Abstract
Background
Lifestyle changes are notoriously difficult. Since women who intend to become pregnant are more susceptible to lifestyle advice, interventions during this time window might be more effective than interventions during any other period in life. We here report the effects of the first large preconception lifestyle intervention RCT on diet and physical activity in obese infertile women.
Methods
In total, 577 women were randomized between a six-month lifestyle intervention program (intervention group; N = 290) or prompt infertility treatment (control group; N = 287). Self-reported dietary behaviors and physical activity were assessed at baseline, three, six and twelve months after randomization. Mixed models were used to analyze differences between groups.
Results
Compared to the control group, the intervention group reduced their intake of sugary drinks at three months (-0.5 glasses/day [95% C.I. = -0.9;-0.2]), of savory snacks at three (-2.4 handful/week [-3.4;-1.4]) and at six months (-1.4 handful/week [-2.6;-0.2]), and of sweet snacks at three (-2.2 portion/week [-3.3;-1.0]) and twelve months after randomization (-1.9 portion/week [-3.5;-0.4]). Also, the intervention group was more moderate to vigorous physically active at three months after randomization compared to the control group (169.0 minutes/week [6.0; 332.1]).
Conclusion
Our study showed that obese infertile women who followed a six-month preconception lifestyle intervention program decreased their intake of high caloric snacks and beverages, and increased their physical activity. These changes in lifestyle may not only improve women’s health but their offspring’s health too.
Introduction
The increasing prevalence of obesity is a major public health problem in women of reproductive age [1]. Besides the association of obesity with increased prevalence of non-communicable diseases [2], it also adversely affects women’s reproductive health [3,4], as well as offspring’s health [5].
A healthy lifestyle is recommended as the first step to control obesity [6]. However, we do know that structurally improving lifestyle is notoriously difficult. Women who intend to become pregnant are known to be more susceptible to lifestyle advice, for example to quit smoking and stop drinking alcohol [7,8]. Therefore, lifestyle interventions prior to conception might be more effective in changing diet and physical activity than interventions during any other period in life.
Up until now, studies mainly focused on intervening during the period of pregnancy [9–14], but currently attention shifts to intervention strategies targeting obese women before pregnancy to improve reproductive, maternal and child health [15–17]. However, no experimental studies assessing the effect of preconception lifestyle interventions in humans have been done yet.
The LIFEstyle study was the first randomized controlled trial (RCT) designed to examine the efficacy of a preconception lifestyle intervention in a large group of obese infertile women on reproductive, gestational and delivery outcomes [18]. The lifestyle intervention resulted in significantly more weight loss [19] and improved cardiometabolic health [20], but it is unclear how the intervention changed lifestyle.
Therefore, we here report the effects of the LIFEstyle preconception intervention program on diet and physical activity in obese infertile women throughout the intervention program and thereafter.
Materials and methods
The LIFEstyle study was a multicenter RCT in obese infertile women (Dutch trial register; NTR 1530; http://www.trialregister.nl/trialreg/admin/rctview.asp?TC=1530). Participants were included in the study between June 9, 2009 and June 22, 2012 and followed for two years. Design and primary results of the LIFEstyle study have been described previously [18,19]. In brief, the original study population consisted of 577 infertile women between 18 and 39 years old, with a BMI of ≥29 kg/m2. Women were eligible for recruitment when presenting with infertility in a general or academic hospital. Infertility was defined as failure to conceive within 12 months of unprotected intercourse in case of an ovulatory cycle, or in case of chronic anovulation according to WHO class I or II. Couples were excluded if suffering from azoospermia or using donor semen, women with endometriosis AFS class III or IV, chronic anovulation WHO class III (premature ovarian failure) or endocrinopathies (such as Cushing syndrome, adrenal hyperplasia and diabetes type I). Women with untreated pre-existent hypertension, preeclampsia, eclampsia or HELLP syndrome in a previous pregnancy were also not eligible.
This study was conducted according to the guidelines laid down in the Declaration of Helsinki. All procedures were approved by the Medical Ethics Committee of the University Medical Center Groningen, the Netherlands (METc 2008/284) and the review board of each participating center. Written informed consent was obtained from all participants.
Intervention
Participants were randomized by a web-based randomization program at a central location, stratified according to trial center and ovulatory status. Blinding was not possible due to the nature of the intervention. Participants randomized into the intervention arm participated in a six-month structured lifestyle program, aiming at a weight loss of 5–10% of the original body weight. After completion of the intervention program, if the target weight reduction of 5–10% was met, or if BMI decreased below 29 kg/m2, infertility treatment was started in accordance with the Dutch infertility guidelines [21]. When becoming pregnant participants discontinued the intervention, but they could re-enter the intervention in case of a miscarriage. The control group promptly started infertility treatment based on the Dutch infertility guidelines. They did not receive any lifestyle advice with the exception of the patient information leaflet containing general information on the adverse effects of overweight and obesity on women’s reproductive health, pregnancy, and pregnancy outcomes.
The lifestyle program combined counselling on diet and physical activity with an individualized behavioral modification plan [22–24]. Intervention nurses, with a background in infertility care, were trained to guide and support the participants during six face-to-face and four telephone consultations [18]. Participants were advised to consume a healthy diet according to the Dutch dietary guidelines of 2006 [25] with a caloric reduction of approximately 600kcal compared to their usual caloric intake, but not below 1200kcal/day. To create awareness of total food intake, participants could receive feedback on food and caloric intake on a daily basis using a web-based food diary of the Netherlands Nutrition Center [26]. Participants brought a copy of these results to the consultations to discuss their dietary intake. In addition, participants were advised to be physically active 2–3 times a week for at least 30 minutes at moderate intensity (60–85% of maximum heart rate frequency), and to increase physical activity in daily life by taking 10.000 steps per day monitored with a pedometer. A diary was kept on these physical activities to establish self-monitoring, which was also used during the consultations to discuss physical activity levels.
