Abstract
Background.
Mancozeb belongs to a group of pesticides known as dithiocarbamates (DTC) that are a non-systemic group of pesticides extensively used in Uganda to protect crops from fungal diseases.
Objectives.
This study was done in 5 selected districts of Central Uganda with a focus on markets and farms to investigate the current mancozeb concentrations on tomatoes and identify key areas of improvement to minimize human exposure.
Methods.
Tomato samples were analyzed for mancozeb residue determined as carbon disulfide (CS2) by gas chromatography—mass spectrometer (GC-MS).
Results.
All the samples analyzed had detectable concentrations of mancozeb residue. It was observed that farm samples had mean concentrations of 1.03±0.28 mg/kg, while market samples had 0.77±0.49 mg/kg. The study also found that farmers applied 3–6 times the dosage of mancozeb recommended by manufacturers. Furthermore, the observed pre-harvest interval after application of mancozeb was 1–2 days as opposed to 3–7 days set by manufacturers.
Conclusions.
The observed practices at farms are likely to put farmers and final consumers at a risk of exposure to dithiocarbamates.
Keywords: dithiocarbamates, Mancozeb, residue, tomatoes, Uganda, fungal disease, fungicide
Introduction
Pesticide residue research supports a number of activities including crop protection, environmental monitoring, consumer protection, and legislative enforcement. In Uganda, there is inadequate scientific evidence to support interventions of the whole lifecycle of chemical management, including specific categories like dithiocarbamates. Furthermore, there is no routine pesticide residue food safety monitoring or surveillance plans. Individual research has reported levels of Dithane M-45, a mancozeb contact fungicide, applied to tomatoes to be 3 to 7 times above the recommended dose.1 It was also noted that the majority of retailers were interested in visible signs of fungicide on tomatoes before purchasing. There are also allegations of substance abuse and misuse of the post-harvest application to prolong the shelf life of fruit.2
It is therefore important to generate scientific evidence for action to minimize impact of human exposure to dithiocarbamate fungicide residues in food and build institutional capacity for routine surveillance of chemical contamination in food.
Unlike most carbamates, dithiocarbamates do not inhibit choline esterases to any significant degree and are relatively non-toxic to humans.3 Mancozeb is practically not acutely toxic via the oral and dermal route of exposure, though it is a mild skin irritant. However, it has been shown that chronic exposure leads to impaired thyroid function, birth defects and cancer. The toxicity of mancozeb, maneb, metiram under the chemical group ethylene bisdithiocarbamate in food is related to the metabolite or its degradation product ethylene thiourea (ETU). ETU is responsible for the toxicity effects during chronic exposure and also known to be carcinogenic and teratogenic in rats.4,5 In addition, laboratory animals that ingested dithiocarbamates were shown to develop neuropathology, thyroid toxicity, and developmental toxicity to the central nervous system. Contact dermatitis has also been reported in workers exposed to mancozeb; the metabolite ETU is suspected to be goitrogenic and teratogenic in humans. Furthermore, several workers with long-term exposure to maneb have developed Parkinsonism, possibly as a result of manganese accumulation.6
Methods
Study Area
The study was carried out in 5 districts of the Central Region of Uganda. These are: Kampala, Mukono, Wakiso, Mityana and Mpigi. The main tomato variety grown in this region is Lycopersicon esculentum. Samples were obtained from markets and farms as indicated in Table 1.
Table 1.
