Abstract
To investigate how aging affects corneal geometry in Japanese adults, and the association between corneal geometry and astigmatism.
We included 421 participants who had undergone systemic and ophthalmological examinations in 2015 in Funagata town, Yamagata, Japan. Corneal topographic data were obtained using anterior-segment optical coherence tomography (CASIA SS-1000). Astigmatism was evaluated using power vector analyses where J0 represents the power of the orthogonal astigmatism. Positive values of J0 indicate with-the-rule astigmatism, while negative values indicate against-the-rule (ATR) astigmatism.
Regarding age-related variations in corneal geometry, the anterior elevations at axis 0° and 180° decreased, and those at axis 90° and 270° increased with increasing age in linear regression analyses, demonstrating horizontal steepening and vertical flattening of the corneal surface. There were no significant age-related variations in posterior elevations and pachymetry findings, including central corneal thickness. Regarding age-related variations in orthogonal astigmatism, the mean values of J0 and corneal J0 (cJ0) decreased by –0.014 and –0.015 per year of increase in age, indicating astigmatic shift toward ATR. Regarding the correlation between corneal geometry and astigmatism, the shift toward ATR was positively correlated with horizontal steepening and vertical flattening, in accordance with the age-related corneal variations. In addition, the posterior surface of the cornea also has an association with this shift to some extent.
The results of our population-based study demonstrated that the age-related variation in astigmatism is associated with geometrical changes in the cornea, especially those in the anterior surface of the cornea.
Keywords: aging, astigmatism, cornea, optical coherence tomography, power vector analysis
1. Introduction
The human eye constructs an optical system that focuses visual images onto the retina. Ocular aberrations including defocus, astigmatism, and higher-order aberrations can deteriorate vision quality in combination with other optical factors (eg, intraocular scattering).[1–4] Aberrations are caused by differential magnification in each principal meridian of the anterior corneal surface and internal optics, posterior corneal surface, and crystalline lens. Particularly, the anterior cornea is the main source of aberrations. Aberrations in the anterior cornea are compensated by internal aberrations.[5–8]
Previous studies that investigated the age-related change in astigmatism reported that the prevalence of astigmatism increases[9–13] and that the axes of astigmatism shift from with-the-rule (WTR) toward against-the-rule (ATR) with aging.[13–19] Our report using power vector analysis also confirmed astigmatic shift toward ATR with increasing age, and showed that older age causes a larger change toward ATR and a larger corneal astigmatic change over the subsequent 5 years.[20] However, the mechanisms and processes involved in the age-related changes in astigmatism are not yet fully understood.
Most studies concerning age-related astigmatic changes have been investigated in terms of optical functions. However, the age-related changes in whole corneal geometry and their influence on astigmatism have been rarely investigated. Thus, the aims of the present study were to investigate how aging affects corneal geometry in Japanese adults and to determine whether there is an association between corneal geometry and astigmatism.
2. Methods
2.1. Subjects
The present study was performed as a part of the Yamagata Study (Funagata), a population-based epidemiologic study examining systemic and ophthalmologic disorders in adult Japanese individuals aged 35 years and older. Details regarding the study participants and research methodology have previously been described.[20–25] Present investigation was conducted using cross-sectional data. Systemic and ophthalmic data were obtained from residents living in Funagata town via study examinations in June 2015. Informed consent was obtained from all study participants and the research adhered to the tenets of the Declaration of Helsinki. The Yamagata Study (Funagata) was approved by the Ethics Committee of the Yamagata University Faculty of Medicine, Yamagata, Japan.
Corneal topographic data from only the right eye were used to avoid the use of interdependent data between two eyes from the same subject. Patients were excluded from the current analyses if they had a history of ocular or corneal surgery, corneal scarring, or other corneal pathologies (eg, pterygium) on slit-lamp examination. Subjects with missing or insufficient data were also excluded.