Diet
Participants in both the intervention and the control group were asked to complete a food frequency questionnaire (FFQ) four times. Once at the start of the intervention, and at three, six and twelve months after randomization. The self-administered FFQ asked about foods and food groups the intervention focused on. It consisted of two parts: the first part includes the standardized questionnaire on food consumption used for the Public Health Monitor in the Netherlands [27]. This first part has been supplemented with a second part, consisting of additional frequency and portion size questions about snack intake and the usage of sugar containing and alcoholic beverages. Frequency of consumption was asked per week or per month. Portion size for all foods and food groups had been asked per standard household measure (e.g. glass or handful). We focused on the intake of vegetables (raw as well as cooked; grams/day), fruits (grams/day), sugary drinks (fruit juice and soda; glasses/day), alcoholic beverages (glasses/day) and the intake of savory snacks (crisps, pretzels, nuts and peanuts; handful/week) and sweet snacks (biscuits, pieces of chocolate, candies or liquorices; portion/week). One portion of sweet snacks included 2 biscuits, or 2 pieces of chocolate, or 5 candies, or 5 pieces of liquorice. Portion sizes and food groups as presented were pre-specified in the questions of the FFQ.
Physical activity
Participants completed the Short QUestionnaire to ASsess Health-enhancing physical activity (SQUASH) four times. Once at the start of the intervention, and at three, six and twelve months after randomization. The SQUASH is a validated questionnaire to rank subjects according to their level of physical activity [28]. Data were collected about commuting activities, leisure time activities, household activities, and activities at work and school, using three main questions: days per week, average time per day/week (hours and/or minutes), and intensity (low, moderate, high). We focused on the outcomes moderate to vigorous leisure time physical activity (minutes/week), moderate to vigorous commuting activities (walking or cycling from/to work or school; minutes/week) and moderate to vigorous total physical activity (MVPA; minutes/week).
Statistical methods
Differences and 95% confidence intervals (95% C.I.) in dietary intake as well as in physical activity between both groups at three, six and twelve months after randomization were analyzed by mixed model analysis, using a random intercept. This method was chosen to account for decreasing response to questionnaires over time. All associations were adjusted for baseline values, using time and an interaction term between time and randomization group in the model. In addition, results are expressed as marginal means per time point, incorporating the dependency of observations within subjects and corrections for baseline. We checked if our data was normally distributed after adjusting for baseline values. To identify potential confounders, we adjusted for pregnancy, education level and smoking, one at the time, because of small, statistically non-significant differences between intervention and control group at baseline. If the effect estimate in the majority of the models changed >10%, we included the variable in the final model. To account for differences in the number of pregnant women in the intervention and control group, we tested for effect modification by adding pregnancy to the model and an interaction term with randomization group. Alcoholic beverages and commuting activities both had a median of zero in combination with a very narrow distribution, therefore we only showed medians and inter quartile rangers (IQR) for these variables (S2 and S3 Tables).
We additionally used univariate regression models to explore if weight change between baseline and six months after randomization (clinically measured weight in kg at 6 months minus clinically measured weight in kg at baseline) was related to changes in diet and physical activity between baseline and six months after randomization (physical activity/diet at 6 months minus physical activity/diet at baseline). Only total MVPA and diet variables that were statistically significant in our mixed model analyses were included. We performed these explorative analyses irrespective of randomization group, using complete cases while pregnant women were excluded.
All questions of the FFQ contained open answer categories for the largest portion size (e.g. more than 5 glasses of soda), with the exception of vegetable intake. As we did not know the exact portion size consumed when this answer was given, we arbitrarily chose to recode the portion size for these categories into X+1 (e.g. 6 glasses of soda). We performed a sensitivity analysis with X+1+30% (e.g. 8 glasses of soda) and found that the associations were robust (S1 Table).
Statistical analyses were performed using the software Statistical Package for the Social Sciences (SPSS) version 22 for Windows (SPSS, Chicago, IL, USA). P-values <0.05 were considered statistically significant.
Results
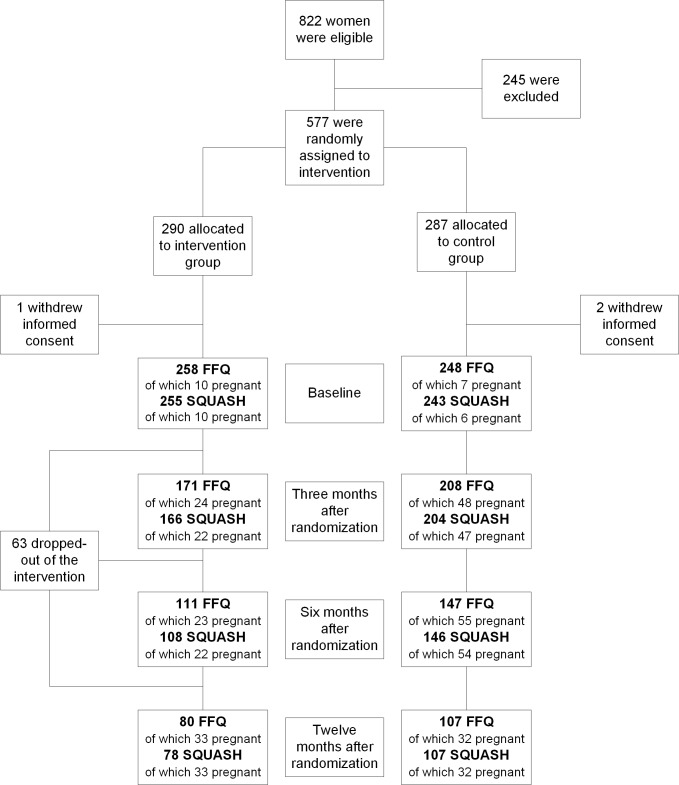
Table 1 shows the characteristics of the study participants who completed the FFQ and/or SQUASH at baseline (N = 510). Characteristics were similar for the intervention group and the control group. There were no differences compared to the LIFEstyle study participants as a whole (N = 574). Response decreased over time for both questionnaires (Fig 1). S2 and S3 Tables show the dietary intakes and physical activity at baseline, three, six and twelve months after randomization. After correction for baseline values, residuals were normally distributed. For diet and physical activity we found no significant interaction effect between pregnancy and randomization group. Therefore, our model does not include an interaction term between pregnancy and randomization group. Results were adjusted for pregnancy, education level and smoking based on their impact on the effect estimates.