Markets and Farms Sampled with Their Main Regional Source of Supplies
| District | Markets | Farm | Main Supply Source |
| Kampala | Kasubi | — | Western |
| Busega | — | West and Central | |
| St.Balikudembe | — | All over | |
| Kireka | — | Central and East | |
| Kalerwe | — | Central | |
| Nateete | — | West and Central | |
| Mpigi | — | Mapeera Estates Farm | — |
| Mpigi | — | Central | |
| Kikunyu | — | Central | |
| Wakiso | Namalyagonja | — | Central |
| Kasangati | — | Central | |
| Kawempe | — | Central and Northern | |
| Majije | — | Central | |
| Mukono | Ssangalyambogo | — | Central |
| — | Nyanja Farm A | — | |
| — | Nyanja Farm B | — | |
| Mityana | — | Kikonge green house | — |
The sampling approach included purchasing tomatoes from various randomly selected vendors in the markets and growers at the farms.7,8 At least 3 replicate samples were selected from each location with each sample consisting of at least 10 tomatoes, as suggested in the Codex guidelines.9–11 These were packaged in new polythene bags that were marked with unique identifier codes and sealed tight to avoid movement that could cause loss of mancozeb surface residues. They were also perforated to avoid sweating that would wash away the residues.8–10 In addition to that, the vendors were interviewed after informed consent. All vendors and farmers that were approached were willing to take part in this study.
Analytical Procedure
The method used to identify mancozeb residues was adapted from Eurofins Agroscience. 12 It is based on the method as originally published, with some modifications which have been validated.13–16 In this method, mancozeb is converted to carbon disulphide (CS2) which is measured by gas chromatography—mass spectrometer (GC-MS) in the electron impact—selected ion monitoring mode.
The analytical standard materials of mancozeb (purity 74.0%) was obtained from Dr. Ehrenstorfer GmbH (Ausburg, Germany). All reagents used were analytical grade. The hydrochloric acid and stannous chloride were obtained from Sigma-Aldrich (St. Louis, USA); iso-octane was purchased from Fisher Scientific (Waltham, USA); and lactose was obtained from LabChemie (Mumbai, India).
Abbreviations
- AR
Analytical reagent
- CS2
Carbon disulfide
- DTC
Dithiocarbamates
- ETU
Ethylene thiourea
- eV
Electron volt
- g
Gram
- GC-MS
Gas chromatography — mass spectrometer
- kg
Kilogram
- LOQ
Limit of quantification
- mg/kg
Milligrams per kilogram
- ml
Milliliter
- ml/min
Milliliters per minute
- MRL
Maximum residue limits
- mz
Mass-to-charge ratio
- NIST/EPA
National Institute of Standards and Technology/Environmental Protection Agency
- SD
Standard deviation
- SIM
Selected ion monitoring method
- SPSS
Statistical Packagefor the Social Sciences
- μg/ml
Microgram per milliliter
- μL
Microliter
The samples were frozen and cut to minimize degradation of mancozeb when in contact with acidic tomato juices. Wedge-shaped portions that included outer surfaces from each tomato were prepared.17 Representative portions were taken by mixing opposite quarters and a 50±0.1 g portion required for analysis was weighed into 250 ml gas-tight reaction Duran bottles.9 Iso-octane (20 ml) was added, followed by stannous (II) chloride (reducing solution) in diluted hydrochloric acid (100 ml), and sealed immediately with a cap. The 2-phase system was incubated at 80ºC in a water bath for 1.5 hours with frequent shaking. The Duran bottles were removed and left at ambient temperature for approximately 1 hour. The bottles were then placed in a freezer for 30 minutes to allow the generated carbon disulphide gas to condense. The samples were shaken and left for 5 minutes. The organic phase (isooctane) was removed and placed in a vial prior to the quantitation of carbon disulphide by GC-MS.
Procedural recoveries were determined concurrently with each batch of analytical extracts by analysing the carbon disulphide evolved after digestion of the spiked tomato samples with mancozeb standard. The spiking was done twice, once at the level of limit of quantitation (LOQ) (0.05 mg/kg) and the other at expected residue level (1.0 mg/kg). These values were obtained from prior runs during instrument optimisation.
Quantitative analysis was done from calibrations using Mancozeb Certified Reference Standard, corrected for purity and prepared in lactose. A 5-point calibration was done, ranging from 0.125–5 μg/ml. The method's LOQ was set at 0.05 mg/kg which equates to the calibration standard of 0.125 μg/ml.12
All extracts were analyzed using GC-MS (Shimadzu QP2010, Kyoto, Japan). The column used was an Agilent (Santa Clara, USA) J&W GC column (GS-GASPRO, length 30 m, diameter 0.32 mm with no film thickness). The system was calibrated daily using perfluorotributylamine. In addition, system blanks and known standards were run to monitor performance and sensitivity.
