Abstract
Background.
Ghana, like many countries in Africa, has a history of heavy metal pollution largely emanating from industrial effluent discharges and anthropogenic deposits on prevailing winds of pollutants from industrial activities. One of the biggest contributors to pollution in the Ghanaian environment is mineral mining.
Objectives.
The aim of this study was to determine the distribution and health risks of heavy metals in surface water from both pristine environments and major mining areas in Ghana.
Methods.
A total of 32 composite samples were collected between September and October, 2014 to assess concentrations of heavy metals and pollution levels, as well as cancer and non-cancer risks to human health from exposure to heavy metals from four major mining regions and four rain forest reserves in the Western, Ashanti, Brong Ahafo and Eastern regions of Ghana. Samples were analyzed using atomic absorption spectrometry.
Results.
The mean concentrations (mg/L) of heavy metals at the pristine sites ranged from 1.747 for iron (Fe) to 0.001 for mercury (Hg) and 0.453 for Fe to 0.002 for Hg at the mining sites. All the metals were found to be below World Health Organization (WHO) and United States Environmental Protection Agency (USEPA) recommended limits except for Hg, which was at the USEPA guideline limit. However, the concentrations of the metals from the mining sites were found to be slightly higher than those from the pristine sites.
Conclusions.
The concentrations of heavy metals in the Nyam, Subri, Bonsa and Birim Rivers from the mining sites and the Atiwa Range, Oda, Ankasa and Bosomkese Rivers from the pristine sites were found to be either below or within the USEPA and WHO's recommended limits for surface water. The health risk assessment values for the hazard quotient for ingestion of water (HQing), dermal contact (HQderm) and chronic daily intake (CDI) indicated no adverse effects as a result of ingestion or dermal contact from the rivers. However, arsenic (As) in both the pristine and mining sites and chromium (Cr) in the pristine sites pose a carcinogenic threat to the local residents.
Keywords: Heavy metal pollution, health risk assessment, source apportionment, distribution, surface water, pristine environments, mining activities
Introduction
Ghana, like many countries in Africa, has a history of heavy metal pollution largely emanating from industrial effluent discharges and anthropogenic deposits from industrial activities due to prevailing winds. One of the biggest contributors to pollution in the Ghanaian environment is mineral mining. In Ghana, both local and foreign investor companies have equal rights to engage in mineral exploration. In 2000, a total of 224 local and foreign companies obtained mineral rights for gold exploration, as well as 600 registered small-scale miners. At the same time, the informal (illegal) sector locally referred to as “galamsey operators” dominated the small-scale gold sector with an estimated number of 200,000 people in 2004, up from a total of approximately 60,000 people in 1997.1
In Ghana, the most common approach adopted by many mining companies is the use of cyanide, which makes it possible for mining companies to make large profits from low grade ores.2 Lately, the use of cyanide in gold extraction has become unattractive as a result of several cyanide spillages which have caused significant damage to the environment. According to Amegbey and Adimado, the number of officially reported cyanide spillages between 1989 and 2003 in Tarkwa and Obuasi alone was 11 cases.3 A separate investigative report issued by the Wassa Association of Communities Affected by Mining (WACAM)4 indicated that cyanide spillages increased from 8 between 1989 and 2002 to about 13 cyanide spillages in 2006. This situation has led to the pollution of major water bodies such as streams and rivers, and over 52% of the population in the mining areas still lacks potable water.5
As gold is the main contributor to Ghana's economy, accounting for 38% of total stock and 95% of total mineral exports,5 small-scale mining as well as improper regulation of large-scale mining pose enormous environmental challenges such as land degradation, subsidence due to gold mining and water pollution, all of which pose risks to human health.6–8 Wastes from mining processes such as tailings can result in the influx of metals and toxic chemicals into the environment. Waste rocks are known to contain arsenic (As), mercury (Hg), cadmium (Cd), lead (Pb) and other toxic metals.9,10 Eventually, the metals attain higher concentrations and accumulate in large quantities in crops and plants, and pose serious health hazards to humans and animals through bio-magnification.11
Abbreviations
- AAS
Atomic absorption spectrophotometer
- ADD
Average daily dose
- ADDing
Quantity of heavy metals ingested per kilogram of body weight
- ANOVA
Analysis of variance
- APHA
American Public Health Association
- As
Arsenic
- At
Averaging time
- Bwt
Average body weight
- C
Concentration of heavy metal in water
- Cd
Cadmium
- CDI
Chronic daily intake
- Cf
Unit conversion factor
- Co
Cobalt
- Cr
Chromium
- CR
Carcinogenic risk
- CRderm
Carcinogenic risk due to dermal contact
- CRing
Carcinogenic risk due to ingestion
- Cu
Copper
- Cw
Concentration of metal in water
- Ed
Exposure duration
- Ef
Exposure frequency
- Et
Exposure time
- FA
Factor analysis
- Fe
Iron
- Hg
Mercury
- HNO3
Nitric acid
- HQ
Hazard quotient
- HQderm
Hazard quotient for dermal contact
- HQing
Hazard quotient for ingestion of water
- IARC
International Agency for Research on Cancer
- KMO
Kaiser-Meyer-Olkin
- Mn
Manganese
- NTU
Nephelometric turbidity unit
- Pb
Lead
- PCA
Principal component analysis
- QC
Quality control
- RfD
Reference dose
- RfDdermal
Dermal reference dose
- RfDoral
Oral reference dose
- SF USEPA
Slope factor United States Environmental Protection Agency
- WACAM
Wassa Association of Communities Affected by Mining
- WHO
World Health Organization
- Zn
Zinc
There have been reports indicating increased concentrations of heavy metals and other pollutants in water bodies around mining communities in Ghana. Reports from studies have shown that from 1947 to 1992, mine effluents were discharged without restriction and treatment into water bodies, soil and air, thereby resulting in the degeneration of the environment.12–14
Rivers are the main surface water resources for domestic, industrial and irrigation purposes, and their contamination as a result of large industrial wastewater discharges and seasonal run-offs from agricultural lands poses a growing threat to the environment.15 In recent years, many rivers, streams and ponds in Ghana have had elevated inputs of heavy metals as a result of an increase in mining and other anthropogenic activities in and around water bodies, raising serious concern about the toxic effects on aquatic organisms and persistence in the environment.16 Huge deposits of mine wastes as well as ore stockpiles and waste rocks are usually seen in large piles around both large- and small-scale mining areas. These deposits and stockpiles are gradually washed through weathering and leaching into far and near surface water bodies, thereby releasing toxic substances into aquatic bodies. The wastes from gold extraction are known to contain metals such as arsenic, mercury, cadmium, lead, and other toxic metals.3,8,10 The latest World Health Organization (WHO) evaluation concluded that arsenic exposure through drinking water is causally related to cancer in the lungs, kidney, bladder and skin. There is also an increased risk of skin cancer and other skin lesions, such as hyperkeratosis and pigmentation changes. Ingestion of inorganic arsenic may induce peripheral vascular disease, which leads to black foot disease.17
Figure 1.
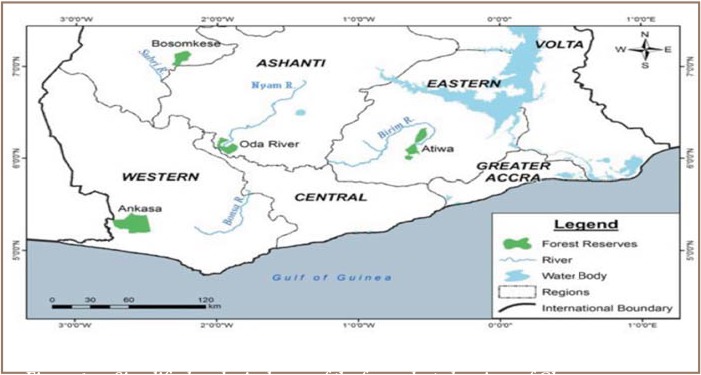
Simplified geological map of the four selected regions of Ghana showing the location of the study areas
Table 1.
