Abstract
High salt, angiotensin II (Ang II), and reactive oxygen species (ROS) enhance progression of chronic kidney disease (CKD). We tested the hypothesis that a high salt intake generates specific ROS to enhance Ang II contractions of afferent arterioles from mice with reduced renal mass (RRM). C57BL/6 mice were subjected to surgical RRM or sham operations and received 6% or 0.4% NaCl salt diet for 3 months. Ang II contractions were measured in perfused afferent arterioles and superoxide (O2.-) and H2O2 by fluorescence microscopy. RRM enhanced the afferent arteriolar gene expression for p47phox and NOX2 and high salt intake in RRM mice enhanced gene expression for AT1 receptors, POLDIP2 and NOX4 and reduced catalase. High salt in mice with RRM enhanced arteriolar O2.- and H2O2 generation and maximal contractions to Ang II (10−6 mol∙L−1) that were dependent on O2.- since they were prevented by gene deletion of p47phox and on H2O2 since they were prevented by transgenic smooth muscle cell expression of catalase (tgCAT-SMC) and POLDIP2 gene deletion. Three months of tempol normalized arteriolar ROS and Ang II contractions. However, arteriolar contractions to lower concentrations of Ang II (10−8 to 10−11 mol·l−1) were paradoxically inhibited by H2O2 and POLDIP2. In conclusion, both O2.- from p47phox/NOX2 and H2O2 from NOX4/POLDIP2 enhance maximal arteriolar Ang II contractions from RRM mice during high salt but H2O2 and NOX4/POLDIP2 reduce the sensitivity to lower concentrations of Ang II by >100-fold. Tempol prevents all of these changes in function.
Keywords: Hydrogen peroxide, superoxide, POLDIP2, p47phox, tempol
INTRODUCTION
Angiotensin II (Ang II) enhances the progression of acute or chronic kidney disease (CKD) in experimental 1, 2 and clinical 3 studies. Some of the adverse effects of Ang II on progression of CKD may relate to renal vasoconstriction that limits renal blood flow and O2 delivery 4–7 since hypoxia promotes the progression of CKD 8, 9. A high salt intake accelerates the progression of CKD in both experimental 2, 8 and clinical studies 10 but paradoxically activates the intrarenal renin-angiotensin system (RAS), despite suppressing systemic renin release 2, perhaps by enhancing the intrarenal generation of reactive oxygen species (ROS) 8, 11, 12 ROS can have opposing effects on afferent arteriolar function 13. Thus, the NOX2 isoform of nicotinamide adenine dinucleotide phosphate (NADPH) oxidase is activated by p47phox/p67phox to generate superoxide (O2.-) that can enhance arteriolar contractility to perfusion pressure 14. However, the NOX4 isoform is activated by POLDIP2 to generate principally hydrogen peroxide (H2O2) 15 that, at low concentrations, reduces contraction to perfusion pressure 14, 16. However, the effects of these two ROS in mediating the effects of high salt intake on the renal afferent arteriolar reactivity to Ang II in models of CKD have not been studied. This was the object of the study.
We tested the hypothesis that a high salt diet increases O2.- and/or H2O2 in afferent arterioles from mice with reduced renal mass (RRM) and that these regulate contractions to Ang II. To assess the sources of ROS on the afferent arteriolar contractions to Ang II, the effects of a high salt intake in mice with RRM were assessed in p47phox or POLDIP2 gene deleted mice or mice transgenic for catalase in vascular smooth muscle cells (TgCAT-SMC). Finally tempol was administered to test the role of prolonged excess ROS and the potential impact of effective antioxidant therapy on Ang II contractility.
METHODS
The data that support the findings of this study are available from the corresponding author upon reasonable request.
Male C57Bl/6 mice aged 2 to 3 months weighing 25 – 31 g (Charles River Inc. Germantown, MD, USA) were randomized to surgical RRM or sham operation (sham) after which both groups were randomized for 3 months to feeding a 0·4g·100g−1 (normal) or a 6g·100g−1 (high) NaCl salt diet (Harlan Teklad, CA). Studies of gene expression were undertaken in RRM and sham mice fed 0.4 or 6% salt diets, but subsequent studies of mechanisms were undertaken in the light of these gene expression studies only in mice fed 6% salt. Additional groups of high salt fed C57Bl/6 mice were randomized to drink tempol (2 mmol∙L−1 in water) or vehicle 8. Further groups of p47phox−/−, 17 TgCAT-SMC 16 and POLDIP2+/− 15, 16 mice, and their wild type (+/+) littermates, were fed a high salt diet and randomized to RRM or sham operations. POLDIP2+/− mice were used since few POLDIP2−/− mice survive 15.
All procedures conformed to the Guide for Care and Use of Laboratory Animals prepared by The Institute for Laboratory Animal Research. Studies were approved by the Georgetown University Animal Care and Use Committee.
Animal surgery and preparation of afferent arterioles:
A two-step surgical 5/6 nephrectomy procedure was used to create RRM as described 8. Sham-operated control mice (sham) were subject to a similar two stage procedure without removal of kidney tissue. Other groups of wild type mice fed a high salt diet were randomized to receive a vehicle (water) or tempol (2 mmol·L−1) in the water. After three months, mice (n = 6 – 12 per group) were euthanized and renal afferent arterioles were dissected, mounted and perfused via a pipette whose pressure at its tip was recorded by a calibrated intra-luminal micropipette, as described 17.
Measurements of afferent arteriolar responses to angiotensin II:
Graded concentrations of Ang II (10−12 to 10−6 mol·l−1) were added to the bath for 2 mins and the arteriolar diameter recorded 17. Arterioles were perfused at 40 mmHg 18.
ROS measurements in afferent arterioles:
Incubation of afferent arterioles with paraquat to generate O2.- increases the fluorescence ratio of ethidium: dihydroethidium (E:DHE) that is >80% prevented by incubation with PEG-superoxide dismutase (SOD). Therefore, PEG-SOD-inhibitable E: DHE fluorescence was selected as a measure of arteriolar O2.−. Further, incubation with H2O2 increases the fluorescence of 2’,7’-dichlorodihydrofluorescein diacetate (H2DCFDA) that is >90% prevented by incubation with PEG-catalase. Therefore, PEG-catalase-inhibitable H2DCFDA fluorescence was selected as a measure of H2O2 14.
Gene expression in afferent arterioles:
These studies were performed in ∼15 afferent arterioles dissected from each mouse, pooled and placed in 2 ml of lysing matrix in a D tube (MP Biochemical) containing QIAzol lysis reagent and homogenized by MP Fast Prep. Total RNA was extracted with an RNeasy Mini Kit (Qiagen Inc., Valencia, CA). Primers and probes for mouse AT1R, AT2R, catalase, p47phox, NOX2, POLDIP-2, NOX4 and 18s rRNA (ID: Mm03928990_g1) were used to quantitate the mRNA expressions (Applied Biosystems, Foster City, CA) using RT/PCR primers as previously described 16, 19, 20.
