Abstract
Objectives:
To assess the antimicrobial effectiveness of multipurpose solutions in regard to the disinfection of silicone hydrogel contact lenses (CL) using a study of clinical bacterial isolates from ocular material.
Methods:
Three multipurpose solutions (solution A: polyhexamethylene biguanide 0.00025 g/100 mL; solution B: polyquaternary-1 0.001% and myristamidopropyl dimethylamine 0.0006%; and solution C: polyaminopropyl biguanide 0.00013% and polyquaternary 0.0001%) were used as a 3-phase disinfection on silicone hydrogel CL contaminated with bacteria from clinical isolates that were divided into five groups (group 1: Pseudomonas aeruginosa; group 2: Staphylococcus aureus; group 3: Staphylococcus epidermidis; group 4: Streptococcus spp; and group 5: enterobacteria).
Results:
No differences were observed between the 24- and 48-hr measurements in any of the samples, and the positivity of microorganisms in T0 was 100% for all solutions; it was 0% in T3. Therefore, only steps T1 (rubbing followed by rinsing) and T2 (rubbing followed by rinsing and immersion of CL into solution) were considered for analysis at the 24-hr measurement time. Throughout the phases, a decrease in the number of bacteria was observed, culminating in the elimination (no recovery) of all microorganisms in the three solutions.
Conclusions:
At the end of the proposed process, the tested solutions were effective.
Key Words: Contact lenses, Hydrogel, Silicone, Disinfectant/Pharmacology, Contact lens solution
Silicone hydrogel contact lenses (CL) have rapidly become one of the main CL options worldwide and are among the CL most prescribed for daily usage.1 Approximately 140 million patients are users of these CL.1,2 The maintenance of these CL ensures their functionality and continuity of use. Nowadays, the primary reason for discontinuation is associated with discomfort, when a great number of contact lens wearers experience this situation.3 Improper conservation of the CL as a result of noncompliant users, can lead to ocular complications that, together with a lack of motivation, are another reasons that patients discontinue their use.4
Incorrect use, maladaptation and contamination of CL, adverse environmental conditions, and previous eye disease could be significant sources of large numbers of corneal infections caused by the proliferation of microorganisms, such as bacteria, fungi, viruses, and parasites, as the use of CL modifies the defensive mechanism of the eye.5 Bacteria have been demonstrated to be the most consistent agents of infectious keratitis; Staphylococcus aureus, Staphylococcus epidermidis, Streptococcus pneumoniae, Strep. spp, Pseudomonas aeruginosa, and Enterobacteriaceae are the most frequently identified causative agents, whereas Neisseria spp, Moraxella spp, Mycobacterium spp, Nocardia spp, and Corynebacterium spp are rare.6–8
Pseudomonas aeruginosa is the bacterium most frequently identified as causing infectious keratitis resulting from to the use of CL. The use of CL increases the risk of infection because it offers a higher bacterial adherence to the ocular surface cells, promoting direct cell invasion through breakage of the tight junctions.2 One way to fight these microorganisms is nonoxidative chemical disinfection through the immersion of CL in solutions containing antimicrobials, called multipurpose solutions when designed for cleansing, rinsing, disinfection, and conditioning.9
Some of the active ingredients in multipurpose solutions are as follows: polyquaternary-1 (a cationic polymer that does not inhibit the growth of human corneal cells but is protective against bacteria); myristamidopropyl dimethylamine (an antifungal agent); polyaminopropyl biguanide (a broad spectrum antibacterial against Gram-positive and Gram-negative bacteria, fungi, spores, and P. aeruginosa); and polyhexamethylene biguanide (responsible for permeability changes in the plasma membrane because of the functional modification of related enzymes).10–12 These solutions can also contain EDTA (edetate disodium), which is not a bacterial agent, but it enhances the action of cationic antimicrobials. This probably occurs by reducing the magnesium and calcium levels in the microbial cell membrane and increasing the cell permeability.13–15
This study aims to assess the antimicrobial effectiveness of multipurpose solutions used to disinfect silicone hydrogel CL by studying clinical bacterial isolates from ocular material.
