Supplemental Digital Content is available in the text.
Keywords: adjuvant therapy, economic burden, melanoma, recurrence
Abstract
Surgery is the mainstay treatment for operable nonmetastatic melanoma, but recurrences are common and limit patients’ survival. This study aimed to describe real-world patterns of treatment and recurrence in patients with melanoma and to quantify healthcare resource utilization (HRU) and costs associated with episodes of locoregional/distant recurrences. Adults with nonmetastatic melanoma who underwent melanoma lymph node surgery were identified from the Truven Health MarketScan database (1 January 2008 to 31 July 2017). Locoregional and distant recurrence(s) were identified on the basis of postsurgery recurrence indicators (i.e. initiation of new melanoma pharmacotherapy, new radiotherapy, or new surgery; secondary malignancy diagnoses). Of 6400 eligible patients, 219 (3.4%) initiated adjuvant therapy within 3 months of surgery, mostly with interferon α-2b (n=206/219, 94.1%). A total of 1191/6400 (18.6%) patients developed recurrence(s) over a median follow-up of 23.1 months (102/6400, 1.6% distant recurrences). Among the 219 patients initiated on adjuvant therapy, 73 (33.3%) experienced recurrences (distant recurrences: 13/219, 5.9%). The mean total all-cause healthcare cost was $2645 per patient per month (PPPM) during locoregional recurrence episodes and $12 940 PPPM during distant recurrence episodes. In the year after recurrence, HRU was particularly higher in patients with distant recurrence versus recurrence-free matched controls: by 9.2 inpatient admissions, 54.4 inpatient days, 8.8 emergency department admissions, and 185.9 outpatient visits (per 100 person-months), whereas all-cause healthcare costs were higher by $14 953 PPPM. It remains to be determined whether the new generation of adjuvant therapies, such as immune checkpoint inhibitors and targeted agents, will increase the use of adjuvant therapies, and reduce the risk of recurrences and associated HRU/cost.
Introduction
Cutaneous melanoma is responsible for the majority of skin cancer morbidity and mortality 1. Melanoma represents a global burden, with Australia/New-Zealand, North America, and Europe presenting the highest incidence and mortality rate 2. In the USA, the incidence of melanoma has increased over the past decades, with 91 270 estimated new cases and 9320 related deaths in 2018 1.
Most patients with cutaneous melanoma are diagnosed at early stages, with mainly localized/regional (i.e. nonmetastatic) disease 3. For patients with cutaneous nonmetastatic melanoma, the mainstay of treatment is local excision of the tumor with wide margins, and lymphadenectomy if regional lymph nodes (LNs) are involved 4. However, up to half of these patients will develop a recurrence, with ∼50% recurrence in regional LNs, 20% local recurrences, and 30% distant recurrences 5,6. In patients with known LN metastasis, the recurrence rates are even higher 7. Postrecurrence survival has historically been poor, with a 3-year survival postdistant recurrence of ∼16% 8, although odds are improving with recent advances in systemic therapy 9.
The treatment landscape for the advanced stages of melanoma has recently changed at a global level. For patients with stage III melanoma, v1.2018 National Comprehensive Cancer Network (NCCN) guidelines added recommendations for adjuvant therapy with nivolumab and dabrafenib/trametinib (for patients with the BRAF V600 mutation), in addition to the older recommendation for adjuvant therapy with high-dose ipilimumab/interferon α 10. The European Medicines Agency has also approved the use of nivolumab (either alone or in combination with ipilimumab) as well as the use of dabrafenib/trametinib (for patients with BRAF V600 mutation) for the treatment of advanced melanoma 11,12.
Although the treatment landscape will likely change with the introduction of adjuvant nivolumab and dabrafenib/trametinib, information on current use of ipilimumab or interferon α in nonmetastatic high-risk melanoma remains scarce. A 2011 study noted a decreasing trend in the use of adjuvant interferon α in melanoma patients with regional diseases [from 26% (1995) to 21% (2001)], suggesting a lack of consensus for adjuvant therapy use in community settings in the interferon adjuvant treatment era 13.
The annual costs of melanoma reportedly range from $45 million among prevalent cases to $933 million among newly diagnosed USA patients 14. However, there is a paucity of recent data on recurrence-related healthcare resource utilization (HRU) and costs among patients with operable nonmetastatic melanoma undergoing melanoma surgery 15. No study to date has assessed HRU and costs associated with episodes of locoregional recurrences in this population. Furthermore, although several studies have assessed HRU and costs among patients with metastatic melanoma 16–21, many are outdated because of major treatment advances for metastatic melanoma since 2011. This represents an important knowledge gap because recurrence is a key outcome for assessing burden of disease and informing decisions about the cost-effectiveness of therapies. Given that most cancer patients are treated in community centers, real-world data on cancer outcomes and costs are crucial 22.
The study aimed to: (a) describe real-world patterns of treatment and recurrence in patients with nonmetastatic melanoma who underwent melanoma surgery; (b) quantify all-cause and melanoma-specific HRU and costs associated with episodes of locoregional and distant recurrence following the initial melanoma surgery; and (c) compare all-cause HRU and costs between recurrence and recurrence-free cohorts in the year following the start of the first locoregional or distant recurrence.
Patients and methods
Data source
This study relied on the Truven Health MarketScan Commercial and Medicare Supplemental database (Truven Health Analytics LLC, an IBM Company, Ann Arbor, Michigan, USA), which contains provider and institutional health services claims for medical and pharmacy services for more than 240 million individuals from ∼100 employers since 1995 (period included: 1 January 2008 to 31 July 2017). Data are representative of all census regions, predominantly the South and North Central (Midwest). Truven is fully compliant with the Health Insurance Portability and Accountability Act of 1996 and its implementing regulations; thus, no ethics board review was required 23.