Statistics:
Data are expressed as mean ± SEM. A 2 × 2 analyses of variance (ANOVA) was applied to assess the effects of RRM and salt intake, genotype, or tempol and the interaction. Changes were analyzed using nonparametric statistics (GraphPad Prism, GraphPad Software). P < 0.05 was considered statistically significant.
RESULTS
1. High dietary salt enhances afferent arteriolar O2.- and H2O2 generation and contractions to high concentrations of Ang II in mice with RRM
Both RRM and high salt enhanced O2.-and H2O2 generation with 10−6 mol·L−1 Ang II in afferent arterioles (Fig 1A and B). Moreover, a high salt intake exaggerated the effects of RRM to increase H2O2 (Fig 1B; positive interaction term). RRM did not change the afferent arteriolar contractions to Ang II in mice fed a normal salt diet (Figure 1C) but enhanced contractions to high concentrations of Ang II (10−7 to 10−6 mol·l−1) in mice fed high salt diet by > 60% (maximum change in diameter, −72 ± 2% versus −45 ± 2%: P<0.005).
Figure 1:
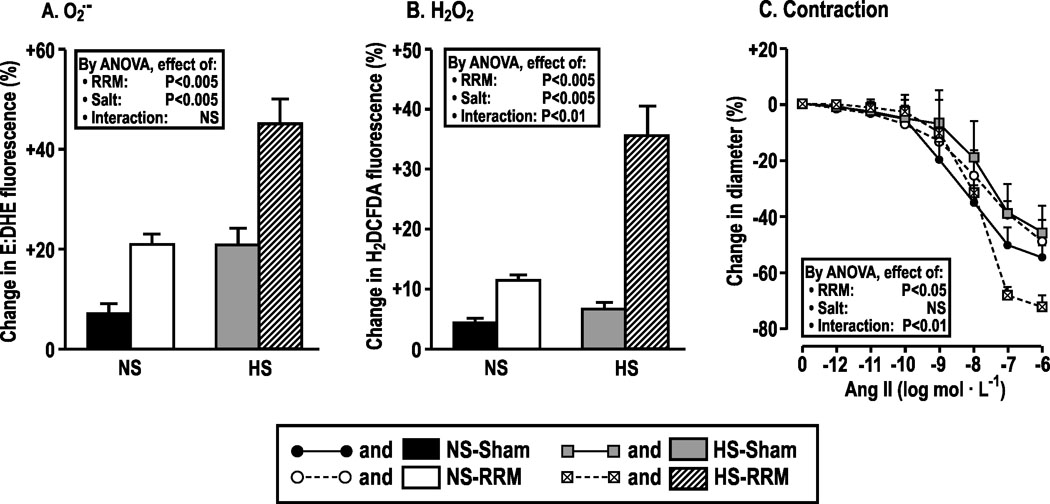
Dietary salt intake increases the ROS production and contractions to angiotensin II in afferent arterioles from mice with RRM. Mean ± SEM values (n = 5 to 6) for O2.-, H2O2 and contractions to angiotensin II of afferent arterioles from C57Bl/6 WT mice fed normal (NS) or high salt (HS) diets for three months after sham surgery (NS-sham, solid boxes or solid circles with continuous line; HS-sham, gray boxes or gray squares with continuous line) or RRM (NS-RRM, open boxes or open circles with broken line; HS-RRM, slash-hatched boxes or crossed boxes with broken line). Data are shown for changes in ethidium: dihydroethidium (E: DHE) ratio (panel A) and H2DCFDA fluorescence (panel B) with angiotensin II (10−6mol·L−1) and changes in diameter in afferent arterioles from sham or RRM mice fed normal or high salt with graded angiotensin II (panel C). Ang II: angiotensin II; E: DHE: ethidium: dihydroethidium; H2DCFDA: 2’,7’-dichlorodihydrofluorescein diacetate.
2. RRM and high dietary salt modulate gene expression in afferent arterioles.
Compared to sham, mice with RRM fed a normal salt diet had an increase in afferent arteriolar mRNA expression for p47phox and NOX2 and a decrease in NOX4. Mice with RRM fed a high salt diet had an increase on afferent arteriolar mRNA expression for AT1R, p47phox, POLDIP2, NOX2, and NOX4 but a reduction in mRNA for catalase (Figure 2). Expression of AT2R was unchanged by salt or RRM (data not shown). Therefore, the roles of p47phox/NOX2, POLDIP2/NOX4 and catalase in ANGII contractions were explored in mice with RRM fed a high salt diet.
Figure 2:
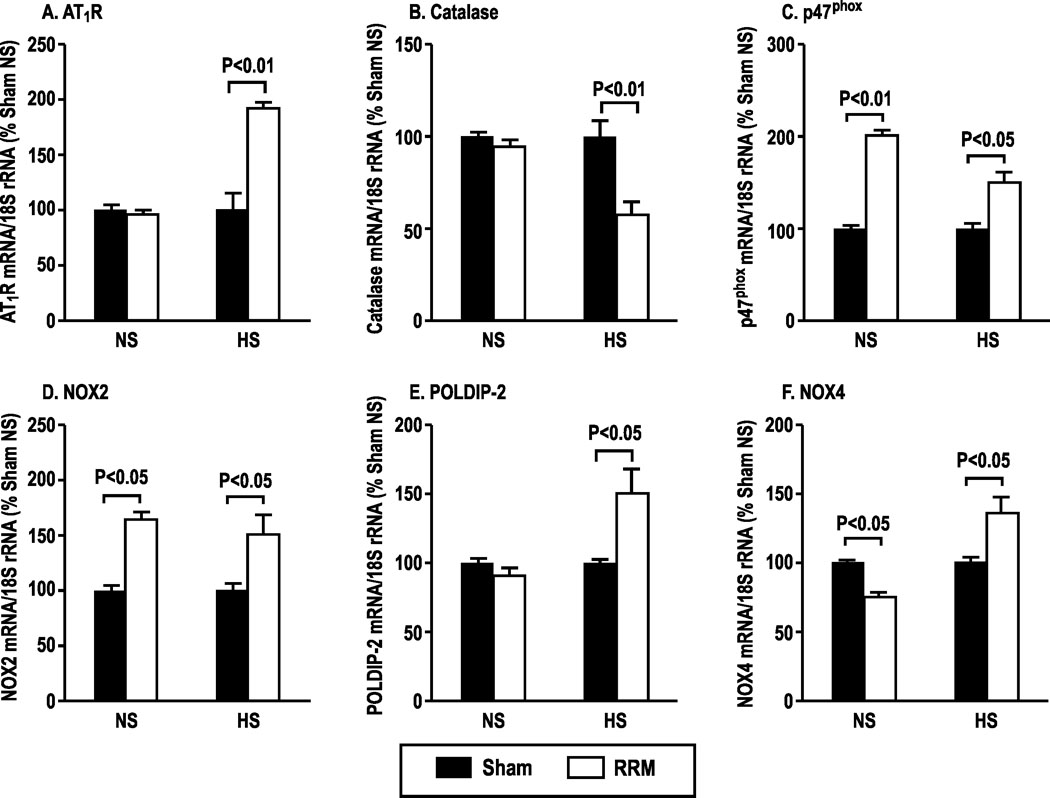
Effects of dietary salt intake and reduced renal mass on gene expression in afferent arterioles. Mean ± SEM values (n=6) for expression of mRNAs for angiotensin type 1 receptor (AT1R), catalase, p47phox, neutrophil oxidase 2 (NOX2), POLDIP-2 and NOX4 in afferent arterioles dissected from C57Bl/6 WT mice fed with normal salt (NS) or high salt (HS) diets for three months after sham (solid boxes) and RRM (open boxes) surgeries.
