately mimic what is found in hypertensive patients. It should also be noted that long-term collateral damage of the surrounding tissue by radiofrequency energy delivery in the branches and the main renal arteries need to be investigated in future studies with longer follow-up. The study was not designed to evaluate different treatment strategies as has been shown in other preclinical studies. Lastly, blood pressure measurements were not performed in this group of normotensive juvenile swine during the study.
In conclusion, the two devices evaluated in this study demonstrated similar histopathological effects (lesion depth, lesion area, nerve necrosis, nerve atrophy) and similar reduction of renal cortical NEPI levels. Ablations performed in the branches improved efficacy and reduced variability in treatment effects. This confirms the importance of anatomic and procedural parameters when performing RDN and deserves further investigation in clinical studies
ACKNOWLEDGEMENTS
Funding: This study was funded by an independent grant from Terumo Corporation.
Conflicts of interest
A.S. is an employee of Terumo Corporation. K.K. reports grants from Medtronic Japan Co. Ltd.; honoraria from Terumo Corp. and Otsuka Pharmaceutical Co. Ltd. during the conduct of the study. M.B. reports Speaker's fees and scientific advice honoraria to Medtronic, Abbot Vascular, Servier, Novartis, Boehringer-Ingelheim. F.M. is supported by Deutsche Hochdruckliga and Deutsche Gesellschaft für Kardiologie and has received speaker honoraria and consultancy fees from Medtronic and Recor.
Footnotes
Abbreviations: ANOVA, analysis of variance; IBB, IberisBloom; NEPI, norepinephrine; QVA, qualitative vascular analysis; RDN, renal denervation; SPY, Symplicity Spyral
Revised 28 May, 2018
REFERENCES
- 1.Lopez AD, Mathers CD, Ezzati M, Jamison DT, Murray CJL. Global and regional burden of disease and risk factors, 2001: systematic analysis of population health data. Lancet 2006; 367:1747–1757. [DOI] [PubMed] [Google Scholar]
- 2.Kearney PM, Whelton M, Reynolds K, Whelton PK, He J. Worldwide prevalence of hypertension: a systematic review. J Hypertens 2004; 22:11–19. [DOI] [PubMed] [Google Scholar]
- 3.Forouzanfar MH, Liu P, Roth GA, Ng M, Biryukov S, Marczak L, et al. Global burden of hypertension and systolic blood pressure of at least 110 to 115 mm Hg, 1990-2015. JAMA 2017; 317:165-182. [DOI] [PubMed] [Google Scholar]
- 4.Krum H, Schlaich MP, Sobotka PA, Böhm M, Mahfoud F, Rocha-Singh K, et al. Percutaneous renal denervation in patients with treatment-resistant hypertension: Final 3-year report of the Symplicity HTN-1 study. Lancet 2014; 383:622–629. [DOI] [PubMed] [Google Scholar]
- 5.Esler MD, Krum H, Sobotka PA, Schlaich MP, Schmieder RE, Böhm M, et al. Renal sympathetic denervation in patients with treatment-resistant hypertension (The Symplicity HTN-2 Trial): a randomised controlled trial. Lancet 2010; 376:1903–1909. [DOI] [PubMed] [Google Scholar]
- 6.Tsioufis C, Ziakas A, Dimitriadis K, Davlouros P, Marketou M, Kasiakogias A, et al. Blood pressure response to catheter-based renal sympathetic denervation in severe resistant hypertension: data from the Greek Renal Denervation Registry. Clin Res Cardiol 2017; 105:322–330. [DOI] [PubMed] [Google Scholar]
- 7.Sharp ASP, Davies JE, Lobo MD, Bent CL, Mark PB, Burchell AE, et al. Renal artery sympathetic denervation: observations from the UK experience. Clin Res Cardiol 2016; 105:544–552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Townsend RR, Mahfoud F, Kandzari DE, Kario K, Pocock S, Weber MA, et al. SPYRAL HTN-OFF MED trial investigators∗. Catheter-based renal denervation in patients with uncontrolled hypertension in the absence of antihypertensive medications (SPYRAL HTN-OFF MED): a randomised, sham-controlled, proof-of-concept trial. Lancet 2017; 390:2160–2170. [DOI] [PubMed] [Google Scholar]