Study design
In this retrospective cohort study, patients had to undergo lymph node surgery (LNS; e.g. sentinel LN biopsy, dissection, lymphadenectomy) as part of their surgical treatment for nonmetastatic melanoma, except for less than 0.2% of patients with a diagnosis of secondary malignancy in the LNs who had skin excision without LNS. Given that some patients had several consecutive surgeries of increasing complexity after the nonmetastatic melanoma diagnosis (e.g. a simpler surgery such as excision, followed within 3 months by a more complex surgery such as LNS), the index surgery was defined as the most complex procedure among surgeries started within 3 months of the first melanoma diagnosis. It is noteworthy that the surgery outcome (i.e. presence or not of LN metastasis) was not available in the Truven data.
Baseline characteristics were measured in the 6 months before the index surgery (baseline period). Treatment patterns were identified from the index surgery until the earliest date between the end of eligibility (due to disenrollment or death) or the end of data availability (31 July 2017; study period). Locoregional and distant recurrence(s) during the study period were identified on the basis of recurrence indicators derived from patterns of therapy for melanoma (initiation of new pharmacotherapy, radiotherapy, or new skin excision/LNS) and secondary malignancy diagnoses after index surgery. Recurrence episodes were defined from 30 days before the first indicator of a locoregional/distant recurrence to 30 days after the last indicator for recurrence (locoregional recurrences) or the end of the study period (distant recurrences). HRU and healthcare cost were reported separately during episodes of locoregional and distant recurrence. Moreover, patients in the recurrence cohorts were matched to recurrence-free controls by propensity score and other covariates. For the matched cohorts, HRU and healthcare costs were also measured in the year following an index date defined as follows: recurrence cohorts – the date of the first recurrence; recurrence-free cohorts – a random date selected from a distribution matching the distribution of time from index surgery to the date of first recurrence.
Sample selection
Patients were included if they (a) had at least one diagnosis of melanoma during a hospitalization or at least two diagnoses of melanoma during outpatient (OP) visits on at least two distinct days; (b) were at least 18 years old at the first melanoma diagnosis; (c) had the first melanoma surgery within 3 months of the first melanoma diagnosis; and (d) had at least 15 months of continuous healthcare coverage, including at least 12 months before the first melanoma diagnosis, and at least 3 months after index surgery (see Study Design section). Also, for the matched cohort comparison, patients in both the recurrence and recurrence-free cohorts needed at least 3 months of continuous healthcare coverage after the index date (see Study Design section). Patients were excluded if they had indicators of (a) other primary cancers in the 5 years before first melanoma diagnosis (i.e. diagnosis or antineoplastic treatment); (b) metastatic melanoma at the index surgery (i.e. diagnosis of secondary malignancy excluding LNs, antineoplastic pharmacotherapy indicated for metastatic melanoma, or stereotactic radiotherapy from first melanoma diagnosis to 3 months after the index surgery); and (c) participation in a clinical trial after the first melanoma diagnosis.
Measurements and outcomes
Patient baseline characteristics: patient baseline characteristics included demographics, melanoma tumor site, HRU, and costs during the baseline period, the Quan–Charlson comorbidity index 24 (excluding cancer diagnosis), and a cancer-specific index 25 (excluding melanoma diagnosis).
Treatment patterns: antineoplastic pharmacotherapies identified during the study period included agents and regimens recommended for melanoma in adjuvant and metastatic setting according to the NCCN guidelines 10. Pharmacotherapies initiated within 3 months of the index surgery were labeled as adjuvant first line (adjuvant 1L; because patients with metastatic melanoma at index surgery were excluded, 1L could only consist of adjuvant therapy). Lines of pharmacological therapy initiated more than 3 months after index surgery were labeled as the second and subsequent line of therapy (2L+), irrespective of whether the treatment received was adjuvant or systemic. Identification of start/end dates for 2L+ and regimens used in 2L+ was adapted from previously published algorithms 26–28. Specifically, the first prescription fill/administration of a therapeutic agent after index surgery (for patients not receiving adjuvant) or after the adjuvant 1L ended was defined as 2L start date, whereas all agents received during the first 28 days of 2L therapy constituted the 2L regimen (irrespective of whether they were administered as single agents or combination therapy). The duration of 2L was based on the days of supply for agents with oral administration or 21 days for agents with intravenous administration. The end of 2L was determined by either discontinuation of all agents in the 2L regimen [i.e. a gap of >60 consecutive days (90 days for ipilimumab) without pharmacotherapy] or switch to a new regimen (i.e. initiation of a new agent not included within the 2L regimen). The start date, end date, and regimens for subsequent lines of therapy (3L+) were identified similar to 2L. The last observed line of pharmacotherapy was censored if the patient remained on treatment at the end of the study period. Other treatments (radiotherapy, new surgery), use of biopsy, use of BRAF tests, and diagnosis of secondary malignancy were reported both during lines of pharmacotherapy (e.g. during 1L, during 2L) and during the gaps between lines of pharmacotherapies (e.g. between 2L end and 3L start).
Locoregional and distant recurrences: melanoma recurrences during the study period were identified on the basis of the following indicators: (a) skin excision within 3 months of index surgery, if index surgery was LNS; (b) new skin excision (excluding shaving of epidermal or dermal lesion, and Moh’s surgery) or LNS at least 3 months after index surgery; (c) antineoplastic pharmacotherapy (except adjuvant therapy initiated within 3 months of the index surgery) after index surgery; (d) radiotherapy at least 3 months after index surgery; and (e) new diagnosis code for secondary malignancy at least 3 months on two distinct dates after index surgery. The sequence of indicators/treatments occurring within 3 months of each other were aggregated in a single recurrence episode. Indicators specific to distant recurrences, used to distinguish episodes of distant recurrence from episodes of locoregional recurrence, included the following: (a) regimens not listed as adjuvant therapies in the NCCN melanoma guidelines (i.e. systemic therapies, including ipilimumab before 2015) 10; (b) stereotactic radiotherapy; and (c) new diagnosis code for secondary malignancy excluding LNs at least 3 months after index surgery on two distinct dates. Patients could have several locoregional recurrence episodes during the study period, but only one distant recurrence episode. Additional details on the duration of recurrence episodes are provided in the Study Design section.
