Abstract
Hypothesis:
Phosphorus and vitamin D (calcitriol) supplementation in the Phex mouse, a murine model for endolymphatic hydrops (ELH), will improve otic capsule mineralization and secondarily ameliorate the postnatal development of ELH and sensorineural hearing loss (SNHL). Background: Male Phex mice have X-linked hypophosphatemic rickets (XLH), which includes osteomalacia of the otic capsule. The treatment for XLH is supplementation with phosphorus and calcitriol. The effect of this treatment has never been studied on otic capsule bone and it is unclear if improving the otic capsule bone could impact the mice’s postnatal development of ELH and SNHL.
Methods:
Four cohortswere studied: 1) wild-type control, 2) Phex control, 3) Phex prevention, and 4) Phex rescue. The control groups were not given any dietary supplementation. The Phex prevention group was supplemented with phosphorus added to its drinking water and intraperitoneal calcitriol frompostnatal day (P) 7–P40. The Phex rescue group was also supplemented with phosphorus and calcium but only from P20 to P40. At P40, all mice underwent auditory brainstem response (ABR) testing, serum analysis, and temporal bone histologic analysis. Primary outcome was otic capsule mineralization. Secondary outcomes were degree of SNHL and presence ELH.
Results:
Both treatment groups had markedly improved otic capsule mineralization with less osteoid deposition. The improved otic capsule mineralized did not prevent the development of ELH or SNHL.
Conclusion:
Supplementation with phosphorus and calcitriol improves otic capsule bone morphology in the Phex male mouse but does not alter development of ELH or SNHL.
1. Introduction
Meniere’s disease (MD) is a debilitating condition characterized by episodic vertigo, tinnitus, aural fullness, and progressive sensorineural hearing loss (SNHL) [1]. First described in 1861 by Prosper Meniere, the etiology of MD remains idiopathic although its relationship to endolymphatic hydrops (ELH) has been well established in both human specimens and animal models [2–7]. Therapeutic options for patients with MD range from a conservative low-salt diet to complete ablation of labyrinthine function. Clinicians’ inability to predict therapeutic response and the disease clinical course adds to both patient and clinician frustration with MD management [8].
Mouse models are commonly used to study human diseases. The Phex mouse had a spontaneous loss-of-function mutation in the phosphate-regulating gene with homology to endopeptidases located on the X chromosome (PHEX gene), resulting in X-linked hypophosphatemic rickets (XLH). In 2004, a new mouse mutation of the Phex gene – PhexHyp-Duk – was described. PhexHyp-Duk arose on the BALB/cAnBomUrd strain at Duke University (PhexHyp-Duk/Y). PhexHyp-Duk/Y (simplify as Phex) has similar pathological ELH features as those seen in ELH involving human Meniere’s disease [9]. Analogous to human XLH, these mice have hypophosphatemia caused by renal phosphate wasting, osteomalacia, mild hypocalcemia, and shortened limbs [10]. In 2008, the inner ear pathology of this mouse was further characterized when the male Phex mice were found to have postnatal development of vestibular dysfunction (circling behavior and/or head bobbing), progressive sensorineural hearing loss (SNHL), and ELH in a characteristic and reproducible manner [7]. Since then, the Phex mouse model has increasingly served as a valuable tool to investigate the pathophysiology of ELH.
The incidence of XLH in humans is 1/20,000 live births, which makes it the most common inheritable form of rickets [11,12]. The bone of XLH demonstrates osteomalacia with thick strands of unmineralized osteoid. The treatment for XLH consists of oral phosphate and calcitriol supplementation [13]. Treatment has shown to improve growth andmineralization in long bones and vertebrae [14–16]. Therapeutic response is assessed using alkaline phosphatase, which serves as amarker for bone turnover and drops to a normal level when a therapeutic dosage has been reached [13].
Otologic manifestations of XLH have been described in humans. Progressive SNHL and Meniere’s-like symptoms of episodic vertigo and tinnitus have been reported in upwards of 76% of patients [17–19]. Electrocochleography (ECochG) in the majority of these patients suggests the presence of ELH [20]. Radiographic studies have also demonstrated dysmorphic temporal bones [21,22]. All of the otologic manifestations associated with XLH in humans have been replicated in the Phex mouse model, particularly the presence of diseased otic capsule bone and ELH [7,23].
The interplay between otic capsule bone and the endolymphatic system is still under investigation. Likewise, the mechanism underlying ELH remains unclear. This project attempts to further characterize the relationship between the otic capsule and ELH. By providing Phex mice with phosphorus and calcitriol supplementation, we investigated whether the standard treatment for XLH could improve the dysmorphic otic capsule bone and secondarily if the bone health would influence the progression of ELH and SNHL.
2. Materials and methods
The Institutional Animal Care and Use Committee of Case Western Reserve University and the Institutional Review Board of University Hospitals Case Medical Center approved this research protocol. The animals usedweremale BALB/cAnBomUrd mice with the PhexHyp-Duk mutation (PhexHyp-Duk/Y) and their male wild-type littermates (+/Y). The mice were originally obtained from The Jackson Laboratory (Bar Harbor, ME) but have since been bred and maintained at Case Western Reserve University in compliance with their Animal Care and Use Committee guidelines.
