A comprehensive literature search suggests that anti–vascular endothelial growth factor therapy is effective in treating eyes with pigment epithelial detachment due to neovascular age-related macular degeneration. This therapy should focus primarily on vision gains because there is no apparent correlation between anatomical and functional improvement in most eyes with pigment epithelial detachment and neovascular age-related macular degeneration.
Key words: age-related macular degeneration, anti–vascular endothelial growth factor, neovascularization, pigment epithelial detachment, retinal pigment epithelium, retinal pigment epithelial tear, visual acuity
Abstract
Purpose:
This review aimed to determine the optimal management of retinal pigment epithelial detachments (PEDs) in neovascular age-related macular degeneration (nAMD) based on review of available evidence in the literature.
Methods:
A comprehensive literature review evaluates previous retrospective and prospective studies that assessed the treatment of PEDs in nAMD.
Results:
Studies illustrated that anti–vascular endothelial growth factor (VEGF) therapy can be effective in eyes with PED secondary to nAMD. Similar visual outcomes are associated with different anti-VEGF treatments. Higher anti-VEGF doses may improve anatomical response, without correlation with vision improvement. Fibrovascular PEDs may be difficult to treat, but even these eyes can gain vision with anti-VEGF therapy. A retinal pigment epithelial tear may develop in 15% to 20% of eyes with PEDs after anti-VEGF therapy, especially in PEDs greater than 500 µm to 600 µm in height; however, vision may stabilize with continued therapy. Atrophy may complicate eyes with PED and nAMD after anti-VEGF therapy, especially in association with complete PED resolution.
Conclusion:
Available literature suggests that anti-VEGF therapy is safe and efficacious for PED and nAMD. Treatment should focus on vision gains rather than PED resolution because there is no apparent correlation between anatomical and functional improvement in most eyes with PED and nAMD.
Retinal pigment epithelial detachments (PEDs) are defined anatomically as a distinct separation of the retinal pigment epithelium (RPE) from the underlying Bruch membrane layer. Pigment epithelial detachments are associated with a number of retinal diseases, most commonly neovascular age-related macular degeneration (nAMD) and may be identified in 63% to 80% of eyes with nAMD.1,2 Currently, there are few prospective studies that demonstrate optimal therapy for PEDs associated with nAMD, and without treatment, significant loss of visual acuity is encountered in 40% to 50% of eyes over a mean of 9 to 10 months.3 In addition, some studies have noted secondary loss of visual acuity after anti–vascular endothelial growth factor (anti-VEGF) treatment in eyes with fibrovascular PED.4,5 We performed a comprehensive review of the literature to evaluate the treatment of PEDs in nAMD to determine the optimal management of this challenging disorder.
Search Methodology
A PubMed search was conducted with the terms “pigment epithelial detachment(s)” in the title. All searches were limited to the English language and to human studies. This review includes subanalyses of clinical trials and prospective and retrospective studies with efficacy results (anatomical and/or visual) with more than 10 eyes over the past 5 years. The search was conducted on September 16, 2016. Additional searches were completed to evaluate 1) RPE tears: “retinal pigment epithelial tear(s)” in the title and 2) atrophy in eyes with PED: “atrophy” and “pigment epithelial detachment(s)” in any field. During study selection, studies were excluded from the present analysis based on number of patients and endpoints. In total, six studies were identified that had too few patients to draw meaningful conclusions or whose endpoints were not efficacy-based.
Retrospective Studies of Patients With Age-related Macular Degeneration and Pigment Epithelial Detachment
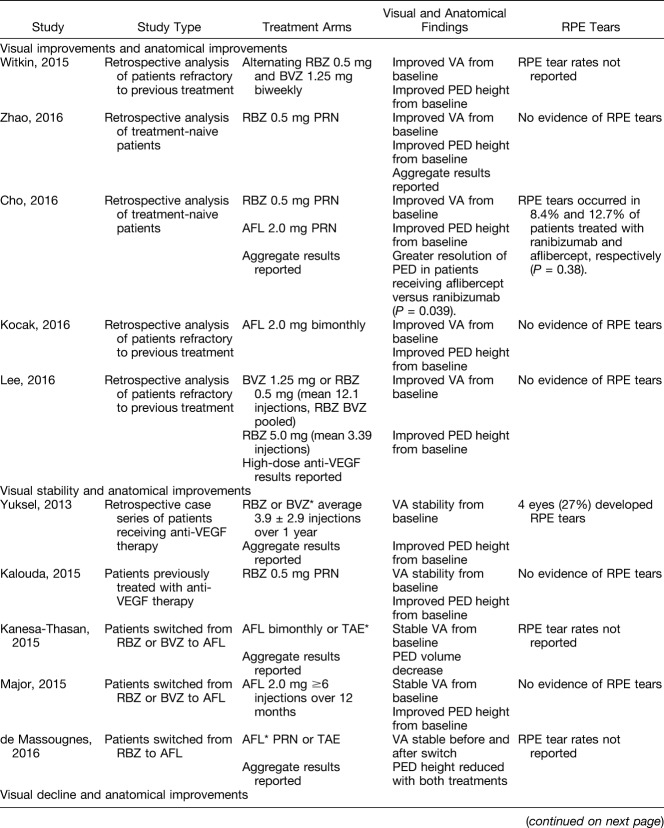
A number of retrospective studies, with differing results, have evaluated eyes with PED and nAMD. Our comprehensive search captured retrospective studies using the current anti-VEGF treatments for nAMD, including ranibizumab (Lucentis; Genentech, Inc, South San Francisco, CA), aflibercept (Eylea; Regeneron, Tarrytown, NY), and bevacizumab therapy, which is off-label (Avastin; off-label use; Genentech, Inc, South San Francisco, CA). Studies identified consisted of varying patient populations, sample sizes, and dosing regimens, although all used anti-VEGF therapy for the treatment of PED secondary to nAMD. These retrospective studies primarily investigated visual acuity as best-corrected visual acuity (BCVA) change from baseline, and anatomical outcomes, as measured by changes in PED structure. Interestingly, this analysis uncovered various visual and anatomical outcomes with no apparent trend (Table 1).
Table 1.
Summary of Retrospective Trials
Five of the retrospective studies analyzed found that treatment yielded both anatomical and visual improvements, whereas another five reported visual stability with anatomical improvement, and one study even found visual decline despite anatomical improvements (Table 1). In summary, all the studies described anatomical improvement with anti-VEGF therapy, whereas several also demonstrated visual improvement. Most of the studies that evaluated eyes with PED that were resistant to previous bevacizumab or ranibizumab therapy did not demonstrate an improvement in vision after a switch to aflibercept therapy, despite improved anatomical outcomes. The studies evaluating a switch did not control for a uniform dosing schedule of the initial agent before switching to a uniform new dosing schedule, which may have confounded the response findings. Furthermore, the definition of a recalcitrant PED was not consistent between studies. Most switching studies did not consider the onset of tachyphylaxis, which can result in suboptimal response to the initial agent. Only one small study (n = 15) noted a visual improvement after the switch to aflibercept.
Taken together, these studies suggest that there is no predictable or reproducible correlation between anatomical and functional improvement in eyes with PED and nAMD, and that no pattern of visual improvement can be attributed to switching anti-VEGF agents, despite anatomical improvements.
Additional retrospective analyses, included in Table 1, stratified patients based on PED type, including stratifications by serous, avascular, vascularized, and fibrovascular PEDs. In the preparation of this review, retrospective studies reporting outcomes by PED type in patients treated with aflibercept therapy were not found; however, a review by Mrejen et al6 summarizing the spectrum of PED types noted the prognostic importance of differentiation using multimodal imaging. Overall, the results of retrospective studies evaluating outcomes by PED type were variable. Some of the analyzed studies demonstrated greater PED reduction and visual acuity improvement in patients with an avascular versus vascularized PED after anti-VEGF therapy. Others, however, demonstrated no differences between PED types. From these analyses, there is no evidence that patients with PED of any type should be excluded from anti-VEGF treatment; however, the identification of associated neovascularization (NV) using multimodal imaging would certainly strengthen the indication for treatment, especially given the more unfavorable natural history of vascularized or fibrovascular PEDs. It is not possible to accurately predict anatomical and/or functional response based solely on features found in baseline PEDs. Further large-scale analyses are required to understand the outcomes in the different PED types. Interestingly, the functional responders reported to have vision improvement by Ersoy et al7 had larger PEDs at baseline and a smaller decrease in PED height after the three loading injections. These results further support the findings that improvement in PED height may not be predictably correlated with positive functional outcomes.