Table 1. Characteristics of participants who completed the FFQ and/or SQUASH at baseline.
| Intervention group (N = 261) | Control group (N = 249) |
P-value | |
|---|---|---|---|
| Age (mean; SD) | 29.8 (4.5) | 29.8 (4.5) | 0.88 |
| Caucasian (%; N) | 89.3 (233) | 89.2 (222) | 0.97 |
| Education (%; N) | |||
| Primary school (4–12 years) | 6.0 (15) | 2.9 (7) | 0.26 |
| Secondary education | 24.0 (60) | 23.4 (56) | |
| Intermediate Vocational Education | 49.2 (123) | 47.7 (114) | |
| Higher Vocational Education and University | 20.8 (52) | 25.9 (62) | |
| Smoking (yes; %; N) | 26.1 (67) | 21.4 (53) | 0.22 |
| Weight (kg; mean; SD) | 103.7 (13.7) | 103.4 (12.3) | 0.80 |
| Body Mass Index (kg/m2; mean; SD) | 36.0 (3.4) | 36.1 (3.4) | 0.85 |
| Anovulation (yes; %; N) | 45.0 (117) | 48.4 (120) | 0.44 |
| PCOS (%; N) | 76.1 (89/117) | 74.2 (89/120) | 0.70 |
| Nulliparous (%; N) | 70.1 (183) | 67.1 (167) | 0.73 |
Baseline characteristics are presented as means and standard deviations (SD) for continuous variables, and as percentages (%) and total number of participants (N) for categorical data. To compare groups, an independent Student’s t-test was used for continuous variables, and a Chi-square test for categorical data; kg/m2 = kilograms per square meter; PCOS = Polycystic ovarian syndrome.
Fig 1. Flow diagram LIFEstyle study for diet and physical activity data.
FFQ = Food Frequency Questionnaire; SQUASH = Short QUestionnaire to ASsess Health-enhancing physical activity; mo. = months.
Diet
Table 2 shows the overall differences in lifestyle between the intervention and control group, which represents the effect of randomization group on the diet and physical activity outcomes irrespective of the effect of time, and the differences in lifestyle per time point after randomization. There were overall group effects for the intake of sugary drinks (-0.4 glasses/day [95% C.I. = -0.6; -0.1]; Table 2), savory snacks (-1.8 handful/week [-2.6; -0.9]), and sweet snacks (-1.8 portion/week [-2.8; -0.9]). The intervention group had a lower intake of sugary drinks at three months after randomization compared to the control group (-0.5 glasses/day [-0.9; -0.2]). They also had a lower intake of savory snacks at three months (-2.4 handful/week [-3.4; -1.4]) and at six months after randomization (-1.4 handful/week [-2.6; -0.2]), and a lower intake of sweet snacks at three months (-2.2 portion/week [-3.3; -1.0]) and twelve months after randomization (-1.9 portion/week [-3.5; -0.4]) compared to the control group.
Table 2. Differences in diet and physical activity in the intervention group compared to the control group.
| Overall (95% C.I.)a |
Time point after randomization | Difference (95% C.I.) |
P-value | ||
|---|---|---|---|---|---|
| Vegetable intake (gram/day) | |||||
| Corrected for baseline | 6.3 (-4.1; 16.6) |
Three months | 5.2 (-6.9; 17.4) | 0.40 | |
| Six months | 13.2 (-1.0; 27.4) | 0.07 | |||
| Twelve months | -3.3 (-19.2; 12.6) | 0.69 | |||
| Corrected for baseline, education, pregnancy and smoking | 4.0 (-6.8; 14.8) |
Three months | 3.1 (-9.5; 15.7) | 0.63 | |
| Six months | 10.7 (-4.1; 25.6) | 0.16 | |||
| Twelve months | -4.9 (-21.6; 11.7) | 0.56 | |||
| Fruit intake (gram/day) | |||||
| Corrected for baseline | -0.5 (-11.8; 10.8) |
Three months | 7.2 (-6.8; 21.2) | 0.32 | |
| Six months | -12.3 (-28.9; 4.2) | 0.14 | |||
| Twelve months | -0.7 (-19.6; 18.2) | 0.94 | |||
| Corrected for baseline, education, pregnancy and smoking | 0.7 (-10.8; 12.3) |
Three months | 8.9 (-5.3; 23.1) | 0.22 | |
| Six months | -8.7 (-25.5; 8.2) | 0.31 | |||
| Twelve months | -5.3 (-24.6; 14.0) | 0.59 | |||
| Sugary drinks (glasses/day) | |||||
| Corrected for baseline | -0.4 (-0.7; -0.1)c |
Three months | -0.5 (-0.9; -0.2) | 0.001 | |
| Six months | -0.5 (-0.8; -0.1) | 0.03 | |||
| Twelve months | 0.02 (-0.4; 0.5) | 0.93 | |||