MATERIALS AND METHODS
This study was performed in the Laboratory of Microbiology, Discipline of Microbiology, Department of Pathological Sciences, Faculty of Medical Sciences of Santa Casa de Misericórdia de São Paulo (F.C.M.S.C.S.P.) from April to May 2014. This study was approved by the Institute of Scientific Commission and Research of the Department of Ophthalmology.
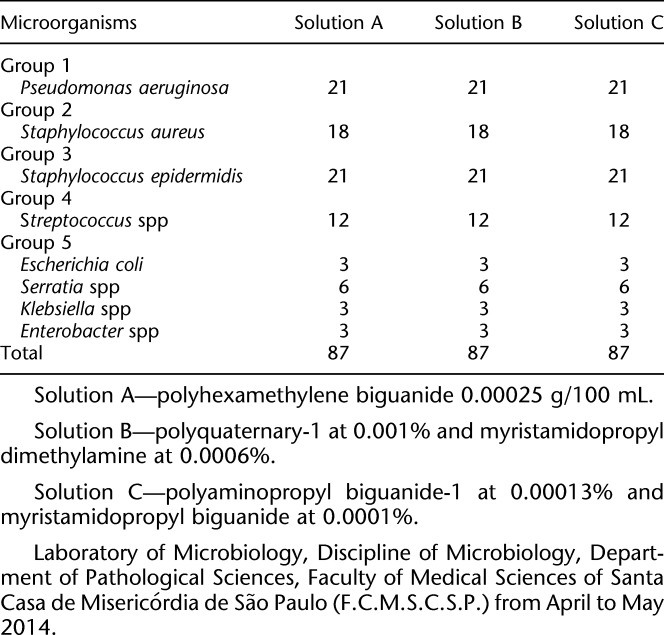
This study investigated 261 silicone hydrogel CL (67% Lotrafilcon B, 33% water, and 1% copolymer 845—Air Optix Aqua, Ciba Vision [Alcon Laboratories, Inc.Fort Worth, TX]), which were contaminated with 29 bacterial strains grown from ophthalmologic clinical isolates (secretion, cornea scrapings, and conjunctival scrapings) and previously kept at the Laboratory of Microbiology of the Discipline of Microbiology, Department of Pathological Sciences of the Faculty of Medical Sciences of Santa Casa de Misericórdia de São Paulo. The clinical isolates were divided into the following five groups: group 1: seven strains of P. aeruginosa; group 2: six strains of S. aureus; group 3: seven strains of S. epidermidis; group 4: four strains of Strep. spp; and group 5: five strains of enterobacteria (two Serratia spp, one Enterobacter spp, one Klebsiella spp, and one Escherichia coli).
To obtain these bacteria, strains were selected and thawed and plated on blood agar at 35°C±2°C. The inoculum was prepared with a 0.5-McFarland dilution (1.5 × 108 UFC/mL), followed by a second dilution of 1:100, resulting in a final inoculum of 1.5 × 106 UFC/mL.16 The CL were submerged in a culture medium for 2 hours with the bacteria strains, which were studied in triplicate, and the experiment was repeated three times with each bacterium to guarantee no deviation from the technique used and reliable results.
Three multipurpose solutions were used and identified as solution A: polyhexamethylene biguanide 0.00025 g/100 mL; solution B: polyquaternary-1 0.001% and myristamidopropyl dimethylamine 0.0006%; solution C: polyaminopropyl biguanide 0.00013% and polyquaternary 0.0001%, and the in vitro antimicrobial effectiveness of these solutions was evaluated (Table 1).
TABLE 1.