Prospective Studies
Six prospective analyses that assessed eyes with nAMD and PED with differing populations and evaluations are reviewed. One such trial studied 36 eyes with vascularized PED caused by AMD, treated with ranibizumab 0.5 mg vs. 2.0 mg either monthly or as needed (PRN) after 4 consecutive monthly loading doses.8 Four eyes treated with ranibizumab 2.0 mg and 1 eye treated with 0.5 mg ranibizumab developed an RPE tear (5/36, 14%) during the course of evaluation. Overall, the median logarithm of the minimum angle of resolution (logMAR) BCVA was 0.57 (approximate Snellen equivalent, 20/80) at baseline, improving to 0.46 (20/63) by Month 12 (P = 0.04). There was no significant difference in letters gained between treatment groups. At 12 months, 33.3% of eyes treated with ranibizumab 0.5 mg monthly (n = 6), 42.8% of eyes treated with 0.5 mg PRN (n = 7), 33.3% of eyes treated with 2.0 mg monthly (n = 12), and 18.2% of eyes treated with 2.0 mg PRN (n = 11) gained 15 or more letters from baseline. Eyes treated with 2.0 mg had a more substantial improvement in vision and decrease in subretinal fluid (SRF) revealed by time-domain optical coherence tomography (TD-OCT) than eyes treated with 0.5 mg, as measured at 4 and 8 weeks. There were, however, no significant differences between regimens in the 12-month outcomes analyzed, including BCVA, PED height, and PED or choroidal NV surface area.
A second prospective study evaluated 32 eyes with predominantly fibrovascular PED-type lesions secondary to nAMD that received intravitreal ranibizumab 0.5 mg injections monthly for 6 months.9 At 6 months, eyes were stratified into responder (n = 24) and nonresponder (n = 5) groups according to visual acuity and spectral domain optical coherence tomography (SD-OCT) findings. Nonresponders did not receive further treatment, but were reevaluated at 12 months. Responders continued with OCT-guided treatment to 12 months. Responders demonstrated mean BCVA improvements from baseline of 7.2 ± 9.8 letters at 6 months and 6.3 ± 8.6 letters at 12 months. Responders had significant improvements in PED height and central retinal thickness at both 6 and 12 months from baseline. Nonresponders demonstrated a mean decrease in BCVA from baseline of 8.2 ± 4.6 letters at 6 months and a mean decrease of 18.6 ± 10.11 letters at 12 months, and improvements in PED height were not statistically significant. Four eyes developed an RPE tear (4/32, 12.5%) during the course of the study, including 2 responders and 2 nonresponders. Three of these tears occurred after the second anti-VEGF injection, whereas the fourth was identified at a nonresponder's 12-month follow-up visit.
In a third prospective analysis, Inoue et al10 examined 57 eyes of Japanese patients with PED and nAMD, but did not correlate PED height with BCVA gains at 12 months in eyes that received 3 consecutive monthly injections of ranibizumab 0.5 mg followed by PRN therapy based on prespecified criteria. At baseline, 11 eyes had serous PED, 28 had fibrovascular PED, 7 had mixed PED, and 10 had hemorrhagic PED. The mean (SD) number of injections over the 12-month study period was 7.4 (3.2) in eyes with serous PED, 5.2 (2.4) in eyes with fibrovascular PED, 7.1 (3.1) in eyes with mixed PED, and 4.4 (2.2) in eyes with hemorrhagic PED (P = 0.063 among the 4 groups). At 12 months, eyes with serous or mixed PED experienced gains in logMAR BCVA of 0.14 ± 0.14 (approximately 7 ± 7 letters) and 0.07 ± 0.29 (approximately 3.5 ± 14.5 letters) from baseline, respectively, whereas eyes with fibrovascular PED or hemorrhagic PED lost vision from baseline (−0.02 ± 0.32 and −0.03 ± 0.26, respectively [approximately −1 ± 16 letters and −1.5 ± 13 letters, respectively]). Pigment epithelial detachment height, measured by SD-OCT, decreased in all eyes with serous or mixed PED, in 60.7% of eyes with fibrovascular PED, and in 90.0% of eyes with hemorrhagic PED. There was no significant difference in BCVA response between eyes in which PED height decreased and those in which PED height remained stable or increased. One eye in this study developed an RPE tear after the first injection.
A fourth study of eyes with PED and nAMD was completed by Panos et al.11 Sixty-one eyes received 3 consecutive monthly injections of ranibizumab 0.5 mg followed by PRN therapy on the basis of visual acuity, SD-OCT, and clinical examination criteria to 12 months. At baseline, 32 eyes had vascularized PED and 29 eyes had serous PED. Over the 12-month study, the mean (SD) number of injections was 6.1 (1.8) for eyes with vascularized PED and 7.1 (2.2) for eyes with serous PED. At baseline, the mean logMAR BCVA was 0.57 ± 0.28 (approximate Snellen equivalent, 20/80) and 0.60 ± 0.30 (20/80) for eyes with vascularized and serous PED, respectively, improving to 0.48 ± 0.32 (20/63) and 0.47 ± 0.28 (20/63) at Month 12. Pigment epithelial detachment height measured by SD-OCT decreased from baseline by 135 µm in eyes with vascularized PED and 180 µm in eyes with serous PED at Month 12. There was no significant difference regarding vision improvement or decrease in PED height between eyes with serous or vascularized PEDs. In addition, there was no correlation between PED height and change in BCVA in eyes with serous or vascularized PEDs. During the course of treatment, 1 eye (1/61, 2%) developed an RPE tear after the second injection.
In a fifth study, completed by Chen et al,12 36 eyes with PED and treatment-naive nAMD received 6 consecutive monthly aflibercept 2.0 mg injections, followed by 6 months of bimonthly injections. During the bimonthly dosing phase, 3 additional injections were allowed 1 month after the most recent injection on a PRN basis for intraretinal fluid (IRF) or SRF. At baseline, 28 eyes with PED illustrated Type 1 NV, originating in the choroid and located beneath the RPE, and eight eyes with PED displayed Type 3 NV, originating from the deep retinal capillary plexus within the retina, on the basis of SD-OCT grading. During the bimonthly dosing period, 79% of eyes with Type 1 NV and 20% of eyes with Type 3 NV required 1 or more PRN aflibercept injections. At Month 12, eyes with Type 1 and Type 3 NV showed a vision improvement from baseline of 4.5 ± 23 letters (P = 0.1665) and 14 ± 11 letters (P = 0.0072), respectively. At baseline, Type 1 and Type 3 eyes had a mean PED height ± SD of 333 ± 199 µm and 357 ± 240 µm, respectively. Although both lesion types illustrated statistically significant decreases from baseline in PED height and PED volume, measured using SD-OCT, after 12 months of anti-VEGF therapy, PEDs with Type 3 NV demonstrated significantly greater PED reduction. Five eyes (5/28, 18%) with Type 1 NV and no eyes with Type 3 NV developed an RPE tear during the course of this study. The eyes with Type 1 NV that developed an RPE tear had a significantly greater maximal PED height and PED volume at baseline than eyes with Type 1 NV that did not develop RPE tears.