2.2. Examination
Corneal topographic data were obtained using anterior-segment optical coherence tomography (AS-OCT), CASIA SS-1000 (Tomey Corp., Aichi, Japan). AS-OCT allows for visualization of the entire anterior segment structures in a single image and for performing the precise quantitative measurements of these structures. SS-1000 facilitates imaging at a high resolution (axial: 10 μm; transverse: 30 μm) and high-speed scanning (30,000 A-scans per second).[26] With a substantial improvement in scan speed, the anterior segment structures can be imaged 360° in 128 cross-sections (each with 512 A-scans) in about 2.4 seconds. We used the topographic data, such as pachymetry findings, anterior elevation, and posterior elevation, to evaluate corneal shape. Those indices measured at the central area of cornea, and peripheral 6 mm diameter areas, along the axes of 0°, 45°, 90°, 135°, 180°, 225°, 270°, and 315°. The anterior and posterior elevations were calculated by the scanner software with respect to the best-fit sphere (the sphere that best adjusts to the anterior or posterior surface of the cornea). Positive values of anterior/posterior elevation indicate that the actual corneal surface is in front of the best-fit sphere (or flattening), while negative values indicate that the actual corneal surface is behind the best-fit sphere (or steepening).
Refractive spherical and cylindrical errors, corneal spherical and cylindrical powers, and intraocular pressure were measured using an auto ref/kerato/tonometer (Tonoref II; Nidek Co., Ltd., Aichi, Japan). Axial length was measured via partial coherence laser interferometry (OA-2000; Tomey Corp.). Body indexes, such as height, weight, and waist size, were measured while subjects were wearing light clothing and no shoes.
2.3. Power vector analysis
In terms of statistical descriptions of optical characteristics, separate analyses of the cylinder power and cylinder axis may be insufficient. Therefore, we used the method of power vector analysis. As described in previous reports,[13,20,27,28] we converted the refractive and keratometric astigmatism from the spherocylinder notation to the power vector notation. J0, one of the power vector components, represents the power of the orthogonal astigmatism. Positive values of J0 indicate WTR astigmatism, while negative values indicate ATR. J0 was calculated by using the following equation:
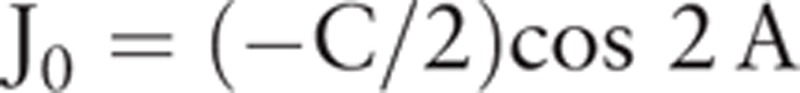 |
C is negative cylinder power, and A is cylinder axis. To analyze keratometric astigmatism, corneal J0 (cJ0) was also calculated.
2.4. Statistical analyses
Data were analyzed using SPSS statistical software (SPSS Statistics version 21.0, IBM Corp., Armonk, NY). Statistical significance was defined as P < .05. Associations among age and topographic data, and power vector components were examined using simple and multiple adjusted linear regression analyses. Sex, height, and weight were included in the multiple regression models as potential confounders, as previously described.[20] Associations of corneal geometry with astigmatism were also examined using linear regression analyses.
3. Results
3.1. Subject characteristics
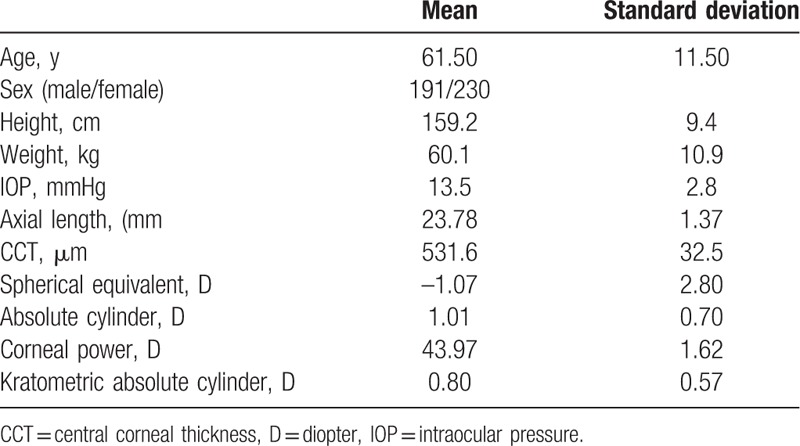
According to the exclusion of participants, due to those corneal pathologies or insufficient data, we included the 421 out of 544 subjects. Table 1 summarizes the characteristics of the subjects (191 males [45.4%], 230 females [54.6%]) included in the current study. The age range of the subjects was 36 to 89 (61.5 ± 11.5) years.