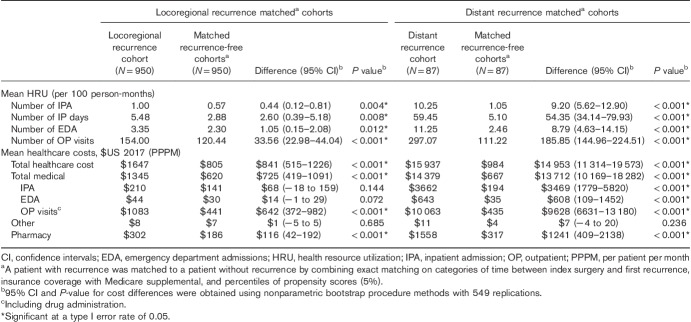
HRU and costs: HRU, reported per 100 person-months, included number of inpatient (IP) admissions, number of IP days, number of emergency department (ED) admissions, and number of OP visits (including OP visits for medication administration). All-cause healthcare costs, reported per patient per month (PPPM), included medical costs [IP, ED, OP (including medication administration), other (e.g. durable medical equipment)], and pharmacy prescription costs. Depending on the objective, HRU and costs were measured either during episodes of recurrence or in the year following the first recurrence (randomly selected index date for the matched controls). Melanoma-specific costs were those associated with medical services for melanoma, including treatments (pharmacological, surgery, radiotherapy) and disease monitoring. Healthcare costs were adjusted for inflation at the claim level to 2017 USA dollars using the medical component of the USA Consumer Price Index. Given that HRU/costs are reported per person-month, all matched patients were included in the comparative HRU/cost analyses, whether they had a full year of observation post-index date or not.
Statistical analyses
Descriptive analyses of patient baseline characteristics and outcomes (i.e. treatment patterns, recurrence rates, and HRU/cost during episodes of locoregional and distant recurrence) relied on frequencies (proportions) for categorical variables and means (SD) for continuous variables. For the comparative HRU/cost analyses, patients in the locoregional and distant recurrence cohorts were matched 1 : 1 to recurrence-free patients on propensity score percentiles 29, insurance coverage with Medicare supplemental, and time between index surgery and index date. The matching enabled balancing baseline covariates between cohorts. Covariate balance before and after matching was assessed with standardized differences 30. Although no consensus exists as to what value of a standardized difference indicates the presence of meaningful confounding, guidelines suggest that differences below |10%| and |20%| represent reasonable cut-offs 31–34. The 95% confidence interval (CI) and the P-value for HRU/cost differences between the matched recurrence and recurrence-free cohorts (i.e. the incremental HRU/cost associated with recurrence) were derived from nonparametric bootstrap resampling (B=549 resamples).
Results
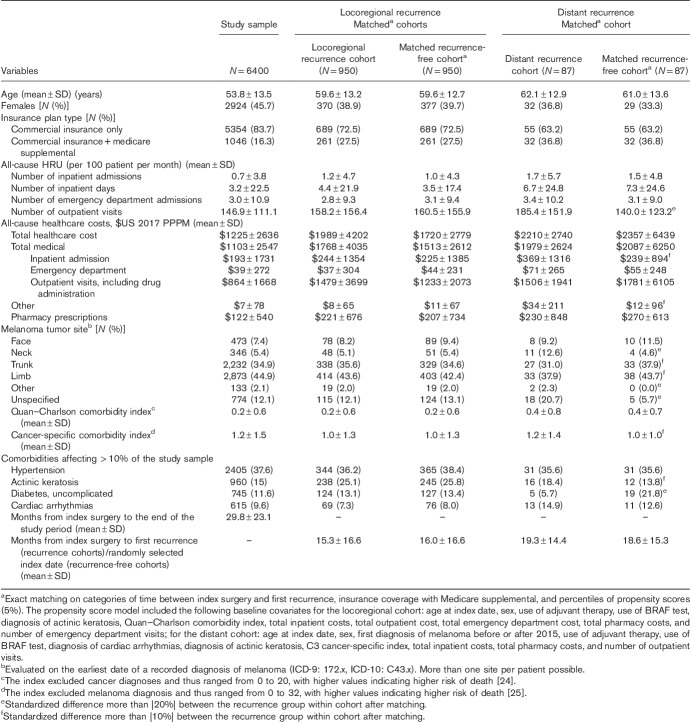
The study sample included 6400 eligible patients. The mean age was 53.8 years; ∼46% were women. The mean study period duration was 29.8 months (Table 1).
Table 1.
Baseline characteristics: study sample, and after matching for the locoregional and distant recurrence cohorts
Treatment patterns and recurrences
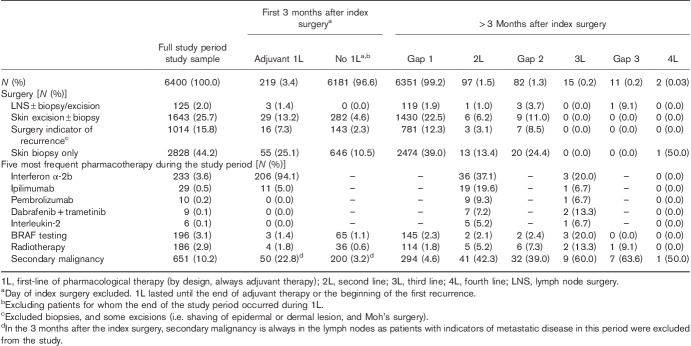
Of 6400 patients with nonmetastatic melanoma undergoing melanoma surgery from 1 January 2008 to 31 July 2017, 219 (3.4%) initiated adjuvant pharmacotherapy within 3 months of index surgery (mean therapy duration=6.4 months). Interferon α-2b was the most common adjuvant regimen (N=206/219, 94.1%). In total, 257/6400 (4.0%) received adjuvant therapy anytime during the study period. Radiotherapy use was rare, but increased with the line of treatment (i.e. 1.8% users concurrently with 1L of pharmacological therapy vs. 13.3% users concurrently with 3L). BRAF testing was uncommon in this patient population (3.1% over the study period; Table 2).