Finally, a post hoc analysis of a prospective, open-label clinical trial of eyes with treatment-resistant nAMD reported by Broadhead et al13 analyzed eyes treated with aflibercept 2.0 mg for 3 initial monthly loading doses followed by treatment every 8 weeks. Forty-three eyes had treatment-resistant PEDs for at least 6 months, despite receiving at least four ranibizumab or bevacizumab injections during that time period. At Week 48, 4 (9.3%) eyes demonstrated PED resolution on the basis of SD-OCT, 18 (42%) eyes had PED improvement (>50 µm reduction in PED height), 24 (56%) had stable PEDs, and 1 (2%) showed PED worsening (>50 µm increase). Changes in PED height correlated with changes in central macular thickness (R2 = 0.36; P < 0.001), but not with visual acuity. At 48 weeks, the change in BCVA from baseline was +4.6 letters (P < 0.001).
Summary: Prospective Studies of Patients with Neovascular Age-related Macular Degeneration and Pigment Epithelial Detachment
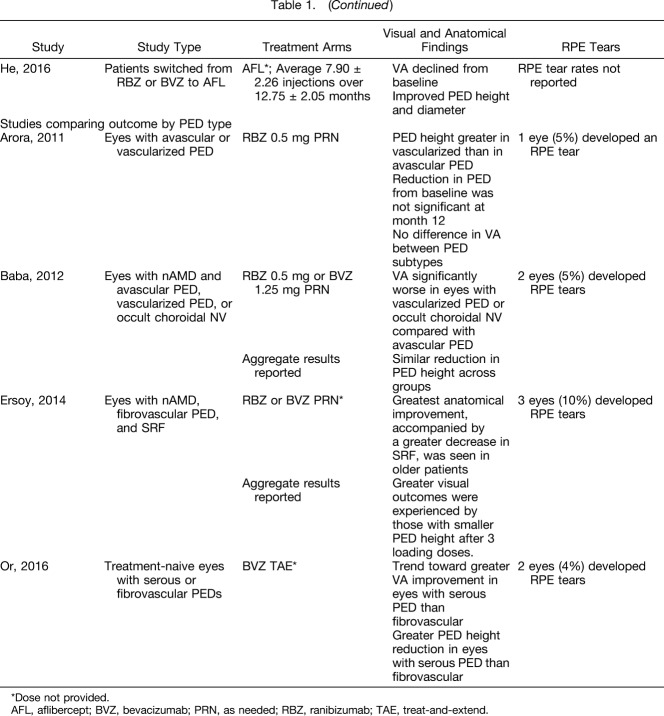
Collectively, these six prospective studies provide important information regarding the optimal management of eyes with PED and nAMD. Vision outcomes from these studies are summarized in Figure 1. Similar to the retrospectives studies discussed earlier, there was no correlation between reduction in PED height and improvements in vision over the course of these studies. These prospective studies suggest that there may not be a correlation between anatomical and functional response in eyes with PED, which was similarly suggested by the results of the retrospective studies. Interestingly, there may be a difference in response to anti-VEGF treatment on the basis of NV subtype. Specifically, certain studies indicate that eyes with Type 3 NV and PED may demonstrate a better response to anti-VEGF therapy and indicate that evaluating baseline NV subtype closely with angiography, and especially SD-OCT, may help to tailor optimal therapy and improve the prediction of anatomical and visual response or lack thereof. In addition, there may be a difference in response by PED subtype. A few studies describe better visual and anatomical outcomes in eyes with serous PEDs.10,14 Although similar to the retrospective studies, the data are conflicting between the two small, prospective studies evaluating outcomes based on PED subtype. These types of studies may have limited comparability owing to the definitions of PED subtypes, dosing frequency, retreatment criteria, and imaging modalities used. Finally, there was evidence that a 2.0 mg dose of ranibizumab produced a more substantial vision improvement at 1 to 2 months versus ranibizumab 0.5 mg, but there was no significant difference at 12 months between these dose groups.
Fig. 1.
Vision outcomes across trials of eyes with PEDs.9,12,13,15,16 AFL, aflibercept; ETDRS, Early Treatment Diabetic Retinopathy Study; LD, loading dose; PRN, as needed; q4w, every 4 weeks; q8w, every 8 weeks; 2q8, every 8 weeks; RBZ, ranibizumab; TR, treatment-resistant; V, vascular.
Some eyes with fibrovascular PED may be considered “nonresponders”; however, it is not clear whether these eyes would have lost less vision if they had continued with more frequent anti-VEGF therapy. It is important to consider how response is measured because some eyes may not respond with a significant anatomical reduction of PED height, but may still sustain visual gains or achieve visual stability with anti-VEGF therapy.
Post Hoc Analyses of Randomized, Clinical Trials
Three post hoc analyses of large, randomized clinical trials evaluated the efficacy of anti-VEGF in patients with PED. One such post hoc analysis by Sarraf et al15 examined the HARBOR trial wherein eyes with PED and nAMD were treated with ranibizumab 0.5 mg or 2.0 mg, either monthly or PRN, after 3 consecutive monthly loading doses. The HARBOR study is a unique multicenter, prospective, randomized, controlled clinical trial in the nAMD landscape because it used SD-OCT to evaluate PED, which is more commonly used than TD-OCT throughout clinical practice. During this trial, PRN retreatment criteria included a ≥5-letter decrease in BCVA from the previous visit or any evidence of disease activity on SD-OCT. It is important to note that in HARBOR, the definition of disease activity included presence of PED. In all treatment groups combined, 54.5% of eyes demonstrated PED at baseline (n = 598). At 24 months, PED resolved more frequently in eyes treated with ranibizumab 2.0 mg monthly (70.4%) versus eyes treated with ranibizumab 0.5 mg monthly (53.2%), or ranibizumab 0.5 mg PRN (44.5%), or ranibizumab 2.0 mg PRN (57.3%). Despite the differences in PED resolution, at 24 months, mean gains in BCVA from baseline were similar between eyes treated with ranibizumab 0.5 mg monthly (9.0 letters), ranibizumab 0.5 mg PRN (+8.4 letters), ranibizumab 2.0 mg monthly (7.1 letters), and ranibizumab 2.0 mg PRN (7.2 letters). At baseline, eyes with PED demonstrated better mean letter scores than eyes without PED (55.7 vs. 51.9 letters, respectively). This difference persisted at Month 24, at which time eyes with PED demonstrated a mean letter score of 64.4 versus 62.0 for eyes without PED (P = 0.03). Eyes with PED that were treated with ranibizumab 0.5 mg and ranibizumab 2.0 mg PRN required 14.0 injections and 11.6 injections, respectively, which was similar to eyes without PED at baseline (12.5 and 10.7 injections, respectively). During the HARBOR study, 28 (4.7%) eyes developed an RPE tear, with the most tears occurring in eyes with the largest PEDs (n = 21; of all eyes that developed RPE tears, 75% occurred in those eyes with PED ≥352 µm). Of all eyes with PED ≥352 µm, 14% developed RPE tears. There was a higher rate of macular atrophy present at Month 24 in eyes with complete flattening or resolution of PED (44%) versus eyes with persistent PED (17%; P < 0.0001). Vision improvement was similar in those patients who developed atrophy and those who did not at Month 24 (mean [SD] change in BCVA from baseline to Month 24: macular atrophy absent at Month 24, +7.8 [15.5] letters vs macular atrophy present at Month 24, +8.8 [13.7] letters; P = 0.53).
Another large study, VIEW 2, was reviewed in a post hoc analysis by Schmidt-Erfurth et al.2 In VIEW 2, eyes with nAMD (n = 1,240) received aflibercept 2.0 mg treatment every 4 or 8 weeks or ranibizumab 0.5 mg treatment every 4 weeks. Of note, PED analysis was performed using TD-OCT because SD-OCT was not used for data collection in the VIEW 2 study. In all treatment groups combined, 80.1% of eyes had PED defined by TD-OCT at baseline. Pigment epithelial detachment resolved in 38% of eyes after the first 3 injections. Over 48 weeks, the presence of PED at baseline was predictive of a negative BCVA outcome (BCVA estimate ± SE, −2.12 ± 0.97 letters; P = 0.0284). Pigment epithelial detachment presence declined during continuous therapy, but the switch to PRN therapy led to a recurrence of PED. The occurrence of intraretinal cysts during the PRN period correlated with the vision decline in eyes that had PED at baseline. In this analysis, rates of macular atrophy and RPE tear development were not reported. Visual acuity outcomes of PED that had resolved during the study compared with those that persisted were not reported. This comparison would be an interesting subanalysis of these data to illustrate the effects of PED resolution during the course of treatment on overall vision gains.