Regions and Locations of Water Sample Collection Around the Major Mining
| Site | Region | Location | Area |
| Oda River | Ashanti | Bekwai* | 633 km2 |
| Nyam River | Ashanti | Obuasi | 162.4 km2 |
| Bosomkese River | Brong Ahafo | Bosomkese* | 138 km2 |
| Subri River | Brong Ahafo | Kenyasi | – |
| Ankasa River | Western | Ankasa* | 509 km2 |
| River Bonsa | Western | Tarkwa | 2354 km2 |
| Atewa River | Eastern | Atewa* | 2,950 km2 |
| Birim River | Eastern | Atewa | 3875 km2 |
*Indicates rivers from forest reserves. The remainder are rivers from mining areas
Lead poisoning can lead to headache, irritability, abdominal pain and various symptoms related to the nervous system. Acute exposure to lead is known to cause proximal renal tubular damage.18 Long-term lead exposure may also give rise to kidney damage. Although the overall evidence for lead as a carcinogen is weak; the most likely associated diseases are lung cancer, stomach cancer and gliomas.19
The International Agency for Research on Cancer (IARC) has classified cadmium (Cd) as a human carcinogen.20 Data on recent studies suggest that relatively low exposure may give rise to skeletal and tubular kidney damage.21–23
The aim of this study is therefore to determine the distribution and health risks of heavy metals in surface water from both pristine environments and major mining areas in Ghana.
Methods
Sampling and Analysis
Four water bodies were selected based on their proximity to the mining areas. Sample collection was undertaken between September and October 2014, the wet season. A total of 32 composite samples were collected from rivers in the four mining areas as well as the four pristine environments. At each sampling site, 8 discrete samples were collected and composited to give a representative sample of that point of the river. The samples were collected 100 m apart at 4 points from downstream to upstream of each of the rivers in duplicate for metal analysis and for physico-chemical measurement.4 Composite surface water samples were collected from each river around the mining communities and the pristine environments. The eight selected sites therefore gave a total of 32 composite samples that were collected from communities across the four major gold mining regions of Ghana. The samples were collected in 1.5 L plastic bottles prewashed with detergent and 1:1 concentrated nitric acid/distilled water solution and eventually rinsed with distilled water. Those samples meant for metal analysis were acidified to a pH of 2 using concentrated nitric acid before being transported to the laboratory in an ice-chest filled with ice chips. The samples were stored in a refrigerator at 4°C upon arrival at the laboratory for further analysis.24,25
Digestion and Analysis of Heavy Metals in Water
Digestion of the water samples involved sample measurement and reagent addition and digestion in a microwave. Then 5 ml of the water samples in three replicates were accurately weighed into TFM Teflon vessels of a microwave digester (Milestone ETHOS 900). Next, 6 ml of nitric acid (HNO3) (65%), 3 ml of hydrogen chloride (35%) and 0.25 ml of hydrogen peroxide (30%) were added to each of the vessels containing the samples. The vessels were swirled gently until thoroughly mixed and then fitted vertically into the microwave digester and digested for 21 minutes.
The Teflon bombs mounted on the microwave carousel were cooled in a water bath for 20 minutes to reduce high temperatures and pressure build-up within the vessels. The digestates were then transferred quantitatively into a volumetric flask and diluted to 20 ml using distilled water. A blank was also prepared in similar fashion, but without the analyte.
All the samples were analysed using a Varian AAS 240FS flame atomic absorption spectrometer coupled with an Atlas Copco LFx MED Superflow compressor at the Nuclear Chemistry and Environmental Research Center, Ghana Atomic Energy. To ensure the reliability of the analytical method during digestion and sample preparation, quality control (QC) and blank samples were digested along with each set of samples and subsequently analyzed for appropriate elements through the same procedure. Reference standards for this work were obtained from Fluka Analytical, Sigma-Aldrich Chemie GmbH, Switzerland.
Determination of Physico-chemical Characteristics
Physico-chemical measurements of pH, conductivity, temperature and turbidity were conducted to identify indications that would affect the results obtained from the study. The American Public Health Association (APHA, 1998) method for preparation and analysis of water samples was employed for the determination of physico-chemical parameters in this study. The pH of the water samples was determined alongside the temperature using a pre-calibrated pH meter. Conductivity was measured using a pre-calibrated Hach SensION5 conductivity meter. The turbidity of the water samples was measured with a Hach turbidimeter. In addition, pH and temperature were measured on site.
Quality Control
Samples were analyzed in triplicates and after every 4 samples, a calibration standard was analyzed to check the response and efficiency of the analytical instrument alongside the blank which was used to constantly check for contamination.
Calibration curves were optimized by the use of quality control standards at every step of sample reading. All glassware were soaked in 10% HNO3 overnight and thoroughly washed with distilled water and dried in an oven overnight at 50–60° C. After oven-drying, the glassware were dried in a desiccator for about 20 minutes before use.
Recovery and Reproducibility Studies
A recovery study was conducted by adding a known amount of certified reference standard material to three different water samples and digested the same way as the other samples and the responses were compared based on values calculated from the standard curve. The recovery study was performed to help determine the validity of the sensitivity and efficiency of the method used. The recovery values for the reference material and the heavy metals are given as follows: the measured concentration for the spiked QC certified standard (Fluka Analytical) ranged between 4.839 and 4.979 mg/L against the certified concentration of 5.000 mg/L. The percent recovery was calculated to be 98.18%. Similar average percent recovery estimates for the metals in the spiked water samples were: zinc (Zn), 100.4%; iron (Fe), 100%; copper (Cu), 100.1%; manganese (Mn), 96.4%; cobalt (Co), 99.9%; Cd, 99.9%; chromium (Cr), 100.1%; As, 92.5%; Hg, 99.3%; and Pb, 95.7%. The recovery values indicated that the error associated with the determination of concentrations of the heavy metals from the mining areas and the pristine environments was negligible.
Data and Statistical Analysis
Data from the study were analyzed using IBM SPSS Statistics version 21 and the Excel Analysis ToolPak. Basic statistics such as mean and standard deviation were computed along with the multivariate statistics. Factor analysis (FA) and principal component analysis (PCA) were computed to identify significant principal components in the data as well as possible loadings and sources of the heavy metals. The PCA was carried out by the Promax normalized rotation method for the results. A scree plot was performed to determine how many important components were present in the data and to provide a visual explanation of the metal loading process which describes loading groups with a reduction in the original data.26,27
Human Health Risk Assessment
The human health risk assessment was used to characterize the nature and magnitude of possible human health risks and ecological receptors from heavy metal contaminants and other stressors that may be present in the environment. The health risk assessment for the heavy metals in surface water from both the mining areas and the pristine environments was estimated via ingestion and dermal contact based on the USEPA risk assessment method.28 Details of the standards employed in the calculation of various parameters are shown above (Table 2).
Table 2.
Standard Values for Calculating Exposure Assessment of Trace Metals in Surface Water Samples28–30
| Dermal Permeability Co-effcient (cm/h) | Values | |
| Zn | 6×10−4 | |
| Cu | 1×10−3 | |
| Mn | 1×10−3 | |
| Fe | 1×10−3 | |
| Cd | 1×10−3 | |
| Cr | 1×10−3 | |
| Pb | 1×10−4 | |
| Hg | 1×10−3 | |
| Co | 4×10−4 | |
| As | 1×10−3 | |
| Exposure Factors | Units | Values |
| Concentration of Metal in Water (Cw) | mg/L | – |
| Water Ingestion Rate | L/day | 2 |
| Exposure Frequency (Ef) | Days/year | 365 |
| Exposure Duration (Ed) | Years | 70 |
| Average Body Weight (Bwt) | Kg | 70 |
| Averaging Time (At) | Days | 25,550 |
| Exposed Skin Area (Sa) | Cm2 | 28000 |
| Exposure Time (Et) | h/day | 0.6 |
| Unit Conversion Factor (Cf) | L/cm3 | 1×10−3 |
In the exposure assessment, the average daily dose (ADD) for the heavy metals level in surface water from the pristine and mining environments was calculated using the following slightly modified equations from USEPA protocol in 1989 and 2004.
