Abstract
Purpose:
Fungal keratitis is a major cause of corneal ulcers, resulting in significant visual impairment and blindness. Fenretinide, a derivative of vitamin A, has been shown to suppress inflammation in a multitude of diseases. In this study, we aimed to characterize the effect of fenretinide in Aspergillus fumigatus keratitis of the eye in a mouse model.
Methods:
In vivo and in vitro experiments were performed in mouse models and THP-1 macrophage cell cultures infected with A. fumigatus, respectively. Experimental subjects were first pretreated with fenretinide, and then the effect of the compound was assessed with clinical evaluation, neutrophil staining, myeloperoxidase assay, quantitative polymerase chain reaction (qRT-PCR), and western blot.
Results:
We confirmed that fenretinide contributed to protection of corneal transparency during early mouse A. fumigatus keratitis by reducing neutrophil recruitment, decreasing myeloperoxidase (MPO) levels and increasing apoptosis. Compared with controls, fenretinide impaired proinflammatory cytokine interleukin 1 beta (IL-1β) production in response to A. fumigatus exposure with contributions by lectin-type oxidized LDL receptor 1 (LOX-1) and c-Jun N-terminal kinase (JNK).
Conclusions:
Together, these findings demonstrate that fenretinide may suppress inflammation through reduced neutrophil recruitment and inflammatory cytokine production in A. fumigatus keratitis.
Key Words: keratitis, Aspergillus fumigatus, fenretinide, neutrophils, IL-1β
Fungal keratitis, which is mostly caused by Fusarium solani and Aspergillus fumigatus, may be caused by ocular trauma with vegetative matter.1 Fungal keratitis has a large negative effect on visual health, with up to 60% of corneal ulcers being attributable to fungal infection in developing countries.1,2 Pattern recognition receptors (PRRs) in the cornea are activated by Aspergillus fumigatus infection; pattern recognition receptors are responsible for chemokine production and neutrophil recruitment into the corneal stroma. Although neutrophils are essential for fungal killing, they also cause tissue damage that can result in loss of corneal clarity.3,4 Transparency is crucial to the function of the cornea; therefore, it is important to eliminate pathogenic fungi in the cornea, while reducing neutrophil infiltration to maintain transparency. Although new therapies for fungal keratitis have been used in clinical settings, this infection remains a challenge for ophthalmologists because of its generally delayed diagnosis and a dearth of effective drugs and treatment methods.5,6
Fenretinide (4-hydroxy (phenyl) retinamide; 4-HPR) is a synthetic vitamin A derivative that inhibits the activation of the proinflammatory transcriptional factor, nuclear factor (NF)-kappaB, by upregulating ceramide. It is believed that it is through this mechanism that fenretinide has been shown to suppress inflammation. For instance, it was well established that the imbalance of phospholipid-bound fatty acid is corrected by fenretinide in macrophages by modulating inflammatory cytokine expression through ERK1/2 phosphorylation.7 In addition, fenretinide decreased lipopolysaccharide (LPS)-induced proinflammatory cytokine production, including tumor necrosis factor-α, interleukin 6 (IL-6), and monocyte chemoattractant protein 1, nitric oxide synthase expression, and nitrogen oxide production through activating peroxisome proliferator-activated receptor γ in macrophages.8 Moreover, inflammation and airway hyperresponsiveness induced by ovalbumin were protected by fenretinide, which completely blocked inflammatory cell recruitment into the airways and distinctly reduced goblet cell proliferation.9
However, the role of fenretinide in fungal keratitis has not been previously explored. To this end, we designed and executed in vivo and in vitro experiments in a mouse model and THP-1 macrophages cell cultures infected with A. fumigatus and pretreated with fenretinide, respectively. We demonstrated that fenretinide contributed to protect corneal transparency of early mouse A. fumigatus keratitis by reducing neutrophil recruitment, decreasing myeloperoxidase (MPO) levels, and increasing apoptosis. Fenretinide further impaired proinflammatory cytokine interleukin 1 beta (IL-1β) production in response to A. fumigatus exposure with the contribution of lectin-type oxidized LDL receptor 1 (LOX-1) and c-Jun N-terminal kinase (JNK). These findings demonstrate that fenretinide may provide a protective effect for cells and organs infected with early fungal keratitis by suppressing inflammation.