Finally, Waldstein et al16 completed a post hoc analysis of both the VIEW 1 and 2 trials. The VIEW 1 and 2 trials investigated the use of aflibercept 2.0 mg every 4 (n = 613) or 8 weeks (n = 607) or ranibizumab 0.5 mg every 4 weeks (n = 595) in the treatment of eyes with nAMD. Of note, the aflibercept 0.5 mg arm of the VIEW trials was not included in this post hoc analysis because this dose is not licensed. Time-domain OCT was performed monthly to assess the change in morphologic features on a monthly basis. Only the VIEW 2 population was included because monthly OCT readings were not mandated per protocol in the VIEW 1 trial. At baseline, the proportion of eyes with PED as identified by TD-OCT was 75.3% in the aflibercept 2.0 mg every 4 weeks group, 75.8% in the aflibercept 2.0 mg every 8 weeks group, and 73.3% in the ranibizumab 0.5 mg every 4 weeks group. Pigment epithelial detachment resolved at 52 weeks in 39.5% in the aflibercept 2.0 mg every 4 weeks group, 34.1% in the aflibercept 2.0 mg every 8 weeks group, and 28.1% in the ranibizumab 0.5 mg every 4 weeks group. The authors did not report whether the differences in anatomical response resulted in any visual acuity benefit. Visual acuity outcomes were evaluated in all treatment groups combined, limiting the ability to compare the effects of the different treatment regimens on BCVA. In eyes with PED present at baseline (n = 1,353), mean (SE) BCVA was 55.42 (0.494; approximate Snellen equivalent, 20/80) compared with 53.43 (0.678; 20/100) in eyes with PED absent at baseline (adjusted difference, 1.99; 95% confidence interval, 0.60–3.39; P = 0.005). At Week 52, mean (SE) BCVA was 65.25 (0.754; 20/50) in eyes with PED present (n = 798) at baseline and 60.35 (0.788; 20/63) in eyes with PED absent at baseline (n = 829; adjusted difference, 4.89; 95% confidence interval, 3.57–6.22; P < 0.001). In a multivariable model, the presence of PED had an impact of −1.88 letters on change in BCVA from baseline to Week 52 compared with absence of PED (SE, 0.75; P = 0.012). The rates of macular atrophy and RPE tear development were not reported nor differentiated between the treatment groups.
Summary: Post Hoc Analyses of Randomized, Clinical Trials
Together, these post hoc analyses suggest that ranibizumab or aflibercept are effective in treating eyes with PED associated with nAMD. The vision outcomes from the prospective and post hoc analyses of clinical trials are summarized in Figure 1 for those studies in which Early Treatment Diabetic Retinopathy Study letter score from baseline to final endpoint were available. The vision gains from baseline to the end of follow-up (1–2 years) range from 4.5 to 14 letters, depending on the population. On average, over 1 or 2 years, eyes with PED at baseline treated with anti-VEGF therapy gained approximately 2 letters fewer when compared with eyes without PED at baseline. However, in both HARBOR and the combined analysis of VIEW 1 and 2, eyes with PED at baseline showed a better mean letter score both at baseline and at the end of the analysis versus eyes without PED at baseline. Pigment epithelial detachment resolution was demonstrated in a significant proportion of eyes treated with ranibizumab or aflibercept therapy. There was additional resolution with a higher dose of anti-VEGF (ranibizumab 2.0 mg) versus a lower dose (ranibizumab 0.5 mg). However, this did not have an impact on visual outcomes in the HARBOR study as final BCVA was similar between groups. The data from the VIEW trials showing BCVA in individual treatment groups were not reported. The VIEW studies used TD-OCT, and therefore, the HARBOR analysis may be more relevant to clinical practice in the current environment, because it is the only prospective nAMD database that captured images using SD-OCT. Most of the community retina clinics use SD-OCT imaging in the routine evaluation of PEDs, and the results of trials collected with TD-OCT may not mirror the results of treatment in modern routine care with patient populations evaluated using SD-OCT. None of the aflibercept analyses evaluated RPE tears or atrophy development.
Overall, the data support the use of ranibizumab or aflibercept therapy for the treatment of eyes with PED and nAMD. Although eyes with PED are often considered a difficult subtype to treat, studies have demonstrated that a greater baseline visual acuity is often associated with better visual outcomes, with or without baseline PED detection.17,18 It is unknown whether the anatomy of a PED is a protective factor in otherwise random and well-matched nAMD populations, but there is a slight predisposition toward better acuity with the presence of a PED. Certain studies19 have noted a favorable long-term visual outcome associated with chronic multilayer fibrovascular PEDs that develop an organized fibrotic scar under the RPE with overlying photoreceptor preservation. In addition, it has been proposed that Type 1 NV associated with PED may recapitulate the choriocapillaris and reduce the risk of RPE atrophy.20,21 Further trials with longer follow-up are needed to confirm this finding. In addition, some lesion types associated with PEDs were excluded from these prospective trials (e.g., retinal angiomatous proliferation and polypoidal choroidal vasculopathy), which may require further evaluation.
Additional Search Results: Retinal Pigment Epithelium Tears
Tears of the RPE occur most commonly in eyes with nAMD and PED, both with and without anti-VEGF therapy. Retinal pigment epithelium tears or rips may be complicated by significant hemorrhage and severe vision loss.22–24 A number of studies have evaluated RPE tears in eyes with PED treated with anti-VEGF therapy, reporting tear rates as high as 27%.25 Cunningham et al26 reported a post hoc analysis of ANCHOR, MARINA, and PIER, which are 3 phase-3 trials of ranibizumab therapy in eyes with nAMD. The pooled rate of tears, based on fluorescein angiography of the 1,298 included patients, was 1.8% with ranibizumab 0.5 mg, 3.0% with ranibizumab 0.3 mg, and 1.6% in the control group. Of note, the analysis conducted by Cunningham et al26 included patients without PED at baseline. Pigment epithelial detachment is a high-risk characteristic that has been shown to precede the development of most RPE tears.27 Cunningham et al26 reported that the majority of tears (76%; 16/21) developed after the first 3 months of initiation of ranibizumab therapy. Eyes that developed an RPE tear that were treated with ranibizumab demonstrated vision gains at Month 24 compared with baseline, whereas eyes that received photodynamic therapy or sham injections had reduced vision at Month 24 compared with baseline.
Another retrospective case series studied 60 eyes of patients with nAMD and PED treated with ranibizumab, bevacizumab, or pegaptanib therapy.28 Ten of 60 (17%) eyes developed an RPE tear during the course of the study. Although the median Snellen visual acuity was not statistically significantly different between eyes with and without a tear at baseline (20/300 vs. 20/80, respectively; P = 0.096), there was a significant difference in median final visual acuity (tear, 20/400 vs. nontear, 20/60; P = 0.042). Several risk factors were identified in eyes that developed an RPE tear, including a greater linear diameter and height of the baseline PED, and a higher percentage of eyes with baseline SRF versus eyes that did not develop an RPE tear during the course of this study.
Sarraf et al23 developed a grading scheme for RPE tears that developed after anti-VEGF therapy and noted that the visual prognosis was best for Grade 1 (greatest linear diameter of 200 μm or less with fluorescein angiography) and Grade 2 tears (greatest linear diameter 200 μm to 1 disk diameter) and worst for Grade 4 RPE tears (greater than 1 disk diameter and involving the fovea) with a guarded visual prognosis for Grade 3 tears (1 disk diameter but not involving the central fovea). The management of RPE tears that develop after anti-VEGF therapy is challenging. In a retrospective case series conducted by Sarraf et al,27 56 eyes with RPE tears secondary to nAMD that received continued anti-VEGF therapy were studied. The majority of these eyes (88%) presented with a fibrovascular PED before RPE tear development. Moreover, the median number of anti-VEGF injections before RPE tear development was 3. At final follow-up (average, 41 months), eyes that had received at least 4 anti-VEGF injections within 12 months of the observation of an RPE tear demonstrated better average logMAR visual acuity than those that had received fewer than 4 injections (1.07 [20/250] vs. 1.71 [counting fingers]; P = 0.04). Overall, in this study, a higher annual injection frequency after the development of an RPE tear correlated with better visual acuity, although the final visual outcome was still suboptimal. Continued anti-VEGF treatment of RPE tears was recommended based on SD-OCT characteristics of fluid and heme to limit the development of a large severe end-stage disciform scar.