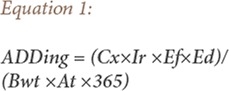 |
Where, Cx is the concentration of toxicant metals in the drinking water (mg/L), Ir is the ingestion rate per unit time (L/day), Ed is the exposure duration (years), Ef is the exposure frequency (days/year), Bwt is the body weight of receptor (kg), and At is the averaging time (years) which is equal to the life expectancy of a resident Ghanaian. For the conversion factor from year to days, 365 was used. In addition, ADDing is the quantity of heavy metals ingested per kilogram of body weight.29,31
In this study, surface water ingestion is assumed to be the main pathway for risk assessment because the rivers are potential sources of drinking water. However, dermal contact is another important pathway, because residents sometimes swim in these rivers and thus may come into contact with the toxic metals.32
Average daily dose for dermal contact was calculated using the formula:
 |
Where, Sa is the total skin surface area (cm3), Cf is the volumetric conversion factor for water (1L/1000 cm3), Et is the exposure duration (h/day), Pc is the chemical-specific dermal permeability constant (cm/h), Ef is the exposure frequency (days/years), Ed is the exposure duration (years), Lt is the human lifetime (defined as 70 years) and Bwt is body weight.
The purpose of hazard assessment is to evaluate whether an agent poses a carcinogenic hazard to humans and under what circumstances an identified hazard may be expressed.33,34 Hazard assessment is performed by comparing the calculated contaminant dose from ingestion and dermal exposure routes with the reference dose (RfD) to develop the hazard quotient (HQ) using equation 3 below:
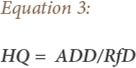 |
Where HQ represents the hazard quotient via ingestion or dermal contact (no units) and RfD is the oral/dermal reference dose (mg/L/day).26
The chronic daily intake (CDI) of the metal was estimated using equation 4 below:
 |
Where C is the concentration of heavy metal in water, DI is the average daily intake rate (2 L) and Bwt is the body weight (70 kg).
Finally, the carcinogenic risks (CRs) of the metals were estimated to assess the probability of an individual developing cancer over a lifetime as a result of exposure to a potential carcinogen. The slope factor (SF) is a toxicity value that quantitatively defines the relationship between dose and response. Potential carcinogenic effect probabilities that an individual will develop cancer over a lifetime of exposure are estimated from projected intakes and slope factor. The range for carcinogenic risk acceptable by the USEPA is 1×10−6 to 1×10−4.
 |
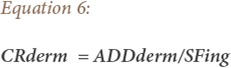 |
Where CRing and CRderm represent carcinogenic risk due to ingestion and dermal exposure routes, respectively, and SF is the slope factor (mg/kg)/day. To demonstrate the lifetime carcinogenic risk that the local population experiences, the CR values were calculated for As, Cd and Cr. In this study, both oral and dermal reference doses (RfDoral/dermal) were employed for the respective toxic metals. The slope factor values for the metals were 1.5 (mg/kg)/day, 6.1×102 (mg/kg)/day, and 5.0×102 (mg/kg)/day, respectively.32 All parameters were calculated by the use of IBM SPSS Statistics version 21 and Excel Analysis ToolPak.
Results
Physico-chemical Variations in the Water Samples
The level of pH in the aquatic environment can affect chemical and biological processes. Even though humans may not be affected directly by pH, an elevated range of pH results in a bitter taste for drinking water.30 The pH ranges for rivers in the study area are shown in Table 3. All rivers fell within the WHO range for potable water of 6.5 to 8.5. Temperatures ranged from 28.0 to 28.7° C with an average of 28.4° C. Conductivity of the water samples from the four mining sites ranged from 442 to 2890 μs/cm, with a mean value of 1666 μs/cm and 228 to 938 μs/cm from pristine sites with a mean value of 583 μs/cm. Turbidity values of water samples from the mining areas in this study ranged from 0 to 92 nephelometric turbidity units (NTU) with a mean of 46 NTU, whiles samples from the pristine areas ranged from 0 to 39 NTU with a mean of 19.5 NTU.
Table 3.
Physico-chemical Parameters for Water Samples from Pristine and Mining Sites
| ID Mining | Conductivity (μs/cm) | pH | Turbidity (NTU) | Temperature (°C) |
| Bonsa River Mean + range | 2790–2890 | 7.0–7.2 | 0–1 | 28.2–28.6 |
| Birim River Mean + range | 442–1890 | 5.7–7.0 | 21–50 | 28.4–28.7 |
| Subri River Mean + Range | 2070–2079 | 6.9–7.2 | 6–52 | 28.1–28.4 |
| Nyem River Mean + range | 470–478 | 6.3–6.5 | 7–92 | 28.1–28.4 |
| ID Pristine | Cond. (μs/cm) | pH | Turb. (NTU) | Temp. (°C) |
| Ankasa Forest River Mean + range | 242–272 | 5.6–6.2 | 1–4 | 28.0–28.6 |
| Atiwa Range River Mean + range | 485–519 | 6.8–7.8 | 0–1 | 28.1–28.4 |
| Bosomkese Forest River Mean + range | 626–938 | 6.7–6.8 | 21–39 | 28.0–28.4 |
| Oda River Mean + range | 228–258 | 6.2–6.6 | 6–8 | 28.0–28.3 |
| US EPA, 2004, 2012 | 6.5–8.5 | |||
| WHO, 2003, 2008 and NAFDAC, 2007 | 500 | 6.5–8.5 | 5 | 29 |
Statistical Evaluation and Source Apportionments of the Heavy Metals
The statistical analysis conducted to identify possible loading on components resulted in 2 main components of metals from the mining sites and 4 components from the pristine sites, of which only three showed clear significance. For the mining sites, component 1 included Zn, Fe, Mn and Cr, and component 2 included As and Cd. Pb and Cu were below detection limits and thus were excluded. For the pristine sites, component 1 consisted of Co, As and Hg, component 2 consisted of Fe and Mn, component 3 consisted of Zn and Cd, and component 4 consisted of Cr. Factor and principal component analysis (FA/PCA) conducted on the results from the mining sites gave two components with total %variance of 78.74% and eigen-values of 3.33 and 2.18 for components 1 and 2, respectively, as shown in Table 5. The FA/PCA on components 1 and 2 accounted for 47.60% and 31.14%, respectively, of the total variance.
Table 5.
Factor and Principal Component Analysis (PCA) Results Showing Loading of Metals on Components in Water Samples from Pristine and Mining Sites
| Elements |
Mining |
Pristine |
||||
| PCA1 | PCA2 | PCA1 | PCA2 | PCA3 | PCA4 | |
| Zn | 0.956 | 0.067 | −0.096 | 0.080 | 0.860 | −0.322 |
| Cd | −0.210 | 0.789 | 0.004 | −0.011 | 0.859 | 0.286 |
| Cr | 0.702 | −0.093 | 0.491 | −0.060 | 0.127 | 0.816 |
| Hg | – | – | 0.828 | −0.339 | −0.045 | −0.109 |
| Fe | 0.913 | 0.122 | 0.267 | 0.942 | 0.110 | −0.073 |
| Mn | 0.948 | 0.152 | 0.193 | 0.945 | −0.173 | −0.082 |
| As | −0.313 | 0.884 | 0.849 | −0.080 | 0.121 | −0.434 |
| Co | 0.221 | 0.851 | 0.848 | −0.056 | −0.052 | 0.036 |
| Eigen Value | 3.332 | 2.180 | 2.485 | 1.915 | 1.555 | 1.066 |
| % Total Variance | 47.597 | 31.144 | 31.057 | 23.933 | 19.436 | 13.327 |
| % Cumulative Variance | 47.597 | 78.741 | 31.057 | 54.990 | 74.425 | 87.753 |
Human Health Risk Evaluation
The evaluation of the heavy metals in the water samples from pristine and mining sites for possible adverse health effects associated with exposure to such chemicals revealed that the level of exposure through ingestion (ADDing) was observed in the order Cr > Cd > As from the pristine and mining sites.28 The observed dermal exposure (ADDderm) was in the order Cr > Cd > As from both sites. In the present study, Zn, Fe, Co and Mn were not considered in the risk and hazard quotient estimation because their slope factors could not be obtained and Pb and Cu were below the detection limit.