MATERIALS AND METHODS
Preparation of A. fumigatus
A. fumigatus strain 3.0772 was purchased from the China General Microbiological Culture Collection Center (Beijing, China). The strain was cultured for 3 to 4 days on Sabouraud agar, and suspensions of fresh conidia were scraped from the surface of the medium. Samples were quantified using a hemacytometer and adjusted to a final concentration of 5 × 104 conidia/μL in phosphate-buffered saline.
In Vivo Experiments
Eight-week-old C57BL/6 female mice were purchased from the Changzhou Cavens Laboratory (Jiangsu, China). Mice were treated in accordance with the Statement for the Use of Animals in Ophthalmic and Vision Research by the Association for Research in Vision and Ophthalmology (ARVO). Mice were anaesthetized with 8% chloral hydrate, and then 1 eye was randomly selected from each mouse to receive a subconjunctival injection (5 μL) containing 100 μM of fenretinide (MedChemExpress) or dimethyl sulfoxide (DMSO) as a control 1 day and 2 hours before infection. Eyes were infected when A. fumigatus conidia (0.5 × 105/μL) were injected into the corneal stroma.
Mice were examined daily with a slit-lamp microscope for corneal opacification and ulceration. Ocular disease was graded using clinical scores ranging from 0 to 12 according to the scoring system proposed by Wu et al.10 Corneas were then harvested for myeloperoxidase assay, quantitative polymerase chain reaction (qRT-PCR), and western blot 1 day after establishment of the mouse models, and eyes were removed at 1 day for immunofluorescence staining.
In Vitro Experiments
THP-1 macrophages purchased from the China Center for Type Culture Collection (Wuhan, China) were differentiated with 100 nM of Phorbol 12-myristate 13-acetate (PMA) (Sigma, St. Louis, MO) for 48 hours and then allowed to recover for 24 hours before infection. Macrophages were cultured in RPMI-1640 medium at a density of 1 × 106/mL. THP-1 macrophages were treated with fenretinide at a final concentration of 10 μM 2 hours before treatment with conidia. Finally, THP-1 macrophages were treated with A. fumigatus conidia in 12-well and 6-well plates at a multiplicity of infection of 1 for 4 hours (qRT-PCR) and 16 hours (western blot).
Immunofluorescence Staining
Neutrophil recruitment in response to A. fumigatus keratitis was visualized with immunofluorescence staining. Mouse eyes were embedded in optimum cutting temperature compound (Tissue-Tek) and frozen in liquid nitrogen until used. The immunofluorescence protocol used in this experiment is described in previous publications.11 The primary antibody used was 10 μg/mL rat anti-mouse NIMP-R14 (Santa Cruz), and Alexa Fluor 488-conjugated goat anti-rat antibody (1:1000; CST) was used as a secondary antibody.
Myeloperoxidase (MPO) Assay
Corneas were cut 1 day after infection. The MPO assay protocol used followed those detailed in a previous publication.12 The slope of the line was determined in relation to the assay units of the MPO/cornea.
RNA Isolation and qRT-PCR
The mRNA levels of LOX-1 and IL-1β of THP-1 macrophages and mouse corneas, as well as the mRNA levels of Bcl-2, BAX, cytochrome C, caspase-8, caspase-9, caspase-3 of corneas were experimentally determined after infection with A. fumigatus. The PCR protocol used in this study is described in previous publications.11 The primer pair sequences were as follows: hLOX-1 F-GCT CCT CCT GAG CGC AAG and R-CAT CTG CTG GAA GGT GGA CA, hIL-1β F-ATG CAC CTG TAC GAT CAC TGA and R-ACA AAG GAC ATG GAG AAC ACC, hβ-actin F-GCT CCT CCT GAG CGC AAG and R-CAT CTG CTG GAA GGT GGA CA, mLOX-1 F-AGG TCC TTG TCC ACA AGA CTG G and R-ACG CCC CTG GTC TTA AAG AAT TG, mIL-1β F-CGC AGC AGC ACA TCA ACA AGA GC and R-TGT CCT CAT CCT GGA AGG TCC ACG, mBcl-2 F-GGA CTT GAA GTG CCA TTG GT and R-AGC CCC TCT GTG ACA GCT TA, mBax F-CGA GCT GAT CAG AAC CAT CA and R-CTC AGC CCA TCT TCT TCC AG, mCyt-c F-AGT CCT TGG GCA CAG CAG TTG and R-GGC ACT GAG CAC ATT TCT GAA CA, mCaspase-9 F-AGT TCC CGG GTG CTG TCT AT and R-GCC ATG GTC TTT CTG CTC AC, mCaspase-8 F-CTC CGA AAA ATG AAG GAC AGA and R-CGT GGG ATA GGA TAC AGC AGA, mCaspase-3 F-TGG GCC TGA AAT ACC AAG TC and R-AAA TGA CCC CTT CAT CAC CA, and mβ-actin F-GAT TAC TGC TCT GGC TCC TAG C and R-GAC TCA TCG TAC TCC TGC TTG C.