Nagiel et al29 reported a decline in median BCVA in a retrospective case study of 8 eyes with an RPE tear (pretear, Snellen VA 20/65; time of tear diagnosis, 20/75). The majority of tears (75%) occurred after the first anti-VEGF injection. In this SD-OCT analysis, risk factors for the development of RPE tear included PED height and the presence of OCT evidence of traction exerted by the Type 1 neovascular membrane along the internal RPE monolayer. The authors noted that the combined hydrostatic forces of a large PED (PED height greater than 550 µm) conspired with the tangential tractional forces of the Type 1 neovascular membrane, exacerbated by anti-VEGF therapy, to cause an RPE tear.
In a retrospective case series of 21 eyes with nAMD that developed an RPE tear and were treated with ranibizumab, Rouvas et al30 reported that 9 eyes developed an RPE tear during the natural course of the disease and 12 eyes developed an RPE tear after anti-VEGF injection, photodynamic therapy, and/or steroid therapy. Of the 21 eyes in this study with RPE tears, 17 were accompanied by PED, whereas 4 had retinal angiomatous proliferation. Over a median of 12 months, BCVA improved in 6 eyes, remained stable in 12, and decreased in 3. The three eyes in which vision decreased over the study demonstrated a fovea-involving tear.
Several other retrospective studies primarily focused on the visual outcome of RPE tear treatment have noted rates of RPE tear development in eyes with PED and nAMD of 10.5% to 27%.19,25,31–33 Doguizi and Ozdek25 reported that there was no significant change in BCVA from 0.92 logMAR (approximate Snellen equivalent, 20/160) at baseline to 0.89 logMAR (20/160) after development of an RPE tear, and 0.96 logMAR (20/200) at the last follow-up visit after continued anti-VEGF therapy. Figurska31 noted visual improvement or stabilization in 87.5% of eyes that developed an RPE tear, although this outcome was not statistically significant. Chan et al32 also reported an improvement, although not significant, in eyes that developed an RPE tear (baseline BCVA, 0.92 logMAR [20/166]; post-RPE tear BCVA was 0.84 logMAR or 20/137 P = 0.25). Introini et al33 described a decline in mean BCVA in eyes that developed an RPE tear (baseline, 0.30 logMAR [20/40]; Month 12, 0.73 logMAR [20/100]; P = 0.007).
Prospective trials focusing on RPE tears are also available to evaluate. In a prospective study of 37 eyes that were randomized to treatment with ranibizumab 0.5 mg or 2.0 mg monthly, or ranibizumab 0.5 mg or 2.0 mg PRN after 3 consecutive monthly loading doses, Sarraf et al34 reported that 5 (14%) eyes developed an RPE tear during the study, and 4 (80%) of these tears were in patients treated with 2.0 mg. Baseline PED height, surface area, and greatest linear diameter were significantly greater in the RPE tear group versus the nontear group (P = 0.018, 0.031, 0.048, respectively). Four of the 5 (80%) tears occurred in eyes with baseline PED height greater than 550 µm. Vision, as measured by Early Treatment Diabetic Retinopathy Study letters, was similar between the tear and nontear group at baseline; however, at 12 months, the nontear group gained 6.88 letters, whereas the tear group lost 13.50 letters.
Summary: Studies of Patients Experiencing Retinal Pigment Epithelium Tears
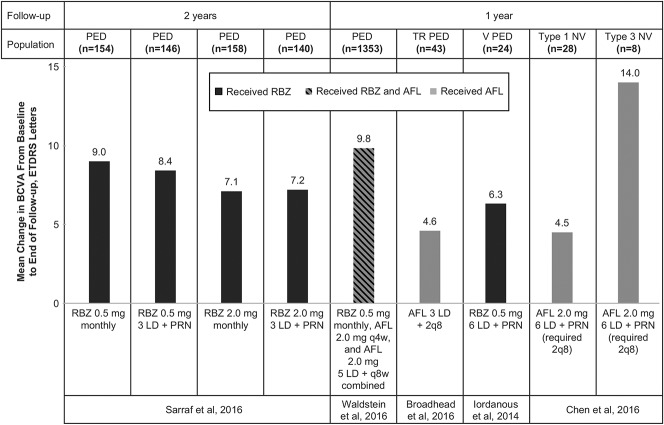
In general, RPE tear rates have varied in PED studies not primarily focused on RPE tear (0%–27%, as reported earlier; Figure 2).9–12,15,35–40 Although the rate of tear development may be very low in studies of nAMD that do not specify lesion type, this rate typically runs 15% or higher in more selective cohorts with high-risk PED. Studies have identified a trend in RPE tears occurring within the first 3 months of treatment, which may indicate a causal effect of the anti-VEGF therapy.26,27,41 Eyes that developed an RPE tear also tended to have larger PEDs (i.e., greater height, linear diameter, or volume) at baseline.15,28,34 Informed by these studies, PEDs 600 µm or greater in height are considered to harbor an increased risk of RPE tear after anti-VEGF therapy. When anti-VEGF treatment is continued in eyes with an RPE tear, BCVA usually stabilizes.
Fig. 2.
Development of RPE tear across trials of eyes with PEDs.9–12,15,19,25,28,31–40 AFL, aflibercept; BVZ, bevacizumab; F, fibrovascular; H, hemorrhagic; M, multilayered; RBZ, ranibizumab; S, serous; TN, treatment-naive; TR, treatment-resistant; V, vascular.
The findings of these trials are further supported by clinical practice. The included case highlights a patient presenting with a baseline vascularized serous PED measuring 826 µm in height (visual acuity 20/80 in the right eye) that was treated with anti-VEGF therapy. With continued intravitreal injections, the PED evolved into a multilayered fibrovascular morphology over 6 months (Figure 3) before developing a Grade 2 RPE tear23 (Figure 4A), as evidenced by the characteristic crescentic shape and hypoautofluorescent nature with fundus autofluorescent imaging (Figure 4B). Additional anti-VEGF therapy resulted in possible reepithelialization of the RPE tear (this mechanism has not been proven) and reduced hypoautofluorescence of the tear (Figure 5). With continued anti-VEGF therapy, the patient has maintained a visual acuity of 20/40 in the affected eye.
Fig. 3.
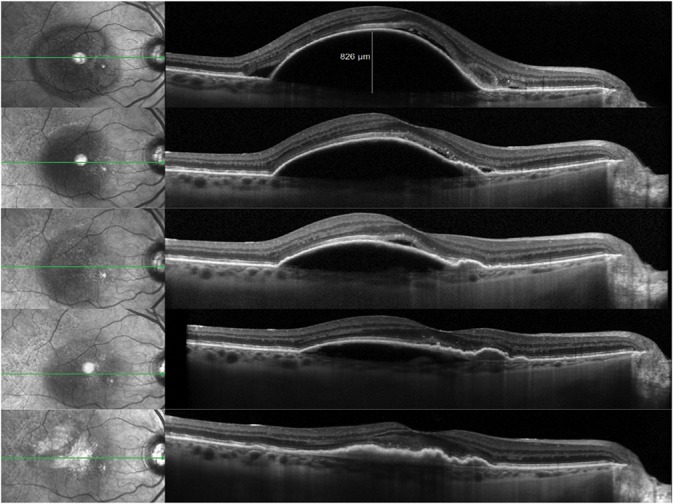
Sequential SD-OCT B-scans during the course of anti-VEGF therapy demonstrating the evolution of a multilayered fibrovascular PED with progressive vascularization and increasing evidence of traction as illustrated by the RPE folds.