Table 1.
Demographic characteristics.
3.2. Evaluation of age-related variations in corneal geometry
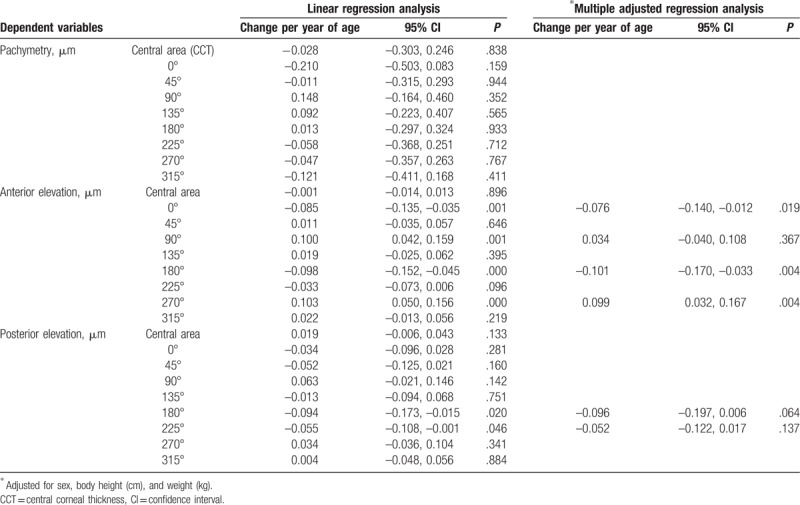
Age-related variations in corneal geometry were analyzed using linear regression and are summarized in Table 2. The anterior elevations at axis 0° and 180° decreased, and those at axis 90° and 270° increased with increasing age in simple regression analyses, demonstrating horizontal steepening and vertical flattening of the corneal surface. In addition, multiple adjusted regressions (adjusted for sex, body height, and weight) confirmed these results. Although the posterior elevations indicated similar tendency, they did not show significance. Pachymetry findings, including central corneal thickness (CCT), did not change significantly except for that at axis 0°.
Table 2.
Evaluation of age-related changes in corneal shape.
3.3. Age-related variations in orthogonal astigmatism
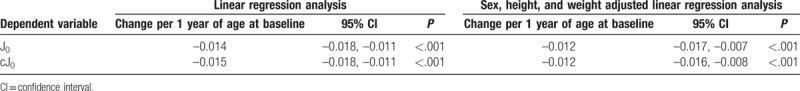
J0 and cJ0 were examined to investigate the degree of the orthogonal astigmatism in the whole eye and cornea. The distributions of J0 and cJ0 are shown with scatter plots in Figure 1, and both J0 and cJ0 decreased with increasing age. In linear regression analyses, the mean values of J0 and cJ0 decreased by –0.014 and –0.015 per year of increase in age, respectively. Sex, body height, and weight adjusted regression analyses also revealed that the mean values of J0 and cJ0 decreased by –0.012 and –0.012 per year of increase in age, respectively (Table 3). These results indicate that increasing age produces a shift toward ATR astigmatism and that this shift is independent of those indices.
Figure 1.