Western Blotting
The western blotting protocol that is used to assay corneas and cells is described in previous publications.11 Membranes were incubated accordingly using the following antibodies: anti-LOX-1 (ProteinTech, Chicago, IL), anti-JNK (ProteinTech), anti-pJNK (CST), anti-mIL-1β (R&D, Minneapolis, MN), anti-hIL-1β (CST, Danvers, MA), anti-Bcl-2 (ProteinTech), anti-Bax (ProteinTech), anti-cytochrome c (ProteinTech), anti-cleaved-CASP9 (ProteinTech), anti-cleaved-CASP8 (ProteinTech), anti-cleaved-CASP3 (ProteinTech), and anti-β-actin (CST). The HRP-tagged secondary anti-bodies were purchased from CST.
Statistical Analysis
An unpaired 2-tailed Student t-test was used to determine the statistical significance of the clinical score, MPO assays, qRT-PCR, and western blotting results. Data were represented as mean ± SD and analyzed using GraphPad 5.0 software. Data were considered significant at P ≤ 0.05.
RESULTS
Disease Response After Fenretinide Pretreatment in the Mouse A. fumigatus Keratitis Model
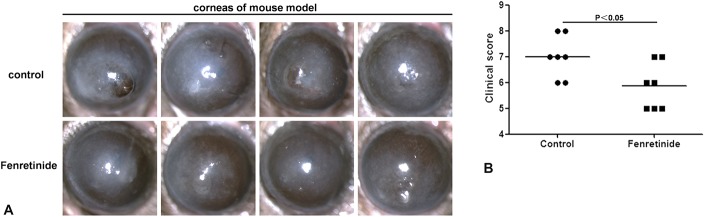
Images captured with the slit lamp at 1 day (Fig. 1A) after infection illustrate the disease response in control versus fenretinide-pretreated mice. The disease response is represented by a clinical score, which was higher at day 1 (Fig. 1B) in controls compared with fenretinide-pretreated mice (P < 0.05).
FIGURE 1.
Disease response with fenretinide pretreatment in a mouse Aspergillus fumigatus keratitis model. A, Images captured with a slit lamp at 1 day after infection illustrate the disease response of control versus fenretinide-pretreated mice. B, Disease response is represented by a clinical score (n = 7/group), which was higher at day 1 for control compared with fenretinide-pretreated mice. Mean values and SDs of 2 independent experiments are shown.
Fenretinide Pretreatment Reduced Neutrophils Recruitment and MPO Activity in the Mouse A. fumigatus Keratitis Model
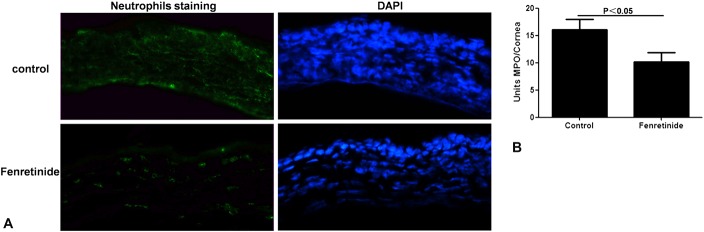
NIMP-R14 was stained in the corneas of control and fenretinide-pretreated mice 1 day after infection (Fig. 2A). The apparent positive staining (green) in the corneas of fenretinide-pretreated mice indicated a decreased amount of neutrophils compared with control mice. Corneas of fenretinide-pretreated mice exhibited a significant decrease (P < 0.05) in MPO levels 1 day after infection compared with the controls (Fig. 2B).
FIGURE 2.
Fenretinide pretreatment reduced neutrophils recruitment and MPO activity in the mouse Aspergillus fumigatus keratitis model. A, Positive staining (green) of NIMP-R14 in the corneas of pretreated mice was indicative of a decreased presence of neutrophils compared with control mice at 1 day after infection (magnification ×400). B, Corneas (n = 7/group) from pretreated mice exhibited a significant decrease in MPO levels at 1 day after infection compared with control mice. The mean values and SDs of 2 independent experiments are shown.