Fig. 4.
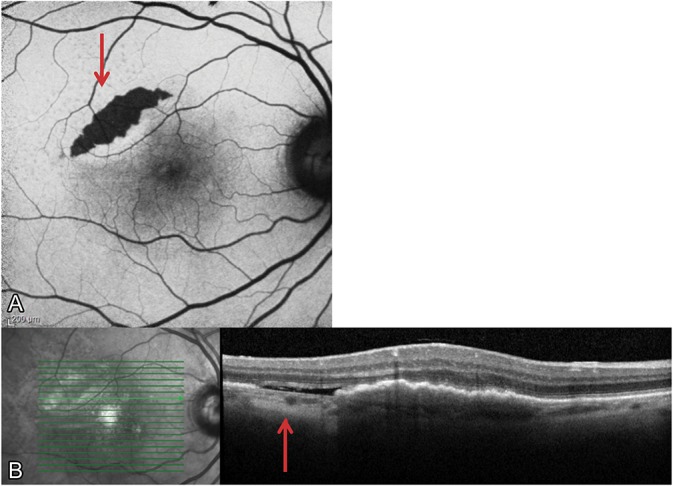
A Grade 2 RPE tear developed after several anti-VEGF injections. A. The fundus autofluorescent image illustrates the characteristic crescentic shape and hypoautofluorescent nature (red arrow) that are typical features of a tear. B. The RPE tear is also visible with SD-OCT, where areas of choroidal hypertransmission denote the absence of RPE (red arrow).
Fig. 5.
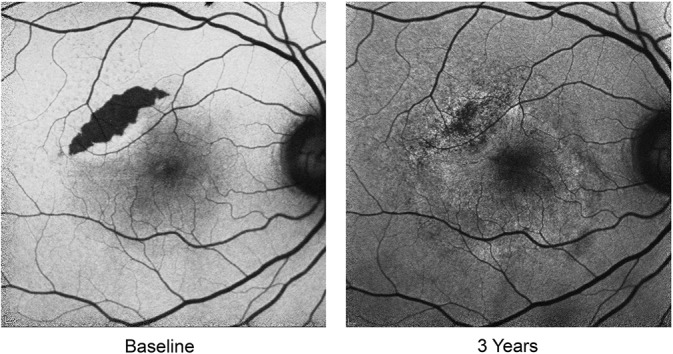
Progressive reduction in the hypoautofluorescence of the RPE tear occurred over several years after many anti-VEGF injections. With continued anti-VEGF therapy, the patient has maintained a visual acuity of 20/40 in the affected right eye.
Additional Search Results: Atrophy Development
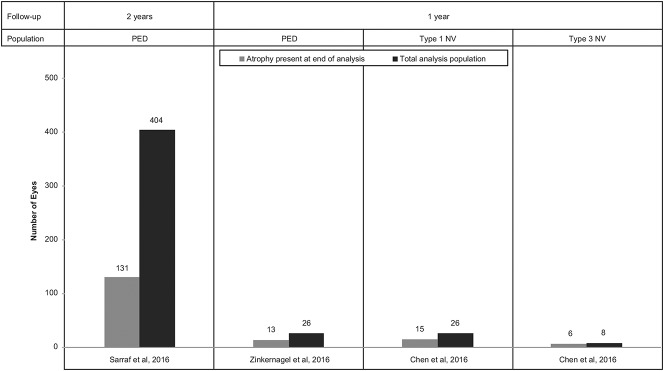
Very limited data are available on the association between PED and atrophy in the setting of nAMD (Figure 6). A study performed by Zinkernagel et al42 evaluated 26 eyes with nAMD and PED that were treated with aflibercept therapy (2.0 mg) for 3 consecutive monthly loading doses and then every other month thereafter. Nine (34%) eyes in this study were complicated by atrophy of the RPE at baseline, whereas at 1 year, 13 eyes (50%) exhibited RPE atrophy within the macula. Eyes without atrophy at 1 year demonstrated significantly better mean BCVA gains from baseline versus eyes with atrophy (P = 0.03).
Fig. 6.
Development of atrophy across studies of eyes with PEDs.12,15,42
Similar 1-year atrophy rates were identified by Chen et al12 in patients who received 6 monthly aflibercept injections followed by 3 bimonthly injections. This study reported the absence of atrophy in all eyes with Type 1 NV at baseline, whereas 15 of 26 (58%) of these eyes developed geographic atrophy by Month 12. One of 8 (12.5%) eyes with Type 3 NV demonstrated geographic atrophy at baseline and 6 out of 8 (75%) showed detectable atrophy by Month 12. In a linear discriminant analysis, the only significant correlation identified was a positive correlation between baseline subretinal hyperreflective material with SD-OCT and atrophy development in Type 3 lesions (P = 0.026). No correlation was seen with baseline PED size or SRF volume in either Type 1 or Type 3 lesions. Type 3 neovascularization has been associated with a greater incidence of macular atrophy in several other studies, whereas Type 1 NV may confer an element of protection.21,43–46
In another study of atrophy rates in patients with PED, Sarraf et al15 reported a higher rate of macular atrophy at Month 24 in eyes with complete flattening of PED (44%) versus eyes with persistent PED (17%; P < 0.0001). This study found that although vision gains were similar between eyes that developed macular atrophy at Month 24 and those that did not, it is unclear how the development of atrophy will affect these eyes in the longer term. There was no significant difference in the detection of macular atrophy at Month 24 in eyes with PED versus without PED at baseline or based on PED size at baseline. It is more likely that RPE atrophy leads to PED collapse, as with drusenoid PEDs,47 and not vice versa, but the association between atrophy and PED needs to be explored further in large studies with long-term follow-up.
Summary: Studies of Patients Experiencing Atrophy
The studies by Zinkernagel et al42 and Chen et al12 both used SD-OCT to evaluate for atrophy and identified this complication in 50% or more of eyes with PED and nAMD by 1 year. The study by Sarraf et al15 analyzed trials that used fluorescein angiography and color fundus photography for the detection of atrophy and identified a lower rate of atrophy over 2 years (32%), but also illustrated a significant difference between those eyes with PED resolution and those without. Taken together, these analyses are linked to an important consideration in the optimal treatment of PEDs: complete anatomical resolution of the PED may not be necessary or even desirable.
The treatment goals for patients presenting with PED should focus on visual acuity and on the presence of IRF and SRF and heme in the absence of correlative vision gains and in the presence of de novo macular atrophy associated with complete resolution of PED.
Conclusion
Intravitreal anti-VEGF therapy is an effective treatment in most of the eyes with PED secondary to nAMD. Similar visual acuity outcomes have been reported with different treatment algorithms. There is limited evidence in both the retrospective and prospective literature supporting the concept that higher dosages of various anti-VEGF agents, delivered either as more frequent dosing or as a greater dosage, may lead to a more rapid or more improved anatomical response. However, there is no evidence that this correlates with an improvement in vision.
As with the administration of higher dosages of anti-VEGF agents, switching anti-VEGF agents may result in additional anatomical improvement, but vision typically remains stable in eyes with treatment-resistant PEDs. Lesions defined as fibrovascular PEDs may be a more difficult subtype to treat, but even these difficult-to-treat eyes can have significant vision improvements with anti-VEGF therapy. Retinal pigment epithelium tears occurs in approximately 0% to 27% of eyes depending on the study population; 15% to 20% of eyes with PED develop RPE tear after anti-VEGF therapy, especially in eyes with PED 600 µm or greater in height. Vision is often stabilized in these eyes with RPE tears if anti-VEGF therapy is continued. Atrophy may develop in eyes with PED and nAMD treated with anti-VEGF therapy. Moreover, there is a higher rate of macular atrophy development in eyes with baseline PED that have complete resolution of PED with anti-VEGF treatment.