Number of Contaminated Contact Lenses by Each Micro—Using A, B, and C Solutions
The contaminated CL were plated in the culture media using the imprint method,17,18 which places the CL anterior and posterior surfaces in contact with the agar surface. The first imprint, named T0, was used as a positive control for contamination; then, cleaning and rinsing of the CL with multipurpose solutions was performed, as indicated by the manufacturer (rub solution for 20 sec in A, B, and C and rinse each side of the CL for 10 sec in A and B and 5 sec in C). After this step, the second imprint, identified as T1, was accomplished. After T1 was performed, the CL were stored in proper cases containing the solutions for the minimum time indicated by the manufacturer: 6 hr for solutions A and B and 4 hr for solution C. Then, a third imprint, identified as T2, completed the process, as indicated by the manufacturers of the multipurpose solutions. After these steps, one more disinfection phase was accomplished, as described in T1 (rubbing and rinsing), and a new imprint, identified as T3, was performed. Aseptic technique was used throughout the study.
The culture media used were Blood Müeller–Hinton agar for CL cultures contaminated with Strep. spp and plates with a 150-mm diameter of Müeller–Hinton agar for the other microorganisms. The plates were incubated at 35°C±2°C for 48 hr and measured at 24 and 48 hr.
The presence or absence of bacterial growth was analyzed; the plates were measured by three examiners to minimize the possibility of α (individual variation) and β (culture media defect or contamination) errors.
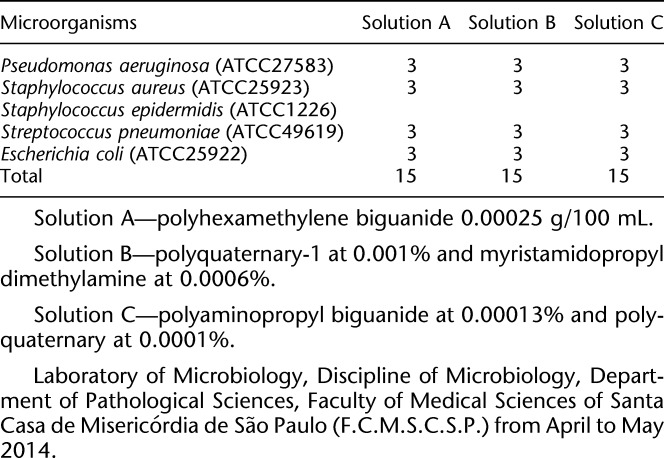
Aiming for reproducibility and validation, the following was a selection of strains with known microbiological profiles that met the “American Type Culture Collection” (ATCC) standards: P. aeruginosa (ATCC 27583), S. aureus (ATCC 25923), S. epidermidis (ATCC 1226), S. pneumoniae (ATCC 49619), and E. coli (ATCC 25922). This procedure involved 45 silicone hydrogel CL (67% Lotrafilcon B, 33% water, and 1% copolymer 845—Air Optix Aqua, Ciba Vision) and a process that was identical across all the clinical isolates (Table 2). The clinical bacterial isolates from eye infection and the ATCCs used for reproducibility were considered in the statistical analysis.
TABLE 2.
Distribution of the Number of Contaminated Contact Lenses to Each Microorganism From Known Epidemiological Profile (ATCC), Using A, B, and C Solutions
The microorganism positivity and negativity on the CL were observed throughout the proposed steps in the three solutions, and the presence of all microorganisms was analyzed in each of the five groups. Then, the performance of the solutions (A × B, A × C, and B × C) was compared when divided into groups and in total.19–21 The tests were performed based on a 5% significance level.
RESULTS
No differences were observed between the 24- and 48-hr measurements in any of the samples, and the positivity of microorganisms in T0 was 100% for all solutions; it was 0% in T3. Therefore, only steps T1 (rubbing followed by rinsing) and T2 (rubbing followed by rinsing and immersion of CL into solution) were considered for analysis at the 24-hr measurement time.