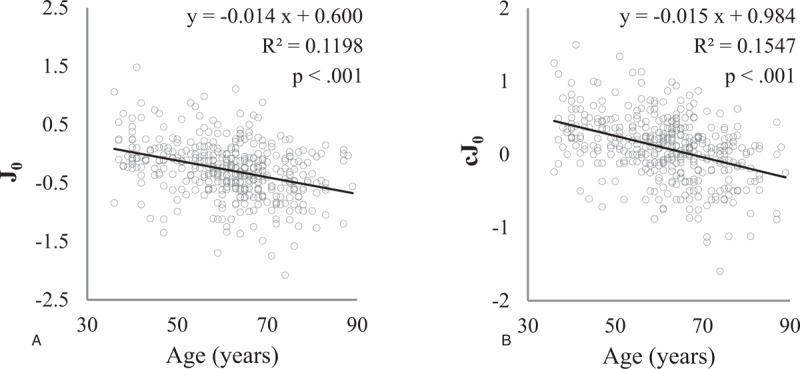
Scatterplots of J0 (A) and cJ0 (B) show that these parameters significantly decrease with age.
Table 3.
Associations between power vector components and age at baseline evaluated via linear regression analysis.
3.4. Associations of corneal geometry with orthogonal astigmatism
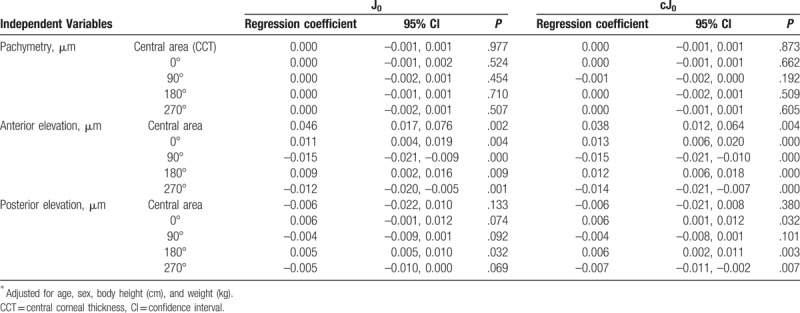
The relationships of corneal geometry indices with J0 and cJ0 are demonstrated in Table 4. All of the orthogonal indices of anterior elevations were associated with J0 and cJ0. J0 and cJ0 increased (shift toward WTR) with an increase in the indices at the angle of 0° and 180° (horizontal flattening). In contrast, J0 and cJ0 decreased with an increase in the indices at the angle of 90° and 270°. The indices of posterior elevations at the angle of 180° were associated with J0, and those at the angle of 0°, 180°, and 270° were associated with cJ0.
Table 4.
Impact of corneal shape on orthogonal astigmatism.
4. Discussion
In the present study, we used data from the Yamagata Study (Funagata), which included Japanese adults, to investigate the association of aging with corneal geometry. To investigate corneal geometry, we used pachymetry findings, including CCT; anterior elevation; and posterior elevation as indices. Because the anterior and posterior elevations were calculated with the best-fit sphere of each subject, estimation of each sphere (Steep/Flat) was lacking. Therefore, we could estimate corneal geometrical variation independent of each corneal sphere.