Discussion
Physico-chemical Variations in the Water Samples
The pH results show that the rivers in the pristine and mining areas are slightly acidic. However, in 2007, Tay and Kortatsi36 reported similar values from their studies in the range of 4.37–7.40 in the Densu Basin and Tay's37 sole study in 2008 reported a pH range of 5.4 to 7.1, with a mean value of 6.4, from the same sampling sites. In 2010, Nkansah et al.38 indicated that pH values lower than 6.5 are considered too acidic for human consumption and can cause health problems such as acidosis which could have adverse effects on the digestive and lymphatic systems of humans. The toxicity of the metals in the aquatic environment is altered depending on the pH of the water body.39 When the pH in water decreases, most metals are soluble and hence become available to aquatic organisms.40 The higher the acidity, the more soluble and mobile the metals become, and the more likely they are to be taken up and accumulated.41 With the pH ranges obtained from this work, the water samples can be considered close to neutral, meeting both drinking and freshwater quality requirements (6.5 to 8.5) of the WHO and the USEPA. The temperature values reported in this work were lower than the 29° C limit set by the WHO in 2003 for potable drinking water.42 Temperature is a factor of great importance for aquatic ecosystems, as it affects water organisms, as well as the physical and chemical characteristics of water. Conductivity results are shown in Table 3. Comparing the mean values of 1666 μs/cm from the mining sites and 583 μs/cm from the pristine sites with the WHO permissible limit for potable water of 500 μs/cm suggests that most values in the present study are higher than the regulatory limit, indicating relatively high salt or ion content of the water bodies, which contradicts the low metal concentrations obtained in this study. The conductivity values were far above the boundary values of the Ghana Raw Water Criteria and Guidelines which ranged from 0 to 70 μs/cm, falling in the ‘noeffect’ range for drinking water.43 However, the low conductivity recorded in samples from the Nyam, Ankasa, and Oda Rivers could be a result of protection of the water bodies from domestic effluent discharges and surface run-off from cultivated fields which contribute to increased concentrations of ions. The results, however, show that water samples from the mining areas contain higher concentrations of salts than those from the pristine environments. The WHO guideline for turbidity in drinking water is 5 NTU. The relatively high measured turbidity values may be attributed to larger particles such as organic matter and dissolved solids. Meanwhile, a study by Akoto and Adiyiah in 200744 indicated that high turbidity could be an indication of the presence of disease-causing organisms such as bacteria, viruses, and parasites that can cause symptoms such as nausea, cramps, diarrhea, and associated headaches (Akoto and Adiyiah, 2007). In 2013, Nkoom et al. measured turbidity ranges of 14.3 to 43 NTU with a mean value of 23.3 NTU around Samreboi in the Wassa Amenfi West District of Ghana.45 In 2010, Schafer et al. also found turbidities in the range of 2 to 266 NTU in most borehole water throughout Ghana.46 Turbidity results from the Bonsa, Ankasa, Atiwa and Oda Rivers in this work can therefore be considered acceptable since they have values equal to or below the WHO's 5 NTU limit. However, turbidity values from the Subri, Birim, Nyam and Bosomkese Rivers were far above the WHO limit and therefore can be considered to be unfit for human consumption. Sample filtration was conducted for samples whose turbidity persisted after sample digestion.
Concentrations and Site Variation of Heavy Metals
The distribution of heavy metals in the rivers from the selected sampling sites was generally low compared to that of national and international standards as shown in Table 4. The low levels of Cd suggest that the source may not necessarily be as a result of the mining activities, but Cd may occur naturally with zinc and sulfide ores.47 The mean concentrations of the heavy metals in samples from both sites were below the WHO standard limit except for Fe, which showed varied metal levels with a few values higher than WHO and USEPA required limits. The high levels of Fe may result from natural geological sources or corroded iron metals carried in seasonal run-offs from illegal mining activities.
Table 4.
Mean Concentrations (mg/L) of Heavy metals in Water Samples from Pristine and Mining Areas BDL Indicates below detection limit
| ID Mining | Zn (mg/L) | Fe (mg/L) | Mn (mg/L) | Co (mg/L) | Cr (mg/L) |
| Bonsa River Mean+SD/Range | 0.015±0.01 | 0.035±0.04 | BDL | 0.016±0.01 | 0.010±0.01 |
| Birim River Mean+SD/Range | 0.010±0.00 | 0.367±0.20 | 0.004±0.00 | 0.011±0.01 | 0.002±0.00 |
| Subri River Mean+SD/Range | 0.012±0.00 | 2.068±0.00 | BDL | 0.020±0.02 | BDL |
| Nyam River Mean+SD/Range | 0.033±0.04 | 4.718±3.23 | 0.012±0.02 | 0.019±0.01 | 0.014±0.00 |
| ID Pristine | Zn (mg/L) | Fe (mg/L) | Mn (mg/L) | Co (mg/L) | Cr (mg/L) |
| Ankasa Forest River Mean+SD/Range | 0.010±0.01 | 0.237±0.16 | BDL | 0.021±0.01 | 0.023±0.01 |
| Atiwa Range River Mean+SD/Range | BDL | BDL | BDL | 0.015±0.01 | 0.002±0.02 |
| Bosomkese Forest River Mean+SD/Range | 0.014±0.00 | 0.378±0.19 | 0.005±0.00 | 0.016±0.01 | 0.012±0.00 |
| Oda River Mean+SD/Range | 0.015±0.01 | 1.193±0.13 | BDL | 0.031±0.02 | 0.008±0.03 |
| US EPA, 2004, 2012 | 5.000 | 0.300 | 0.050 | 0.110 | 0.100 |
| WHO, 1998, 2006 | 5.000 | 1.0 | – | – | – |
| ID Mining | As (mg/L) | Cd (mg/L) | Pb (mg/L) | Cu (mg/L) | Hg (mg/L) |
| Bonsa River Mean+SD/Range | 0.002±0.00 | BDL | BDL | BDL | BDL |
| Birim River Mean+SD/Range | 0.002±0.00 | BDL | BDL | BDL | BDL |
| Subri River Mean+SD/Range | 0.003±0.00 | 0.005±0.01 | BDL | BDL | BDL |
| Nyam River Mean+SD/Range | BDL | BDL | BDL | BDL | BDL |
| ID Pristine | As (mg/L) | Cd (mg/L) | Pb (mg/L) | Cu (mg/L) | Hg (mg/L) |
| Ankasa Forest River Mean+SD/Range | BDL | BDL | BDL | BDL | 0.002±0.00 |
| Atiwa Range River Mean+SD/Range | BDL | BDL | BDL | BDL | BDL |
| Bosomkese Forest River Mean+SD/Range | 0.003±0.00 | 0.004±0.00 | BDL | BDL | 0.002±0.00 |
| Oda River Mean+SD/Range | 0.003±0.00 | BDL | BDL | BDL | BDL |
| US EPA, 2004, 2012 | 0.010 | 0.005 | 0.015 | 1.000 | 0.002 |
| WHO, 1998, 2006 | 0.010 | 0.005 | 0.010 | 2.000 | 0.006 |
In Ghana, the presence of Hg in mining communities is linked with the use of Hg in gold mining. Average Hg levels were therefore expected to be high in water samples from the mining sites. Hg was detected in the Ankasa and Bosomkese Rivers, but the levels (0.002 mg/L) were below WHO recommended guideline limits.39 The Hg presence in the pristine environment could be linked with illegal mining activities in the forest reserves or anthropogenic deposition of this metal. However, similar low concentrations of Cd, Hg, As, Mn, Cu and Zn in water samples from the Samre River in the Wassa Amenfi West District of Ghana were reported.45 In a separate study in 2013, Cobbina et al. found relatively low concentrations of heavy metals in surface water and borehole samples from Tinga, in the Bole-Bamboi District of Ghana.48
Omolara et al., in similar study in 201449 during the wet season in Nigeria, reported concentrations of Cd and As below detection limits, and levels of Pb, Cr, Co and Cu ranging from 0.003 to 0.009 mg/L. The low concentrations of heavy metals in this study may be attributed to the fact that water levels increase during rainy seasons (wet season), resulting in a decrease in the concentration of metals. Most of the pristine sites are located in remote mountains and as a result, the concentrations of some of the measured metals were low.