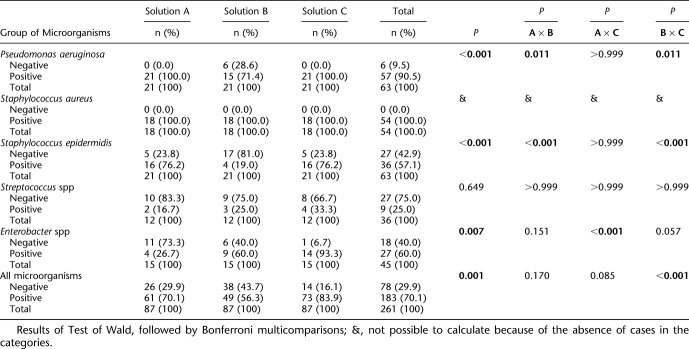
As shown in Table 3, in an analysis of T1 for the presence of P. aeruginosa, after standard cleansing application as indicated by the manufacturers, solution B presented with a statistically lower percentage of positivity than the other solutions (P=0.011 and P=0.011, in solutions A and C, respectively). Staphylococcus epidermidis positivity was statistically significant for all the solutions (P<0.001), and it was lower in solution B than in the other solutions (P<0.05). In addition, positivity for enterobacteria had a statistically significant difference among the solutions (P=0.007); positivity in solution A was lower than in solution C (P<0.001). In addition, when considering all microorganisms used in this study, there was a statistically significant difference among the solutions used (P<0.001), wherein positivity in solution B was lower than that in C (P<0.001). It can also be observed that the S. aureus positivity was 100% in all solutions after CL cleansing.
TABLE 3.
Analysis of the Results in T1 (First Step of Antissepsis)
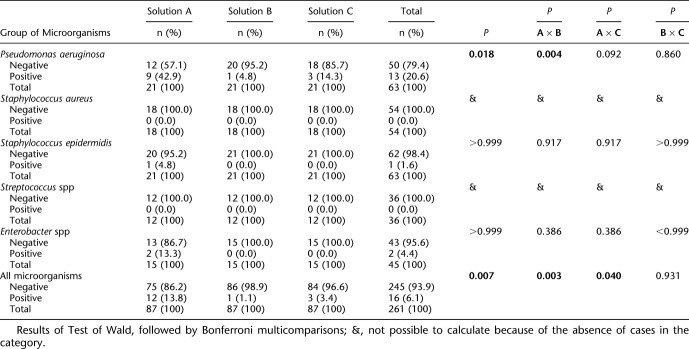
Table 4 shows that there was a statistically significant difference among the solutions used after T2 with rubbing followed by rinsing and immersion of the CL in solution; P=0.018 and P=0.007 for P. aeruginosa positivity and for the presence of all assessed microorganisms, respectively. Pseudomonas aeruginosa was statistically less present in solution B than in solution A (P=0.004) and, as for all microorganisms, the positivity was significantly higher in solution A than in solutions B and C (P=0.003 and P=0.040, respectively). After this step, the S. aureus and S. spp microorganisms were eliminated from all solutions.
TABLE 4.
Analysis of Results in T2 (Second Step of Antissepsis)
DISCUSSION
Analysis of the results shows that, after the first disinfection (T1), there was a statistically significant difference between the solutions, wherein the performance of B was better than that of C. This observation is relevant because a significant number of individuals fail to properly clean their CL as indicated on the packages of multipurpose solutions. Instead, some only partially clean their CL; therefore, effectiveness of the solution is necessary even when incomplete cleaning procedures occur. According to a study, 54.2% of the people who were interviewed considered themselves to be bad users of CL because of their improper cleansing of the CL and cases.22
After the first rubbing and rinsing procedure followed by the immersion of the CL into the solutions during the time defined by the manufacturers (T2), P. aeruginosa was statistically more concentrated in solution A (polihexametil-biguanide 0.00025 g/100 mL) than in solution B (poliquaternary-1 a 0.001% e miristamidopropil dimetilamina a 0.0006%), whereas all S. aureus and Strep. spp were eliminated in the three solutions (A, B, and C). It is relevant to point the three manufacturers' guidelines regarding the disinfection process covered up to step T2 and only abrogated S. aureus and S. spp in the three solutions.