3. Enhanced responses of afferent arterioles to high concentrations of Ang II in mice with RRM fed high dietary salt are prevented by metabolism of O2.- or H2O2.
Compared to sham, incubation of arterioles from mice with RRM fed a high salt diet with Ang II increased the generation of O2.- (especially at high Ang II concentrations; positive interaction term in Fig 3A) and H2O2 (Fig 3C). PEG-SOD and PEG-catalase reduced the contractions to high concentrations of Ang II (10−7 and 10−6 mol·l−1) in arterioles from mice with RRM fed high dietary salt to levels of sham mice (Figure 3B and 3D) but PEG-catalase enhanced the contractions to lower concentrations of Ang II (10−11 to 10−9 mol·l−1; Figure 3D).
Figure 3:
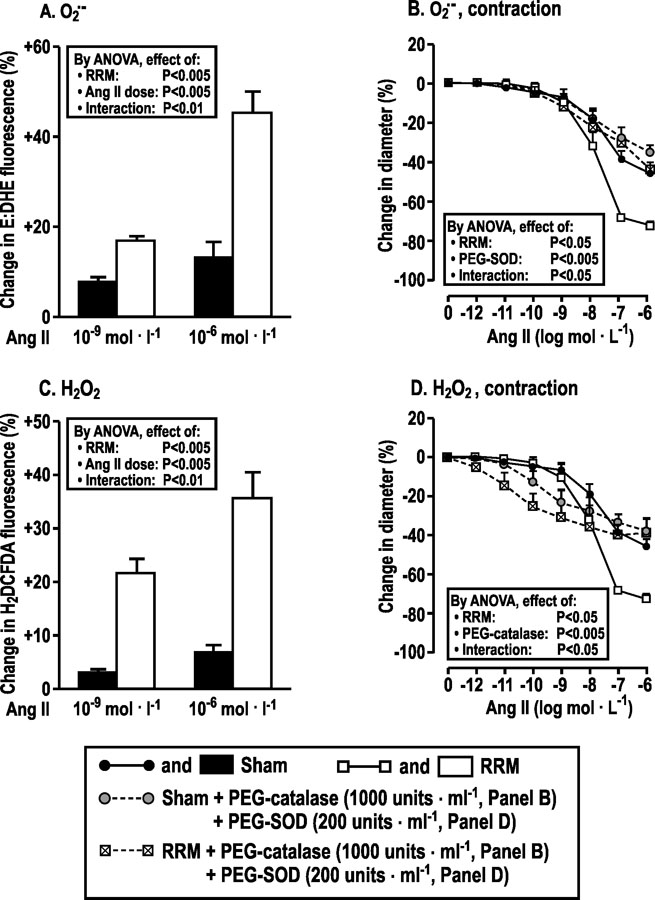
Reduced renal mass in mice fed a high salt diet increases the afferent arteriolar O2.-and H2O2 generation with angiotensin II that contributes to angiotensin II contractions. Mean ± SEM values (n = 6) for changes in O2.-, H2O2 and responses to angiotensin II of afferent arterioles from C57Bl/6 wild type (WT) mice fed a high salt diet for three months after sham surgery (solid boxes, solid circles with continuous line) or RRM (open boxes, open squares with continuous lines) and after addition of PEG-catalase or PEG-SOD to the bath of perfused afferent arterioles from mice after sham (gray filled cycles with broken line) or RRM (crossed boxes with broken lines). Data are shown for changes in ethidium: dihydrithydium (E: DHE) fluorescence (Panel A) or H2DCFDA fluorescence (panel C) with 10−9 or 10−6mol·L−1 of angiotensin II, and changes in diameters (panels B, D) in response to graded angiotensin II.
4. Enhanced afferent arteriolar O2.- and contractions to high concentrations of Ang II in afferent arterioles from mice with RRM fed high dietary salt are prevented in mice with deletion of the p47phox gene or by 3 months of oral tempol.