Statistical Evaluation of the Heavy Metals
Results from the statistical analysis conducted to identify possible loading on components are shown in Table 5. The results suggest that only the first two components have eigen-values greater than one (> 1.00), and together these explain over 79% of the total variability in the data. Also, Bartlett's test of sphericity gave a p-value of 0.000, which is below the recommended value of < 0.001, and therefore a good indication for a valid FA/PCA to be performed on the data. Looking at results from the pristine sites, the factor analysis resulted in 4 components with total %variance of 87.75% and eigenvalues of 2.49, 1.92, 1.56 and 1.07 for components 1 to 4, respectively. The FA/PCA on components 1 to 4 accounted for 31.06%, 23.93%, 19.44% and 13.33% of the total variance. The results indicated that all four components have eigen-values greater than one (> 1.00), and together these explain over 87.75% of the total variability in the data. The Kaiser-Meyer-Olkin (KMO) measure of sampling adequacy gave a p-value at 0.35 and Bartlett's test of sphericity gave a p-value less than the recommended value of 0.001. The KMO value (0.35) indicates that the results are poor and are not fit for principal component analysis. However, Bartlett's test of sphericity with an associated p-value of <0.001 gave a good indication that factor analysis can be performed on the results.35
The correlation analysis showed that a stronger correlation was observed at the mining sites than the pristine sites. Correlations at the mining sites are shown in Table 5. The correlation results suggest that Zn, Mn, Fe and Cr may be coming from the same input sources, while As and Cd may be coming from different input sources. The correlation coefficients as well as the extracted component loading values of Cd and Co (r=0.425) and As and Co (r=0.653) indicate a closer association of Cd with As than with Co. The component plot for this phenomenon is shown in Figures 2 and 3. A similar trend of association of metals was observed in the pristine sites with a majority of the correlation coefficients falling below r=0.4, representing a weak correlation. The moderate correlation between Zn and Cd is not surprising since Cd occurs in the natural environment, typically in association with zinc ores (an impurity in zinc). Cobalt correlated positively and moderately with As (r=0.625) and Hg (r=0.545). There was strong and positive correlation between Hg and As (r=0.768) which could be due to the fact that along with mercury, arsenic is part of the toxic residue of gold mining. As, Hg and Co associations were confirmed by the metals' strong loading on component 1 in the FA/PCA results. However, most of the metals from the mining sites may result from anthropogenic deposition and the use of pesticides and fertilizers by farmers in the vicinity of the selected water bodies, which are eventually washed off into the rivers.2
Figure 2.
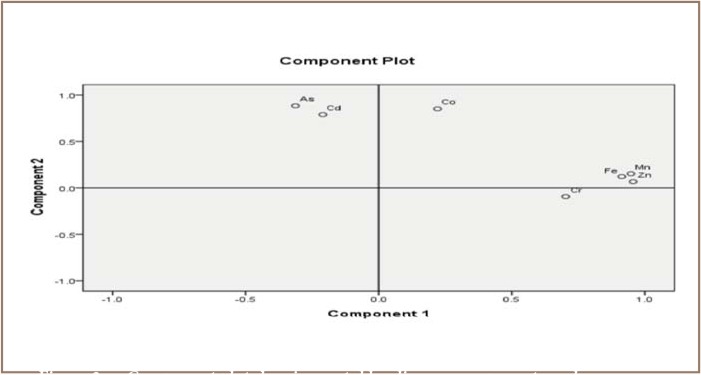
Component plot showing metal loading on components and source apportionment of the metals from the mining sites
Figure 3.
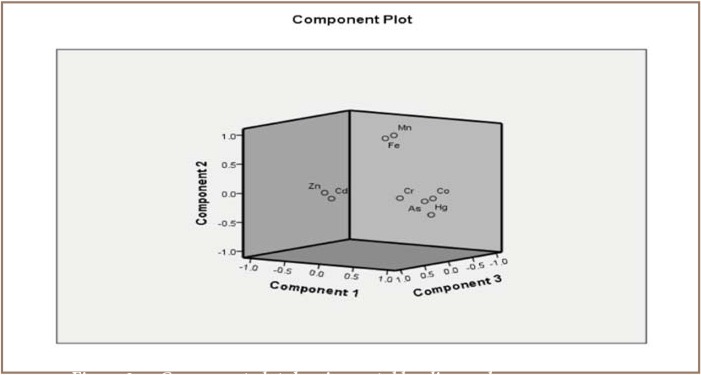
Component plot showing metal loading and source apportionment of metals from the pristine sites
Analysis of Variance Estimation
Two-way analysis of variance (ANOVA) conducted to compare the means and determine if the concentrations of any of the heavy metals had any significant influence on the final result indicated no significant difference in the concentrations of metals between the various sites since the probability associated with the p-value was greater than 0.05 and the F-value was less than the Fcrit (p=0.45, F=1.01, Fcrit=1.74). The results are shown in Table 6 and 7 for the pristine and mining sites, respectively. However, a significant difference was found between the concentrations of the metals, giving a p-value far less than 0.05 and an F-value greater than the F-crit (p-1.1×10–9, F=8.21, Fcri=1.95). These differences were confirmed by FA/PCA and the correlation results, where some of the metals had a stronger correlation than others and also had different loading on the components.
Table 6.
Pristine Sites — Two-way ANOVA Showing Differences Between Sites and Metals
| Source of Variation | SS | df | MS | F | P-value | F crit |
| Between Sites | 2.238675 | 15 | 0.14924498 | 1.010545 | 0.44821 | 1.741066 |
| Between Metals | 10.90729 | 9 | 1.21192068 | 8.205977 | 1.14E-09 | 1.94988 |
| Error | 19.93782 | 135 | 0.14768756 | |||
| Total | 33.08378 | 159 |
Table 7.
Mining Sites — Two-way ANOVA Showing Differences Between Sites and Metals
| Source of Variation | SS | df | MS | F | P-value | F crit |
| Between Sites | 15.40263 | 15 | 1.026842 | 1.013586 | 0.451115 | 1.801825 |
| Between Metals | 24.95044 | 5 | 4.990088 | 4.925668 | 0.000595 | 2.336576 |
| Error | 75.98088 | 75 | 1.013078 | |||
| Total | 116.34 | 95 |
Human Health Risk Evaluation
In addition, HQing and HQderm for Cr, Hg and Cd from the mining and the pristine sites were below 1.0, indicating their minimum hazard effect on the local residents who utilize the rivers. However, the HQing for As and Cd values of 0.76 and 0.71, respectively, were a bit near unity, which indicates the potential of these metals posing a hazard threat should their levels continue to accumulate. From the pristine and mining sites, the average levels of HQing for Cr, Cd, and As were found to be 0.64 and 0.55, respectively, as shown in Table 8, while the observed values for HQderm were 0.17 and 0.11, respectively. According to USEPA risk assessment guidelines, when the value of the hazard quotient is greater than 1.0, the probability of adverse health effects due to exposure is high.28,45 In the current study, the hazard quotients were below 1.0, which suggests that communities which depend on the rivers for drinking or swimming may not be at high risk of illnesses associated with high levels of consumption of contaminants.