2.1. Phex and X-linked hypophosphatemia background
The development of the Phex mouse model and its otologic manifestations has previously been described [7,9,24]. In brief, XLH is caused by a genetic defect in the Phex gene that leads to a dysfunctional Phex protein. The Phex protein is a zinc-metalloendopeptidase located in the cell membrane of osteoblasts and chondrocytes [25]. The protein’s normal function is to bind to pro-mineralization factors, like matrix extracellular phosphoglycoprotein (MEPE) and dentin matrix protein-1 (DMP-1), to support healthy bone mineralization. The defective Phex protein leads to unmineralized osteoid deposition and soft bone, termed osteomalacia and rickets. Additionally, the impaired Phex-MEPE binding leads to an increase in fibroblast growth factor-23 (FGF-23), which is directly responsible for renal phosphate wasting and decreased 1,25-dihydroxyvitamin D production [24,26,27].
Humans with XLH are treated with supplemental phosphate and calcitriol (1,25-dihydroxyvitamin D3). Early initiation of treatment leads to improved bone mineralization and growth. Alkaline phosphatase, a marker of bone turnover, is elevated in patients with XLH. Therapeutic response to the dietary supplementation is in part measured by normalization of alkaline phosphatase levels. Therapy benefit can be attenuated by elevation of the down-stream molecule FGF-23, which disrupts the sodium-phosphate co-transporter in the proximal renal tubule. Anti-FGF-23 medications are in development, but the standard of care for XLH remains phosphate and calcitriol supplementation [13,15,28,29].
Male mice with the PhexHyp-Duk mutation have XLH. Multiple studies have characterized the postnatal development and disease manifestations in the Phex mouse [7,9,23,24,30,31]. At birth, the mice have normal inner ear histology including no ELH, spiral ganglion degeneration, or endolymphatic duct obstruction. Circling behavior and head bobbing, signs of vestibular dysfunction, start between postnatal day (P) 15 and 20. Bilateral hearing loss, documented with auditory brainstem response (ABR), starts around P20 and progresses to profound loss as early as P40. Evidence of apical hydrops and dysmorphic bone begins at P25 and becomes severe in most cases by P90. Spiral ganglion neuron apoptosis precedes inner ear hair cell loss, but both lag behind the onset of hearing loss. The endolymphatic duct remains patent throughout this progression. Phenotypically, the Phex mice have a small body size, short tail, and soft bones. The PhexHyp-Duk mutation on the BALB/cAnBomUrd background leads to ELH, while on the C57BL/6 (B6) or BALB/cByJ background, there is no ELH.
2.2. Study design
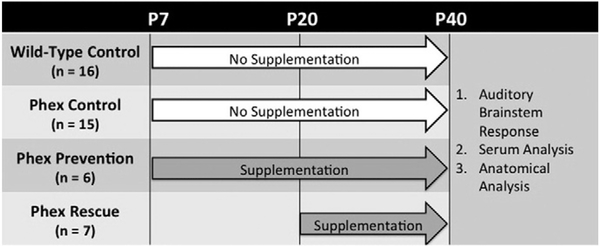
There were four mouse populations: 1) wild-type control (+/Y), 2) Phex control (control PhexHyp-Duk/Y), 3) Phex prevention (prevention PhexHyp-Duk/Y), and 4) Phex rescue (rescue PhexHyp-Duk/Y). All mice were maintained on standard chow(Iso Pro Rodent 3000 by LabDiet, St. Louis, MO, U.S.A.) that contains 1.11% calcium, 0.8% phosphorus, and 2.5 IU/g of VitaminD3. The wild-type and Phex control groups did not receive any supplemental phosphorus or calcitriol. The Phex prevention and Phex rescue groups were supplemented with phosphorus and calcitriol only during a specified treatment period. For the Phex prevention group, that supplementation began at P7, the age of weaning, and continued through P40. The Phex rescue group started supplementation at P20 and continued therapy until P40. Mice from all groups were sacrificed at P40. Phosphorus-supplemented water (1.9 g elemental phosphorus per liter) replaced the normal drinking water and was available ad libitum during the treatment periods. Calcitriol, which is an active metabolite of 1,25-dihydroxyvitamin D3, was given via intraperitoneal (IP) injections at a dose of 0.4 μg/kg per injection. The injections were performed three times a week during the treatment periods. The phosphorus and calcitriol dosages were based on a study from Marie et al. (1982) that showed improved vertebrae mineralization in a PhexHyp (Hyp/Y) hypophosphatemic mouse model [32].
2.3. Auditory-evoked brainstem response (ABR)
All mice underwent ABR testing at P40. The ABR technique has previously been described [33]. In brief, mice aged P40 were anesthetized with an IP injection of ketamine, xylazine, and acepromazine at doses of 40, 5, and 1 mg/kg, respectively. Body temperature was maintained at 37–38 °C by placing the mice on a homeothermic heating pad (Harvard apparatus, Holliston, MA) within a soundproof chamber. ABR testing was carried out using the SmartEP system from Intelligent Hearing Systems (Miami, FL). Platinum subdermal needle electrodes were inserted at the skull vertex (ground electrode) and ventrolateral to the right and left ears. Pure-tone stimuli at 8 kHz, 16 kHz, and 32 kHz of 100-ms duration were presented for at least 700 sweeps to each ear (one at a time) through high-frequency transducers (closed system). ABR thresholds were obtained from both ears for each animal by reducing the stimulus intensity from 90 dB SPL in 5 or 10 dB steps until the lowest intensity that could evoke a reproducible ABR pattern was detected.