Table 2.
Treatment patterns
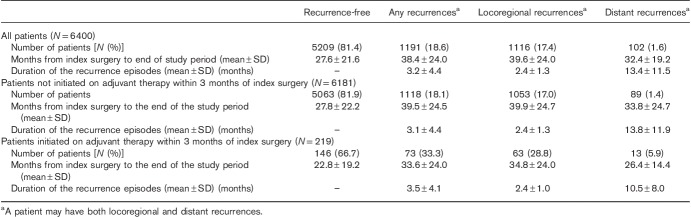
Of 6400 patients, 1191 (18.6%) had recurrence(s) over a median follow-up of 23.1 months: 1116 (17.4%) patients had locoregional recurrence(s), and 102 (1.6%) had a distant recurrence [27 (0.4%) patients had both]. Of 6181 patients who did not initiate adjuvant 1L, 1118 (18.1%) experienced recurrences during the study period, with 89 cases of distant recurrence (1.4%). Of 219 patients who initiated adjuvant 1L, 73 (33.3%) experienced recurrences, with 13 cases of distant recurrence (5.9%; Table 3). The first recurrence occurred within a year of the index surgery for 51/73 (69.9%), within 2 years for 54/73 (74.0%), and within 3 years for 66/73 (90.4%).
Table 3.
Recurrences during the study perioda
Healthcare resource utilization and costs during episodes of recurrence
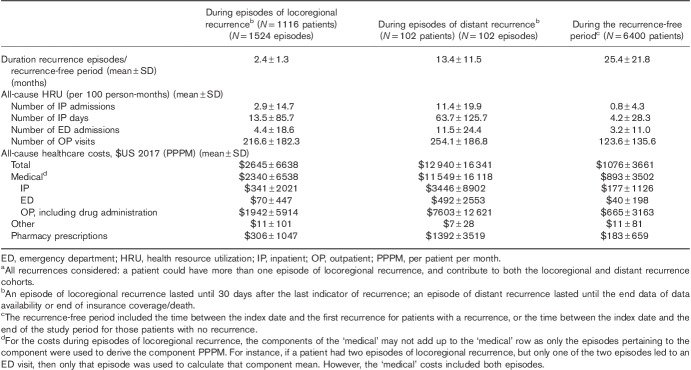
The mean duration of locoregional recurrence episodes was 2.4 months (Table 3). During these episodes, patients had on average 2.9 all-cause IP admissions, 13.5 all-cause IP days, 4.4 all-cause ED admissions, and 216.6 all-cause OP visits per 100 person-months. The mean total all-cause healthcare cost PPPM during locoregional recurrence episodes was $2645, of which 88.4% ($2340/$2645) was attributed to medical costs (Table 4). In terms of melanoma-specific HRU and cost, skin surgery and monitoring (laboratory) were the most important components of melanoma-specific HRU during episodes of locoregional recurrences [used by 944/1116 (84.6%) and 446/1116 (40.0%) of the patients, respectively; Supplementary Fig. (Supplemental digital content 1, http://links.lww.com/MR/A64)], which shows HRU during episodes]; the mean melanoma-specific cost was $1537 PPPM during locoregional recurrence episodes, mostly attributable to surgery [$730/$1537 (47.5%); Fig. 1].
Table 4.
All-cause healthcare resource utilization and healthcare costs during episodes of recurrence and during the recurrence-free perioda
Fig. 1.
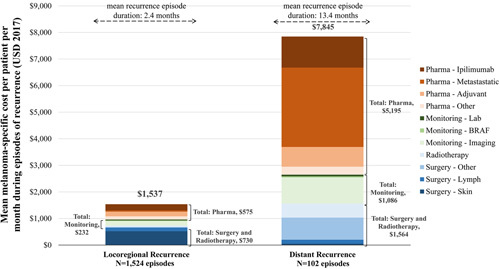
Melanoma specific costs during episodes of recurrence [melanoma specific costs included costs related to treatment (pharmacological, surgery, radiotherapy) and disease monitoring].
For distant recurrence, the mean duration of an episode was 13.4 months (Table 3). During these episodes, patients had on average 11.4 all-cause IP admissions, 63.7 all-cause IP days, 11.5 all-cause ED admissions, and 254.1 all-cause OP visits per 100 person-months. The mean total all-cause healthcare cost PPPM during distant recurrence episodes was $12 940, of which 89.3% ($11 549/$12 940) was attributed to medical costs (Table 4). In terms of melanoma-specific HRU and cost, monitoring (e.g. imaging) and pharmacological treatments were the most important components of melanoma-specific HRU/cost during episodes of distant recurrences [used by 95/102 (93.1%) and 75/102 (73.5%) of the patients, respectively; Supplementary Fig. (Supplemental digital content 1, http://links.lww.com/MR/A64)]; the mean melanoma-specific cost was $7845 PPPM during distant recurrence episodes, mostly attributable to pharmacotherapy [$5195/$7845 (66.2%); Fig. 1].
HRU and costs were numerically higher during episodes of recurrence, especially distant episodes, compared with the recurrence-free period between the index surgery and first recurrence (for patients with recurrence) or end of the study period (for patients without recurrence). For example, the total all-cause healthcare cost was $1076 PPPM in the recurrence-free study period (i.e. 12 times lower than the total all-cause healthcare costs during a distant episode), whereas the number of IP admissions was 0.8 per 100 person-months (14 times lower than the number of IP admissions during a distant episode; Table 4).
Healthcare resource utilization and costs in the year following first recurrence
Of 977 patients with locoregional recurrence(s) and 90 patients with distant recurrence with more than 3 months of continuous healthcare coverage after the beginning of the first recurrence, 950 and 87, respectively, were matched 1 : 1 to recurrence-free patients on the basis of the criteria described in the Statistical Analysis section. Most baseline covariates were balanced after matching (i.e. standardized differences < |20%|), with a few exceptions for the distant recurrence versus recurrence-free cohorts, where the number of OP visits, some melanoma sites, and diabetes presented a standardized difference more than |20%|, and the IP admission costs and some comorbidities showed a standardized difference more than |10%| (Table 1).