Table 8.
| Element | Cr | As | Cd |
| RfDing | 3.00×10−3 | 3.00×10−4 | 5.00×10−4 |
| RfDderm | 7.50×10−5 | 8.00×10−4 | 2.50×10−4 |
| Pristine sites | |||
| ADDing | 1.29×10−3 | 2.28×10−4 | 2.86×10−4 |
| ADDderm | 3.61×10−5 | 6.40×10−6 | 8.00×10−6 |
| CDI | 1.29×10−3 | 2.29×10−4 | 2.86×10−4 |
| CRing | 2.57×10−4 | 1.52×10−4 | 4.68×10−7 |
| CRderm | 7.20×10−8 | 4.27×10−6 | 1.31×10−8 |
| HQing | 0.43 | 0.76 | 0.71 |
| HQderm | 0.48 | 0.01 | 0.03 |
| Mining sites | |||
| ADDing | 7.71×10−4 | 2.29×10−4 | 3.14×10−4 |
| ADDderm | 2.16×10−5 | 6.40×10−6 | 8.80×10−6 |
| CDI | 7.71×10−4 | 2.29×10−4 | 3.14×10−4 |
| CRing | 1.50×10−6 | 1.52×10−4 | 5.15×10−7 |
| CRderm | 4.80×10−8 | 4.27×10−6 | 1.44×10−8 |
| HQing | 0.26 | 0.76 | 0.63 |
| HQderm | 0.29 | 0.01 | 0.04 |
The chronic daily intake (CDI) values for the metals from the pristine and mining sites are shown in Table 8. The low CDI indices indicate that mining activities and agricultural practices like fertilization and run-off are not impacting the heavy metals load in the river bodies in the present study and also do not affect the water quality of the rivers.
Carcinogenic risk through ingestion of heavy metals (CRing) for Cr, Cd and As were estimated to be 2.57×10–6, 4.68×10–7 and 1.52×10–4, respectively, from the pristine sites, and 1.50×10–6, 5.15×10–7 and 1.52×10–4, respectively, from the mining sites. The CRderm from the pristine and mining sites ranged from 10–6 to 10–8, which is below the remedial target goal of 10–6. The USEPA has estimated the CR from projected intakes and slope factor. The range for carcinogenic risk acceptable by the USEPA50 is 1×10–6 to 1×10–4. The CRing results from this study for Cr and Cd were within the USEPA's acceptable limits, with most of the values falling within the upper boundary. The CRing for As was above the remedial goal target of 1×10–6 in both the pristine and mining sites as well as Cr in the pristine sites, therefore raising carcinogenic concerns for the local residents around the catchment areas.51 Inorganic arsenic and chromium (VI) are known human carcinogens. High levels of arsenic can cause cancer of the skin, lungs, liver and bladder. Lower intakes of As may cause nausea and vomiting, abnormal heart rhythm, and damage to blood vessels. Chromium (VI) compounds are toxins and known human carcinogens, and breathing elevated levels can cause irritation to the lining of the nose and nose ulcers.52
The results also show that the carcinogenic risks were found to be higher than the non-carcinogenic risks to the residents through ingestion of water from water bodies around the mining areas.
Conclusions
The concentrations of heavy metals in the Nyam, Subri, Bonsa and Birim Rivers from the mining sites and the Atiwa Range, Oda, Ankasa and Bosomkese Rivers from the pristine sites were found to be either below or within the USEPA and WHO's recommended limits for surface water. The health risk assessment values for HQing, HQderm and CDI, were found to be below 1.0 in the pristine and mining sites, indicating no adverse effects as a result of ingestion or dermal contact of the rivers. However, CRing and CRderm values for As in both the pristine and mining sites and Cr in the pristine site were above the remedial goal target of 1×10–6, and therefore pose a carcinogenic threat to the local residents.
Inorganic arsenic is a known carcinogen and can cause cancer of the skin, lungs, liver and bladder. At low concentrations, As exposure can cause nausea and vomiting, decreased production of red and white blood cells, and damage to blood vessels.53 Multivariate statistical analysis (PCA/FA) has confirmed both geogenic and anthropogenic sources of metal introduction into the rivers. This study is the first of its kind undertaken to compare metal pollution levels in areas believed to be highly polluted (mining areas) with areas believed to be out of metal pollution range (pristine locations). Even though further studies are needed, the current study nevertheless provides preliminary information on heavy metal levels in rivers from mining areas and pristine environments in Ghana which can be used for future water pollution monitoring.
Acknowledgments
This work was funded in part by a grant from Pure Earth. The authors are also grateful to staff of the Ghana Atomic Energy Commission (Chemistry and Environmental section) for making available their laboratory equipment for this work.
References
- 1.Koranteng DO. Statement by WACAM on the cyanide spillage by Bogoso Gold Limited [Internet] Tarkwa, Ghana: Wassa Association of Communities Affected by Mining; 2004 Oct 23 [cited 2015 May 21]. [about 2 screens]. Available from: http://www.miningwatch.ca/statement-wacam-cyanide-spillagebogoso-gold-limited. [Google Scholar]
- 2.Obiri S. Determination of heavy metals in boreholes in Dumasi in the Wassa West District of western region of the Republic of Ghana. Environ Monit Assess [Internet] 2007 Jul [cited 2015 Nov 9];130(1–3):455–63. doi: 10.1007/s10661-006-9435-y. Available from: http://link.springer.com/article/10.1007%2Fs10661-006-9435-y Subscription required to view. [DOI] [PubMed] [Google Scholar]
- 3.Adimado AA, Amegbey NA. Incidents of cyanide spillage in Ghana. Mineral Process Extractive Metall [Internet] 2003 Aug 1 [cited 2015 Nov 9];112(2):126–30. Available from: http://www.maneyonline.com/doi/abs/10.1179/037195503225002808 Subscription required to view. [Google Scholar]
- 4.Owusu-Koranteng H. Presentation on the Social Impact of Gold Mining in Ghana Un-equal Distribution of Burdens and Benefits and its implications on Human Rights at the 11th EADI General Conference [Internet] 2005 [cited 2015, May 21] Available from www.wacamghana.com. [Google Scholar]
- 5.Aryee BN. Ghana's mining sector: its contribution to the national economy. Resour Policy [Internet] 2001 Jun [cited 2015 Nov 9];27(2):61–75. Available from: http://www.sciencedirect.com/science/article/pii/S0301420700000428 Subscription required to view. [Google Scholar]
- 6.Manaf MH. The environmental impact of smallscale mining in Indonesia. Third Environmental Co-operation Workshop for Sustainable Development on Mining Activities; 1999 Oct 5–8; Cairns, Austria. [place unknown]: [publisher unknown]; [date unknown] [Google Scholar]
- 7.Hilson G. Small-scale mining in Africa: tackling pressing environmental problems with improved strategy. J Environ Dev [Internet] 2002 Jun [cited 2015 Nov 9];11(2):149–74. Available from: http://jed.sagepub.com/content/11/2/149.abstract Subscription required to view. [Google Scholar]
- 8.Akabzaa TM, Yakubo BK, Seyire JS. Impact of mining activities on water in the vicinity of the Obuasi mine. West Afr J ApplEcol [Internet] 2007 [cited 2015 Nov 9];11(1):101–10. Available form: http://www.ajol.info/index.php/wajae/article/view/45719/29197. [Google Scholar]