2.4. Serum analysis
Immediately following ABR testing, the anesthetized mice were sacrificed. Blood was collected via cardiac puncture, spun down in a centrifuge for 10 min at 10,000 rpm (RPM), and then the serum was extracted. Serum phosphorus, calcium, creatinine, and alkaline phosphatase assays were processed with the Dimensions VISTA® system from Siemens Medical Solutions, Inc. (Malvern, PA) in the University Hospitals Case Medical Center Core Laboratory. Serum FGF-23 concentration was determined using an enzyme-linked immunosorbent assay (ELISA) kit fromImmutopics International (San Clemente, CA) that was processed in the Dahms Clinical Research Unit at University Hospitals Case Medical Center. The mouse FGF-23 (C-Term) assay is a two-site ELISA that detects epitopes within the carboxyl-terminal (C-Term) region of mouse FGF-23 [34].
2.5. Anatomical analysis
After obtaining serum, a microscope was used for temporal bone dissection and exposure of the inner ear. The stapes footplate was removed. Using a fine needle, the oval window and round window were delicately punctured to facilitate perfusion of the cochlea. The inner ear specimens were then immersed in 4% paraformaldehyde (PFA) for 1 week at 4 °C. Next, the specimens were rinsed in 0.1 M sodium phosphate buffer (pH 7.4) and decalcified in 0.35 M ethylenediaminetetraacetic acid (EDTA) for 1 week. The inner ears were then dehydrated with serial alcohol rinses and embedded in paraffin. Tissue sections were cut to a thickness of 5–8 μm using a cold microtome and plated on glass slides. The tissue was then stained with hematoxylin and eosin to facilitate morphologic analysis under a light microscope. The otic capsule bone, membranous cochlea including Reissner’s membrane, and spiral ganglion were analyzed. The study design is summarized in Fig. 1.
Fig. 1 –
Summary of the study design. There were four cohorts consisting of two control groups (wild-type control and Phex control) and two treatment groups (Phex prevention and Phex rescue). The control groups did not receive any dietary supplementation. The Phex prevention group was supplemented with phosphorus and calcitriol immediately after weaning on postnatal day (P) 7. The Phex rescue group started supplementation with phosphorus and calcitriol at P20. At P40, all mice were analyzed with auditory brainstem response, serum analysis (calcium, phosphorus, alkaline phosphatase, fibroblast growth factor-23, creatinine), and histology.
2.6. Statistical analysis
Numerical data were collected, entered into Excel (Microsoft, Redmond, WA), and imported into GraphPad Prism (GraphPad, San Diego, CA). Mean values and standard deviations or confidence intervals were calculated for continuous variables. Percentages or frequencies were calculated for categorical variables. Both parametric and non-parametric statistics were used as appropriate. A one-way analysis of variance (ANOVA) was used to compare serum data and ABR thresholds between the four groups. An independent Student’s t-test was used for weight comparisons. The criterion for statistical analysis was set at p < 0.05, two-tailed.
3. Results
There were 16 mice in the wild-type control group, 15 mice in the Phex control group, 6 mice in the Phex prevention group, and 7 mice in the Phex rescue. Supplementation with phosphorus and calcitriol had no effect on the Phex phenotype, which includes small body size, vestibular dysfunction characterized by circling behavior/head bobbing, shortened tails, and soft bones. The wild-type control mice had a mean weight of 21.1 g (SD 1.7), which was significantly larger than those of the Phex control (16.1 g, SD 1.7), Phex prevention (15.2 g, SD 1.7), and Phex rescue (15.1 g, SD 4.3) (p < 0.001, <0.001, and <0.001, respectively). There were no weight differences among the Phex groups.
3.1. Otic capsule and cochlea morphology
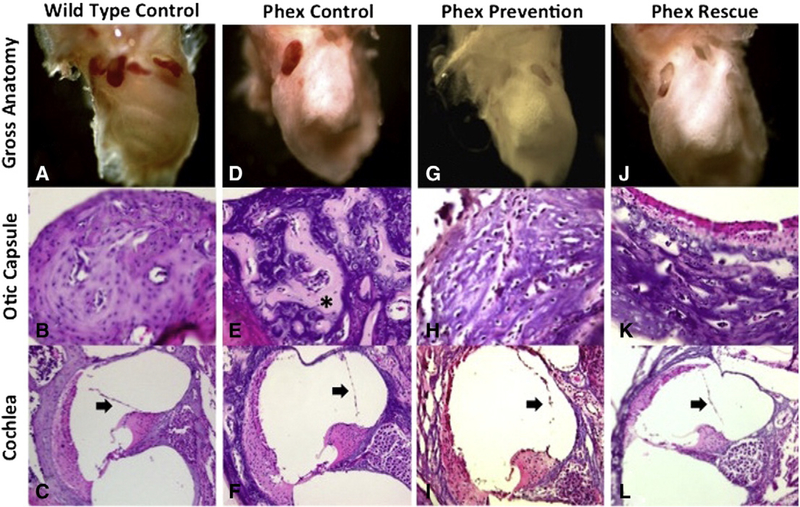
The anatomical findings are summarized in Fig. 2. A LEICA stereomicroscope and a DC500 camera were used to analyze the gross anatomy of the cochleae. The wild-type control showed a well-defined cochlea structure with clear delineation of the basal, middle, and apical turns. The bone was solid with an expected firmness typical of the dense otic capsule bone. In contrast, all three Phex groups demonstrated grossly malformed cochleae with loss of the well-defined cochlear architecture. The Phex bones all had a soft, chalk-like, consistency. The Phex prevention and Phex rescue groups did not differ from the Phex control group (Fig. 2A, D, G, J).