Fenretinide Inhibited A. fumigatus-Induced Production of IL-1β Through Blockade of the LOX-1/JNK Pathway in the Mice Cornea
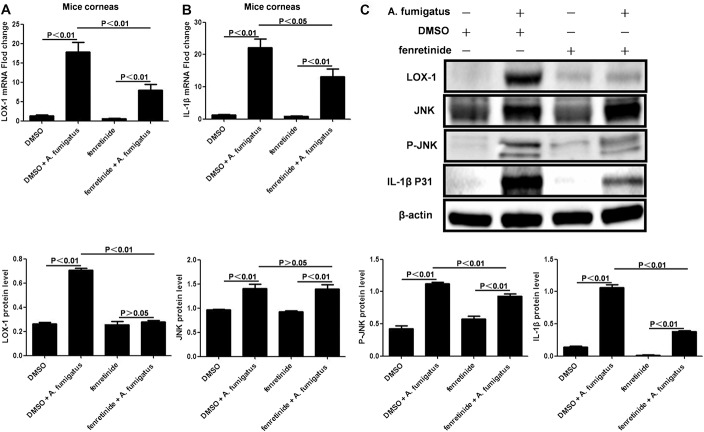
After fenretinide pretreatment, expression of LOX-1 (Fig. 3A; P < 0.01) and IL-1β (Fig. 3B; P < 0.05) in mRNA levels was significantly downregulated in A. fumigatus-infected mouse corneas. Compared with infection controls, LOX-1, phosphorylated JNK, and pro-IL-1β protein levels in corneas were significantly lower (P < 0.01, respectively) at 1 day after infection in fenretinide-pretreated mice (Fig. 3C). Interestingly, JNK was not affected by fenretinide pretreatment (Fig. 3C; P > 0.05).
FIGURE 3.
Fenretinide inhibited Aspergillus fumigatus-induced production of IL-1β through blockade of the LOX-1/JNK pathway in the mice cornea. A, After fenretinide pretreatment, mRNA expression of LOX-1 was significantly downregulated in A. fumigatus-infected mouse corneas (n = 6/group) at 1 day after infection. B, mRNA expression of IL-1β was also downregulated. C, Compared with infected controls, LOX-1, phosphorylated JNK, and pro-IL-1β protein levels in corneas were significantly lower at 1 day after infection in fenretinide-pretreated mice. JNK protein levels were not affected by fenretinide pretreatment (P > 0.05). The mean values and SDs of 2 independent experiments are shown.
Fenretinide Inhibited A. fumigatus-Induced Production of IL-1β Through Blockade of the LOX-1/JNK Pathway in THP-1 Macrophages
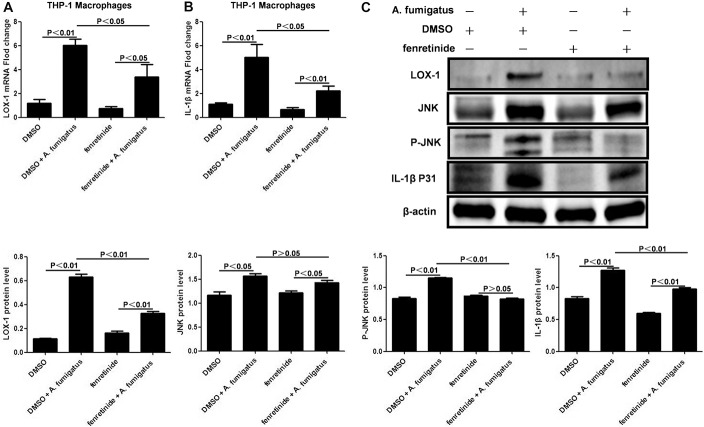
After fenretinide pretreatment, expression of LOX-1 (Fig. 4A; P < 0.05) and IL-1β (Fig. 4B; P < 0.05) mRNA was significantly downregulated in A. fumigatus THP-1 macrophages stimulated with A. fumigatus. Compared with stimulated controls, LOX-1, JNK, phosphorylated JNK, and pro-IL-1β protein levels were significantly lower in fenretinide-pretreated THP-1 macrophages at 16 hours after A. fumigatus stimulation (P < 0.01, respectively) (Fig. 4C). Similar to earlier results, JNK was not affected by pretreatment with fenretinide (Fig. 4C; P > 0.05).
FIGURE 4.