Based on the available literature, anti-VEGF therapy is efficacious in treating eyes with PED and nAMD. Treatment should focus on achieving improvements in visual acuity and not necessarily complete resolution of PED because there is no apparent correlation between anatomical and functional improvement in most eyes with PED and nAMD.
Often, protocol-mandated treatment regimens in clinical trials differ from those used in the real-world setting. As such, clinical trial findings may not be truly representative of the treating physician experience; however, it is important to gain knowledge and insight from clinical trial results. Although PEDs were treated until resolution in HARBOR, it is not a universal practice to continue treatment once visual acuity and PEDs have stabilized and SRF or IRF have resolved.48 Despite the additional treatment received by patients during the HARBOR trial, PED reduction was not correlated with better visual acuity outcomes.15 In fact, collapsed PED has been associated with de novo macular atrophy48 and a worse overall visual acuity outcome, suggesting that treatment to resolution may not be beneficial to all patients.8,34
The landscape of treatment for PED secondary to nAMD is ever-evolving, with additional evidence emerging from both clinical and real-world settings. Based on the findings of the present comprehensive review of anti-VEGF therapies for PED in eyes with nAMD, the authors recommend the treatment of PED cases in which IRF and/or SRF are concurrently detected as biomarkers of exudative activity. Moreover, cases in which visual acuity and PED stabilize in the absence of fluid may be observed without additional treatment, or treatment may be continued. In special circumstances, as with a documented increase in size or volume of the PED, or associated vision loss, treatment of a PED without clear SRF and/or IRF can be considered. Other biomarkers of NV may prove to be useful to guide therapy in these challenging situations in the future.49
Footnotes
Third-party writing assistance was provided by Jack W. Pike, PhD, of Envision Scientific Solutions, and funded by Genentech, Inc.
A. M. Khanani: Supported Research: Aerpio, Alcon, Allergan, DigiSight, Genentech, Inc, Novartis, Ophthotech, ThromboGenics; Consultant/Advisor: Aerpio, Alcon, Alimera, Allergan, Genentech, Inc, Novartis, ThromboGenics; Speaker: Allergan, Genentech, Inc, Novartis Pharma, D. Eichenbaum: Consultant/Advisor: Alimera, Allergan, Genentech, Inc, Regeneron, ThromboGenics; Supported Research: Alcon, Alimera, Allergan, Clearside, Genentech, Inc, Ophthotech, River Vision, ThromboGenics, TOGA Trial; Speaker: Allergan, Genentech, Inc; Equity: Boston Image Reading Center, Hemera Biopharmaceuticals, USRetina, P. Schlottmann: Advisor: Bayer, Novartis, Roche; Travel grants: Allergan, Bayer, Novartis. L. Tuomi: Employee of Genentech, Inc. D. Sarraf: Consultant: Amgen, Bayer, Genentech, Inc, Novartis, Nuvelution, Optovue; Research support: Allergan, Genentech, Inc, Heidelberg, Optovue, Regeneron; Speaker: Novartis, Optovue.
Genentech, Inc participated in the design and conduct of the study; data collection, analysis, and interpretation of results; and preparation, review, and approval of the manuscript, but had no role in the conduct of this research or final clinical interpretation of the data.
References
- 1.Coscas F, Coscas G, Souied E, et al. Optical coherence tomography identification of occult choroidal neovascularization in age-related macular degeneration. Am J Ophthalmol 2007;144:592–599. [DOI] [PubMed] [Google Scholar]
- 2.Schmidt-Erfurth U, Waldstein SM, Deak GG, et al. Pigment epithelial detachment followed by retinal cystoid degeneration leads to vision loss in treatment of neovascular age-related macular degeneration. Ophthalmology 2015;122:822–832. [DOI] [PubMed] [Google Scholar]
- 3.Pauleikhoff D, Loffert D, Spital G, et al. Pigment epithelial detachment in the elderly. Clinical differentiation, natural course and pathogenetic implications. Graefes Arch Clin Exp Ophthalmol 2002;240:533–538. [DOI] [PubMed] [Google Scholar]
- 4.Mariani A, Deli A, Ambresin A, Mantel I. Characteristics of eyes with secondary loss of visual acuity receiving variable dosing ranibizumab for neovascular age-related macular degeneration. Graefes Arch Clin Exp Ophthalmol 2011;249:1635–1642. [DOI] [PubMed] [Google Scholar]
- 5.Nagai N, Suzuki M, Uchida A, et al. Non-responsiveness to intravitreal aflibercept treatment in neovascular age-related macular degeneration: implications of serous pigment epithelial detachment. Sci Rep 2016;6:29619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mrejen S, Sarraf D, Mukkamala SK, Freund KB. Multimodal imaging of pigment epithelial detachment: a guide to evaluation. Retina 2013;33:1735–1762. [DOI] [PubMed] [Google Scholar]
- 7.Ersoy L, Ristau T, Kirchhof B, Liakopoulos S. Response to anti-VEGF therapy in patients with subretinal fluid and pigment epithelial detachment on spectral-domain optical coherence tomography. Graefes Arch Clin Exp Ophthalmol 2014;252:889–897. [DOI] [PubMed] [Google Scholar]
- 8.Chan CK, Abraham P, Sarraf D, et al. Earlier therapeutic effects associated with high dose (2.0 mg) Ranibizumab for treatment of vascularized pigment epithelial detachments in age-related macular degeneration. Eye (Lond) 2015;29:80–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Iordanous Y, Powell AM, Mao A, et al. Intravitreal ranibizumab for the treatment of fibrovascular pigment epithelial detachment in age-related macular degeneration. Can J Ophthalmol 2014;49:367–376. [DOI] [PubMed] [Google Scholar]
- 10.Inoue M, Arakawa A, Yamane S, Kadonosono K. Variable response of vascularized pigment epithelial detachments to ranibizumab based on lesion subtypes, including polypoidal choroidal vasculopathy. Retina 2013;33:990–997. [DOI] [PubMed] [Google Scholar]
- 11.Panos GD, Gatzioufas Z, Petropoulos IK, et al. Effect of ranibizumab on serous and vascular pigment epithelial detachments associated with exudative age-related macular degeneration. Drug Des Devel Ther 2013;7:565–569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chen X, Al-Sheikh M, Chan CK, et al. Type 1 versus type 3 neovascularization in pigment epithelial detachments associated with age-related macular degeneration after anti-vascular endothelial growth factor therapy: a prospective study. Retina 2016;36(S1):S50–S64. [DOI] [PubMed] [Google Scholar]
- 13.Broadhead GK, Hong T, Zhu M, et al. Response of pigment epithelial detachments to intravitreal aflibercept among patients with treatment-resistant neovascular age-related macular degeneration. Retina 2015;35:975–981. [DOI] [PubMed] [Google Scholar]
- 14.Baba T, Kitahashi M, Kubota-Taniai M, et al. Two-year course of subfoveal pigment epithelial detachment in eyes with age-related macular degeneration and visual acuity better than 20/40. Ophthalmologica 2012;228:102–109. [DOI] [PubMed] [Google Scholar]
- 15.Sarraf D, London NJ, Khurana RN, et al. Ranibizumab treatment for pigment epithelial detachment secondary to neovascular age-related macular degeneration: post hoc analysis of the HARBOR study. Ophthalmology 2016;123:2213–2224. [DOI] [PubMed] [Google Scholar]
- 16.Waldstein SM, Simader C, Staurenghi G, et al. Morphology and visual acuity in aflibercept and ranibizumab therapy for neovascular age-related macular degeneration in the VIEW trials. Ophthalmology 2016;123:1521–1529. [DOI] [PubMed] [Google Scholar]
- 17.Shona O, Gupta B, Vemala R, Sivaprasad S. Visual acuity outcomes in ranibizumab-treated neovascular age-related macular degeneration; stratified by baseline vision. Clin Exp Ophthalmol 2011;39:5–8. [DOI] [PubMed] [Google Scholar]