In the year after recurrence, HRU and costs were significantly higher in the locoregional recurrence cohort compared with the recurrence-free matched cohort. Notably, the mean number of OP visits was 154 per 100 person-months during the year after recurrence versus 120 (difference=34 OP visits, 95% CI=23–44, P<0.001), whereas total all-cause healthcare costs PPPM during the year after recurrence were $1647 versus $805 (difference=$841, 95% CI=515–1226, P<0.001; Table 5).
Table 5.
All-cause healthcare resource utilization and healthcare costs in the year following recurrence
The same pattern was observed when HRU and costs in the year after recurrence were compared between the distant recurrence cohort and the recurrence-free matched cohort, but with larger differences. Notably, the mean number of OP visits was 297 per 100 person-months during the year postrecurrence versus 111 (difference=186 OP visits, 95% CI=145–225, P<0.001). The total all-cause healthcare costs were $15 937 PPPM during the year after recurrence versus $984 (difference=$14 953, 95% CI=11 314–19 573, P<0.001; Table 5).
Discussion
This study documents real-world patterns of treatment and recurrence, and quantifies the economic burden of recurrences among patients with nonmetastatic melanoma following surgery (mostly LNS). Nearly one-fifth of these patients developed recurrences, mostly locoregional. Few patients initiated adjuvant therapy after surgery, with one-third of these patients developing recurrences during follow-up. The most common adjuvant regimen was interferon α-2b. Melanoma-specific costs were markedly higher during episodes of distant recurrence than during episodes of locoregional recurrences. Patients with recurrences, particularly distant recurrences, incurred significantly higher HRU and costs compared with recurrence-free matched controls in the first year after recurrence.
Although this study’s recurrence rate of 19% among patients with nonmetastatic melanoma coincides with some previous community-based studies 8, other studies, including those based on clinical trial data, have reported higher recurrence rates 35,36. In the placebo arm of the recent COMBI-AD phase 3 trial, the recurrence rate was 57% among patients with BRAF-mutated melanoma 35. In Europe, a systematic literature review reported that the recurrence rate, on the basis of recurrence-free survival in clinical trials, was 56–72% for stage III patients 37–39. In Brazil, a similar value was 44% for patients in different stages in a retrospective registry study 40.
Several factors could explain the lower recurrence rates in the current study, notably in comparison with recent clinical trials. First, COMBI-AD only included patients with stage III melanoma, whereas this study may have included patients with earlier stages of melanoma since for instance the outcome of sentinel LN biopsy (LN metastasis) was unknown. Second, patients are monitored more closely in clinical trial settings than in real-world practice, perhaps accelerating the diagnosis of some recurrences in COMBI-AD. Finally, patients with recurrences who did not receive treatment and did not have a secondary malignancy diagnosis recorded were not captured by the claims-based algorithm used in this study.
The recurrence rate was higher among patients who initiated adjuvant 1L after index surgery than those who did not (33 vs. 18%). This could be partly explained by a potential indication bias if high-risk patients were more likely to receive adjuvant therapy than low-risk patients.
Only 3.4% of patients in this study received adjuvant therapy following melanoma surgery. The rare use of adjuvant therapy has also been reported by a previous USA population-based study using data from 1995, 1996, and 2001, in which more than 98% of patients with localized melanoma and more than two-thirds of patients with regional disease received surgery as their only treatment 13. Similar to the present study, interferon α was the most frequent adjuvant agent used for the treatment of regional melanoma 13. Low rates of adjuvant therapy in the interferon era that overlap considerably with the period in the present study are unsurprising, given interferon’s controversial efficacy and poor tolerability 41,42. Nevertheless, somewhat higher rates of adjuvant interferon use were reported by two USA claims-based studies. In one (years 2004–2008), 8.4% of patients were treated with interferon α-2b and 6.6% with other chemotherapies following melanoma surgery 15. In another (years 2007–2011), 9% of patients with melanoma surgery were treated with adjuvant interferon α-2b 43. Differences in study samples may explain the differences in adjuvant therapy rates between different claims-based studies. In the current study, patients had at least 12 months of insurance coverage before the first melanoma diagnosis, without any indicator of metastatic melanoma and/or other primary cancer in the 5 years before melanoma diagnosis. The two other claims studies had different requirements and may have selected patients already in a recurrence stage, perhaps explaining their higher rate of adjuvant use. Moreover, use of adjuvant therapy was recorded up to 120 days after surgery 43 versus 90 days in this study.
To our knowledge, the present study is the first to quantify HRU and costs during recurrence episodes and in the year after recurrence (allowing the estimation of savings associated with 1 year of recurrence-free survival) in patients with nonmetastatic melanoma undergoing melanoma surgery. The matched comparison between patients with and without recurrence suggested significantly higher HRU and costs associated with recurrence, particularly distant recurrence (with melanoma treatment the main contributor to costs in the first year after recurrence). Patients with distant recurrences had 186 more OP visits per 100 person-months in the year after recurrence compared with recurrence-free patients, whereas all-cause healthcare costs were higher by $14 953 PPPM in the year after recurrence. Previous studies in real-world USA populations have reported a range of $6773–62 859 PPPM for patients with metastatic melanoma 16,20,21,44. Outside of the USA, including in developing countries, the cost per patient with melanoma was reported to range between $9162 and $86 875 14,45–47.
The wide range may be explained by these studies’ data sources, methodological differences, and various foci (notably, inclusion of patients using only certain treatments). Nevertheless, the estimate of $12 940 PPPM during episodes of distant recurrence in the current study is within the range of past studies, especially when considering real-world USA population.