The GC temperature program was as follows: initial temperature was 60°C held for 2.5 minutes and then increased to 260°C at a rate of 15°C/min. The total run time was 15.83 minutes. Sample volumes of 1.0 μL were injected in a spitless mode with a solvent cut of 3 minutes. Initially, a standard at a high concentration was run in full scan acquisition mode, the MS was in positive electron impact mode at 70 eV and mass detection range was a mass-to-charge ratio (mz) of 40–550. Ion source was set at 200°C and interface temperature was 260°C. The peaks were confirmed with NIST/EPA Mass Spectral library. The carrier gas was helium (purity 99.999%) at flow rate of 2.0 ml/min. From this, a selected ion monitoring (SIM) method was developed with the target ion for carbon disulfide being mz 76 along with 44 and 78 as reference ions.
The data was analyzed using Microsoft Excel (Redmond, USA) and IBM SPSS v21 (Armonk, USA).
Results
Using the GC-MS technique, the mancozeb detected as CS2 was identified at a retention time of 6.7 minutes and confirmed with the corresponding selected ions in the mass spectrum as shown in Figure 1.
Figure 1.
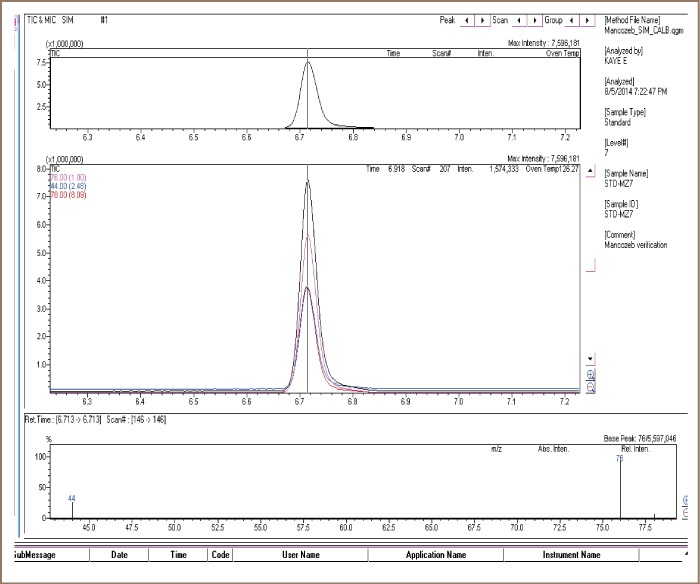
Sample of the GC-MS chromatogram showing identification and confirmation of CS2
A total of 57 samples obtained from 4 farms and 13 markets were analyzed. The findings are summarized in Tables 2 and 3. Satisfactory recoveries of between 70 and 110% were obtained and therefore no corrections to the concentrations were made.
Table 2.
Mean Concentration of Mancozeb Per Sampling Location
| Concentration of Mancozeb in mg/kg | ||
| Sampling Location | Description | Mean ± SD |
| Farm | Nyanja Farm A | 0.83±0.38 |
| Nyanja Farm B | 0.89±0.64 | |
| Mapeera Estate | 0.95±0.63 | |
| Kikonge green house | 1.45±0.01 | |
| Market | Kasubi | 0.78±0.58 |
| Mpigi | 0.22±0.12 | |
| Namalyagonja | 0.95±0.01 | |
| Kasangati | 0.39±0.13 | |
| Kawempe | 0.73±0.30 | |
| Busega | 0.42±0.26 | |
| Majije | 0.39±0.04 | |
| Kikunyu | 0.33±0.06 | |
| St. Balikuddembe | 1.13±0.21 | |
| Kireka | 1.52±0.51 | |
| Kalerwe | 1.29±0.23 | |
| Nateete | 1.64±0.66 | |
| Ssangalyambogo | 0.27±0.13 | |
SD = Standard deviation
Table 3.