- 9.Chapman D, editor. 2nd ed. London: E&FN Spon; 1996 [cited 2015 Nov 10]. Water quality assessments: a guide to the use of biota, sediment and water in environmental monitoring [Internet] p. 651. editor. p. Available from: http://www.who.int/water_sanitation_health/resourcesquality/wqa/en/ [Google Scholar]
- 10.Donato DB, Nichols O, Possingham H, Moore M, Ricci PF, Noller BN. A critical review of the effects of gold cyanide-bearing tailings solutions on wildlife. Environ Int [Internet] 2007 Oct [cited 2015 Nov 10];33(7):974–84. doi: 10.1016/j.envint.2007.04.007. Available from: http://www.sciencedirect.com/science/article/pii/S0160412007000815 Subscription required to view. [DOI] [PubMed] [Google Scholar]
- 11.Malabika R. Accumulation of heavy metals in plants grown in industrial areas. Indian Biol XXII. 1990;(2):33–8. [Google Scholar]
- 12.Carboo D, Armah YS. Arsenic in stream and sediments in Obuasi area [Internet] :114–9. Proceeding of the symposium on the mining industry and the environment; 1997 Apr 14–15; Kumasi, Ghana. Kumasi, Ghana: UST/IDRC Environmental Research Group; 1997[cited 2015 Nov 10]. p. Available from: https://idl-bnc.idrc.ca/dspace/bitstream/10625/26841/1/122374.pdf. [Google Scholar]
- 13.Tufour K. Mining degradation of forest lands resources and rehabilitation [Internet] :43–8. Proceeding of the symposium on the mining industry and the environment; 1997 Apr 14–15; Kumasi, Ghana: UST/IDRC Environmental Research Group; 1997[cited 2015 Nov 10]. p. Available from: https://idlbnc.idrc.ca/dspace/bitstream/10625/1/122374.pdf. [Google Scholar]
- 14.Tsidzi KE, editor. Kumasi, Ghana: University of Science and Technology; 1993. Proceedings of the national seminar on current developments in the minerals industry of Ghana, Kumasi/Ghana 6 &7 May; p. 131. editor. p. [Google Scholar]
- 15.Obiri S. Risk assessment of toxic chemicals in mining operations in Ghana [master's thesis] :30. [Kumasi, Ghana]: Department of Chemistry, Kwame Nkrumah University of Science and Technology; 2005. p. [Google Scholar]
- 16.MacFarlane GR, Burchette MD. Cellular distribution of copper, lead and zinc in the grey mangrove, Avicenna marina (Forsk) Vierh. Aquat Bot [Internet] 2000 Sep 1 [cited 2014 Sep 23];68(1):45–59. Available form: http://www.sciencedirect.com/science/article/pii/S0304377000001054 Subscription required to view. [Google Scholar]
- 17.Caminero AG, Howe P, Hughes M, Kenyon E, Lewis DR, Moore M, Ng J, Aitio A, Becking G. 2nd ed. Geneva, Switzerland: World Health Organization; 2001 [cited 2015 Nov 11]. Environmental health criteria 224: arsenic and arsenic compounds [Internet] p. 512. p. Available from: http://www.who.int/ipcs/publications/ehc/ehc_224/en/ [Google Scholar]
- 18.Geneva, Switzerland: World Health Organization; 1995 [cited 2015 Nov 11]. Environmental health criteria 165: inorganic lead [Internet] p. 300. p. Available from: http://www.inchem.org/documents/ehc/ehc/ehc165.htm. [Google Scholar]
- 19.Steenland K, Boffetta P. Lead and cancer in humans: where are we now? Am J Ind Med [Internet] 2000 Sep [cited 2015 Nov 11];38(3):295–9. doi: 10.1002/1097-0274(200009)38:3<295::aid-ajim8>3.0.co;2-l. Available from: http://onlinelibrary.wiley.com/doi/10.1002/1097-0274(200009)38:3%3C295::AID-AJIM8%3E3.0.CO;2-L/abstract Subscription required to view. [DOI] [PubMed] [Google Scholar]
- 20.Beryllium, cadmium, mercury and exposure in the glass manufacturing industry [Internet] Vol. 58. Lyon, France: International Agency for Research on Cancer Monographs on the Evaluation of Carcinogenic Risks to Humans; 1993 [cited 2015 Nov 11]. Cadmium and cadmium compounds; pp. 119–237. In. p. Available from: http://monographs.iarc.fr/ENG/Monographs/vol58/mono58-7.pdf. [Google Scholar]
- 21.Staessen JA, Roels HA, Emelianov D, Kuznetsova T, Thijs L, Vangronsveld J, Fagard R. Environmental exposure to cadmium, forearm bone density, and risk of fractures: prospective population study. Lancet [Internet] 1999 Apr 3 [cited 2015 Nov 11];353(9159):1140–4. doi: 10.1016/s0140-6736(98)09356-8. Available from: http://www.thelancet.com/journals/lancet/article/PIIS0140-6736(98)09356-8/abstract Subscription required to view. [DOI] [PubMed] [Google Scholar]
- 22.Alfven T, Elinder CG, Carlsson MD, Grubb A, Hellstrom L, Persson B, Pettersson C, Spang G, Schutz A, Jarup L. Low-level cadmium exposure and osteoporosis. J Bone Miner Res [Internet] 2000 Aug [cited 2015 Nov 11];15(8):1579–86. doi: 10.1359/jbmr.2000.15.8.1579. Available from: http://onlinelibrary.wiley.com/doi/10.1359/jbmr.2000.15.8.1579/abstract Subscription required to view. [DOI] [PubMed] [Google Scholar]
- 23.Nishijo M, Nakagawa H, Morikawa Y, Tabata M, Senma M, Miura K, Takahara H, Kawano S, Nishi M, Mizukoshi K. Mortality of inhabitants in an area polluted by cadmium: 15 year follow up. Occup Environ Med [Interne] 1995 Mar [cited 2015 Nov 11];52(3):181–4. doi: 10.1136/oem.52.3.181. Available from: http://www.ncbi.nlm.nih.gov/pmc/articles/PMC1128184/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Clescerl LS, Greenberg AE, Eaton AD, . 20th ed. Washington, D.C.: American Public Health Association; 1999 Jan.. Standard methods for the examination of water and wastewater; p. 1325. editors. p. [Google Scholar]
- 25.Naveedullah, Hashmi MZ, Yu C, Shen H, Duan D, Shen C, Lou L, Chen Y. Concentration and human health risk assessment of selected heavy metals in surface water of the Siling Reservoir watershed in Zhejiang Province, China. Pol J Environ Stud [Internet] 2014 [cited 2015 Nov 13];23(3):801–11. Available from: http://www.pjoes.com/pdf/23.3/Pol.J.Environ.Stud.Vol.23.No.3.801-811.pdf. [Google Scholar]
- 26.Bartholomew DJ, Steele F, Moustaki I, Galbraith J. 2nd ed. Boca Raton, FL: CRC press; 2008 Jun 4. Analysis of multivariate Social Science data; p. 384. p. [Google Scholar]
- 27.Risk assessment guidance for superfund: human health evaluation manual. Vol. 1, Pt A [Internet] Washington, D.C.: United States Environmental Protection Agency; 1989 Dec [cited 2015 Nov 13]. p. 291. p. Available from: http://rais.ornl.gov/documents/HHEMA.pdf. [Google Scholar]
- 28.Risk assessment guidance for superfund: human health evaluation manual. Vol. 1, Pt E, supplemental guidance for dermal risk assessment [Internet] Washington, D.C.: United States Environmental Protection Agency; 2004 Jul [cited 2015 Nov 13]. p. 156. p. Available from: http://www2.epa.gov/sites/production/files/2015. [Google Scholar]
- 29.Health effects assessment summary tables: FY 1997 uptake [Internet] Washington, D.C.: United States Environmental Protection Agency; 1997 Jul 31 [cited 2015 Nov 13]. p. 404. p. Available from: http://www.id.energy.gov/Groundwater/PDF/MOL/MOL.20010724.0301.pdf. [Google Scholar]