The infrequent use of adjuvant therapies and the relatively high rate of postsurgery recurrences observed in this study suggest an unmet treatment need in patients with nonmetastatic melanoma undergoing surgery. Furthermore, as melanoma recurrence is associated with increased HRU and costs, strategies to prevent or delay recurrences are highly desired. The results of this study suggest that preventing a single episode of distant recurrence would lead to savings of ∼$180 000 in the year after recurrence ($14 953 PPPM); similarly, preventing a single episode of locoregional recurrence would lead to saving of ∼$10 000 in the year after recurrence ($841 per month PPPM). In the USA and Europe, adjuvant therapy for advanced melanoma is recommended or there are new indications 10–12, but the optimal treatment strategy for melanoma patients after melanoma surgery has remained elusive because of the difficult trade-off between long-term recurrence prevention and short-term toxicities with interferon and ipilimumab. However, the treatment landscape for melanoma in the adjuvant setting will soon change as results from phase 3 clinical trials of new-generation targeted agents and immune checkpoint inhibitors will offer melanoma patients additional adjuvant treatment options with demonstrated efficacy 35,48. In the COMBI-AD trial, adjuvant use of combination dabrafenib and trametenib in patients with stage III BRAF-mutated melanoma was associated with a lower risk of recurrence (37 vs. 57% in the placebo group) 35. In the CheckMate 238, a higher rate of recurrence free-survival was observed among patients treated with adjuvant nivolumab (70.5 vs. 60.8% in the placebo group) 48. Whether these new treatment options lead to consensus on the use of adjuvant therapies in patients with stage III melanoma remains to be determined.
This study was subject to common limitations of studies on the basis of healthcare claims data, including occasional coding errors or inaccurate/missing data on prescriptions, procedures, or diagnoses. Because information on recurrence is unavailable in claims databases, this study relied on an algorithm to infer recurrence and duration of recurrence episodes. Although previous studies have reported that claims-based algorithms can be used reliably to identify lines of treatment and cancer recurrences 26–28,49–51, potential misclassifications cannot be excluded. In particular, patients with recurrences who did not receive treatment or a diagnosis code for secondary malignancy were not detected. Also, despite the requirement of 12 months of insurance coverage before the first melanoma diagnosis, for some patients, the first melanoma diagnosis captured in the current study could have corresponded to a recurrence rather than the first melanoma diagnosis in the patient’s lifetime. However, the mean age of patients at first melanoma diagnosis (53.8 years) was comparable to patient characteristics reported at diagnosis in retrospective studies (i.e. 48.6–53.4 years old) 36,52, suggesting that this limitation may have limited impact. Finally, the study was a retrospective comparative analysis and may be subject to residual confounding because of unmeasured confounders.
Notwithstanding these limitations, the current study shows that the use of adjuvant therapy among patients with nonmetastatic melanoma undergoing surgery was low during the period 1 January 2008 to 31 July 2017. The considerable economic burden associated with postsurgery recurrences, along with poor prognosis, underscores the need to prevent recurrences in these patients. New generations of adjuvant therapies such as immune-checkpoint inhibitors and targeted agents have the potential to change the treatment landscape at a global level. Future studies are needed to determine whether use of novel adjuvant therapies for nonmetastatic melanoma will reduce the risk of recurrences and whether this in turn translates to reduced HRU and costs.
Supplementary Material
Supplemental Digital Content is available for this article. Direct URL citations appear in the printed text and are provided in the HTML and PDF versions of this article on the journal's website, www.melanomaresearch.com.
Acknowledgements
Assistance in preparing figures and tables was provided by Ahmed Kazi and Allie Briggs, whereas medical writing assistance was provided by Sara Kaffashian and Samuel Rochette. All were employees of Analysis Group Inc.
Conflicts of interest
This study was funded by Novartis Pharmaceuticals Corporation. The study sponsor was involved in all stages of the study research, including study design; collection, analysis, and interpretation of data; writing of the report; and the decision to submit the report for publication.
Analysis Group Inc. received consulting fees from Novartis Pharmaceuticals Corporation for the conduct of this study.
Ahmad Tarhini: consultant role with Novartis Pharmaceuticals Corporation. Sameer R. Ghate, Briana Ndife, and Antonio Nakasato are employees of Novartis Pharmaceuticals Corporation and may own stock/stock options. Raluca Ionescu-Ittu, Ameur M. Manceur, Philippe Jacques, François Laliberté, Rebecca Burne, and Mei Sheng Duh are employees of Analysis Group Inc., which has received consultancy fees from Novartis Pharmaceuticals Corporation
References
- 1.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2018. CA Cancer J Clin 2018; 68:7–30. [DOI] [PubMed] [Google Scholar]
- 2.Karimkhani C, Green AC, Nijsten T, Weinstock MA, Dellavalle RP, Naghavi M, et al. The global burden of melanoma: results from the Global Burden of Disease Study 2015. Br J Dermatol 2017; 177:134–140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Pollack LA, Li J, Berkowitz Z, Weir HK, Wu XC, Ajani UA, et al. Melanoma survival in the United States, 1992 to 2005. J Am Acad Dermatol 2011; 65 (Suppl 1):S78–S86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wong SL, Faries MB, Kennedy EB, Agarwala SS, Akhurst TJ, Ariyan C, et al. Sentinel lymph node biopsy and management of regional lymph nodes in melanoma: American Society of Clinical Oncology and Society of Surgical Oncology Clinical Practice Guideline Update. J Clin Oncol 2018; 36:399–413. [DOI] [PubMed] [Google Scholar]
- 5.Benvenuto-Andrade C, Oseitutu A, Agero AL, Marghoob AA. Cutaneous melanoma: surveillance of patients for recurrence and new primary melanomas. Dermatol Ther 2005; 18:423–435. [DOI] [PubMed] [Google Scholar]
- 6.Leiter U, Meier F, Schittek B, Garbe C. The natural course of cutaneous melanoma. J Surg Oncol 2004; 86:172–178. [DOI] [PubMed] [Google Scholar]
- 7.Romano E, Scordo M, Dusza SW, Coit DG, Chapman PB. Site and timing of first relapse in stage III melanoma patients: implications for follow-up guidelines. J Clin Oncol 2010; 28:3042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dalal KM, Patel A, Brady MS, Jaques DP, Coit DG. Patterns of first-recurrence and post-recurrence survival in patients with primary cutaneous melanoma after sentinel lymph node biopsy. Ann Surg Oncol 2007; 14:1934–1942. [DOI] [PubMed] [Google Scholar]
- 9.Tarhini AA. The future of systemic therapy of melanoma: combinations, predictive biomarkers. Oncology 2015; 29:94–94. [PubMed] [Google Scholar]
- 10.National Comprehensive Cancer Network. NCCN Clinical Practice Guidelines in Oncology, Melanoma 2017. Version I. 2018. 11 October 2017.