| Corrected for baseline, education, pregnancy and smoking | -0.4 (-0.7; -0.1)c |
Three months | -0.6 (-0.9; -0.2) | 0.001 | |
| Six months | -0.4 (-0.8; 0.02) | 0.07 | |||
| Twelve months | -0.04 (-0.5; 0.4) | 0.86 | |||
| Savory snacks (handful/week) | |||||
| Corrected for baseline | -1.8 (-2.7; -1.0)d |
Three months | -2.4 (-3.4; -1.4) | <0.001 | |
| Six months | -1.5 (-2.7; -0.3) | 0.01 | |||
| Twelve months | -0.8 (-2.1; 0.5) | 0.25 | |||
| Corrected for baseline, education, pregnancy and smoking | -1.7 (-2.6; -0.9)d |
Three months | -2.5 (-3.5; -1.5) | <0.001 | |
| Six months | -1.4 (-2.6; -0.2) | 0.03 | |||
| Twelve months | -0.4 (-1.8; 0.9) | 0.52 | |||
| Sweet snacks (portion/week)b | |||||
| Corrected for baseline | -1.9 (-2.8; -1.0)d |
Three months | -2.3 (-3.4; -1.1) | <0.001 | |
| Six months | -1.4 (-2.8; -0.1) | 0.04 | |||
| Twelve months | -1.8 (-3.3; -0.2) | 0.03 | |||
| Corrected for baseline, education, pregnancy and smoking | -1.8 (-2.8; -0.9)d |
Three months | -2.2 (-3.3; -1.0) | <0.001 | |
| Six months | -1.2 (-2.6; 0.2) | 0.08 | |||
| Twelve months | -1.8 (-3.4; -0.2) | 0.03 | |||
| Total moderate to vigorous physical activity (min/week) | |||||
| Corrected for baseline | 132.0 (5.5; 258.6)c |
Three months | 172.7 (14.9; 330.5) | 0.03 | |
| Six months | 91.8 (-94.9; 278.5) | 0.34 | |||
| Twelve months | 57.5 (-155.5; 270.6) | 0.60 | |||
| Corrected for baseline, education, pregnancy and smoking | 133.6 (3.0; 264.3)c |
Three months | 169.0 (6.0; 332.1) | 0.04 | |
| Six months | 93.2 (-102.0; 288.4) | 0.35 | |||
| Twelve months | 81.0 (-141.8; 303.8) | 0.48 | |||
| Leisure time moderate to vigorous physical activity (min/week) | |||||
| Corrected for baseline | 82.4 (-0.2; 165.0) |
Three months | 107.0 (-2.3; 216.2) | 0.06 | |
| Six months | 74.1 (-56.3; 204.5) | 0.27 | |||
| Twelve months | 19.0 (-130.9; 168.9) | 0.80 | |||
| Corrected for baseline, education, pregnancy and smoking | 63.8 (-21.5; 149.1) |
Three months | 88.6 (-24.0; 201.3) | 0.12 | |
| Six months | 49.9 (-86.2; 186.1) | 0.47 | |||
| Twelve months | 12.8 (-143.8; 169.4) | 0.87 | |||
Differences and 95% confidence intervals (95% CI) were analyzed by mixed model analysis, including all women with at least one value (range N = 511 for sugary drinks; N = 535 for fruit intake), using a random intercept. Time and an interaction term between time and randomization group was used in all models. As all women had different dietary intakes and physical activity levels at baseline, we corrected by default for baseline values. The fully corrected model included correction for the confounders education, pregnancy and smoking; C.I. = confidence interval; min/week = minutes per week.
a The overall effect represents the effect of randomization group on the diet and physical activity outcomes irrespective of the effect of time. The linear mixed model included randomization group, baseline dietary intake/physical activity, and in case of the fully corrected model, education level and pregnancy as independent fixed effect variables. Time was not added to this model.
b One portion of sweet snacks included 2 biscuits, or 2 pieces of chocolate, or 5 candies, or 5 pieces of liquorice.
c P-value <0.05
d P-value <0.001
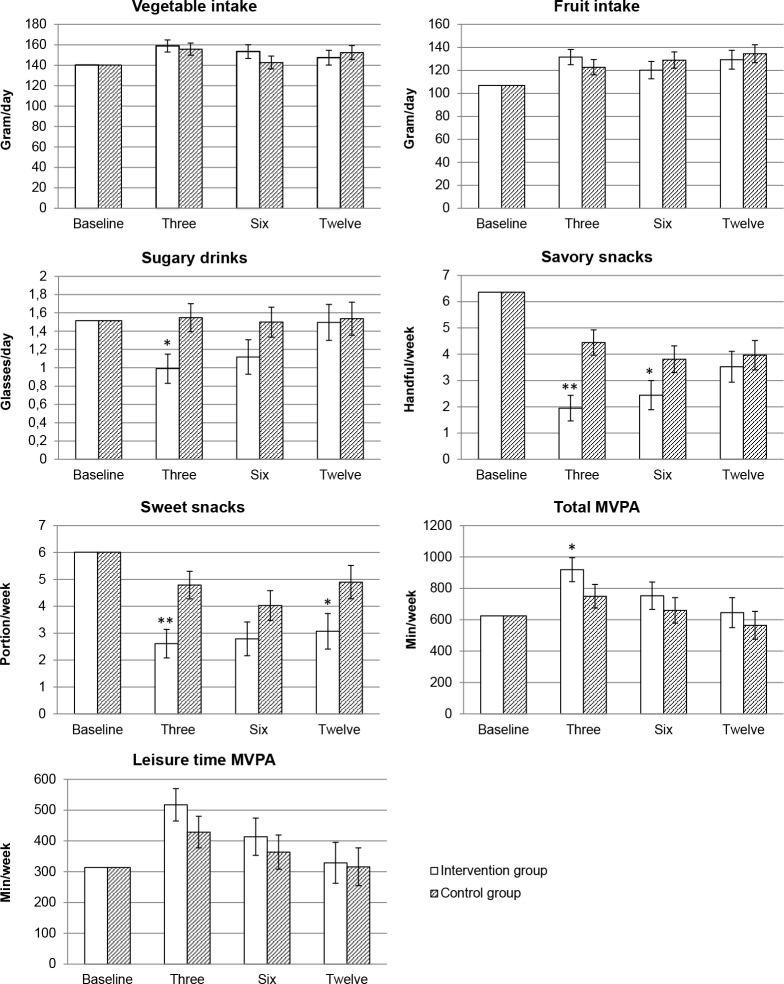
Fig 2 shows the estimated marginal means for dietary intake and physical activity in the intervention and control group over the different time points. We tested if the effects of the intervention on the dietary intake and physical activity outcomes differed over time by adding an interaction term between time and randomization group into our model. Interaction effects between time and randomization group showed no significant results, with exception of savory snacks (p = 0.01). This is due to the large decrease in savory snack intake in the intervention group compared to the control group at three months after randomization (Fig 2).