Fenretinide inhibited Aspergillus fumigatus-induced production of IL-1β through blockade of the LOX-1/JNK pathway in THP-1 macrophages. A, After fenretinide pretreatment, mRNA expression of LOX-1 was downregulated in A. fumigatus stimulated THP-1 macrophages (P < 0.05). B, mRNA expression of IL-1β was also observed to be downregulated (P < 0.05). C, Compared with stimulated controls, LOX-1, JNK, phosphorylated JNK, and pro-IL-1β protein levels in fenretinide-pretreated THP-1 macrophages were significantly lower at 16 hours with A. fumigatus stimulation (P < 0.01, respectively). JNK was not affected by fenretinide pretreatment (P > 0.05). The mean values and SDs of 2 independent experiments are shown.
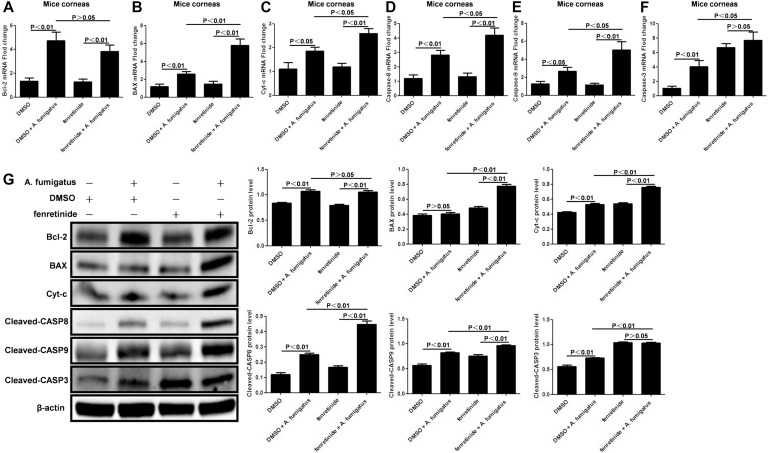
Fenretinide Pretreatment Increased Apoptosis in the Mouse A. fumigatus Keratitis Model
The levels of Bcl-2 mRNA (Fig. 5A; P > 0.05) and protein (Fig. 5G; P > 0.05) were not affected by fenretinide pretreatment. As determined by qRT-PCR, compared with infection controls, the mRNA levels of BAX (Fig. 5B; P < 0.01), cytochrome c (Fig. 5C; P < 0.05), cleaved-caspase-8 (Fig. 5D; P < 0.05), cleaved-caspase-9 (Fig. 5E; P < 0.05), and cleaved-caspase-3 (Fig. 5F; P < 0.05) in fenretinide-pretreated mice corneas were significantly increased. Finally, western blot of the protein levels of BAX, cytochrome c, cleaved-caspase-8, cleaved-caspase-9, and cleaved-caspase-3 in fenretinide-pretreated mice corneas showed significantly increased levels (Fig. 5G; P < 0.01, respectively).
FIGURE 5.
Fenretinide pretreatment increased apoptosis in mouse Aspergillus fumigatus keratitis. A, mRNA levels of Bcl-2 were not affected by fenretinide pretreatment (P > 0.05) (n = 6/group). B–F, Compared with infection controls, the mRNA levels of BAX (P < 0.01), cytochrome c (P < 0.05), cleaved-caspase-8 (P < 0.05), cleaved-caspase-9 (P < 0.05), and cleaved-caspase-3 (P < 0.05) in fenretinide-pretreated mice corneas were significantly increased. G, BAX, cytochrome c, cleaved-caspase-8, cleaved-caspase-9, and cleaved-caspase-3 protein levels in fenretinide-pretreated mice corneas were found to be significantly increased (P < 0.01, respectively); however, Bcl-2 levels were not affected by fenretinide pretreatment (P > 0.05). Mean values and SDs from 2 independent experiments are shown.
DISCUSSION
Our results demonstrate that fenretinide may suppress inflammation through reduced neutrophil recruitment and inflammatory cytokine production in A. fumigatus keratitis. Thus, can conclude that fenretinide may have a protective effect in early fungal keratitis. Although the role of fenretinide in fungal keratitis is still not well understood, several studies have indicated its role in suppressing inflammation. For example, Carocci et al13 showed that oral administration of fenretinide reduced dengue viremia by inhibiting steady-state viral genomic RNA accumulation in a mouse dengue virus infection model. Moreover, Lachance et al14 demonstrated that fenretinide pretreatment significantly improved survival of the acute phase in Streptococcus suis infection mouse models by reducing the expression of inflammatory mediators. Finally, Haraoui et al15 suppressed the acute and chronic stages of a streptococcal cell wall-induced arthritis rat model with orally administered fenretinide in a dose- and time-dependent manner. Together, these observations are consistent with our findings that fenretinide may suppress inflammation caused by infectious diseases.