Our results did not show an association between sex and CCT. Although the correlation between sex and CCT has been pointed out in the literature, it is still controversial.[29–32] Previous studies considered that the hormonal changes in women may influence CCT. It was shown that CCT was lowest in women at the beginning of the menstrual cycle and highest at the end of the menstrual cycle and during ovulation.[33] In addition, CCT was significantly decreased in postmenopausal women.[34]
The findings of our study do not indicate a significant association between age and pachymetry findings, including CCT. Some reports have indicated age-related CCT decreases in individuals of several ethnicities.[32,35,36] Foster et al[37]demonstrated that CCT decreased by 5 μm in men and 6 μm in women every 10 years of age. Similarly, Eballe et al[32] and Aghaian et al[36] reported a decrease of 4.2 μm and 3 μm over the same period, in spite of differences in ethnicity among individuals enrolled in this study. Nevertheless, a few studies have concluded that CCT was not associated with age.[37–39] Although the association between age and CCT remains controversial, we believe that our results must have persuasiveness to a certain extent because of absence of a certain direction in the pachymetry map. The results in the anterior elevations manifest horizontal steepening and vertical flattening in the corneal surface. In contrast, the posterior elevations did not indicate any significance. Although the mechanism is still uncertain, some hypotheses relating to histology have been proposed. Germundsson et al[39] reported age-related thinning of Bowman's layer. A few researchers have indicated an age-related reduction in keratocyte density, especially in the anterior stroma.[39–42] Although the results regarding this continue to remain controversial,[43,44] reduction in keratocyte density may result in the destruction of collagen fibers and affect corneal structure and rigidity.[45] Other studies have shown that diminished eyelid tension caused by age-related dermatochalasis may affect corneal geometry.[46,47]
Regarding age-related variations in orthogonal astigmatism, both J0 and cJ0 decreased with age. This observation indicates that increasing age has an association with a shift toward ATR astigmatism, which is consistent with our previous report.[20] Although other groups have also published results regarding age-related ATR shift, there seems to be a variation in the amount of change. Liu et al[13] reported that J0 changed by –0.016 per year of age, and Guan et al[14] showed a change of –0.017 per year of age. The results of the study by Sanfilippo et al. demonstrated a smaller shift toward ATR (–0.003 per year of age in J0) than that was observed in ours.[15] This difference may likely be due to differences in the age distribution or ethnicity of the subjects enrolled in these studies.
We also investigated the correlation between corneal geometry and astigmatism. Positive values of J0 and cJ0 were positively correlated with horizontal flattening and vertical steepening at the anterior surface of the cornea. Therefore, an astigmatic shift toward ATR (negative value of J0 and cJ0) is positively correlated with horizontal steepening and vertical flattening, which are demonstrated as age-related variations in the present study. In addition, the posterior surface of the cornea also has an association with this shift to some extent. The results of our population-based study suggest that aging has an association with astigmatism through corneal geometrical variations. However, corneal thickness might not be associated with this shift. Although several previous studies have shown age-related increase in shifting toward ATR,[13–20] only a small number of studies have assessed corneal geometry, including posterior surface geometry, in conjunction with astigmatism.[48,49]
Our study confirms that age-related alterations of the cornea occur, and that these are associated with a change in astigmatism. Age-related changes in visual function have been rarely investigated in terms of both corneal structure and optics. The present study provides baseline data regarding visual correction, including refractive surgery. In addition, the present study is a population-based study. As this study was a part of the Yamagata Study (Funagata), we could reduce a correction bias. For evaluation of age-related changes in geometry, we took potential confounders into consideration.
Our study also had some limitations. First, because present investigation is cross-sectional, we are not able to estimate age-related geometrical changes in same individuals. Second, owing to a lack of comprehensive medical examination data, we might not be able to exclude some conditions. Those who had medical conditions or a history of surgeries that may have an influence on astigmatism were excluded on account of information gathered during history taking. However, information regarding daily wear of contact lenses, use of eye drops, or history of glaucoma could not be obtained. Third, there were unmeasured factors that are potentially associated with astigmatism, such as biological measures excluded from serological tests, eyelid/cornea interactions, or occupations.[46,47,50]
In conclusion, aging has associations with corneal geometry, in particular, horizontal steepening and vertical flattening, especially in the anterior cornea. In addition, these geometrical alterations of the cornea are associated with astigmatic shift toward ATR. Although we believe our study will improve the understanding of astigmatism, the mechanism of astigmatic change is not fully described. Further studies are needed.
Acknowledgments
The authors thank the participants, and the associate staff of the Yamagata Study (Funagata).
Author contributions
Conceptualization: Hiroyuki Namba.
Formal analysis: Hiroyuki Namba, Tsuneo Konta.
Investigation: Hiroyuki Namba, Akira Sugano, Katsuhiro Nishi, Takanori Murakami.
Project administration: Koichi Nishitsuka, Takamasa Kayama, Hidetoshi Yamashita.
Writing – original draft: Hiroyuki Namba.