Fig. 2 –
Otic capsule and cochleamorphology. Wild-type control specimens (A–C) demonstrate normal mineralization and morphology of the otic capsule and cochlea. There is no distension of Reissner’smembrane (arrow). Phex control specimens (D–F) demonstrate dysmorphic grossmorphology and poor otic capsule mineralization with thick osteoid strands (*). Reissner’smembrane is distended, which is consistent with endolymphatic hydrops (arrow). The Phex prevention (G–I) and Phex rescue (J–L) groups still demonstrate altered grossmorphology and distention of Reissner’smembrane (arrow). Compared to the Phex control group, the two treatment arms show improved otic capsule mineralization with less osteoid deposition. Gross anatomy images were taken with low magnification on a stereomicroscope. The otic capsule and cochlea images were taken with a light microscope at 100× and 10× magnification, respectively.
After the bone was processed and stained as detailed above, light microscopy was used to analyze the inner ear morphology at 10× and 100× magnification. The wild-type control group demonstrated normal mineralization of the otic capsule and normal inner ear morphology without evidence of ELH (Fig. 2B, C). The Phex control group demonstrated dysmorphic otic capsule bone with thick osteoid seams characteristic of poor mineralization. Severe ELH was seen in all specimens (Fig. 2E, F). A formal spiral ganglion neuron count was not performed but there appeared to be no obvious dearth of neurons in the Phex control compared to the wild-type control. Spiral ganglion neuron loss in the Phex model has previously been shown to occur at an age later than P40 [7,31]. The Phex prevention group (Fig. 2H, I) and the Phex rescue group (Fig. 2K, L) both showed significant improvement in otic capsule mineralization with less osteoid deposition than the Phex control. The bone of the treatment groups still lacked the organization of the wild-type control. Overall, neither treatment group had improvement in the severity of the ELH. While there were a few specimens in both treatment groups that may have had a slight reduction in the degree of Reissner’s membrane distention (Fig. 2L), the specimen fragility precluded any global volumetric analysis. An assumption of ELH improvement in the treatment groups could not be made based on the available evidence.
3.2. Auditory brainstem response
Pure-tone ABR thresholds were performed on all mice at frequencies of 8000 Hz, 16,000 Hz, and 32,000 Hz. The mean average of the right and left ears was calculated for each mouse. At all frequencies, the wild-type control group had significantly better hearing thresholds than the Phex groups. Specifically, the wild-type control mean thresholds were 38.5 dB ± 12.2 at 8 kHz, 31.7 dB ± 10.1 at 16 kHz, and 36.5 dB ± 8.8 at 32 kHz; the mean Phex control thresholds were 87.9 dB ± 5.4 at 8 kHz, 84.3 dB ± 9.6 at 16 kHz, and 81.8 dB ± 9.3 at 32 kHz; the mean Phex prevention thresholds were 90.0 dB ± 0 at 8 kHz, 86.7 dB ± 4.1 at 16 kHz, and 86.7 dB ± 4.1 at 32 kHz; and the mean Phex rescue thresholds were 87.9 dB ± 3.9 at 8 kHz, 79.3 dB ± 8.9 at 16 kHz, and 84.3 dB ± 4.5 at 32 kHz (p < 0.0001 comparing wild-type control to all three Phex groups using a one-way ANOVA). There was no difference in hearing among the Phex groups.
3.3. Serum analysis
Phosphate, calcium, creatinine, alkaline phosphatase, and FGF-23 values are summarized in Table 1. The serum diluent had to be separated to perform the different biochemistry assays, thus, every lab test could not be performed on every mouse. The number of samples for each test is indicated in Table 1. All analyses were performed using a one-way ANOVA.