- 9.Mahfoud F, Lüscher TF. Renal denervation: Symply trapped by complexity? Euro Heart J 2015; 36:199–202. [DOI] [PubMed] [Google Scholar]
- 10.Bhatt DL, Kandzari DE, O’Neill WW, D’Agostino R, Flack JM, Katzen BT, et al. A controlled trial of renal denervation for resistant hypertension. N Engl J Med 2014; 370:1393–1401. [DOI] [PubMed] [Google Scholar]
- 11.Mahfoud F, Edelman ER, Böhm M. Catheter-based renal denervation is no simple matter: lessons to be learned from our anatomy? J Am Coll Cardiol 2014; 64:644–646. [DOI] [PubMed] [Google Scholar]
- 12.Mahfoud F, Pipenhagen CA, Boyce Moon L, Ewen S, Kulenthiran S, Fish JM, et al. Comparison of branch and distally focused main renal artery denervation using two different radio-frequency systems in a porcine model. Int J Cardiol 2017; 241:373–378. [DOI] [PubMed] [Google Scholar]
- 13.Sakaoka A, Terao H, Nakamura S, Hagiwara H, Furukawa T, Matsumura K, et al. Accurate depth of radiofrequency-induced lesions in renal sympathetic denervation based on a fine histological sectioning approach in a porcine model. Circ Cardiovasc Interv 2018; 11:e005779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ewen S, Meyer MR, Cremers B, Laufs U, Helfer AG, Linz D, et al. Blood pressure reductions following catheter-based renal denervation are not related to improvements in adherence to antihypertensive drugs measured by urine/plasma toxicological analysis. Clin Res Cardiol 2015; 104:1097–1105. [DOI] [PubMed] [Google Scholar]
- 15.Donazzan L, Mahfoud F, Ewen S, Ukena C, Cremers B, Kirsch CM, et al. Effects of catheter-based renal denervation on cardiac sympathetic activity and innervation in patients with resistant hypertension. Clin Res Cardiol 2016; 105:364–371. [DOI] [PubMed] [Google Scholar]
- 16.Mahfoud F, Böhm M, Azizi M, Pathak A, Durand Zaleski I, Ewen S, et al. Proceedings from the European clinical consensus conference for renal denervation: Considerations on future clinical trial design. Euro Heart J 2015; 36:2219–2227. [DOI] [PubMed] [Google Scholar]
- 17.Ewen S, Ukena C, Linz D, Kindermann I, Cremers B, Laufs U, et al. Reduced effect of percutaneous renal denervation on blood pressure in patients with isolated systolic hypertension. Hypertension 2015; 65:193–199. [DOI] [PubMed] [Google Scholar]
- 18.Sakakura K, Ladich E, Cheng Q, Otsuka F, Yahagi K, Fowler DR, et al. Anatomic assessment of sympathetic peri-arterial renal nerves in man. J Am Coll Cardiol 2014; 64:635–643. [DOI] [PubMed] [Google Scholar]
- 19.De Jager RL, Sanders MF, Bots ML, Lobo MD, Ewen S, Beeftink MMA, et al. Renal denervation in hypertensive patients not on blood pressure lowering drugs. Clin Res Cardiol 2016; 105:755–762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hering D, Lambert EA, Marusic P, Walton AS, Krum H, Lambert GW, et al. Substantial reduction in single sympathetic nerve firing after renal denervation in patients with resistant hypertension. Hypertension 2013; 61:457–464. [DOI] [PubMed] [Google Scholar]
- 21.Mahfoud F, Moon LB, Pipenhagen CA, Jensen JA, Pathak A, Papademetriou V, et al. Catheter-based radio-frequency renal nerve denervation lowers blood pressure in obese hypertensive swine model. J Hypertens 2016; 34:1854–1862. [DOI] [PubMed] [Google Scholar]
- 22.Böhm M, Mahfoud F, Ukena C, Hoppe UC, Narkiewicz K, Negoita M, et al. GSR Investigators. First report of the global SYMPLICITY registry on the effect of renal artery denervation in patients with uncontrolled hypertension. Hypertension 2015; 65:766–774. [DOI] [PubMed] [Google Scholar]
- 23.Kandzari DE, Böhm M, Mahfoud F, Townsend RR, Weber MA, Pocock S, et al. SPYRAL HTN-ON MED Trial Investigators. Effect of renal denervation on blood pressure in the presence of antihypertensive drugs: 6-month efficacy and safety results from the SPYRAL HTN-ON MED proof-of-concept randomised trial. Lancet 2018; [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]