Writing – review & editing: Koichi Nishitsuka, Tsuneo Konta, Kenichi Ishizawa, Takamasa Kayama, Hidetoshi Yamashita.
Footnotes
Abbreviations: AS-OCT = anterior-segment optical coherence tomography, ATR = against-the-rule, CCT = central corneal thickness, cJ0 = corneal J0, WTR = with-the-rule.
HY received a patent from Senju Pharmaceutical Co., Ltd.
The authors declare no conflicts of interest.
References
- [1].Liang J, Williams DR. Aberrations and retinal image quality of the normal human eye. J Opt Soc Am A Opt Image Sci Vis 1997;14:2873–83. [DOI] [PubMed] [Google Scholar]
- [2].Morlet N, Minassian D, Dart J. Astigmatism and the analysis of its surgical correction. Br J Ophthalmol 2001;85:1127–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Pérez GM, Manzanera S, Artal P. Impact of scattering and spherical aberration in contrast sensitivity. J Vis 2009;9:1–0. [DOI] [PubMed] [Google Scholar]
- [4].Sawides L, Marcos S, Ravikumar S, et al. Adaptation to astigmatic blur. J Vis 2010;10:22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Artal P, Guirao A, Berrio E, et al. Compensation of corneal aberrations by internal optics in the human eye. J Vis 2001;1:1–8. [DOI] [PubMed] [Google Scholar]
- [6].Artal P, Guirao A. Contributions of the cornea and the lens to the aberrations of the human eye. Opt Lett 1998;23:1713–5. [DOI] [PubMed] [Google Scholar]
- [7].Dubbelman M, Sicam VA, Van der Heijde GL. The shape of the anterior and posterior surface of the aging human cornea. Vision Res 2006;46:993–1001. [DOI] [PubMed] [Google Scholar]
- [8].Sun M, Birkenfeld J, de Castro A, et al. OCT 3-D surface topography of isolated human crystalline lenses. Biomed Opt Express 2014;5:3547–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Sawada A, Tomidokoro A, Araie M, et al. Refractive errors in an elderly Japanese population: the Tajimi Study. Ophthalmology 2008;115:363–70. [DOI] [PubMed] [Google Scholar]
- [10].Cheng CY, Hsu WM, Liu JH, et al. Refractive errors in an elderly Chinese population in Taiwan: the Shihpai Eye Study. Invest Ophthalmol Vis Sci 2003;44:4630–8. [DOI] [PubMed] [Google Scholar]
- [11].He M, Huang W, Li Y, et al. Refractive error and biometry in older Chinese adults: the Liwan Eye Study. Invest Ophthalmol Vis Sci 2009;50:5130–6. [DOI] [PubMed] [Google Scholar]
- [12].Guzowski M, Wang JJ, Rochtchina E, et al. Five-year refractive changes in an older population: the Blue Mountains Eye Study. Ophthalmology 2003;110:1364–70. [DOI] [PubMed] [Google Scholar]
- [13].Liu YC, Chou P, Wojciechowski R, et al. Power vector analysis of refractive, corneal, and internal astigmatism in an elderly Chinese population: the Shihpai Eye Study. Invest Ophthalmol Vis Sci 2011;52:9651–7. [DOI] [PubMed] [Google Scholar]
- [14].Guan Z, Yuan F, Yuan YZ, et al. Analysis of corneal astigmatism in cataract surgery candidates at a teaching hospital in Shanghai. China J Cataract Refract Surg 2012;38:1970–7. [DOI] [PubMed] [Google Scholar]
- [15].Sanfilippo PG, Yazar S, Kearns L, et al. Distribution of astigmatism as a function of age in an Australian population. Acta Ophthalmol 2015;93:e377–85. [DOI] [PubMed] [Google Scholar]
- [16].Baldwin WR, Mills D. A longitudinal study of corneal astigmatism and total astigmatism. Am J Optom Physiol Opt 1981;58:206–10. [DOI] [PubMed] [Google Scholar]