Table 1 –
Serum analysis.
| Phosphate (mg/dl) | Calcium (mg/dl) | Alk. Phos. (mg/dl) | Creatinine (mg/dl) | FGF-23 (mg/dl) | |
|---|---|---|---|---|---|
| Wild-type control | 12.0 ± 1.3 | 9.5 ± 0.3 | 241.7 ± 34.6 | 0.2 ± 0.0 | 180 ± 54 |
| n = 14 | n = 14 | n = 16 | n = 14 | n = 6 | |
| Phex control | 8.1 ± 1.3* | 9.2 ± 0.6 | 747 ± 109.5 + | 0.2 ± 0.0 | 1840 ± 223 ^ |
| n = 13 | n = 11 | n = 13 | n = 11 | n = 6 | |
| Phex prevention | 8.6 ± 1.1** | 8.8 ± 0.4 | 647.6 ± 99.6 ++ | 0.2 ± 0.0 | 2934 ± 883 ^^,! |
| n = 6 | n = 5 | n = 5 | n = 4 | n = 3 | |
| Phex rescue | 9.9 ± 1.5*** | 8.3 ± 0.4 #,⌘ | 566.8 ± 72.1 +++,‡ | 0.2 ± 0.1 | 3239 ± 718 ^^^,† |
| n = 5 | n = 6 | n = 6 | n = 6 | n = 2 |
Data are expressed as mean ± SD. Serum samples were collected at P40.
Statistics calculated with a one-way ANOVA using multiple comparisons. Alk. Phos. = alkaline phosphatase. CI = 95% confidence interval of the mean differences. FGF-23 = fibroblast growth factor-23.
p < 0.0001 Phosphate wild-type control vs. Phex control (CI: 2.5 to 5.3).
p = 0.0001 Phosphate wild type vs. Phex prevention (CI: 1.5 to 5.3).
p = 0.003 Phosphate wild type vs. Phex rescue (CI: 0.8 to 4.6).
p < 0.0001 Calcium wild-type control vs. Phex rescue (CI: 0.6 to 1.7).
p = 0.002 Calcium Phex prevention vs. Phex rescue (CI: 0.3 to 1.5). −585 to −426).
p < 0.0001 Alk. Phos. wild-type control vs. Phex control (CI: −546 to −307).
p < 0.0001 Alk. Phos. wild-type control vs. Phex prevention (CI: −427 to −223).
p < 0.0001 Alk. Phos. wild-type control vs. Phex rescue (CI: −2379 to −941).
p = 0.0003 Alk. Phos. Phex control vs. Phex rescue (CI: 74.8 to 285).
p < 0.0001 FGF-23 wild-type control vs. Phex control (CI: −3635 to −1874).
p < 0.0001 FGF-23 wild-type control vs. Phex prevention (CI: −4076 to −2043).
p < 0.0001 FGF-23 wild-type control vs. Phex rescue (CI: −1975 to −214).
p = 0.014 FGF-23 Phex control vs. Phex prevention (CI: −2416 to −383).
p = 0.007 FGF-23 Phex control vs. Phex rescue (CI:−2416 to-383).
Phosphate by definition is low in patients who suffer from hypophosphatemia, as well as the Phex mouse model. The wild-type control phosphate level was 12.0 mg/dl (SD 1.3). The Phex control (8.1 mg/dl, SD 1.3), Phex prevention (8.6 mg/dl, SD 1.1), and Phex rescue (9.9 mg/dl, SD 1.5) groups each had significantly lower phosphate levels than the wild-type control (wild-type vs. Phex control, p < 0.0001; wild-type vs. Phex prevention, p = 0.0001; wild-type vs. Phex rescue, p = 0.003, respectively). Despite the treatment groups having higher mean phosphate levels than the Phex control, this did not reach a statistically significant elevation.
Patients with XLH can have normal or slightly decreased calcium levels. This trend was replicated in our study, with no significant difference between the wild-type control group (9.5 mg/dl, SD 0.3) and the Phex control (9.2 mg/dl, SD 0.6) or Phex prevention (8.8 mg/dl, SD 0.4) groups. The Phex rescue group (8.3 mg/dl, SD 0.4) did have a significantly lower calcium level than the wild-type control group (p < 0.0001) and Phex control group (p = 0.002) but not the Phex prevention group (p = 0.20).
Alkaline phosphatase is a marker of bone turnover. It is significantly elevated in patients with XLH and should normalize with phosphorus and calcitriol treatment [13]. The wild-type control had an alkaline phosphatase level of 241.7 mg/dl (SD 34.6), which was significantly lower than those of the Phex control (747 mg/dl, SD 109.5, p < 0.0001), Phex prevention (647.6 mg/dl, SD 99.6, p < 0.0001), and Phex rescue (566.8 mg/dl, SD 72.1, p < 0.0001) groups. The Phex control group was significantly higher than the Phex rescue group (p = 0.0003) but did not reach statistical significance against the Phex prevention group (p = 0.32).
A potential complication of XLH treatment is nephrocalcinosis and kidney injury [13]. There were no differences between the four groups in creatinine level (wild-type control 0.2 mg/dl, SD 0.0; Phex rescue 0.2 mg/dl, SD 0.0; Phex prevention 0.2 mg/dl, SD 0.0; and Phex rescue 0.2 mg/dl, SD 0.1).