Compared with the normal disease course of C57BL/6 mice, fenretinide pretreatment contributed to remarkable corneal clarity in early fungal keratitis. Decreased corneal neutrophil recruitment and MPO activity are believed to be responsible for this phenomenon.16 Kanagaratham et al9 identified that fenretinide prevents recruitment of inflammatory cells to the airways after ovalbumin challenge. Similarly, Dong et al17 demonstrated that fenretinide treatment blocked tumor angiogenesis with fewer M2-like macrophages in tumor tissues. These findings indicate that fenretinide may reduce inflammatory cell recruitment during the course of an immune response.
Similar to cellular infiltration, fenretinide-treated mice corneas exhibited impaired proinflammatory cytokine IL-1β production compared with controls. As a critical mediator of the host response to microbial infections,18,19 IL-1β is involved in a variety of cellular activities, including cell proliferation, apoptosis, and differentiation. Our results are consistent with reports that proinflammatory cytokine IL-1β, IL-6, cyclooxygenase-2, and prostaglandin E2 levels were found to be decreased in response to A. actinomycetemcomitans by treatment with fenretinide in Raw 264.7 cells.20
In addition, we observed in vitro that fenretinide inhibited A. fumigatus-induced production of IL-1β through blockade of the LOX-1/JNK pathway in mouse corneas and THP-1 macrophages. In addition to its role as an oxLDL receptor, LOX-1 is a multiligand receptor that binds to activated platelets, apoptotic cells, C-reactive protein, and bacteria.21 Previous research has found that the expression of IL-1β, IL-6, CXCL1, and tumor necrosis factor-α was regulated by LOX-1 through p38MAPK and that the cross-talk between TLR4 and LOX-1 elevated ROS levels in A. fumigatus keratitis.22,23 JNK is one of MAPKs, includes ERK1/2, JNK, and p38, and plays an important role in the cellular response to proinflammatory cytokines.24,25 Previous research has found that production of IL-1β induced by A. fumigatus is JNK dependent, and thus, JNK has an essential role in TLR2-induced corneal inflammation 11.26 These findings support the conclusion that fenretinide may suppress inflammation through the LOX-1/JNK/IL-1β pathway as part of the antifungal immune response.
Infection and apoptosis act as a combined inflammatory trigger to clear pathogens and repair host tissue damage during infection.27,28 Inflammatory immune responses induce necrotic cell death of immunocytes and release damage-associated molecular patterns, amplifying inflammation. Apoptosis during infection has been shown to shape a suppressive, autoreactive, or protective immune response.29,30 For this reason, fenretinide has been suggested as a potential treatment for endometriosis because it promotes apoptosis and increases STRA6 expression potentially reversing pathological loss of retinoid availability.31 Li et al32 identified that fenretinide induced apoptosis through activation of caspase-9, caspase-8, and caspase-3 inhibiting the growth of multiple myeloma cells. Raguénez et al33 observed that apoptosis was induced by the addition of fenretinide through upregulation of caspase-8 expression and activation, increasing apoptotic cell death in metastatic neuroblasts. Fenretinide was identified by Ulukaya et al34 to induce apoptosis by releasing cytochrome c and activating caspase-9 through a p53-independent pathway. Furthermore, Mohan et al35 demonstrated that a combination of fenretinide and apigenin promotes apoptosis of malignant neuroblastoma cells by upregulating Bax, downregulating Bcl-2, and activating caspase-3. Consistent with each of these findings, our results demonstrated that fenretinide promoted apoptosis by upregulating proapoptotic Bax, releasing cytochrome c, activating caspase-9, caspase-8, and caspase-3 in our mouse model of A. fumigatus keratitis.
In conclusion, our results demonstrate that fenretinide, a nontoxic vitamin A derivative, may suppress inflammation by reducing neutrophil recruitment, decreasing inflammatory cytokine production and inducing apoptosis during A. fumigatus keratitis. These findings were supported with results from a range of experiments using an A. fumigatus keratitis mouse model and cellular methods. As a synthetic all-trans retinoic acid molecule, fenretinide may be a potential treatment to control lesion severity in fungal keratitis.36
Footnotes
Supported by the National Natural Science Foundation of China (Grant No. 81300730, 81470609), China Postdoctoral Science Foundation (Grant No. 2018M630482), Key Research Project of Shandong (Grant No. 2018GSF118193), and Applied Basic Research Project of Qingdao (Grant No. 16-5-1-65-jch).