Summary of Findings
| Concentration of Mancozeb in mg/kg | |||||
| District | N | Mean ± SD | Min | Max | Range |
| Farm | 4 | 1.03±0.28 | 0.8336 | 1.448 | — |
| Market | 13 | 0.77±0.49 | 0.219 | 1.6404 | 1.4214 |
| Total | 17 | 0.83±0.46 | 0.219 | 1.6404 | 1.4214 |
N = number of sampling locations; SD = Standard deviation
The data was found to be normally distributed using the Kolmogorov-Smirnov test with a P-value of 0.703 at a 95% confidence level. Therefore, parametric tests were performed to compare results.
The interquartile distribution of the DTC concentrations within the samples is shown in Figure 2.
Figure 2.
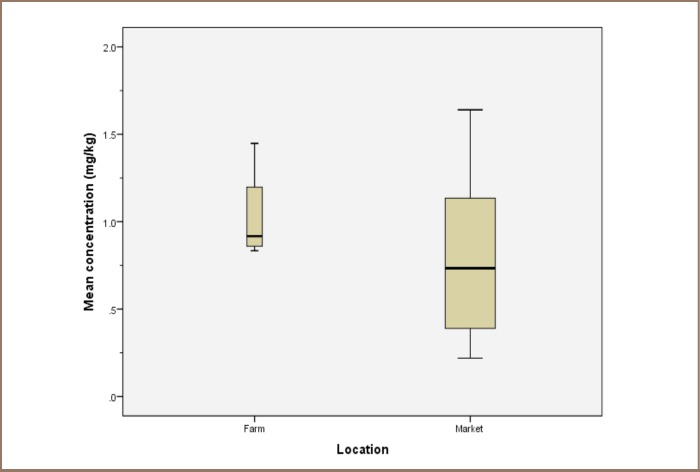
Boxplot showing mancozeb concentrations against sampling location
Discussion
This study was carried out in the Central Region of Uganda, where most of the final tomato transactions are carried out. The sampling was focused on the markets to look at what final consumers take to their homes, and farms to look at the practice and what goes to the markets.
Farm visits and interviews revealed that 3 out of the 4 farmers had received some training on pesticide use from the National Agricultural Advisory Services, a body responsible for enhancing agricultural production in Uganda. The common dithiocarbamate applied was mancozeb (concentration: 80% wettable powder) and its preharvest application interval was 1–2 days, as opposed to the manufacturers' recommendations of 3–7 days.18,19 The reason given for this short pre-harvest interval was to prolong the shelf-life of tomatoes; also, vendors required visible signs of mancozeb. The visible signs included off-white powder on the tomatoes, which gave confidence to vendors that the tomatoes would last longer. It was also observed that none of the farmers followed package label instructions for dilution of the powder. Every farmer confessed to adding more powdered mancozeb per unit volume of water than advised. Information obtained from mancozeb packets found at farms indicated that the recommended dosages ranged from 40–50 g mancozeb per 20 liters of water. However, the actual dosages applied ranged from 125–300 g mancozeb per 20 liters of water, showing that farmers applied 3 to 6 times more mancozeb than recommended by the manufacturers. Some farmers used more than the recommended dosages because they assumed that some of the products had been diluted prior to sale. This arose from past experience of recommended dosages resulting in very dilute solutions that did not serve the intended purpose.
It was observed that none of the farmers used personal protection equipment during the application of macozeb nor during harvesting. This could be attributed to the low level of awareness about the toxicity of mancozeb and the sheer lack of personal protective equipment.
From the 13 markets sampled, it was observed that some vendors cleaned their tomatoes, while others did not. This tallied with the relatively low mean concentrations of mancozeb observed at Ssangalyambogo and Mpigi markets with 0.27±0.13 mg/kg and 0.22±0.12 mg/kg, respectively. On the other hand, the highest mean concentrations of mancozeb were observed at Kireka and Nateete markets with 1.52±0.51 mg/kg and 1.64±0.66 mg/kg respectively. This could be attributed to vendors who confessed that they preferred leaving visible traces of mancozeb, which was perceived to prolong the shelf life of tomatoes.2
This study revealed that the farms had a higher mean mancozeb concentration of 1.03±0.28 mg/kg than the markets which had a mean concentration of 0.77±0.49 mg/kg. However, a two-tailed t-test performed at a 95% confidence level, obtained; t = 0.971, df = 15 and P = 0.347, implying that there was no significant difference between the concentrations of mancozeb obtained from the markets and the farms.