A higher adherence of P. aeruginosa to silicone hydrogel CL has already been demonstrated in comparison with S. aureus, as described in another study that a higher rate of positivity was found for P. aeruginosa compared with the other bacteria such as S. aureus, S. epidermidis, S. spp, and enterobacteria in T2.1 By contrast, another report presented a 100% in vitro growth reduction of ATCCs for P. aeruginosa, S. epidermidis, Klebsiella pneumoniae, S. aureus, and Candida albicans after the use of multipurpose solutions to cleanse silicone hydrogel CL as well as a possible reduction in the effectiveness of the disinfection process in clinical isolates.23
Some authors have shown that Gram-negative organisms presented better resistance to microbicides compared with Gram-positive bacteria (except mycobacteria), possibly because of the structure and lipopolysaccharide composition of the outer cell membrane.24 It is believed that P. aeruginosa is able to create structured aggregates known as biofilms characterized by a self-produced matrix, protecting them from antimicrobial attack and the host immune response.25 Another point is that P. aeruginosa is reported to be hydrophobic with a surface water contact angle of 132° compared with that of various S. aureus strains which ranges from 20° to 36° and it is known that organisms with greater surface hydrophobicity adhere in higher numbers than hydrophilic organisms. Both of these characteristics are possible reasons for the finding that P. aeruginosa has more adherence than S. aureus, and it is a predominant causative agent in contact lens–induced infectious keratitis.26,27
In addition, there was no statistically significant difference between B and C, whereas solution A performed less favorably than the other solutions in T2, when considering the presence of all investigated microorganisms. One possibility is the function of the polyquaternary in multipurpose solutions' efficacy. This cationic polymer can be bound covalently to the material surface or integrated in the original polymer used for the medical device killing fungi, Gram-positive, and Gram-negative bacteria.24 It enables ionic interactions between their cationic polymer chains and the negatively charged phospholipids of the bacterial cell surface and displacement of stabilizing calcium ions.28 A previous study with device-related infections in otolaryngology showed the use of microbicides polyquaternary polymer inhibited S. aureus biofilm formation under the conditions tested and related to P. aeruginosa, also showed a reduction, but less significant24 in agreement with our results.
In the last step of this study, sterilization was reached after the last proposed step (T3), which shows that the solutions used were effective although the manufacturers' instructions were incomplete. According with this result, a study involving the cleaning of lens cases contaminated with P. aeruginosa 122, Serratia marcescens ATCC 13880, and S. aureus ATCC 6538 demonstrated that mechanical rubbing and wiping of lens cases were the most effective cleaning regimen tested in reducing biofilm,29 suggesting how much mechanical cleaning assists the disinfection process.
It is important to consider that bacterial adherence is influenced by the properties of the CL surface, which modifies the composition of the tear-absorbed elements, such as proteins and multipurpose solutions.30 For example, a study showed that P. aeruginosa could help to provide colonization of contact lens surfaces in presence of dying neutrophils in vitro. They show that commercial contact lens care solutions fail to fully remove cellular debris from contact lens surfaces using recommended rub and rinse cleaning practices, and this residual debris may represent a new risk factor for microbial recolonization of CL.31 This article suggests an attempt at approximating the interaction among the microorganisms, CL, and multipurpose solutions in clinical isolates.
This study has some limitations. First, the sample size is small, and the five groups of clinical isolates are relatively heterogeneous, which is justified by the availability of the microbiology files (e.g., the bacterial strain banking). In addition, the disinfection process for the three solutions varied because the methods were set by the manufacturers and they were slightly different.
In conclusion, the three solutions were effective in disinfecting the bacterial strains in clinical isolates but only after the last proposed step was completed that means after T3 (after a rub, rinse and soak followed by a rub and rinse before reinsertion).