Compared to sham, afferent arterioles from p47phox wild type (+/+) mice with RRM fed a high salt diet had increased generation of O2.- (Fig 4A) and increased contractions to high concentrations of Ang II (10−7 to 10−6 mol·l−1) (Fig 4B), similar to C57Bl/6 mice (Fig 1). However, arterioles from littermate p47phox knockout (−/−) mice with RRM fed a high salt diet did not generate more O2.- (Fig 4A) and did not have stronger contractions with Ang II (Fig 4B) than arterioles from sham mice. These effects of p47phox −/− were similar to incubation of arterioles from C57Bl/6 WT mice with PEG-SOD (Fig 3A and B). Indeed, three months of oral tempol (2 mmol·l−1) vs vehicle in C57Bl/6 mice with RRM fed a high salt diet prevented the increases in O2.- generation (Fig 4C) and in contractions to high concentrations of Ang II (Fig 4D). These results of tempol in mice with RRM fed a high salt diet were similar to mice with RRM fed a normal salt diet (Fig 1C)
Figure 4:
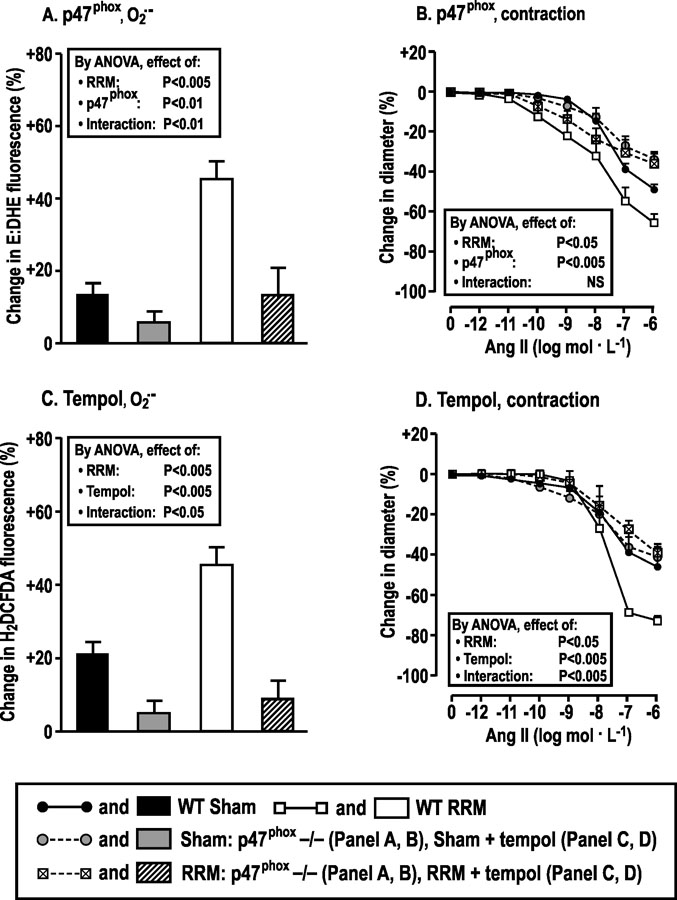
Superoxide generated by p47phox/NOX2 is required for enhanced contractions to high concentrations of Ang II in arterioles from mice with reduced renal mass fed a high salt diet. Mean ± SEM values (n = 5 – 7) for changes in O2.- and responses to angiotensin II of afferent arterioles from mice fed a high salt diet for three months after sham surgery (p47phox +/+: solid boxes, or solid circles with continuous lines; p47phox −/−: gray filled boxes or gray filled circles with broken lines) or RRM (p47phox +/+: open boxes, open squares with continuous lines; p47phox −/−: slash hatched boxes, crossed boxes with broken lines) surgeries. In other studies, sham or RRM mice fed a high salt diet were administered tempol (2 mmol∙L−1) or vehicle for 3 months. Data are shown in panels A and C for changes in ethidium: dihydroethidium (E: DHE) ratio with 10−6 mol·L−1 of angiotensin II, and in panels B and D for changes in diameter in response to graded angiotensin II.
5. Differential modulation of afferent arteriolar H2O2 and contractions to high and low concentrations of Ang II in mice with RRM fed high dietary salt in TgCAT-SMCand POLDIP2 +/− mouse strains
Incubation of afferent arterioles from mice with RRM from TgCAT-SMC(vs wt) (Fig 5A and B) and POLDIP2 +/− (vs +/+) strains (Fig 5C and D) fed a high a salt diet with high concentrations of Ang II (10−7 and 10−6 mol·l-1) failed to increase H2O2 generation or contractions, yet both had increased contractions to lower concentrations of Ang II (10−10 to 10−9 mol·l−1). These effects of TgCAT-SMC and POLDIP2 +/− were similar to arterioles from C57Bl/6 WT mice incubated with PEG-catalase (Fig 3C and D).
Figure 5:
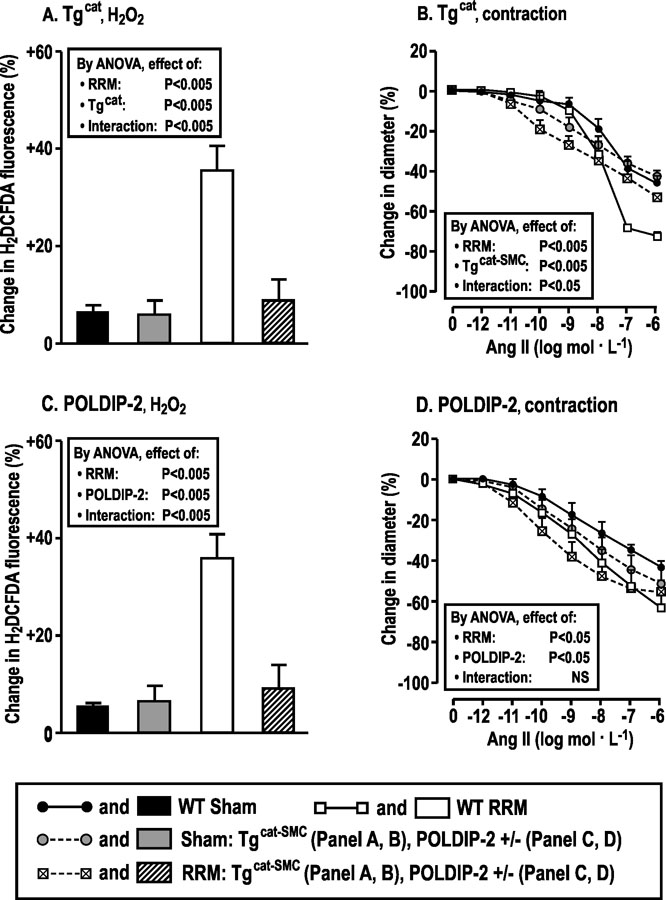
Hydrogen peroxide generated by POLDIP2/NOX4 modulates contractions to angiotensin II in afferent arterioles from mice with reduced renal mass fed a high salt diet. Mean ± SEM values (n =6) for changes in H2O2 and responses to angiotensin II of afferent arterioles from WT and transgenic mice over-expressing catalase in smooth muscle cells (TgCAT-SMC) or POLDIP2+/− mice fed a high salt diet for three months after sham surgery (WT-sham: solid boxes, solid circles with continuous lines; sham TgCAT-SMC or sham POLDIP2+/−: gray filled boxes, gray filled circles with broken lines) or RRM (WT-RRM: open boxes, open squares with continuous lines; RRM TgCAT-SMC or RRM POLDIP2+/− slash hatched boxes, crossed boxes with broken lines). Data are shown for changes in H2DCFDA fluorescence with 10−6mol·L−1 of angiotensin II (panel A, C), and changes in diameters (Panel B, D) with graded angiotensin II.
DISCUSSION
The present study confirms that high dietary salt increases O2-, and especially H2O2, in afferent arterioles from mice with RRM but extends this from ROS generation with perfusion pressure 8, 14, 16 to ROS generation with Ang II.