Fibroblast growth factor-23 inhibits both phosphate reabsorption from the kidney and bone mineralization. It is elevated in XLH and becomes further elevated during treatment, which may counteract the effects of supplemental phosphorus and calcitriol. It remains a target for future XLH therapy [13,28,29]. The wild-type control level was 180 mg/dl (SD 54), which was significantly lower than those of the Phex control (1840 mg/dl, SD 223, p < 0.0001), Phex prevention (2934 mg/dl, SD 883, p < 0.0001), and Phex rescue (3239 mg/dl, SD 718, p < 0.0001) groups. Both treatment groups also had an elevated FGF-23 level when compared to the Phex control group (Phex control vs. Phex prevention, p = 0.014; Phex control vs. Phex rescue, p = 0.007).
4. Discussion
Since 2004, the Phex mouse has served as a valuable model to study the pathophysiology of ELH [7,9,24]. In addition to ELH, the Phex gene mutation causes XLH and its characteristic osteomalacia. Treatment for XLH is repletion of phosphorus and vitamin D, typically in the form of calcitriol [13]. The present study represents the first investigation of how that standard therapy for XLH impacts the dysmorphic otic capsule of the Phex mouse and secondarily how improvement in the otic capsule bone alters the postnatal development of ELH and SNHL.
The impact of otic capsule bone and the endolymphatic system remains a topic of debate. Early human studies in XLH noted that many patients had a Meniere’s-like presentation with progressive SNHL, episodic vertigo, and tinnitus. They proposed that the temporal bone osteomalacia caused ELH via either obstruction of the endolymphatic duct or altered metabolism of stria vascularis and periductal channels [21,22]. Analysis of the Phex mice endolymphatic ducts has shown that they maintained patency despite severe osteomalacia in the otic capsule [7]. This suggests that development of ELHin the Phexmice and XLHmay be influenced by ametabolic interplay between the endolymphatic space and the otic capsule bone rather than secondary to obstruction.
Other authors have suggested that the otic capsule and, in particular, the periductal channels adjacent to the endolymphatic duct play an active role in the homeostasis of the endolymphatic system. Many small bone channels containing blood vessels surround the endolymphatic duct [35]. Use of electron microscopy has also identified a complex network of periductal connective tissue that contacts the epithelium of the endolymphatic duct with the nearby vasculature [36]. Connective tissue and its associated fibroblasts and pericytes are thought to play an active role in controlling interstitial fluid pressure and volume, which further supports the endolymphatic duct as the site of endolymph reabsorption [37,38]. Disruption of the connective tissue network adjacent to the endolymphatic duct has been proposed as a contributing factor to the development of ELH in MD [39]. Linthicum et al. (2014) recently demonstrated that human temporal bones with disturbances of the periductal channels lead to ELH. Through immunohistochemical staining, they also recognized similarities between the periductal channels and the spiral ligament, which has a known active role in potassium circulation and endolymph homeostasis within the cochlea [40].
Histology from the present study demonstrates that supplementation with phosphorus and calcitriol can greatly improve otic capsule mineralization in a manner similar to the descriptions in studies of long bones and vertebrae [16,32]. Despite healthier otic capsule bone, there does not appear to be a definite improvement in the severity of ELH (Fig. 2). Onset of therapy also did not appear to make a difference in the degree of bone mineralization or ELH. Furthermore, treatment had no impact on hearing or the phenotypic expression of vestibular dysfunction.
The serum analysis is limited by small sample sizes and lack of normative data. Still, there is evidence that the phosphorus and calcitriol supplementation had an effect. Alkaline phosphatase should normalize with XLH therapy [13]. The Phex rescue group showed a statistically significant decline in alkaline phosphatase compared to the Phex control (p = 0.003), but it remained elevated compared to the wild-type control (p < 0.0001). The Phex prevention group, despite having a lower mean alkaline phosphatase value, did not reach statistical significance compared to the Phex control (p = 0.32). These numbers suggest that the mice in both treatment groups may have been underdosed. It is also unclear why the Phex rescue group, which received therapy for 20 days, had a more robust response than the Phex prevention group that received therapy for 33 days.
Finally, the serum analysis confirms the expected increase in FGF-23 with the initiation of therapy (Table 1). FGF-23 is a down-stream molecule of the Phex protein and it is directly responsible for much of the morbidity associated with XLH [41,42]. Treatment for XLH with phosphorus and calcitriol merely circumvents the detrimental actions of FGF-23 and causes an increase in FGF-23 that may attenuate the therapeutic benefit of the dietary supplementation [28]. It is unclear if newer anti-FGF-23 drugs may have a more pronounced effect on bone mineralization and possibly ELH or SNHL. Future studies with the Phex mouse may utilize anti-FGF-23 agents or optimize dosing and timing of phosphorus and calcitriol to see if further cessation of the morphologic changes driven by this therapy will eventually lead to cessation or amelioration of ELH.
5. Conclusion
Supplementation with phosphorus and calcitriol improves otic capsule bone morphology in the Phex male mouse but does not alter development of ELH or SNHL.
Acknowledgments
1. Awarded first place at the combined American Otolaryngological Society and American Neurotology Society poster competition at the Combined Otolaryngological Spring Meeting (COSM), Las Vegas, NV, May 2014.
2. Thomas Carpenter, MD (Professor of Pediatrics and Orthopedics at Yale School of Medicine) for his assistance in research design and background.
3. Paul Hartman, Beth Smith, Sarah Scott, and Sarah Dawson from the Dahms Clinical Research Unit at University Hospitals Case Medical Center for their assistance in serum analysis.