The authors have no conflicts of interest to disclose.
W. Zhao, C. Che, and K. Liu contributed equally to this work.
REFERENCES
- 1.Agarwal S, Iyer G, Srinivasan B, et al. Clinical profile of pythium keratitis: perioperative measures to reduce risk of recurrence. Br J Ophthalmol. 2018;102:153–157. [DOI] [PubMed] [Google Scholar]
- 2.Leal SM, Jr, Cowden S, Hsia YC, et al. Distinct roles for Dectin-1 and TLR4 in the pathogenesis of Aspergillus fumigatus keratitis. PLoS Pathog. 2010;6:e1000976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Pearlman E, Sun Y, Roy S, et al. Host defense at the ocular surface. Int Rev Immunol. 2013;32:4–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Underhill DM, Pearlman E. Immune interactions with pathogenic and commensal fungi: a two-way street. Immunity. 2015;43:845–858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zhu Z, Zhang H, Yue J, et al. Antimicrobial efficacy of corneal cross-linking in vitro and in vivo for Fusarium solani: a potential new treatment for fungal keratitis. BMC Ophthalmol. 2018;18:65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Austin A, Lietman T, Rose-Nussbaumer J. Update on the management of infectious keratitis. Ophthalmology. 2017;124:1678–1689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lachance C, Wojewodka G, Skinner TA, et al. Fenretinide corrects the imbalance between omega-6 to omega-3 polyunsaturated fatty acids and inhibits macrophage inflammatory mediators via the ERK pathway. PLoS One. 2013;8:e74875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lin CH, Lee SY, Zhang CC, et al. Fenretinide inhibits macrophage inflammatory mediators and controls hypertension in spontaneously hypertensive rats via the peroxisome proliferator-activated receptor gamma pathway. Drug Des Devel Ther. 2016;10:3591–3597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kanagaratham C, Kalivodová A, Najdekr L, et al. Fenretinide prevents inflammation and airway hyperresponsiveness in a mouse model of allergic asthma. Am J Respir Cell Mol Biol. 2014;51:783–792. [DOI] [PubMed] [Google Scholar]
- 10.Wu TG, Wilhelmus KR, Mitchell BM. Experimental keratomycosis in a mouse model. Invest Ophthalmol Vis Sci. 2003;44:210–216. [DOI] [PubMed] [Google Scholar]
- 11.Yuan K, Zhao G, Che C, et al. Dectin-1 is essential for IL-1β production through JNK activation and apoptosis in Aspergillus fumigatus keratitis. Int Immunopharmacol. 2017;52:168–175. [DOI] [PubMed] [Google Scholar]
- 12.Li C, Zhao G, Che C, et al. The role of LOX-1 in innate immunity to Aspergillus fumigatus in corneal epithelial cells. Invest Ophthalmol Vis Sci. 2015;56:3593–3603. [DOI] [PubMed] [Google Scholar]
- 13.Carocci M, Hinshaw SM, Rodgers MA, et al. The bioactive lipid 4-hydroxyphenyl retinamide inhibits flavivirus replication. Antimicrob Agents Chemother. 2015;59:85–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lachance C, Segura M, Dominguez-Punaro MC, et al. Deregulated balance of omega-6 and omega-3 polyunsaturated fatty acids following infection by the zoonotic pathogen Streptococcus suis. Infect Immun. 2014;82:1778–1785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Haraoui B, Wilder RL, Allen JB, et al. Dose-dependent suppression by the synthetic retinoid, 4-hydroxyphenyl retinamide, of streptococcal cell wall-induced arthritis in rats. Int J Immunopharmacol. 1985;7:903–916. [DOI] [PubMed] [Google Scholar]
- 16.Boff D, Crijns H, Teixeira MM, et al. Neutrophils: beneficial and harmful cells in septic arthritis. Int J Mol Sci. 2018;19:E468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dong R, Gong Y, Meng W, et al. The involvement of M2 macrophage polarization inhibition in fenretinide-mediated chemopreventive effects on colon cancer. Cancer Lett. 2017;388:43–53. [DOI] [PubMed] [Google Scholar]
- 18.Tosello Boari J, Acosta Rodriguez EV. IL-1β/CD14 pathway induces IL-17 production: dendritic cells activated with IL-1β set Th17 cells on fire by CD14-mediated mechanisms. Immunol Cell Biol. 2016;94:903–904. [DOI] [PubMed] [Google Scholar]