The new findings, summarized in Figure 6, are that mice with RRM fed a normal or high dietary salt had enhanced mRNA expression in their afferent arterioles for p47phox and NOX2. However only mice with RRM fed high dietary salt had enhanced mRNA expression for AT1R, POLDIP2 and NOX4 and decreased mRNA expression for catalase. The increased arteriolar expression of p47phox/NOX2, was a source of increased O2- since the increased O2- was prevented in p47phox −/− mouse arterioles. Moreover, the increased expression of POLDIP2/NOX4 and decreased expression of catalase were sources of increased H2O2 since the increased H2O2 was prevented in POLDIP2 +/− and in TgCAT-SMC mouse arterioles. Both the increased O2- and contractions to maximal concentrations of Ang II in mice with RRM fed a high dietary salt were prevented in arterioles from p47phox (−/−) mice, similar to the effects of metabolism of O2- with PEG-SOD. Likewise, both the increased H2O2 and contractions to high concentrations of Ang II in RRM mice fed high dietary salt were prevented in arterioles from tgCAT-SMC and POLDIP2 (+/−) mice, similar to the effect of metabolism of H2O2 with PEG-catalase. However, the increased H2O2 in arterioles from mice with RRM fed high dietary salt paradoxically reduced contractions to lower concentrations of Ang II (10−10 to 10−9 mol∙L−1). Indeed, in POLDIP2 +/− mouse arterioles or after metabolism of H2O2, the sensitivity of the arterioles to Ang II was increased 100 to 1000-fold. Three months of oral tempol prevented the increased generation of O2- and normalized the contractions to Ang II.
Figure 6:
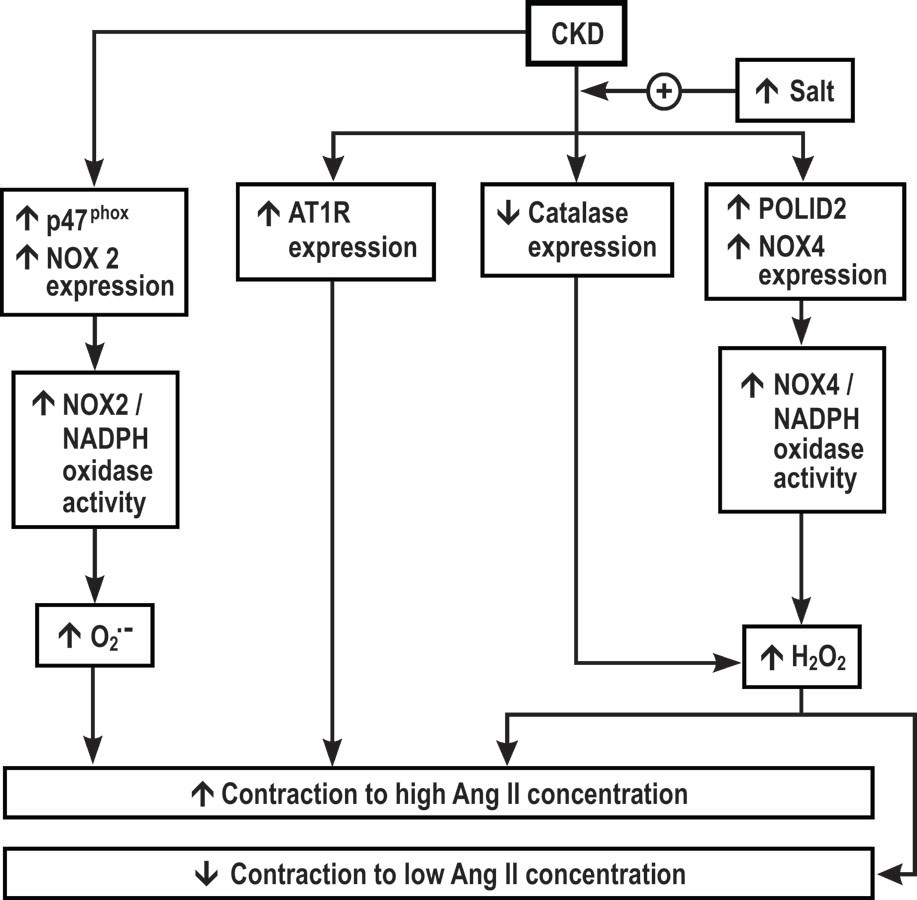
Hypothesis for effects of dietary salt and CKD on angiotensin II contractions of renal afferent arterioles. CKD, chronic kidney disease;AT1R , angiotensin type 1 receptor; NOX, neutrophil oxidase; NADPH oxidase, nicotinamide adenine dinucleotide phosphate oxidase. The effects of CKD to increase afferent arteriolar expression ofAT1R, POLDIP2 and NOX4 and to decrease catalase (and hence to increase H2O2) are enhanced by a high salt diet whereas CKD itself enhances expression of p47phox and NOX2 that increase superoxide (O2.-). Superoxide, AT1R and H2O2 all increase maximal concentrations with angiotensin II whereas H2O2 reduces contractions to lower concentrations of angiotensin II.
The p47phox/ NOX2 isoform of NADPH generates primarily O2- 13, 16, 19. Therefore, the effects of Ang II to generate increased O2- in arterioles from mice with RRM is consistent with the effect of RRM to enhance p47phox and NOX2 gene expression. Moreover, gene deletion of p47phox prevented any increase in O2- with Ang II in arterioles from mice with RRM fed a high salt diet.
The POLDIP2/NOX4 isoform of NADPH oxidase generates primarily H2O2 13, 15, 16. Therefore, the effect of Ang II to generate increased H2O2 in arterioles from mice with RRM fed high dietary salt is consistent with the effect of RRM to increase the arteriolar expression of POLDIP2 and NOX4, and to reduce the expression of catalase. Moreover, deletion of POLDIP2 or enhanced expression of SMC catalase prevented any increase in H2O2 with Ang II in arterioles from mice with RRM fed high dietary salt.
There was internal consistency in the results of the effects of O2- and H2O2 in this model of RRM and high dietary salt. Thus, both metabolism of O2- with PEG-SOD or tempol and reduction of O2- in p47phox knockout reduced maximal Ang II contractions. Likewise, both metabolism of H2O2 with PEG-catalase and reduction of H2O2 in TgCAT-SMC and POLDIP2 +/− mice enhanced contraction to lower concentrations of Ang II but reduced contractions to high concentrations of Ang II.