Fig 2. Estimated marginal means for diet and physical activity corrected for baseline, education level, pregnancy and smoking.
Marginal means were estimated by mixed model analysis and time was added as a categorical variable into the model. Time points are at baseline, three months, six months and twelve months after randomization in both groups; MVPA = moderate to vigorous physical activity; min/week = minutes per week; * P<0.05, ** P<0.001.
Explorative univariate regression analyses showed that weight loss during the first six months is related to decreased savory snack intake during the first six months after randomization (mean predicted value = -2.60 handful/week; P = 0.01; total N = 127). No other statistically significant associations between change in body weight and change in lifestyle behaviors were seen.
Physical activity
There was an overall group effect for total MVPA (133.6 minutes/week [3.0; 264.3]), but not for leisure time MVPA (Table 2). For total MVPA the difference between the intervention group and the control group was statically significant at three months after randomization (169.0 minutes/week [6.0; 332.1]). Thereafter, differences between the intervention group and the control group decreased, although the intervention group was more physically active compared to the control group at all points in time. A similar pattern was seen in leisure time MVPA, but there were no statistically significant differences between the intervention and control group. Interaction effects between time and randomization group showed no significant results.
Discussion
The six-month structured preconception lifestyle intervention decreased the intake of sugary drinks, sweet and savory snacks in obese infertile women while it did not affect intake of fruit and vegetables. This decreased intake of sweet snacks persisted up to six months after the intervention program ended. Women in the intervention group were more physically active than the women in the control group. Although our study showed modest effects on diet and physical activity outcomes, cardiometabolic health of women improved by halving the odds of metabolic syndrome [20].
The LIFEstyle study was the first large RCT studying the effects of a lifestyle intervention program that starts prior to conception in obese women. We observed the largest intervention effects on diet and physical activity at three months after randomization. A reason for this finding could be that during these first three months, participants had more close contact with the intervention nurse compared to the last three months of the intervention period (6 visits of which 4 face-to-face vs. 4 visits of which 2 face-to-face respectively). Women who attended a greater number of scheduled visits with the intervention nurse more often successfully lost ≥5% of their original bodyweight [29]. Therefore, it seems that the higher intensity of guidance in the first three months of the intervention program encouraged healthy changes in diet and physical activity. In our explorative regression analyses, we found that weight loss during the first six months after randomization was associated with a decreased savory snack intake during these first six months, suggesting that the intervention was mainly effective in achieving weight loss through reduced snacking. Since the focus of our intervention program was weight loss, and therefore to eat less calories and increase physical activity, we hypothesize this could explain the decreased intake of snacks and sugary drinks and the lack of intervention effect on the intake of vegetables and fruit. The lack of maintenance in lifestyle changes at twelve months after randomization (six months after the intervention ended) are in line with studies examining long-term weight loss by diet, exercise or combined diet and exercise programs [30,31].
Studies on lifestyle changes, including diet and physical activity, in women of reproductive age mostly focused on the pregnancy period to improve maternal health and to improve pregnancy outcomes [9–14,32]. Reviews and meta-analyses on these studies show positive effects of lifestyle interventions on restricting gestational weight gain [9,11–13] and trends towards [11], or slightly reduced prevalence of gestational diabetes [14]. Recent RCT’s of lifestyle interventions in pregnant women, the RADIEL, UPBEAT, DALI and LIMIT trial, showed that interventions during pregnancy were effective in altering diet and physical activity [33–38].
Our population consisted of infertile women visiting the gynecologist to start infertility treatment. Therefore, motivations and barriers for changing physical activity and diet might be different than in pregnant women. An important motivation for lifestyle changes during pregnancy is having the responsibility for the health of the unborn child besides personal health [39]. As the women included in the LIFEstyle study were not pregnant yet, we expected that an important motivation for them was that overweight negatively influenced the chances of becoming pregnant [3,4], but the struggle with infertility may have made lifestyle changes more difficult.
The most important strength of the current study was the data collection at four points in time within the frame of a RCT design using mixed models to analyze the data. By taking into account the within person dependency of the data, we were able to use all available data and not only data of the complete cases. Therefore, we have a study sample representing the whole study population instead of a selection.
The first limitation of our study is the use of a control group who promptly started with infertility treatment after randomization. This could influence our results in different directions. The patient information leaflet of the LIFEstyle study contained information on the adverse effects of overweight and obesity on women’s reproductive health, pregnancy, and pregnancy outcomes. This could explain the improvements in diet and physical activity in the control group. In addition, infertility treatment is associated with stress [40–42] and hormonal changes [43], which can influence diet and physical activity in different directions [44,45]. A second limitation is the use of self-reported questionnaires instead of objective measurements. Participation in the intervention could lead to social desirability bias, leading to over-reporting healthy behavior and underreporting unhealthy behavior [46–50]. If social desirability bias is present it is likely that it affected the results of the intervention group to a larger extent than of the control group, since women in the intervention group were actively motivated and educated on a healthier lifestyle. However, the intervention group lost significantly more weight compared to the control group [19]. It is therefore unlikely that the intervention effect on diet and physical activity is caused by social desirability bias alone. A third limitation is that the FFQ only asked about the food products the intervention was targeted on. Although we were able to evaluate whether the dietary intervention goals were achieved, we were not able to assess whether women replaced their sugary drinks and snacks with other (unhealthy) foods. Nor were we able to assess whether the intervention group lowered total energy intake compared to the control group or to correct for energy intake, since we have no data on caloric intake of the women randomized into the control group. It is however very likely that the intervention group did lower total energy intake since body weight decreased significantly compared to the control group.