- 18.Ross AH, Donachie PH, Sallam A, et al. Which visual acuity measurements define high-quality care for patients with neovascular age-related macular degeneration treated with ranibizumab? Eye (Lond) 2013;27:56–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rahimy E, Freund KB, Larsen M, et al. Multilayered pigment epithelial detachment in neovascular age-related macular degeneration. Retina 2014;34:1289–1295. [DOI] [PubMed] [Google Scholar]
- 20.Grossniklaus HE, Green WR. Perspective: choroidal neovascularization. Am J Ophthalmol 2004;137:496–503. [DOI] [PubMed] [Google Scholar]
- 21.Christenbury JGP, Gilani N, Freund F, et al. Progression of macular atrophy in eyes with type 1 neovascularization and age-related macular degeneration receiving long-term intravitreal anti–vascular endothelial growth factor therapy. Retina 2017. DOI: 10.1097/IAE.0000000000001766 [DOI] [PubMed] [Google Scholar]
- 22.Chang LK, Sarraf D. Tears of the retinal pigment epithelium: an old problem in a new era. Retina 2007;27:523–534. [DOI] [PubMed] [Google Scholar]
- 23.Sarraf D, Reddy S, Chiang A, Yu F, et al. A new grading system for retinal pigment epithelial tears. Retina 2010;30:1039–1045. [DOI] [PubMed] [Google Scholar]
- 24.Varshney N, Jain A, Chan V, et al. Anti-VEGF response in macular hemorrhage and incidence of retinal pigment epithelial tears. Can J Ophthalmol 2013;48:210–215. [DOI] [PubMed] [Google Scholar]
- 25.Doguizi S, Ozdek S. Pigment epithelial tears associated with anti-VEGF therapy: incidence, long-term visual outcome, and relationship with pigment epithelial detachment in age-related macular degeneration. Retina 2014;34:1156–1162. [DOI] [PubMed] [Google Scholar]
- 26.Cunningham ET, Jr, Feiner L, Chung C, et al. Incidence of retinal pigment epithelial tears after intravitreal ranibizumab injection for neovascular age-related macular degeneration. Ophthalmology 2011;118:2447–2452. [DOI] [PubMed] [Google Scholar]
- 27.Sarraf D, Joseph A, Rahimy E. Retinal pigment epithelial tears in the era of intravitreal pharmacotherapy: risk factors, pathogenesis, prognosis and treatment (an American Ophthalmological Society thesis). Trans Am Ophthalmol Soc 2014;112:142–159. [PMC free article] [PubMed] [Google Scholar]
- 28.Chiang A, Chang LK, Yu F, Sarraf D. Predictors of anti-VEGF-associated retinal pigment epithelial tear using FA and OCT analysis. Retina 2008;28:1265–1269. [DOI] [PubMed] [Google Scholar]
- 29.Nagiel A, Freund KB, Spaide RF, et al. Mechanism of retinal pigment epithelium tear formation following intravitreal anti-vascular endothelial growth factor therapy revealed by spectral-domain optical coherence tomography. Am J Ophthalmol 2013;156:981–988 e982. [DOI] [PubMed] [Google Scholar]
- 30.Rouvas AA, Ladas ID, Georgalas I, et al. Ranibizumab for the treatment of exudative age-related macular degeneration associated with retinal pigment epithelial tear. Retina 2011;31:1083–1088. [DOI] [PubMed] [Google Scholar]
- 31.Figurska M. Retinal pigment epithelial tears following ranibizumab therapy for fibrovascular retinal pigment epithelial detachment due to occult age-related macular degeneration. Med Sci Monit 2012;18:CR32–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chan CK, Abraham P, Meyer CH, et al. Optical coherence tomography-measured pigment epithelial detachment height as a predictor for retinal pigment epithelial tears associated with intravitreal bevacizumab injections. Retina 2010;30:203–211. [DOI] [PubMed] [Google Scholar]
- 33.Introini U, Torres Gimeno A, Scotti F, et al. Vascularized retinal pigment epithelial detachment in age-related macular degeneration: treatment and RPE tear incidence. Graefes Arch Clin Exp Ophthalmol 2012;250:1283–1292. [DOI] [PubMed] [Google Scholar]
- 34.Sarraf D, Chan C, Rahimy E, Abraham P. Prospective evaluation of the incidence and risk factors for the development of RPE tears after high- and low-dose ranibizumab therapy. Retina 2013;33:1551–1557. [DOI] [PubMed] [Google Scholar]
- 35.Cho HJ, Kim KM, Kim HS, et al. Response of pigment epithelial detachment to anti-vascular endothelial growth factor treatment in age-related macular degeneration. Am J Ophthalmol 2016;166:112–119. [DOI] [PubMed] [Google Scholar]
- 36.Kalouda P, Anastasakis A, Tsika C, Tsilimbaris KM. The effect of intravitreal anti-VEGF on the pigment epithelial detachment in eyes with the exudative type of age-related macular degeneration. Semin Ophthalmol 2015;30:6–10. [DOI] [PubMed] [Google Scholar]
- 37.Major JC, Jr, Wykoff CC, Croft DE, et al. Aflibercept for pigment epithelial detachment for previously treated neovascular age-related macular degeneration. Can J Ophthalmol 2015;50:373–377. [DOI] [PubMed] [Google Scholar]
- 38.Yuksel H, Turkcu FM, Sahin A, et al. One year results of anti-VEGF treatment in pigment epithelial detachment secondary to macular degeneration. Arq Bras Oftalmol 2013;76:209–211. [DOI] [PubMed] [Google Scholar]
- 39.Zhao C, Zhang Z, Chen L, et al. Effectiveness of intravitreal injection of ranibizumab for neovascular age-related macular degeneration with serous pigment epithelial detachment. Med Sci Monit 2016;22:833–839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kocak I. Intravitreal aflibercept in treatment-resistant pigment epithelial detachment. Int Ophthalmol 2016;37:531–537. [DOI] [PubMed] [Google Scholar]
- 41.Sarraf D, Hill L, Lu N, et al. Efficacy of ranibizumab therapy in eyes with neovascular AMD and pigment epithelial detachment (PED): a HARBOR subanalysis study. Paper presented at: The Association for Research in Vision and Ophthalmology 2016 Annual Meeting; May 1–5, 2016; Seattle, WA.
- 42.Zinkernagel MS, Wolf S, Ebneter A. Fluctuations in pigment epithelial detachment and retinal fluid using a bimonthly treatment regimen with aflibercept for neovascular age-related macular degeneration. Ophthalmologica 2016;235:42–48. [DOI] [PubMed] [Google Scholar]
- 43.Dhrami-Gavazi E, Balaratnasingam C, Lee W, Freund KB. Type 1 neovascularization may confer resistance to geographic atrophy amongst eyes treated for neovascular age-related macular degeneration. Int J Retina Vitreous 2015;1:15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Group TCR. Ranibizumab and bevacizumab for neovascular age-related macular degeneration. N Engl J Med 2011;364:1897–1908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Chakravarthy U, Harding SP, Rogers CA, et al. Alternative treatments to inhibit VEGF in age-related choroidal neovascularization: 2-year findings of the IVAN randomised controlled trial. Lancet 2013;382:1258–1267. [DOI] [PubMed] [Google Scholar]
- 46.Xu L, Mrejen S, Jung JJ, et al. Geographic atrophy in patients receiving anti-vascular endothelial growth factor for neovascular age-related macular degeneration. 2015;35:177–186. [DOI] [PubMed] [Google Scholar]
- 47.Ferris FL, III, Wilkinson CP, Bird A, et al. Clinical classification of age-related macular degeneration. Ophthalmology 2013;120:844–851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ho AC, Busbee BG, Regillo CD, et al. Twenty-four-month efficacy and safety of 0.5 mg or 2.0 mg ranibizumab in patients with subfoveal neovascular age-related macular degeneration. Ophthalmology 2014;121:2181–2192. [DOI] [PubMed] [Google Scholar]
- 49.Xu D, Davila JP, Rahimi M, et al. Long-term progression of type 1 neovascularization in age-related macular degeneration using optical coherence tomography angiography. Am J Ophthalmol 2017;187:10–20. [DOI] [PubMed] [Google Scholar]