The physiological concentrations of Ang II in the renal afferent arteriole are unknown but may be uniquely high since the juxtaglomerular cells of the afferent arteriole are the site of most of the kidney renin production 21. The concentration of Ang II in the circulation is approximately 2 × 10−11 mol∙L−1 and is increased by approximately 4-fold in CKD 22 while the Ang II concentrations in the renal parenchyma are higher than in plasma 23 and the renin and Ang II concentrations of renal lymph are significantly higher than plasma reflecting higher levels in renal interstitial fluid 24, 25. Whereas high dietary salt in rats reduces circulating levels of Ang II, it paradoxically increases renal parenchymal levels of Ang II 2. The concentrations of Ang II in proximal tubule fluid and efferent arteriolar plasma are 3 × 10−8 and 10−7 mol ∙ L−1 respectively 26. Thus, although it is difficult to predict the endogenous concentrations of Ang II in or around afferent arterioles, it is likely to be high and to be enhanced further by a high salt intake and by CKD. It may be within the range of Ang II concentration of 10−11 to 10−8 mol∙L−1 that contracted afferent arterioles after metabolism of H2O2 or in POLDIP2 gene deleted mice, but it may even be within the range of Ang II concentration of 10−7 to 10−6 mol∙L−1 that contracted afferent arterioles in this study of mice with RRM fed high dietary salt diet.
It is not clear why three months of RRM, even with a high salt diet, did not lead to severe CKD despite increased systemic and renal levels of ROS 8. It is possible that the time was not sufficient or that this represents the resistance of this C57Bl/6 mouse strain to develop CKD, or that an addition stressor such as Ang II infusion is required 27.
High dietary salt intake selectively enhanced the mRNA expression for AT1R (but not AT2R) in afferent arterioles from mice with RRM despite the report that intrarenal Ang II concentrations are enhanced in this model 2 and should thereby downregulate receptor expression. The present findings are consistent with our earlier report that prolonged Ang II infusion enhanced AT1R expression in rabbit afferent arterioles. The enhanced AT1R expression in these two models may contribute to the findings of increased contractions to Ang II in this and prior studies 28, 29. The increased AT1R expression may relate to the increased afferent arteriolar ROS since this was increased by high dietary salt in RRM 2 and by Ang II infusion 28, 29 in both these models and ROS can enhance vascular AT1R expression 12. However, mice with RRM fed a normal salt intake also had increased afferent arteriolar O2∙- and H2O2 (Figure 1A and B) yet AT1R expression was not increased (Figure 2A). It is possible that a more augmented increase in ROS produced by RRM and a high salt diet (Figure 1A and B) is required to increase AT1R expression. In a similar mouse model of RRM and high salt diet, tempol prevented any increase in AT1R expression in the kidneys and prevented any increase in ROS as indexed by the excretion of 8-isoprostane F2α or H2O2 8.
Tempol is a well-established SOD mimetic 30, but also acts as a catalase mimetic 8, 31, 32 Tempol abrogated excessive O2.- and, in parallel, abrogated excessive Ang II contractions in mice with RRM fed high dietary salt yet it preserved afferent arteriolar myogenic contractions and renal parenchymal PO2 in similar mouse models 8. This combination of reduced Ang II contractility but preserved myogenic contractility and renal parenchymal oxygenation could contribute to the beneficial effects of nitroxides such as tempol in preventing progression of CKD 8, 32.
Prior studies have reported that O2.- can enhance Ang II contractions of afferent arterioles 28, 29, 33, 34 consistent with the findings in this study. Low concentration of H2O2 (10 µ mol∙L−1) reduces Ang II contraction of afferent arterioles from mice with renal ischemia-reperfusion injury but higher concentrations of H2O2 (25 µ mol∙L−1) 35 can enhance contractions consistent with the findings in this study. The mechanism of enhanced Ang II contractions with higher concentrations of H2O2 has been related to activation of vascular L-type calcium channels 36 and tyrosine kinase 37. The reports of variable effects of H2O2 on Ang II contractions 35–39 may be explained by our finding of biphasic effects of H2O2: reduced contractions to lower concentrations of Ang II yet enhanced contractions to higher concentration of Ang II in mice with RRM fed high dietary salt.
We acknowledge some limitations. First, the BP was not measured. However, in a prior study with this model neither RRM, salt intake nor tempol changed the mean arterial pressure (MAP) measured telemetrically over 3 months 8. Second, we acknowledge that mice in this RRM model in which the BP is maintained even during high salt intake, and the glomerular filtration rate (GFR) is well maintained despite removal of 5/6 of kidney tissue, show greater ability to adapt than human subject with CKD who typically develop salt-sensitive hypertension and have a more limited ability to maintain their GFR after severe loss of nephrons. 40. Third, the mechanisms whereby O2.- and H2O2 modulate arteriolar contractility with Ang II were not evaluated. However, these mechanisms have been studied by us previously 14. Fourth, tempol has effects beyond its well-established role as a superoxide dismutase mimetic, notably acting as a catalase mimetic during long term studies 8 and actions on a host of other ROS 30 that could have contributed to its beneficial effects.
In conclusion, feeding high dietary salt to mice with RRM increased afferent arteriolar O2.- from p47phox/ NOX2 and H2O2 from POLDIP2/ NOX4, which contributed to enhanced contractions to 10−7 to 10−6 mol·L−1 Ang II. In contrast, metabolism of H2O2 by catalase or prevention of H2O2 generation by POLDIP2 gene deletion increased the sensitivity of the afferent arterioles to Ang II by 100–1000 fold. Tempol administration prevented excessive ROS generation in arterioles from mice with RRM fed high dietary salt and normalized the Ang II contractions.
PERSPECTIVE
Mice with CKD fed high dietary salt have increased renal parenchymal ROS and Ang II that elicit reflex increases in renal inflammation and damage 2. The present findings demonstrate that mice with RRM fed high dietary salt also have enhanced afferent arteriolar ROS and contractile responses to Ang II. Ang II and high dietary salt in normal rats both increase renal ROS 11, 41 that contributes to renal parenchymal hypoxia 6, 7. These findings suggest that high dietary salt may impair renal function in a damaged kidney 1, 2, 10 by increasing afferent arteriolar contractions to Ang II and reducing renal blood flow and O2 delivery while increasing renal O2 demand 4–7 and renal parenchymal hypoxia 8. Hypoxia can enhance the progression of CKD 42, 43. This could explain the renal protection provided by renin-angiotensin system blockade or antioxidants and reduced dietary salt in the RRM model 44 or in subjects with CKD 3, 8, 45. Thus, effective blockade of ROS and/or of Ang II may prevent these adverse effects of high dietary salt on CKD where dietary advice is not effective. However, this remains to be tested.
NOVELTY AND SIGNIFICANCE.
What is New? A high dietary salt intake in a mouse model of CKD transcribes genes that generate O2∙- and H2O2 with Ang II in afferent arterioles. The increased H2O2 reduces the sensitivity to Ang II contractions, but O2∙- and H2O2 enhance the responsiveness.
What is Relevant? Progression of CKD in patients or animal models is accelerated by a high salt intake, and is slowed in many patients by drugs that block the renin angiotensin system. Progression of CKD is dependent on reduced renal parenchymal oxygenation. Therefore, enhanced Ang II vasoconstriction of the afferent arterioles of mice with CKD during a high salt intake may contribute to CKD progression by limiting renal blood flow and oxygenation.