- [17].Fledelius HC, Stubgaard M. Changes in refraction and corneal curvature during growth and adult life. A cross sectional study. Acta Ophthalmol 1986;64:487–91. [DOI] [PubMed] [Google Scholar]
- [18].Hayashi K, Hayashi H, Hayashi F. Topographic analysis of the changes in corneal shape due to aging. Cornea 1995;14:527–32. [PubMed] [Google Scholar]
- [19].Namba H, Kawasaki R, Sugano A, et al. Age-related changes in ocular aberrations and the Yamagata Study (Funagata). Cornea 2017;36:S34–40. [DOI] [PubMed] [Google Scholar]
- [20].Namba H, Kawasaki R, Sugano A, et al. Cross-sectional and longitudinal investigation of the power vector in astigmatism: the Yamagata Study (Funagata). Cornea 2018;37:53–8. doi: 10.1097/ICO.0000000000001418. [DOI] [PubMed] [Google Scholar]
- [21].Tominaga M, Eguchi H, Manaka H, et al. Impaired glucose tolerance is a risk factor for cardiovascular disease, but not impaired fasting glucose. The Funagata diabetes study. Diabetes Care 1999;22:920–4. [DOI] [PubMed] [Google Scholar]
- [22].Kawasaki R, Wang JJ, Rochtchina E, et al. Cardiovascular risk factors and retinal microvascular signs in an adult Japanese population: the Funagata study. Ophthalmology 2006;113:1378–84. [DOI] [PubMed] [Google Scholar]
- [23].Nishitsuka K, Kawasaki R, Kanno M, et al. Determinants and risk factors for central corneal thickness in Japanese persons: the Funagata study. Ophthalmic Epidemiol 2011;18:244–9. [DOI] [PubMed] [Google Scholar]
- [24].Saito K, Tanabe Y, Kawasaki R, et al. Is retinal vasculature change associated with risk of obesity? Longitudinal cohort study in Japanese adults: the Funagata study. J Diabetes Investig 2011;2:225–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Namba H, Kawasaki R, Narumi M, et al. Ocular higher-order wavefront aberrations in the Japanese adult population: the Yamagata Study (Funagata). Invest Ophthalmol Vis Sci 2015;56:90–7. [DOI] [PubMed] [Google Scholar]
- [26].Angmo D, Nongpiur ME, Sharma R, et al. Clinical utility of anterior segment swept-source optical coherence tomography in glaucoma. Oman J Ophthalmol 2016;9:3–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Thibos LN, Wheeler W, Horner D. Power vectors: an application of Fourier analysis to the description and statistical analysis of refractive error. Optom Vis Sci 1997;74:367–75. [DOI] [PubMed] [Google Scholar]
- [28].Miller JM. Clinical applications of power vectors. Optom Vis Sci 2009;86:599–602. [DOI] [PubMed] [Google Scholar]
- [29].Suzuki S, Suzuki Y, Iwase A, et al. Corneal thickness in an ophthalmologically normal Japanese population. Ophthalmology 2005;112:1327–36. [DOI] [PubMed] [Google Scholar]
- [30].Xu L, Zhang H, Wang YX, et al. Central corneal thickness and glaucoma in adult Chinese: the Beijing Eye Study. J Glaucoma 2008;17:647–53. [DOI] [PubMed] [Google Scholar]
- [31].Hashemi H, Yazdani K, Mehravaran S, et al. Corneal thickness in a population based, cross sectional study: the Tehran Eye Study. Cornea 2009;28:395–400. [DOI] [PubMed] [Google Scholar]
- [32].Eballe AO, Koki G, Ellong A, et al. Central corneal thickness and intraocular pressure in the Cameroonian non glaucomatous population. Clin Ophthalmol 2010;4:717–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Goldich Y, Barkana Y, Pras E, et al. Variations in corneal biomechanical parameters and central corneal thickness during the menstrual cycle. J Cataract Refract Surg 2011;37:1507–11. [DOI] [PubMed] [Google Scholar]