Funding: This work was supported in part by a grant from the American Otological Society to Dr. Cliff A. Megerian and by the NIH grants (R01DC007392 and R01DC015111) to Dr. Qing Y. Zheng.
Footnotes
Conflicts of interests: None.
IRB: Animal experiments were carried out in accordance with NIH Animal Care and Use Committee (DC009246) and Case Western Reserve University Institutional Animal Care and Use Committee guidelines.
Copyright transfer: In consideration of the American Journal of Otolaryngology’s reviewing and editing my submission, “Treatment of ear and bone disease in the Phex mouse mutant with dietary supplementation”, the author(s) undersigned transfers, assigns and otherwise conveys all copyright ownership to Elsevier Inc. in the event that such work is published in the American Journal of Otolaryngology.
REFERENCES
- [1].Committee on Hearing and Equilibrium. Committee on Hearing and Equilibrium guidelines for the diagnosis and evaluation of therapy in Meniere’s disease. Otolaryngol Head Neck Surg 1995;13:181–5. [DOI] [PubMed] [Google Scholar]
- [2].Ménière P Maladies de l’oreille interne offrant les symptomes de la congestion cérébrale apoplectiforme. Gaz Méd Paris 1861;16:88. [Google Scholar]
- [3].Hallpike CS, Cairns H. Observations on the pathology of Meniere’s syndrome. J Laryngol Otol 1938;53:625–55. [PMC free article] [PubMed] [Google Scholar]
- [4].Arenberg IK, Marovitz WF, Shambaugh GE Jr. The role of the endolymphatic sac in the pathogenesis of endolymphatic hydrops in man. Acta Otolaryngol 1970;275:1–49. [PubMed] [Google Scholar]
- [5].Schuknecht HF. Pathophysiology of endolymphatic hydrops. Arch Otolaryngol 1976;212:253–62. [DOI] [PubMed] [Google Scholar]
- [6].Kimura RS. Experimental blockage of the endolymphatic duct and sac and its effect on the inner ear of the guinea pig. Ann Otol Rhinol Laryngol 1967;76:664–87. [DOI] [PubMed] [Google Scholar]
- [7].Megerian CA, Semaan MT, Aftab S, et al. A mouse model with postnatal endolymphatic hydrops and hearing loss. Hear Res 2008;237:90–105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Semaan MT, Megerian CA. Meniere’s disease: a challenging and relentless disorder. Otolaryngol Clin N Am 2011;44: 383–403. [DOI] [PubMed] [Google Scholar]
- [9].Lorenz-Depiereux B, Guido VE, Johnson KR, et al. New intragenic deletions in the Phex gene clarify X-linked hypophosphatemia-related abnormalities in mice. Mamm Genome 2004;15:151–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Hyp_Consortium. A gene (PEX) with homologies to endopeptidases is mutated in patients with X-linked hypophosphatemic rickets. The HYP Consortium. Nat Genet 1995;11:130–6. [DOI] [PubMed] [Google Scholar]
- [11].Davies M, Stanbury SW. The rheumatic manifestations of metabolic bone disease. Rheum Dis Clin 1981;7:595–646. [Google Scholar]
- [12].Alizadeh Naderi AS, Reilley RF. Hereditary disorders of renal phosphate wasting. Nat Rev Nephrol 2010;6:657–65. [DOI] [PubMed] [Google Scholar]
- [13].Carpenter TO, Imel EA, Holm IA, et al. A clinician’s guide to X-linked hypophosphatemia. J Bone Miner Res 2011;26: 1381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Harrell RM, Lyles KW, Harrelson JM, et al. Healing of bone disease in X-linked hypophosphatemic rickets/osteomalacia. Induction and maintenance with phosphorus and calcitriol. J Clin Invest 1985;75:1858–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Verge CF, Lam A, Simpson JM, et al. Effects of therapy in X-linked hypophosphatemic rickets. N Engl J Med 1991;325: 1843–68. [DOI] [PubMed] [Google Scholar]
- [16].Sullivan W, Carpenter T, Glorieux F, et al. A prospective trial of phosphate and 1,25-dihydroxyvitamin D3 therapy in symptomatic adults with X-linked hypophosphatemic rickets. J Clin Endocrinol Metab 1992;75:879–85. [DOI] [PubMed] [Google Scholar]
- [17].Davies M, Kane R, Valentine J. Impaired hearing in X-linked hypophosphatemic (vitamin D-resistant) osteomalacia. Ann Intern Med 1984;100:230–2. [DOI] [PubMed] [Google Scholar]
- [18].Boneh A, Reade TM, Scriver CR, et al. Audiometric evidence for two forms of X-linked hypophosphatemia in humans, apparent counterparts of Hyp and Gy mutations in mouse. Am J Med Genet 1987;27:997–1003. [DOI] [PubMed] [Google Scholar]
- [19].Fishman G, Miller-Hansen D, Jacobsen C, et al. Hearing impairment in familial X-linked hypophosphatemic rickets. Eur J Pediatr 2004;163:622–3. [DOI] [PubMed] [Google Scholar]