- 19.Ilarregui JM, van Beelen AJ, Fehres CM, et al. New roles for CD14 and IL-β linking inflammatory dendritic cells to IL-17 production in memory CD4+ T cells. Immunol Cell Biol. 2016;94:907–916. [DOI] [PubMed] [Google Scholar]
- 20.Yu H, Valerio M, Bielawski J. Fenretinide inhibited de novo ceramide synthesis and proinflammatory cytokines induced by Aggregatibacter actinomycetemcomitans. J Lipid Res. 2013;54:189–201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wu Z, Sawamura T, Kurdowska AK, et al. LOX-1 deletion improves neutrophil responses, enhances bacterial clearance, and reduces lung injury in a murine polymicrobial sepsis model. Infect Immun. 2011;79:2865–2870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gao X, Zhao G, Li C, et al. LOX-1 and TLR4 affect each other and regulate the generation of ROS in A. fumigatus keratitis. Int Immunopharmaco. 2016;40:392–399. [DOI] [PubMed] [Google Scholar]
- 23.He K, Yue LH, Zhao GQ, et al. The role of LOX-1 on innate immunity against Aspergillus keratitis in mice. Int J Ophthalmol. 2016;9:1245–1250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Salvador-Bernáldez M, Mateus SB, Del Barco Barrantes I, et al. p38α regulates cytokine-induced IFNγ secretion via the Mnk1/eIF4E pathway in Th1 cells. Immunol Cell Biol. 2017;95:814–823. [DOI] [PubMed] [Google Scholar]
- 25.Zheng S, Hedl M, Abraham C. TAM receptor-dependent regulation of SOCS3 and MAPKs contributes to proinflammatory cytokine downregulation following chronic NOD2 stimulation of human macrophages. J Immunol. 2015;194:1928–1937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Adhikary G, Sun Y, Pearlman E. C-Jun NH2 terminal kinase (JNK) is an essential mediator of toll-like receptor 2-induced corneal inflammation. J Leukoc Biol. 2008;83:991–997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bliss-Moreau M, Chen AA, D'Cruz AA, et al. A motive for killing: effector functions of regulated lytic cell death. Immunol Cell Biol. 2017;95:146–151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Vasilikos L, Spilgies LM, Knop J, et al. Regulating the balance between necroptosis, apoptosis and inflammation by inhibitors of apoptosis proteins. Immunol Cell Biol. 2017;95:160–165. [DOI] [PubMed] [Google Scholar]
- 29.Campisi L, Cummings RJ, Blander JM. Death-defining immune responses after apoptosis. Am J Transpl. 2014;14:1488–1498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Brault M, Oberst A. Controlled detonation: evolution of necroptosis in pathogen defense. Immunol Cell Biol. 2017;95:131–136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Pavone ME, Malpani SS, Dyson M, et al. Fenretinide: a potential treatment for endometriosis. Reprod Sci. 2016;23:1139–1147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Li X, Ling W, Pennisi A, et al. Fenretinide inhibits myeloma cell growth, osteoclastogenesis and osteoclast viability. Cancer Lett. 2009;284:175–181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Raguénez G, Mühlethaler-Mottet A, Meier R, et al. Fenretinide-induced caspase-8 activation and apoptosis in an established model of metastatic neuroblastoma. BMC Cancer. 2017;9:97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ulukaya E, Pirianov G, Kurt MA, et al. Fenretinide induces cytochrome c release, caspase 9 activation and apoptosis in the absence of mitochondrial membrane depolarisation. Cell Death Differ. 2003;10:856–859. [DOI] [PubMed] [Google Scholar]
- 35.Mohan N, Banik NL, Ray SK. Combination of N-(4-hydroxyphenyl) retinamide and apigenin suppressed starvation-induced autophagy and promoted apoptosis in malignant neuroblastoma cells. Neurosci Lett. 2011;502:24–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zhou HY, Zhong W, Zhang H, et al. Potential role of nuclear receptor ligand all-trans retinoic acids in the treatment of fungal keratitis. Int J Ophthalmol. 2015;8:826–832. [DOI] [PMC free article] [PubMed] [Google Scholar]