Summary: A high dietary salt intake in a mouse model of CKD increases the expression of the NOX2 isoform of NADPH oxidase that generates O2∙- and the NOX4 isoform that generates H2O2 in the kidney’s main resistance arteriole both of which modulate the responsiveness of the arteriole to angiotensin II.
ACKNOWLEDGEMENTS
None
SOURCES OF FUNDING
This work was supported by grants from the National Institute for Diabetes, Digestive Disorders and Kidney Disease (DK49870 and DK36079) and the National Heart, Lung and Blood Institute (HL68086) of the NIH, and by funds from the George E. Schreiner Chair of Nephrology, the Smith-Kogod Family Foundation and the Georgetown University Hypertension Research Center, and by grants to En Yin Lai from National Nature Science Foundation of China (31471100 and 31671193).
Footnotes
CONFLICT OF INTEREST
None
References
- 1.Cao W, Li A, Li J, Wu C, Cui S, Zhou Z, Liu Y, Wilcox CS, Hou FF. Reno-cerebral reflex activates the renin-angiotensin system, promoting oxidative stress and renal damage after ischemia-reperfusion injury. Antioxidants & redox signaling 2017;27:415–432 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cao W, Li A, Wang L, Zhou Z, Su Z, Bin W, Wilcox CS, Hou FF. A salt-induced reno-cerebral reflex activates renin-angiotensin systems and promotes ckd progression. Journal of the American Society of Nephrology : JASN 2015;26:1619–1633 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hou FF, Zhang X, Zhang GH, Xie D, Chen PY, Zhang WR, Jiang JP, Liang M, Wang GB, Liu ZR, Geng RW. Efficacy and safety of benazepril for advanced chronic renal insufficiency. The New England journal of medicine 2006;354:131–140 [DOI] [PubMed] [Google Scholar]
- 4.Palm F, Onozato M, Welch WJ, Wilcox CS. Blood pressure, blood flow, and oxygenation in the clipped kidney of chronic 2-kidney, 1-clip rats: Effects of tempol and angiotensin blockade. Hypertension (Dallas, Tex. : 1979) 2010;55:298–304 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Welch WJ, Blau J, Xie H, Chabrashvili T, Wilcox CS. Angiotensin-induced defects in renal oxygenation: Role of oxidative stress. American journal of physiology. Heart and circulatory physiology 2005;288:H22–28 [DOI] [PubMed] [Google Scholar]
- 6.Welch WJ, Mendonca M, Aslam S, Wilcox CS. Roles of oxidative stress and at1 receptors in renal hemodynamics and oxygenation in the postclipped 2k,1c kidney. Hypertension (Dallas, Tex. : 1979) 2003;41:692–696 [DOI] [PubMed] [Google Scholar]
- 7.Welch WJ, Baumgartl H, Lubbers D, Wilcox CS. Renal oxygenation defects in the spontaneously hypertensive rat: Role of at1 receptors. Kidney international 2003;63:202–208 [DOI] [PubMed] [Google Scholar]
- 8.Lai EY, Luo Z, Onozato ML, Rudolph EH, Solis G, Jose PA, Wellstein A, Aslam S, Quinn MT, Griendling K, Le T, Li P, Palm F, Welch WJ, Wilcox CS. Effects of the antioxidant drug tempol on renal oxygenation in mice with reduced renal mass. American journal of physiology. Renal physiology 2012;303:F64–74 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Norman JT, Fine LG. Intrarenal oxygenation in chronic renal failure. Clinical and experimental pharmacology & physiology 2006;33:989–996 [DOI] [PubMed] [Google Scholar]
- 10.Lipkowitz MS, Wilcox CS. What level of sodium intake worsens renal outcomes? American journal of hypertension 2014;27:1243–1244 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kitiyakara C, Chabrashvili T, Chen Y, Blau J, Karber A, Aslam S, Welch WJ, Wilcox CS. Salt intake, oxidative stress, and renal expression of nadph oxidase and superoxide dismutase. Journal of the American Society of Nephrology : JASN 2003;14:2775–2782 [DOI] [PubMed] [Google Scholar]
- 12.Saleem M, Pokkunuri I, Asghar M. Superoxide increases angiotensin ii at1 receptor function in human kidney-2 cells. FEBS open bio 2016;6:1273–1284 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Araujo M, Wilcox CS. Oxidative stress in hypertension: Role of the kidney. Antioxidants & redox signaling 2014;20:74–101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Li L, Lai EY, Wellstein A, Welch WJ, Wilcox CS. Differential effects of superoxide and hydrogen peroxide on myogenic signaling, membrane potential, and contractions of mouse renal afferent arterioles. American journal of physiology. Renal physiology 2016;310:F1197–1205 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Brown DI, Lassegue B, Lee M, Zafari R, Long JS, Saavedra HI, Griendling KK. Poldip2 knockout results in perinatal lethality, reduced cellular growth and increased autophagy of mouse embryonic fibroblasts. PloS one 2014;9:e96657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Li L, Lai EY, Luo Z, Solis G, Griendling KK, Taylor WR, Jose PA, Wellsten A, Welch WJ, Wilcox CS. Superoxide and hydrogen peroxide counterregulate myogenic contractions in renal afferent arterioles from a mouse model of chronic kidney disease. Kidney international 2017;92:625–633 [DOI] [PubMed] [Google Scholar]
- 17.Lai EY, Wellstein A, Welch WJ, Wilcox CS. Superoxide modulates myogenic contractions of mouse afferent arterioles. Hypertension (Dallas, Tex. : 1979) 2011;58:650–656 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lai EY, Onozato ML, Solis G, Aslam S, Welch WJ, Wilcox CS. Myogenic responses of mouse isolated perfused renal afferent arterioles: Effects of salt intake and reduced renal mass. Hypertension (Dallas, Tex. : 1979) 2010;55:983–989 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lai EY, Solis G, Luo Z, Carlstrom M, Sandberg K, Holland S, Wellstein A, Welch WJ, Wilcox CS. P47(phox) is required for afferent arteriolar contractile responses to angiotensin ii and perfusion pressure in mice. Hypertension (Dallas, Tex. : 1979) 2012;59:415–420 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Li L, Feng D, Luo Z, Welch WJ, Wilcox CS, Lai EY. Remodeling of afferent arterioles from mice with oxidative stress does not account for increased contractility but does limit excessive wall stress. Hypertension (Dallas, Tex. : 1979) 2015;66:550–556 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rosivall L Intrarenal renin-angiotensin system. Molecular and cellular endocrinology 2009;302:185–192 [DOI] [PubMed] [Google Scholar]