- 11.European Society for Medical Oncology. EMA Recommends Extension of Indications for Nivolumab. 2018. Available at: https://www.esmo.org/Oncology-News/EMA-Recommends-Extension-of-Indications-for-Nivolumab2. [Accessed 20 July 2018].
- 12.European Society for Medical Oncology. EMA Recommends Extensions of Indications for Dabrafenib and Trametinib. 2017. Available at: https://www.esmo.org/Oncology-News/EMA-Recommends-Extensions-of-Indications-for-Dabrafenib-and-Trametinib. [Accessed 20 July 2018].
- 13.Harlan LC, Lynch CF, Ballard-Barbash R, Zeruto C. Trends in the treatment and survival for local and regional cutaneous melanoma in a US population-based study. Melanoma Res 2011; 21:547–554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Guy GP, Jr, Ekwueme DU, Tangka FK, Richardson LC. Melanoma treatment costs: a systematic review of the literature, 1990-2011. Am J Prev Med 2012; 43:537–545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hackshaw MD, Krishna A, Mauro DJ. Retrospective US database analysis of drug utilization patterns, health care resource use, and costs associated with adjuvant interferon alfa-2b therapy for treatment of malignant melanoma following surgery. Clinicoecon Outcomes Res 2012; 4:169–176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chang CL, Schabert VF, Munakata J, Donga P, Abhyankar S, Reyes CM, et al. Comparative healthcare costs in patients with metastatic melanoma in the USA. Melanoma Res 2015; 25:312–320. [DOI] [PubMed] [Google Scholar]
- 17.Harries M, Mohr P, Grange F, Ehness R, Benjamin L, Siakpere O, et al. Treatment patterns and outcomes of Stage IIIB/IIIC melanoma in France, Germany and the UK: a retrospective and prospective observational study (MELABIS). Int J Clin Pract 2017; 71:12946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Johnston K, Levy AR, Lorigan P, Maio M, Lebbe C, Middleton M, et al. Economic impact of healthcare resource utilisation patterns among patients diagnosed with advanced melanoma in the United Kingdom, Italy, and France: results from a retrospective, longitudinal survey (MELODY study). Eur J Cancer 2012; 48:2175–2182. [DOI] [PubMed] [Google Scholar]
- 19.McKendrick J, Gijsen M, Quinn C, Barber B, Zhao Z. Estimating healthcare resource use associated with the treatment of metastatic melanoma in eight countries. J Med Econ 2016; 19:587–595. [DOI] [PubMed] [Google Scholar]
- 20.Reyes C, DaCosta Byfield S, Linke R, Satram-Hoang S, Teitelbaum AH. The burden of metastatic melanoma: treatment patterns, healthcare use (utilization), and costs. Melanoma Res 2013; 23:159–166. [DOI] [PubMed] [Google Scholar]
- 21.Toy EL, Vekeman F, Lewis MC, Oglesby AK, Duh MS. Costs, resource utilization, and treatment patterns for patients with metastatic melanoma in a commercially insured setting. Curr Med Res Opin 2015; 31:1561–1572. [DOI] [PubMed] [Google Scholar]
- 22.Warren JL, Yabroff KR. Challenges and opportunities in measuring cancer recurrence in the United States. J Natl Cancer Inst 2015; 107:djv134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hansen LG, Changj S. Health Research Data for the Real World: The MarketScan Databases- White Paper. 2011. Available at: http://truvenhealth.com/portals/0/assets/PH_11238_0612_TEMP_MarketScan_WP_FINAL.pdf. [Accessed 20 July 2018].
- 24.Quan H, Li B, Couris CM, Fushimi K, Graham P, Hider P, et al. Updating and validating the Charlson comorbidity index and score for risk adjustment in hospital discharge abstracts using data from 6 countries. Am J Epidemiol 2011; 173:676–682. [DOI] [PubMed] [Google Scholar]
- 25.Sarfati D, Gurney J, Stanley J, Salmond C, Crampton P, Dennett E, et al. Cancer-specific administrative data-based comorbidity indices provided valid alternative to Charlson and National Cancer Institute Indices. J Clin Epidemiol 2014; 67:586–595. [DOI] [PubMed] [Google Scholar]
- 26.Hurvitz S, Guerin A, Brammer M, Guardino E, Zhou ZY, Latremouille Viau D, et al. Investigation of adverse-event-related costs for patients with metastatic breast cancer in a real-world setting. Oncologist 2014; 19:901–908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ramsey S, Henk H, Smith G, Sollano J, Chen C. First-, second- and third-line lung cancer treatment patterns and associated costs in a US healthcare claims database. Lung Cancer Manag 2015; 1:131–143. [Google Scholar]
- 28.Seal BS, Sullivan SD, Ramsey S, Shermock KM, Ren J, Kreilick C, et al. Medical costs associated with use of systemic therapy in adults with colorectal cancer. J Manag Care Pharm 2013; 19:461–467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Austin PC. An introduction to propensity score methods for reducing the effects of confounding in observational studies. Multivariate Behav Res 2011; 46:399–424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cohen J. Statistical power analysis for the behavioral sciences. Toronto: Academic; 1977. [Google Scholar]
- 31.Yang D, Dalton JE. A unified approach to measuring the effect size between two groups using SAS. SAS Global Forum 2012; 22–25 April 2012; Orlando, FL, USA.