In conclusion, we demonstrated that a six-month structured preconception lifestyle intervention in obese infertile women decreased the intake of unhealthy, high caloric foods and beverages and increased physical activity compared to the control group receiving prompt infertility treatment. These improvements in lifestyle, together with the improved cardiometabolic health, may in the future have beneficial effects on health of women and their offspring.
Supporting information
(DOCX)
(DOCX)
(DOCX)
(PDF)
(PDF)
(SAV)
Acknowledgments
We would like to thank the women who participated in this study. We would like to acknowledge Mrs. A. Bolster (University Medical Center Groningen) as senior trainer of the intervention coaches. We thank all lifestyle coaches, research nurses, research midwives and office members of the Dutch Consortium 2.0 (www.studies-obsgyn.nl) for their hard work and dedication.
Group authorship of The LIFEstyle study group: In addition to the listed authors J.M. Burggraaff (Scheper Ziekenhuis, Emmen), W.K.H. Kuchenbecker (Isala, Zwolle), D.A.M. Perquin (Medisch Centrum Leeuwarden, Leeuwarden), C.A.M. Koks (Maxima Medisch Centrum, Veldhoven), R. van Golde (Maastricht Universitair Medisch Centrum, Maastricht), E.M. Kaaijk (OLVG, Amsterdam), J.M. Schierbeek (Deventer Ziekenhuis, Deventer), G.J.E. Oosterhuis (St. Antonius Ziekenhuis, Nieuwegein), F.J. Broekmans (Universitair Medisch Centrum Utrecht, Utrecht), N.E.A. Vogel (Martini Ziekenhuis, Groningen), J.A. Land (Univerisiteit Groningen, Groningen), C.B. Lambalk (VU medisch centrum, Amsterdam), F. van der Veen (Academisch Medisch Centrum, Amsterdam), N.F. Klijn (Leiden Universitair Medisch Centrum, Leiden), P.E.A.M. Mercelina (Atrium Medisch Centrum, Heerlen), Y.M. van Kasteren (Noordwest Ziekenhuisgroep, Alkmaar), A.W. Nap (Rijnstate Ziekenhuis, Arnhem), R.J.A.B. Mulder (Laurentius Ziekenhuis, Roermond), E.T.C.M. Gondrie (Zuyderland Medisch Centrum, Sittard) and J.P. de Bruin (Jeroen Bosch Ziekenhuis, Den Bosch) are members of the LIFEstyle study group and collaborated on this study.
Data Availability
All relevant data are within the paper and its Supporting Information files.
Funding Statement
The LIFEstyle study was funded by ZonMw, the Dutch Organization for Health Research and Development, grant number: 50-50110-96-518. TvE is supported by grants from the Dutch Heart Foundation (2013T085) and the European Commission (Horizon2020 project 633595 DynaHealth). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Kim SY, Dietz PM, England L, Morrow B, Callaghan WM. Trends in Pre-pregnancy Obesity in Nine States, 1993–2003. Obesity. 2007;15(4):986–93. 10.1038/oby.2007.621 [DOI] [PubMed] [Google Scholar]
- 2.Huxley R, Mendis S, Zheleznyakov E, Reddy S, Chan J. Body mass index, waist circumference and waist:hip ratio as predictors of cardiovascular risk—a review of the literature. Eur J Clin Nutr. 2010;64(1):16–22. 10.1038/ejcn.2009.68 [DOI] [PubMed] [Google Scholar]
- 3.Yilmaz N, Kilic S, Kanat-Pektas M, Gulerman C, Mollamahmutoglu L. The Relationship between Obesity and Fecundity. J Women’s Heal. 2009;18(5):633–6. [DOI] [PubMed] [Google Scholar]
- 4.Ramlau-Hansen CH, Thulstrup AM, Nohr EA, Bonde JP, Sørensen TIA, Olsen J. Subfecundity in overweight and obese couples. Hum Reprod. 2007;22(6):1634–7. 10.1093/humrep/dem035 [DOI] [PubMed] [Google Scholar]
- 5.Gluckman PD, Hanson MA, Cooper C, Thornburg KL. Effect of In Utero and Early-Life Conditions on Adult Health and Disease. N Engl J Med. 2008;359(1):61–73. 10.1056/NEJMra0708473 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jensen MD, Ryan DH, Apovian CM, Ard JD, Comuzzie AG, Donato KA, et al. 2013 AHA/ACC/TOS Guideline for the Management of Overweight and Obesity in Adults. Circulation. 2014;129(25 suppl 2):S102–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Altfeld S, Handler A, Burton D, Berman L. Wantedness of pregnancy and prenatal health behaviors. Women Health. 1997;26(4):29–43. [DOI] [PubMed] [Google Scholar]
- 8.Hotham E, Ali R, White J, Robinson J. Pregnancy-related changes in tobacco, alcohol and cannabis use reported by antenatal patients at two public hospitals in South Australia. Aust New Zeal J Obstet Gynaecol. 2008;48(3):248–54. [DOI] [PubMed] [Google Scholar]
- 9.Muktabhant B, Lawrie TA, Lumbiganon P, Laopaiboon M. Diet or exercise, or both, for preventing excessive weight gain in pregnancy. Cochrane Database Syst Rev. 2015;(6):CD007145 10.1002/14651858.CD007145.pub3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bain E, Crane M, Tieu J, Han S, Crowther CA, Middleton P. Diet and exercise interventions for preventing gestational diabetes mellitus. Cochrane Database Syst Rev. 2015;(4):CD010443 10.1002/14651858.CD010443.pub2 [DOI] [PubMed] [Google Scholar]
- 11.Oteng-Ntim E, Varma R, Croker H, Poston L, Doyle P. Lifestyle interventions for overweight and obese pregnant women to improve pregnancy outcome: systematic review and meta-analysis. BMC Med. 2012;10(1):47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Streuling I, Beyerlein A, von Kries R. Can gestational weight gain be modified by increasing physical activity and diet counseling? A meta-analysis of interventional trials. Am J Clin Nutr. 2010. October 1;92(4):678–87. 10.3945/ajcn.2010.29363 [DOI] [PubMed] [Google Scholar]