- 30.Obiri S, Dodoo DK, Essumang DK, Armah FA. Cancer and non-cancer risk assessment from exposure to arsenic, copper, and cadmium in borehole, tap, and surface water in the Obuasi municipality, Ghana. Hum Ecol Risk Assess [Internet] 2010 Jun 7 [cited 2015 Nov 13];16(3):651–65. Available from: http://www.tandfonline.com/doi/abs/10.1080/10807031003788907 Subscription required to view. [Google Scholar]
- 31.Moya J, Phillips L, Schuda L, Wood P, Diaz A, Lee R, Clickner R, Birch RJ, Adjei N, Blood P, Chapman K, Castro RD, Mahaffey K. Exposures factors handbook: 2011 edition. [Internet] Washington, D.C.: United States Environmental Protection Agency; 2011 Sep [cited 2015 Nov 13]. p. 1436. p. Available from: http://cfpub.epa.gov/ncea/risk/recordisplay.cfm?deid=236252. [Google Scholar]
- 32.Science and judgment in risk assessment [Internet] Washington, D.C.: National Academy Press; 1994 [cited 2015 May 15]. p. 652. p. Available from: http://www.nap.edu/openbook.php?isbn=030904894X. [Google Scholar]
- 33.Ogunfowokan AO, Okoh EK, Adenuga AA, Asubiojo OI. An assessment of the impact of point source pollution from a university sewage treatment oxidation pond on a receiving stream: a preliminary study. J ApplSci [Internet] 2005 [cited 2015 Nov 13];5(1):36–43. Available from: http://scialert.net/qredirect.php?doi=jas.2005.36.43&linkid=pdf. [Google Scholar]
- 34.Guidelines for drinking-water quality: health criteria and other supporting information. no. 11 Vol. 23. Washington D.C.: World Health Organization; 2003. pp. 145–96. p. [Google Scholar]
- 35.Hibbert DB, Gooding JJ. Data analysis for chemistry: an introductory guide for students and laboratory scientists. 1st ed. Oxford, UK: Oxford University Press; 2005 Nov 3. p. 192. p. [Google Scholar]
- 36.Tay C, Kortatsi B. “Groundwater quality studies. a case study of Densu Basin, Ghana”. West Africa J ApplEcol 2007. 12:81–99. [Google Scholar]
- 37.Tay CK. “Chemical characteristics of groundwater in the Akatsi and Ketu Districts of the Volta Region, Ghana,”. WestAfrican Journal of Applied Ecology. 2008;11(1):1–23. pp. [Google Scholar]
- 38.Nkansah MA, Boadi MO, Badu M. “Assessment of the Quality of Water from Hand-Dug Wells in Ghana”. Environmental Health Insights. 2010;4:7–12. doi: 10.4137/EHI.S3149. Available from: http://www.la-press.com. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Campbel PG, Stokes PM. Acidification and toxicity of metals to aquatic biota. Can J Fish AquatSci [Internet] 1985 [cited 2015 Nov 13];42(12):2034–49. Available from: http://www.nrcresearchpress.com/doi/abs/10.1139/f85-251?journalCode. [Google Scholar]
- 40.Atlanta, GA: Agency for Toxic Substances and Disease Registry; 2001 Mar 23 [cited 2015 Nov 13]. Summary report for the ATSDR expert panel meeting on tribal exposures to environmental contaminants in plants [Internet] p. 121. p. Available from: http://www.engg.ksu.edu/chsr/outreach/tosnac/docs/NAreport_fnl032301.pdf. [Google Scholar]
- 41.Guidelines for carcinogen risk assessment [Internet] Washington, D.C.: United States Environmental Protection Agency; 2005 Mar [cited 2015 Nov 13]. p. 166. p. Available from: http://www3.epa.gov/airtoxics/cancer_guidelines_final_3-25-05.pdf. [Google Scholar]
- 42.Delince G. The ecology of the fish pond ecosystem with special reference to Africa. :230. In. Addo MA. Probable impact of the West African gas pipeline project at Tema New-Town, Ghana [master's thesis]. [ Legon, Ghana]: Environmental Science Programme, University of Ghana; 2002. p. [Google Scholar]
- 43.Ghana raw water criteria and guidelines. Vol. 1, Domestic water. Accra, Ghana: CSIR-Water Research Institute; 2003. [Google Scholar]
- 44.Akoto O, Adiyiah J. Chemical analysis of drinking water from some communities in the BrongAhafo region. Int. J. Environ. Sci Tech. 2007;4(2):211–214. [Google Scholar]
- 45.Nkoom M, Cobbina JS, Kumi M. Assessment of endocrine disrupting trace metals in River Samre at Samreboi in the WassaAmenfi west district of the western region of Ghana. J Water ResourProt [Internet] 2013 Oct [cited 2015 Nov 13];5(10):983–992. Available from: http://www.scirp.org/journal/PaperInformation.aspx?PaperID=38715. [Google Scholar]
- 46.Schafer AI, Rossiter HM, Owusu PA, Richards BS, Awuah E. Physico-chemical water quality in Ghana: prospects for water technology implementation. Desalination [Internet] 2009 [cited 2015 Nov 13];248(1–3):193–203. Available from: http://www.research.ed.ac.uk/portal/files/4183865/J71_ERA.pdf. [Google Scholar]
- 47.Olatunji OS, Osibanjo O. Determination of selected heavy metals in inland fresh water of lower River Niger drainage in North Central Nigeria. Afr J Environ SciTechnol [Internet] 2012 Oct [cited 2015 Nov 13];6(10):403–408. Available from: http://www.ajol.info/index.php/ajest/article/view/88654/78246. [Google Scholar]
- 48.Cobbina SJ, Nkuah D, Dery DT, Obiri S. Noncancer risk assessment from exposure to mercury (Hg), cadmium (Cd), arsenic (As), copper (Cu) and lead (Pb) in boreholes and surface water in Tinga, in the Bole-Bamboi District. J Toxicol Environ Health Sci [Internet] 2013 Feb [cited 2015 Nov 13];5(2):29–36. Available from: http://www.academicjournals.org/journal/JTEHS/article-fulltext-pdf/6BB3EED4497. [Google Scholar]
- 49.Omolara T, Aladesanmi Isaac F, Adeniyi Ibukun M. Adesiyanomparative Assessment and Source Identification of Heavy Metals in Selected Fishpond Water, Sediment and Fish Tissues/Organs in Osun State, Nigeria. J Health Pollution 2014. 7(4):42–53. [Google Scholar]
- 50.Drinking water standards and health advisories [Internet] 12th ed. Washington, D.C.: United States Environmental Protection Agency; 2012 Spring [cited 2015 Nov 13]. p. 12. p . Available from: http://water.epa.gov/action/advisories/drinking/upload/dwstandards2012.pdf. [Google Scholar]
- 51.Iqbal J, Shah MH. Health risk assessment of metals in surface water from freshwater source lakes, Pakistan. Hum Eco Risk AssessInt J [Internet] 2013 [cited 2015 Nov 13];19(6):1530–43. Available from: http://www.tandfonline.com/doi/full/10.1080/10807039.2012.716681 Subscription required to view. [Google Scholar]
- 52.ToxFAQs [Internet] Atlanta, GA: Agency for Toxic Substances and Disease Registry; [date unknown] - [updated 2014 Jun 24; cited 2015 Nov 13]. [about13 screens]. Available from: http://www.atsdr.cdc.gov/toxfaqs/index.asp#bookmark01. [Google Scholar]
- 53.Toxicological profile for arsenic [Internet] Atlanta, GA: Agency for Toxic Substances and Disease Registry; 2007 Aug [cited 2015 Nov 13]. p. 559. p. Available from: http://www.atsdr.cdc.gov/toxprofiles/tp2.pdf. [PubMed] [Google Scholar]