- 32.Austin PC. Using the standardized difference to compare the prevalence of a binary variable between two groups in observational research. Commun Stat Simul Comput 2009; 38:1228–1234. [Google Scholar]
- 33.Harder VS, Stuart EA, Anthony JC. Propensity score techniques and the assessment of measured covariate balance to test causal associations in psychological research. Psychol Methods 2010; 15:234–249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Stuart EA, Lee BK, Leacy FP. Prognostic score-based balance measures can be a useful diagnostic for propensity score methods in comparative effectiveness research. J Clin Epidemiol 2013; 66 (Suppl):S84–S90.e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Long GV, Hauschild A, Santinami M, Atkinson V, Mandala M, Chiarion-Sileni V, et al. Adjuvant dabrafenib plus trametinib in stage III BRAF-mutated melanoma. N Engl J Med 2017; 377:1813–1823. [DOI] [PubMed] [Google Scholar]
- 36.Salama AK, de Rosa N, Scheri RP, Pruitt SK, Herndon JE, 2nd, Marcello J, et al. Hazard-rate analysis and patterns of recurrence in early stage melanoma: moving towards a rationally designed surveillance strategy. PLoS One 2013; 8:e57665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Svedman FC, Pillas D, Taylor A, Kaur M, Linder R, Hansson J. Stage-specific survival and recurrence in patients with cutaneous malignant melanoma in Europe – a systematic review of the literature. Clin Epidemiol 2016; 8:109–122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Eggermont AM, Suciu S, Testori A, Santinami M, Kruit WH, Marsden J, et al. Long-term results of the randomized phase III trial EORTC 18991 of adjuvant therapy with pegylated interferon alfa-2b versus observation in resected stage III melanoma. J Clin Oncol 2012; 30:3810–3818. [DOI] [PubMed] [Google Scholar]
- 39.Garbe C, Radny P, Linse R, Dummer R, Gutzmer R, Ulrich J, et al. Adjuvant low-dose interferon α2a with or without dacarbazine compared with surgery alone: a prospective-randomized phase III DeCOG trial in melanoma patients with regional lymph node metastasis. Ann Oncol 2008; 19:1195–1201. [DOI] [PubMed] [Google Scholar]
- 40.Vazquez Vde L, Silva TB, Vieira Mde A, de Oliveira AT, Lisboa MV, de Andrade DA, et al. Melanoma characteristics in Brazil: demographics, treatment, and survival analysis. BMC Res Notes 2015; 8:4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lens M. Cutaneous melanoma: interferon alpha adjuvant therapy for patients at high risk for recurrent disease. Dermatol Ther 2006; 19:9–18. [DOI] [PubMed] [Google Scholar]
- 42.Petrella T, Verma S, Spithoff K, Quirt I, McCready D. Adjuvant interferon therapy for patients at high risk for recurrent melanoma: an updated systematic review and practice guideline. Clin Oncol (R Coll Radiol) 2012; 24:413–423. [DOI] [PubMed] [Google Scholar]
- 43.Zhang Y, Le TK, Shaw JW, Kotapati S. Retrospective analysis of drug utilization, health care resource use, and costs associated with IFN therapy for adjuvant treatment of malignant melanoma. Clinicoecon Outcomes Res 2015; 7:397–407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Vekeman F, Cloutier M, Yermakov S, Amonkar MM, Arondekar B, Duh MS. Economic burden of brain metastases among patients with metastatic melanoma in a USA managed care population. Melanoma Res 2014; 24:602–610. [DOI] [PubMed] [Google Scholar]
- 45.Souza RJ, Mattedi AP, Corrêa MP, Rezende ML, Ferreira AC. Estimativa do custo do tratamento do câncer de pele tipo não-melanoma no Estado de São Paulo – Brasil [Estimation of the cost of non-melanoma skin cancer treatment in Sao Paulo – Brazil]. An Bras Dermatol 2011; 86:657–662. [DOI] [PubMed] [Google Scholar]
- 46.Almazán-Fernández FM, Serrano-Ortega S, Moreno-Villalonga JJ. Descriptive study of the costs of diagnosis and treatment of cutaneous melanoma. Actas Dermosifiliogr 2009; 100:785–791. [PubMed] [Google Scholar]
- 47.Leiter U, Marghoob AA, Lasithiotakis K, Eigentler TK, Meier F, Meisner C, et al. Costs of the detection of metastases and follow-up examinations in cutaneous melanoma. Melanoma Res 2009; 19:50–57. [DOI] [PubMed] [Google Scholar]
- 48.Weber J, Mandala M, Del Vecchio M, Gogas HJ, Arance AM, Cowey CL, et al. Adjuvant nivolumab versus ipilimumab in resected stage III or IV melanoma. N Engl J Med 2017; 377:1824–1835. [DOI] [PubMed] [Google Scholar]
- 49.Chubak J, Yu O, Pocobelli G, Lamerato L, Webster J, Prout MN, et al. Administrative data algorithms to identify second breast cancer events following early-stage invasive breast cancer. J Natl Cancer Inst 2012; 104:931–940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Deshpande AD, Schootman M, Mayer A. Development of a claims-based algorithm to identify colorectal cancer recurrence. Ann Epidemiol 2015; 25:297–300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Livaudais-Toman J, Egorova N, Franco R, Prasad-Hayes M, Howell EA, Wisnivesky J, et al. A validation study of administrative claims data to measure ovarian cancer recurrence and secondary debulking surgery. EGEMS 2016; 4:1208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Dong XD, Tyler D, Johnson JL, DeMatos P, Seigler HF. Analysis of prognosis and disease progression after local recurrence of melanoma. Cancer 2000; 88:1063–1071. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Digital Content is available for this article. Direct URL citations appear in the printed text and are provided in the HTML and PDF versions of this article on the journal's website, www.melanomaresearch.com.