- 13.Tanentsapf I, Heitmann BL, Adegboye AR. Systematic review of clinical trials on dietary interventions to prevent excessive weight gain during pregnancy among normal weight, overweight and obese women. BMC Pregnancy Childbirth. 2011;11(1):81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Russo LM, Nobles C, Ertel KA, Chasan-Taber L, Whitcomb BW. Physical Activity Interventions in Pregnancy and Risk of Gestational Diabetes Mellitus. Obstet Gynecol. 2015;125(3):576–82. 10.1097/AOG.0000000000000691 [DOI] [PubMed] [Google Scholar]
- 15.Fleming TP, Watkins AJ, Velazquez MA, Mathers JC, Prentice AM, Stephenson J, et al. Origins of lifetime health around the time of conception: causes and consequences. Lancet. 2018;391(10132):1842–52. 10.1016/S0140-6736(18)30312-X [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Stephenson J, Heslehurst N, Hall J, Schoenaker DAJM, Hutchinson J, Cade JE, et al. Before the beginning: nutrition and lifestyle in the preconception period and its importance for future health. Lancet. 2018;391(10132):1830–41. 10.1016/S0140-6736(18)30311-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Barker M, Dombrowski SU, Colbourn T, Fall CHD, Kriznik NM, Lawrence WT, et al. Intervention strategies to improve nutrition and health behaviours before conception. Lancet. 2018;391(10132):1853–64. 10.1016/S0140-6736(18)30313-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mutsaerts MA, Groen H, ter Bogt NC, Bolster JH, Land JA, Bemelmans WJ, et al. The LIFESTYLE study: costs and effects of a structured lifestyle program in overweight and obese subfertile women to reduce the need for fertility treatment and improve reproductive outcome. A randomised controlled trial. BMC Womens Health. 2010;10(1):22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mutsaerts MAQ, van Oers AM, Groen H, Burggraaff JM, Kuchenbecker WKH, Perquin DAM, et al. Randomized Trial of a Lifestyle Program in Obese Infertile Women. N Engl J Med. 2016;374(20):1942–53. 10.1056/NEJMoa1505297 [DOI] [PubMed] [Google Scholar]
- 20.van Dammen L, Wekker V, van Oers AM, Mutsaerts MAQ, Painter RC, Zwinderman AH, et al. Effect of a lifestyle intervention in obese infertile women on cardiometabolic health and quality of life: A randomized controlled trial. Stepto NK, editor. PLoS One. 2018;13(1):e0190662 10.1371/journal.pone.0190662 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dutch Society of Obstetrics and Gynaecology (NVOG). Voortplantingsgeneeskunde. Available from: https://www.nvog.nl/kwaliteitsdocumenten/richtlijnen/voortplantingsgeneeskunde/ Cited 17 May 2018.
- 22.Clinical Guidelines on the Identification, Evaluation, and Treatment of Overweight and Obesity in Adults—The Evidence Report. National Institutes of Health. Obes Res. 1998;6 Suppl 2:51S–209S. [PubMed] [Google Scholar]
- 23.CBO guideline. Richtlijn Diagnostiek en behandeling van obesitas bij volwassenen en kinderen. 2008. Available from: https://www.nhg.org/themas/publicaties/richtlijn-diagnostiek-en-behandeling-van-obesitas-bij-volwassenen-en-kinderen Cited 17 May 2018.
- 24.Zelissen PMJ, Mathus-Vliegen EMH. Treatment of overweight and obesity in adults: proposal for a guideline. Ned Tijdschr Geneeskd. 2004;148(42):2060–6. [PubMed] [Google Scholar]
- 25.Health Council of the Netherlands (Gezondheidsraad). Guidelines for a healthy diet 2006. 2006. Available from: https://www.gezondheidsraad.nl/sites/default/files/200621E_0.pdf Cited 17 May 2018.
- 26.The Netherlands Nutrition Centre. Eetmeter—Mijn Voedingscentrum. Available from: http://mijn.voedingscentrum.nl/nl/eetmeter/ Cited 17 May 2018.
- 27.van den Brink C, Ocké M, Houben A, van Nierop P, Droomers M, RIVM rapport 260854008/2005. Validation of a Community Health Services food consumption questionnaire in the Netherlands. Available from: https://www.rivm.nl/dsresource?objectid=e370650a-dd54-4ccc-b4f1-1f26c0494215&type=org&disposition=inline Cited 17 May 2018.
- 28.Wendel-Vos GCW, Schuit AJ, Saris WHM, Kromhout D. Reproducibility and relative validity of the short questionnaire to assess health-enhancing physical activity. J Clin Epidemiol. 2003;56(12):1163–9. [DOI] [PubMed] [Google Scholar]
- 29.Karsten MDA, van Oers AM, Groen H, Mutsaerts MAQ, van Poppel MNM, Geelen A, et al. Determinants of successful lifestyle change during a 6-month preconception lifestyle intervention in women with obesity and infertility. Eur J Nutr. 2018. 10.1007/s00394-018-1798-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Curioni CC, Lourenço PM. Long-term weight loss after diet and exercise: a systematic review. Int J Obes. 2005;29(10):1168–74. [DOI] [PubMed] [Google Scholar]
- 31.Wu T, Gao X, Chen M, van Dam RM. Long-term effectiveness of diet-plus-exercise interventions vs. diet-only interventions for weight loss: a meta-analysis. Obes Rev. 2009;10(3):313–23. 10.1111/j.1467-789X.2008.00547.x [DOI] [PubMed] [Google Scholar]
- 32.Weight management before, during and after pregnancy | Guidance and guidelines | NICE guidelines; Appendix D: Gaps in the evidence. Available from: https://www.nice.org.uk/guidance/ph27/chapter/Appendix-D-Gaps-in-the-evidence Cited 17 May 2018.