Conclusions
The study revealed that Mancozeb is extensively used on tomatoes in farms in the central region of Uganda. It was observed that all the samples analyzed had detectable levels of mancozeb. Furthermore, farms had higher concentrations of mancozeb compared to markets: 1.03±0.28 mg/kg and 0.77±0.49 mg/kg, respectively, although the difference was not statistically significant. The observed practices at farms were likely to put the farmers and final consumers at a risk of exposure to dithiocarbamates.
Limitations
This study had some limitations which include: lack of data on the degradation rates of mancozeb in the Ugandan climate in an open environment versus in a green house, and degradation during transportation and storage of tomatoes. Furthermore, the sample preparation technique based on acid digestion to liberate CS2 does not distinguish between the subclasses of dithiocarbamates; if another subclass apart from mancozeb was present, a false positive would be registered.20 If more than one subclass were present, a higher concentration would be recorded.
Acknowledgements
This work was funded in part from a grant from Pure Earth. Gratitude also goes to the Directorate of Government Analytical Laboratory staff for their technical input.
References
- 1.Integrated pest management collaborative research support program: annual workplan for year ten (September 29, 2002 to September 28, 2003) [Internet] Blacksburg, VA: Virginia Tech; 2002 Sep [cited 2014 Mar]. pp. 10–12. p. Available from: http://www.oired.vt.edu/ipmil/wp-content/uploads/2013/03/yr10-wp.pdf. [Google Scholar]
- 2.Kaaya AN, Kyamanywa S, Akemo C, Kagezi E, Warren H, Kyamuhangire W, Hakiza JJ. Dithiocarbamate fungicide residues in Ugandan tomato fruits and their effects on postharvest quality [Internet] Columbus, OH: Ohio State University; 2004 [cited 2014 Mar]. Available from: http://crsps.net/resource/dithiocarbamate-fungicide-residues-in-ugandan-tomato-fruits-and-their-effects-on-postharvest-quality/ [Google Scholar]
- 3.Moffat AC. Clarke's analysis of drugs and poisons. 4th rev. ed. London: Pharmaceutical Press; 2011 May 17. p. 2100. p. [Google Scholar]
- 4.Extension toxicology network: pesticide information profiles [Internet] Corvallis, OR: Oregon State University; 1996 Jun [cited 2014 Oct]. [about 3 screens]. Available from: http://extoxnet.orst.edu/pips/mancozeb.htm. [Google Scholar]
- 5.Caldas ED, Miranda MC, Conceicao MH, Souza LC. Dithiocarbamates residues in Brazilian food and the potential risk for consumers. Food Chem Toxicol [Internet] 2004 Nov [cited 2015 May 20];42(11):1877–83. doi: 10.1016/j.fct.2004.07.006. Available from: http://www.sciencedirect.com/science/article/pii/S0278691504002157. [DOI] [PubMed] [Google Scholar]
- 6.Baselt RC. Disposition of toxic drugs and chemicals in man. 9th ed. Seal Beach, CA: Biomedical Publication; 2011 Nov. p. 2350. p. [Google Scholar]
- 7.Guidelines for pesticide residue trials to provide data for the registration of pesticides and the establishment of maximum residue [Internet] Rome: Food and Agriculture Organization of the United Nations; 1986 [cited 2015 May 20]. Available from: http://www.bvsde.paho.org/bvstox/i/fulltext/fao06/fao06.pdf. [Google Scholar]
- 8.Client guildlines: field sampling for pesticide analysis [Internet] Silver Spring, MD: U.S. Food and Drug Administration; 2008 [cited 2015 May 20]. p. 6. p. Available from: http://www.primuslabs.com/services/CG-FieldSamplingforPesticideAnalysis.pdf. [Google Scholar]