- [34].Keskin N, Cantürk S, Aydin S, et al. An objective method to determine corneal changes during menopause. Clin Exp Obstet Gynecol 2009;36:176–8. [PubMed] [Google Scholar]
- [35].Foster PJ, Baasanhu J, Alsbirk PH, et al. Central corneal thickness and intraocular pressure in a Mongolian population. Ophthalmology 1998;105:969–73. [DOI] [PubMed] [Google Scholar]
- [36].Aghaian E, Choe JE, Lin S, et al. Central corneal thickness of Caucasians, Chinese, Hispanics, Filipinos, African Americans, and Japanese in a glaucoma clinic. Ophthalmology 2004;111:2211–9. [DOI] [PubMed] [Google Scholar]
- [37].Prasad A, Fry K, Hersh PS. Relationship of age and refraction to central corneal thickness. Cornea 2011;30:553–5. [DOI] [PubMed] [Google Scholar]
- [38].Zhang H, Xu L, Chen C, et al. Central corneal thickness in adult Chinese. Association with ocular and general parameters. The Beijing Eye Study. Graef Arch Clin Exp Ophthalmol 2008;246:587–92. [DOI] [PubMed] [Google Scholar]
- [39].Germundsson J, Karanis G, Fagerholm P, et al. Age-related thinning of Bowman's layer in the human cornea in vivo. Invest Ophthalmol Vis Sci 2013;54:6143–9. [DOI] [PubMed] [Google Scholar]
- [40].Berlau J, Becker HH, Stave J, et al. Depth and age-dependent distribution of keratocytes in healthy human corneas: a study using scanning-slit confocal microscopy in vivo. J Cataract Refract Surg 2002;28:611–6. [DOI] [PubMed] [Google Scholar]
- [41].Niederer RL, Perumal D, Sherwin T, et al. Age-related differences in the normal human cornea: a laser scanning in vivo confocal microscopy study. Br J Ophthalmol 2007;91:1165–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Zheng T, Le Q, Hong J, et al. Comparison of human corneal cell density by age and corneal location: an in vivo confocal microscopy study. BMC Ophthalmol 2016;16:109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Mustonen RK, McDonald MB, Srivannaboon S, et al. Normal human corneal cell populations evaluated by in vivo scanning slit confocal microscopy. Cornea 1998;17:485–92. [DOI] [PubMed] [Google Scholar]
- [44].Vanathi M, Tandon R, Sharma N, et al. In-vivo slit scanning confocal microscopy of normal corneas in Indian eyes. Indian J Ophthalmol 2003;51:225–30. [PubMed] [Google Scholar]
- [45].Daxer A, Misof K, Grabner B, et al. Collagen fibrils in the human corneal stroma: structure and aging. Invest Ophthalmol Vis Sci 1998;39:644–8. [PubMed] [Google Scholar]
- [46].Read SA, Collins MJ, Carney LG. The influence of eyelid morphology on normal corneal shape. Invest Ophthalmol Vis Sci 2007;48:112–9. [DOI] [PubMed] [Google Scholar]
- [47].Moon NJ, Lee HI, Kim JC. The changes in corneal astigmatism after botulinum toxin-a injection in patients with blepharospasm. J Korean Med Sci 2006;21:131–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Ho JD, Tsai CY, Liou SW. Accuracy of corneal astigmatism estimation by neglecting the posterior corneal surface measurement. Am J Ophthalmol 2009;147:788–95. [DOI] [PubMed] [Google Scholar]
- [49].Miyake T, Shimizu K, Kamiya K. Distribution of posterior corneal astigmatism according to axis orientation of anterior corneal astigmatism PLoS One 2015 2015;10:e0117194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Puntenney I, Schoch D. Studies on the mechanism of lens injury in radiation cataract. Trans Am Ophthalmol Soc 1953;51:285–300. [PMC free article] [PubMed] [Google Scholar]