Footnotes
The authors have no funding or conflicts of interest to disclose.
REFERENCES
- 1.Keir N, Jones L. Wettability and silicone hydrogel lenses: A review. Eye Contact Lens 2013;39:100–108. [DOI] [PubMed] [Google Scholar]
- 2.Cavanagh HD, Robertson DM, Petroll WM, et al. Castroviejo lecture 2009: 40 years in search of the perfect contact lens. Cornea 2010;29:1075–1085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Nichols JJ, Willcox MD, Bron AJ, et al. The TFOS International workshop on contact lens discomfort: Executive summary. Invest Ophthalmol Vis Sci 2013;54:TFOS7–TFOS13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lipener C, Ray CBM. Sistemas atuais de cuidados e manutenção de lentes de contato. Arq Bras Oftalmol 2008;71(6 Suppl):9–13. [DOI] [PubMed] [Google Scholar]
- 5.Robertson DM, Petroll WM, Jester JV, et al. The role of contact lens type, oxygen transmission, and care-related solutions in mediating epithelial homeostasis and pseudomonas binding to corneal cells: An overview. Eye Contact Lens 2007; 33:394–398; discussion 399–400. [DOI] [PubMed] [Google Scholar]
- 6.Souza LB, Hofling-Lima AL. Doenças infecciosas bacterianas. In: Höfling-Lima AL, Dantas MCN, Alves MR, et al. , eds. Doenças externas oculares e córneas. Rio de Janeiro, Brazil: Guanabara Koogan; 2011;137–146. [Série Oftalmologia Brasileira]. [Google Scholar]
- 7.Stamler JF. Complication of contact lens wear. In: Krachmer JH, Mannis MJ, Holland EJ, eds. Cornea: Fundamentals, Diagnosis and Management. 2nd ed. St. Louis, MO: Elsevier Mosby; 2005;1:1329–1331. [Google Scholar]
- 8.Vermeltfoort PB, Rustema-Abbing M, de Vries J, et al. Influence of day and night wear on surface properties of silicone hydrogel contact lenses and bacterial adhesion. Cornea 2006;25:516–523. [DOI] [PubMed] [Google Scholar]
- 9.Sobrinho Andrade. Manutenção e manuseio das lentes de contato. In: Netto AL, Coral-Ghanem C, Oliveira PC, eds. Lentes de contato. 2a ed Rio de Janeiro, Brazil, Guanabara Koogan, 2011, pp 339–351. [Google Scholar]
- 10.Codling CE, Maillard JY, Russell AD. Aspects of the antimicrobial mechanisms of action of a polyquaternium and an amidoamine. J Antimicrob Chemother 2003;51:1153–1158. [DOI] [PubMed] [Google Scholar]
- 11.McDonnell G, Russell AD. Antiseptics and disinfectants: Activity, action, and resistance. Clin Microbiol Rev 1999;12:147–179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lui ACF, Netto AL, Silva CB, et al. Antimicrobial efficacy assessment of multi-use solution to disinfect hydrophilic contact lens. Vitro Arq Bras Oftalmol 2009;72:626–630. [DOI] [PubMed] [Google Scholar]
- 13.Coral-Ghanem C, Stein HA, Freeman MI. Manutenção e manuseio das lentes de contato. In: Coral-Ghanem C, Stein HA, Freeman MI, eds. Lentes de contato do básico ao avançado. 2a ed Joinville, France, Soluções e Informática; 2005, pp 27–29. [Google Scholar]
- 14.Moreira SMB, Moreira H, Moreira LB. Limpeza e assepsia das lentes hidrofílicas. In: Moreira SMB, Moreira H, Moreira LB, eds. Lente de contato. 3a ed. Rio de Janeiro, Brazil: Cultura Médica; 2004;187–197. [Google Scholar]