- 9.Zaher TR, Nasr IN, Mahmoud HA. Behavior of some pesticide residues in and on tomato and kidney beans fruits grown in open field. Am-Eurasian J Toxicol Sci. [Internet] 2011 [cited 2015 May];3(3):213–8. Available from: http://www.idosi.org/aejts/3(3)11/20.pdf. [Google Scholar]
- 10.Recommended methods of sampling for pesticide residues for the determination of compliance with MRLs: CAC/GL 33-1999 [Internet] Rome: Codex Alimentarius International Food safety Standards; 1999 [cited 2014 Oct]. p. 18. p. Available from: http://www.codexalimentarius.org/standards/list-of-standards/en. [Google Scholar]
- 11.Bojaca CR, Arias LA, Ahumada DA, Casilimas HA, Schrevens E. Evaluation of pesticide residues in open field and greenhouse tomatoes from Colombia. Food Control [Internet] 2013 Apr [cited 2015 May 20];30(2):400–3. Available from: http://www.sciencedirect.com/science/article/pii/S095671351200463X Subscription required to view. [Google Scholar]
- 12.Method mancozeb/crops/DB/12/1. UK: Eurofins Agrosciences Services Limited; [updated 2012] [Google Scholar]
- 13.Keppel GE. Modification of the carbon disulfide evolution method for dithiocarbamate residues. J Assoc Off Anal Chem. 1969;52:162–7. [PubMed] [Google Scholar]
- 14.Keppel GE. Collaborative study of the determination of dithiocarbamate residues by a modified carbon disulfide evolution method. J Assoc Off Anal Chem. 1971 May;54(3):528–32. [PubMed] [Google Scholar]
- 15.Cesnik HB, Gregorcic A. Validation of the method for the determination of dithiocarbamates and thiuram disulphide on apple, lettuce, strawberry, potato and tomato matrix. Acta Chimica Slovenica [Internet] 2006 [cited 2015 May 20];53:100–4. Available from: http://acta-arhiv.chem-soc.si/53/53-1-100.pdf. [Google Scholar]
- 16.Rao TN, Sreenivasulu D, Patrudu TB, Sreenivas KM, Parvatamma B. A GC-MS method for the determination of mancozeb and metiram (as CS2) residues in aquatic tox medium. Scholars Acad J Pharm [Internet] 2013 [cited 2015 May 20];2(2):41–6. Available from: http://saspublisher.com/wp-content/uploads/2013/03/SAJP2241-46.pdf. [Google Scholar]
- 17.Crnogorac G, Schwack W. Residue analysis of dithiocarbamate fungicides. Trends Anal Chem [Internet] 2009 [cited 2015 May 20];28(1):40–50. Available from: http://www.sciencedirect.com/science/article/pii/S0165993608002367 Subscription required to view. [Google Scholar]
- 18.Indofil Industries Limited [Internet] Maharashtra, India: c2015 [cited 2015 May 20]. [publisher unknown] Available from: https://indofilcc.com. [Google Scholar]
- 19.Sinochem International Corp [Internet] Beijing, China: [publisher unknown]; c1996–2014 [cited 2015 May 20]. Available from: http://www.sinochem.com/g860/s1805/t4415.aspx. [Google Scholar]
- 20.Schmidt B, Christensen HB, Petersen A, Sloth JJ, Poulsen ME. Method validation and analysis of nine dithiocarbamates in fruits and vegetables by LC-MS/MS. Food Addit Contam Part A Chem Anal Control Expo Risk Assess [Internet] 2013 [cited 2015 May 20];30(7):1287–98. doi: 10.1080/19440049.2013.801083. Available from: http://www.tandfonline.com/doi/abs/10.1080/19440049.2013.801083?url_ver=Z39.88-2003&rfr_id=ori:rid:crossref.org&rfr_dat=cr_pub%3dpubmed Subscription required to view. [DOI] [PubMed] [Google Scholar]