- 33.Valkama A, Koivusalo S, Lindström J, Meinilä J, Kautiainen H, Stach-Lempinen B, et al. The effect of dietary counselling on food intakes in pregnant women at risk for gestational diabetes: a secondary analysis of a randomised controlled trial RADIEL. Eur J Clin Nutr. 2016;70(8):912–7. 10.1038/ejcn.2015.205 [DOI] [PubMed] [Google Scholar]
- 34.Flynn AC, Seed PT, Patel N, Barr S, Bell R, Briley AL, et al. Dietary patterns in obese pregnant women; influence of a behavioral intervention of diet and physical activity in the UPBEAT randomized controlled trial. Int J Behav Nutr Phys Act. 2016;13(1):124 10.1186/s12966-016-0450-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hayes L, Mcparlin C, Kinnunen TI, Poston L, Robson SC, Bell R, et al. Change in level of physical activity during pregnancy in obese women: findings from the UPBEAT pilot trial. BMC Pregnancy Childbirth. 2015;15(1):52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Dodd JM, Cramp C, Sui Z, Yelland LN, Deussen AR, Grivell RM, et al. The effects of antenatal dietary and lifestyle advice for women who are overweight or obese on maternal diet and physical activity: the LIMIT randomised trial. BMC Med. 2014;12(1):161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Moran LJ, Flynn AC, Louise J, Deussen AR, Dodd JM. The effect of a lifestyle intervention on pregnancy and postpartum dietary patterns determined by factor analysis. Obesity. 2017;25(6):1022–32. 10.1002/oby.21848 [DOI] [PubMed] [Google Scholar]
- 38.Simmons D, Devlieger R, van Assche A, Jans G, Galjaard S, Corcoy R, et al. Effect of physical activity and/or healthy eating on GDM risk: The DALI Lifestyle Study. J Clin Endocrinol Metab. 2016;102(3):jc.2016–3455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Phelan S. Pregnancy: a “teachable moment” for weight control and obesity prevention. Am J Obstet Gynecol. 2010;202(2):135.e1–135.e8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Cousineau TM, Domar AD. Psychological impact of infertility. Best Pract Res Clin Obstet Gynaecol. 2007;21(2):293–308. 10.1016/j.bpobgyn.2006.12.003 [DOI] [PubMed] [Google Scholar]
- 41.Cwikel J, Gidron Y, Sheiner E. Psychological interactions with infertility among women. Eur J Obstet Gynecol Reprod Biol. 2004;117(2):126–31. 10.1016/j.ejogrb.2004.05.004 [DOI] [PubMed] [Google Scholar]
- 42.Turner K, Reynolds-May MF, Zitek EM, Tisdale RL, Carlisle AB, Westphal LM. Stress and Anxiety Scores in First and Repeat IVF Cycles: A Pilot Study. Baradaran HR, editor. PLoS One. 2013;8(5):e63743 10.1371/journal.pone.0063743 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Csemiczky G, Landgren BM, Collins A. The influence of stress and state anxiety on the outcome of IVF-treatment: psychological and endocrinological assessment of Swedish women entering IVF-treatment. Acta Obstet Gynecol Scand. 2000;79(2):113–8. [DOI] [PubMed] [Google Scholar]
- 44.Torres SJ, Nowson CA. Relationship between stress, eating behavior, and obesity. Nutrition. 2007;23(11–12):887–94. 10.1016/j.nut.2007.08.008 [DOI] [PubMed] [Google Scholar]
- 45.Wijndaele K, Matton L, Duvigneaud N, Lefevre J, De Bourdeaudhuij I, Duquet W, et al. Association between leisure time physical activity and stress, social support and coping: A cluster-analytical approach. Psychol Sport Exerc. 2007;8(4):425–40. [Google Scholar]
- 46.Hebert JR, Hurley TG, Peterson KE, Resnicow K, Thompson FE, Yaroch AL, et al. Social Desirability Trait Influences on Self-Reported Dietary Measures among Diverse Participants in a Multicenter Multiple Risk Factor Trial. J Nutr. 2008;138(1):226S–234S. 10.1093/jn/138.1.226S [DOI] [PubMed] [Google Scholar]
- 47.Kristal AR, Andrilla CHA, Koepsell TD, Diehr PH, Cheadle A. Dietary Assessment Instruments are Susceptible to Intervention-associated Response Set Bias. J Am Diet Assoc. 1998;98(1):40–3. 10.1016/S0002-8223(98)00012-1 [DOI] [PubMed] [Google Scholar]
- 48.Hebert JR, Clemow L, Pbert L, Ockene IS, Ockene JK. Social desirability bias in dietary self-report may compromise the validity of dietary intake measures. Int J Epidemiol. 1995;24(2):389–98. [DOI] [PubMed] [Google Scholar]
- 49.Adams SA, Matthews CE, Ebbeling CB, Moore CG, Cunningham JE, Fulton J, et al. The Effect of Social Desirability and Social Approval on Self-Reports of Physical Activity. Am J Epidemiol. 2005;161(4):389–98. 10.1093/aje/kwi054 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Crutzen R, Göritz AS. Does social desirability compromise self-reports of physical activity in web-based research? Int J Behav Nutr Phys Act. 2011;8(1):31. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(DOCX)
(DOCX)
(DOCX)
(PDF)
(PDF)
(SAV)
Data Availability Statement
All relevant data are within the paper and its Supporting Information files.