- 22.Schulz A, Jankowski J, Zidek W, Jankowski V. Absolute quantification of endogenous angiotensin ii levels in human plasma using esi-lc-ms/ms. Clinical proteomics 2014;11:37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Navar LG. Translational studies on augmentation of intratubular renin-angiotensin system in hypertension. Kidney international supplements 2013;3:321–325 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wilcox CS, Roddis S, Peart WS, Gordon D, Lewis GP. Intrarenal prostaglandin release: Effects of arachidonic acid and hyperchloremia. Kidney international 1985;28:43–50 [DOI] [PubMed] [Google Scholar]
- 25.Dzau VJ, Wilcox CS, Sands K, Dunckel P. Dog inactive renin: Biochemical characterization and secretion into renal plasma and lymph. Am J Physiol 1986;250:E55–E61 [DOI] [PubMed] [Google Scholar]
- 26.Seikaly MG, Arant BS Jr., Seney FD Jr. Endogenous angiotensin concentrations in specific intrarenal fluid compartments of the rat. The Journal of clinical investigation 1990;86:1352–1357 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Leelahavanichkul A, Yan Q, Hu X, Eisner C, Huang Y, Chen R, Mizel D, Zhou H, Wright EC, Kopp JB, Schnermann J, Yuen PS, Star RA. Angiotensin ii overcomes strain-dependent resistance of rapid ckd progression in a new remnant kidney mouse model. Kidney international 2010;78:1136–1153 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wang D, Chabrashvili T, Wilcox CS. Enhanced contractility of renal afferent arterioles from angiotensin-infused rabbits: Roles of oxidative stress, thromboxane prostanoid receptors, and endothelium. Circulation research 2004;94:1436–1442 [DOI] [PubMed] [Google Scholar]
- 29.Wang D, Chen Y, Chabrashvili T, Aslam S, Borrego Conde LJ, Umans JG, Wilcox CS. Role of oxidative stress in endothelial dysfunction and enhanced responses to angiotensin ii of afferent arterioles from rabbits infused with angiotensin ii. Journal of the American Society of Nephrology : JASN 2003;14:2783–2789 [DOI] [PubMed] [Google Scholar]
- 30.Wilcox CS, Pearlman A. Chemistry and antihypertensive effects of tempol and other nitroxides. Pharmacological reviews 2008;60:418–469 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chen Y, Pearlman A, Luo Z, Wilcox CS. Hydrogen peroxide mediates a transient vasorelaxation with tempol during oxidative stress. American journal of physiology. Heart and circulatory physiology 2007;293:H2085–2092 [DOI] [PubMed] [Google Scholar]
- 32.Wilcox CS. Effects of tempol and redox-cycling nitroxides in models of oxidative stress. Pharmacology & therapeutics 2010;126:119–145 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Carlstrom M, Lai EY, Ma Z, Steege A, Patzak A, Eriksson UJ, Lundberg JO, Wilcox CS, Persson AE. Superoxide dismutase 1 limits renal microvascular remodeling and attenuates arteriole and blood pressure responses to angiotensin ii via modulation of nitric oxide bioavailability. Hypertension (Dallas, Tex. : 1979) 2010;56:907–913 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wang D, Jose P, Wilcox CS. Beta(1) receptors protect the renal afferent arteriole of angiotensin-infused rabbits from norepinephrine-induced oxidative stress. Journal of the American Society of Nephrology : JASN 2006;17:3347–3354 [DOI] [PubMed] [Google Scholar]
- 35.Huang Q, Wang Q, Zhang S, Jiang S, Zhao L, Yu L, Hultstrom M, Patzak A, Li L, Wilcox CS, Lai EY. Increased hydrogen peroxide impairs angiotensin ii contractions of afferent arterioles in mice after renal ischaemia-reperfusion injury. Acta physiologica (Oxford, England) 2016;218:136–145 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Chaplin NL, Amberg GC. Hydrogen peroxide mediates oxidant-dependent stimulation of arterial smooth muscle l-type calcium channels. American journal of physiology. Cell physiology 2012;302:C1382–1393 [DOI] [PubMed] [Google Scholar]
- 37.Patel RJ, Patel PD, Patel MM, Patel NJ, Thyagarajan B. Mechanisms of potentiation of angiotensin ii-induced contractile response of isolated rat aorta by hydrogen peroxide and tert-butyryl hydroperoxide. Indian journal of pharmacology 2009;41:140–143 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Grover AK, Samson SE, Fomin VP, Werstiuk ES. Effects of peroxide and superoxide on coronary artery: Ang ii response and sarcoplasmic reticulum ca2+ pump. The American journal of physiology 1995;269:C546–553 [DOI] [PubMed] [Google Scholar]
- 39.Torrecillas G, Boyano-Adanez MC, Medina J, Parra T, Griera M, Lopez-Ongil S, Arilla E, Rodriguez-Puyol M, Rodriguez-Puyol D. The role of hydrogen peroxide in the contractile response to angiotensin ii. Molecular pharmacology 2001;59:104–112 [DOI] [PubMed] [Google Scholar]
- 40.Saran R, Padilla RL, Gillespie BW, Heung M, Hummel SL, Derebail VK, Pitt B, Levin NW, Zhu F, Abbas SR, Liu L, Kotanko P, Klemmer P. A randomized crossover trial of dietary sodium restriction in stage 3–4 ckd. Clinical journal of the American Society of Nephrology : CJASN 2017;12:399–407 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Chabrashvili T, Kitiyakara C, Blau J, Karber A, Aslam S, Welch WJ, Wilcox CS. Effects of ang ii type 1 and 2 receptors on oxidative stress, renal nadph oxidase, and sod expression. American journal of physiology. Regulatory, integrative and comparative physiology 2003;285:R117–124 [DOI] [PubMed] [Google Scholar]
- 42.Fine LG, Norman JT. Chronic hypoxia as a mechanism of progression of chronic kidney diseases: From hypothesis to novel therapeutics. Kidney international 2008;74:867–872 [DOI] [PubMed] [Google Scholar]
- 43.Orphanides C, Fine LG, Norman JT. Hypoxia stimulates proximal tubular cell matrix production via a tgf-beta1-independent mechanism. Kidney international 1997;52:637–647 [DOI] [PubMed] [Google Scholar]
- 44.Brenner BM, Goldszer RC, Hostetter TH. Glomerular response to renal injury. Contributions to nephrology 1982;33:48–66 [DOI] [PubMed] [Google Scholar]
- 45.de Zeeuw D, Lewis EJ, Remuzzi G, Brenner BM, Cooper ME. Renoprotective effects of renin-angiotensin-system inhibitors. Lancet (London, England) 2006;367:899–900; author reply 900–892 [DOI] [PubMed] [Google Scholar]