- [20].O’Malley S, Ramsden RT, Latif A, et al. Electrocochleographic changes in the hearing loss associated with X-linked hypophosphataemic osteomalacia. Acta Otolaryngol 1985; 100:13–8. [DOI] [PubMed] [Google Scholar]
- [21].Weir N Sensorineural deafness associated with recessive hypophosphataemic rickets. J Laryngol Otol 1977;91:717–22. [DOI] [PubMed] [Google Scholar]
- [22].O’Malley SP, Adams JE, Davies M, et al. The petrous temporal bone and deafness in X-linked hypophosphataemic osteomalacia. Clin Radiol 1988;39:528–30. [DOI] [PubMed] [Google Scholar]
- [23].Melki SJ, Li Y, Semaan MT, et al. A mouse model validates the utility of electrocochleography in verifying endolymphatic hydrops. J Assoc Res Otolaryngol 2014;15:413–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Wick CC, Semaan MT, Zheng QY, et al. A genetic murine model of endolymphatic hydrops: the Phex mouse. Curr Otorhinolaryngol Rep 2014;2:144–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Liu S, Guo R, Quarles LD. Cloning and characterization of the proximalmurine Phex promoter. Endocrinology 2001;142:3987–95. [DOI] [PubMed] [Google Scholar]
- [26].Jonsson KB, Zahradnik R, Larsson T, et al. Fibroblast growth factor 23 in oncogenic osteomalacia and X-linked hypophosphatemic rickets. N Engl J Med 2003;348:1656–63. [DOI] [PubMed] [Google Scholar]
- [27].Rowe P The chicken or the egg: PHEX, FGF23, and SIBLINGs unscrambled. Cell Biochem Funct 2012;30:355–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Imel EA, DeMeglio LA, Hui SL, et al. Treatment of X-linked hypophosphatemia with calcitriol and phosphate increases circulating fibroblast growth factor 23 concentrations. J Clin Endocrinol Metab 2010;95:1846–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Aono Y, Hasegawa H, Yamazaki Y, et al. Anti-FGF-23 neutralizing antibodies ameliorate muscle weakness and decreased spontaneous movement of Hyp mice. J Bone Miner Res 2011;26:803–10. [DOI] [PubMed] [Google Scholar]
- [30].Sheykholeslami K, Megerian CA, Zheng QY. Vestibular evoked myogenic potentials in normal mice and Phex mice with spontaneous endolymphatic hydrops. Otol Neurotol 2009;30:535–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Semaan MT, Zheng QY, Han F, et al. Characterization of neuronal cell death in the spiral ganglia of a mouse model with endolymphatic hydrops. Hear Res 2008;237:90–105.18289812 [Google Scholar]
- [32].Marie PJ, Travers R, Glorieux FH. Bone response to phosphate and vitamin D metabolites in the hypophosphatemic male mouse. Calcif Tissue Int 1982;34:158–64. [DOI] [PubMed] [Google Scholar]
- [33].Zheng QY, Yu H, Washington JL III, et al. A new spontaneous mutation in the mouse protocadherin 15 gene. Hear Res 2006; 219:1120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Zhang MYH, Ranch D, Pereira RC, et al. Chronic inhibition of ERK1/2 signaling improves disordered bone and mineral metabolism in hypophosphatemic (Hyp) mice. Endocrinology 2012;153:1806–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Ogura Y, Clemis JD. A study of the gross anatomy of the human vestibular aqueduct. Ann Otol Rhinol Laryngol 1971;80:813–25. [DOI] [PubMed] [Google Scholar]
- [36].Hultgard-Ekwall AKH, Couloigner V, Rubin K, et al. Network organization of interstitial connective tissue cells in the human endolymphatic duct. J Histochem Cytochem 2003;51:1491–500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Wig H, Rubin K, Reed RK. New and active role of the interstitium in control of interstitial fluid pressure: potential therapeutic consequences. Acta Anaesthesiol Scand 2003;47:111–21. [DOI] [PubMed] [Google Scholar]
- [38].Rask-Andersen H, Bredberg G, Lyttkens L, et al. The function of the endolymphatic duct – an experimental study using ionic lanthanum as a tracer: a preliminary report. Ann N Y Acad Sci 1981;374:11–9. [DOI] [PubMed] [Google Scholar]
- [39].Friberg U, Rask-Andersen H. Vascular occlusion in the endolymphatic sac in Meniere’s disease. Ann Otol Rhinol Laryngol 2002;111:237–45. [DOI] [PubMed] [Google Scholar]
- [40].Linthicum FH, Doherty J, Webster P, et al. The periductal channels of the endolymphatic duct, hydrodynamic implications. Otolaryngol Head Neck Surg 2014;150:441–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Benet-Pagès A, Lorenz-Depiereux B, Zischka H, et al. FGF23 is processed by proprotein convertases but not by PHEX. Bone 2004;35:455–62. [DOI] [PubMed] [Google Scholar]
- [42].Shimada T, Hasegawa H, Yamazaki Y, et al. FGF-23 is a potent regulator of vitamin D metabolism and phosphate homeostasis. J Bone Miner Res 2004;19:429–35. [DOI] [PubMed] [Google Scholar]