- 15.Moreira SMB, Moreira H, Moreira LB. Limpeza e assepsia das lentes rígidas. In: Moreira SMB, Moreira H, Moreira LB, eds. Lente de contato. 3a ed Rio de Janeiro, Brazil: Cultura Médica, 2004, pp 119–122. [Google Scholar]
- 16.Rosenthal RA, Sutton SV, Schlech BA. Review of standard for evaluating the effectiveness of contact lens disinfectants. PDA J Pharm Sci Technol 2002;56:37–50. [PubMed] [Google Scholar]
- 17.Hacek DM, Trick WE, Collins SM, et al. Comparison of the Rodac imprint method to selective enrichment broth for recovery of vancomycin-resistant enterococci and drug-resistant Enterobacteriaceae from environmental surfaces. J Clin Microbiol 2000;38:4646–4648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Galvin S, Dolan A, Cahill O, et al. Microbial monitoring of the hospital environment: Why and how? J Hosp Infect 2012;82:143–151. [DOI] [PubMed] [Google Scholar]
- 19.Kirkwood BR, Sterne JAC. Essential Medical Statistics. 2nd ed. Malden, MA: Blackwell Science; 2003;501. [Google Scholar]
- 20.McCullagh P, Nelder JA. Generalized Linear Models. 2nd ed London, United Kingdom, Chapman and Hall, 1989;511. [Google Scholar]
- 21.Neter J, Kutner MH, Nachtsheim CJ, et al. Applied linear statistical models. 4th ed Boston, MA: WCB McGraw-Hill; 1996;1408. [Google Scholar]
- 22.Oliveira PR, Kara-José N, Alves MR, et al. Observância da orientação médica pelo usuário de lentes de contato. Arq Bras Oftalmol 2004;67:607–612. [Google Scholar]
- 23.Fávaro A, Netto AL, Lui ACF, et al. Avaliação da ação antimicrobiana de soluções multiuso para desinfecção de lentes de contato silicone-hidrogel, in vitro. São Paulo: Irmandade da Santa Casa de Misericórdia de São Paulo; 2011. Available on: http://oftalmologiausp.com.br/ePoster/PDF/i12.pdf. Accessed 2009. [Google Scholar]
- 24.Dirain CO, Silva RC, Antonelli PJ, et al. Prevention of biofilm formation by polyquaternary polymer. Int J Pediatr Otorhinolaryngol 2016;88:157–162. [DOI] [PubMed] [Google Scholar]
- 25.Maunders E, Welch M. Matrix exopolysaccharides; the sticky side of biofilm formation. FEMS Microbiol Lett 2017;1–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Dutta D, Willcox MD. A laboratory assessment of factors that affect bacterial adhesion to contact lenses. Biology 2013;2:1268–1281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Dutta D, Cole N, Willcox M. Factors influencing bacterial adhesion to contact lenses. Mol Vis 2012;18:14–21. [PMC free article] [PubMed] [Google Scholar]
- 28.Murata H, Koepsel RR, Matyjaszewski K, et al. Permanent, nonleachingantibacterial surface-2: How high density cationic surfaces kill bacterial cells. Biomaterials 2007;28:4870–4879. [DOI] [PubMed] [Google Scholar]
- 29.Wu YT, Zhu H, Willcox M, et al. Removal of biofilm from contact lens storage cases. Invest Ophthalmol Vis Sci 2010;51: 6329–6333. [DOI] [PubMed] [Google Scholar]
- 30.Souza MB, Alves MR, Medeiros FW. Yamane ÍS. Doenças do segmento anterior ocular associadas a lentes de contato. Arq Bras Oftalmol 2008;71:14–18. [DOI] [PubMed] [Google Scholar]
- 31.Hinojosa JA, Patel NB, Zhu M, et al. 2017. Antimicrobial efficacy of contact lens care solutions against neutrophil-enhanced bacterial biofilms. Translational Vis Sci Technology 2017;6:11. [DOI] [PMC free article] [PubMed] [Google Scholar]