Abstract
Background:
This study was designed to evaluate the efficiency and tolerability of empagliflozin (EMPA) as monotherapy or add-on to existing therapy in patients with type 2 diabetes mellitus (T2DM).
Methods:
Randomized controlled trials (RCTs) comparing efficacy and safety of EMPA vs placebo or EMPA plus other antidiabetes drugs vs placebo plus other oral antidiabetes drugs (OADs) in T2DM were recruited from electronic database Pubmed, Web of Knowledge, and Cochrane Central Register of Controlled Trials (CENTRAL), supplemented by a hand search of the reference lists of selected articles. Main effect sizes were change from baseline on glycemia control, body weight, blood pressure, and complications (i.e., incidence of urinary and genital tract infections, and morbidity of hypoglycemia and hyperglycemia). Random-effects model was used to account for clinical or methodologic heterogeneity across studies.
Results:
Fifteen RCTs with a total number of 7891 individuals (5374 in EMPA group and 2517 in control group) were suitable for this meta-analysis. The results demonstrated that significant improvements in glycemia control, body weight, and blood pressure were associated with EMPA application (i.e., monotherapy and add-on therapy) in patient with T2DM when compared with placebo. Meanwhile, EMPA 10 and 20 mg improved glycemia, body weight, and blood pressure control for patients with T2DM. There was no significant difference in incidence of hypoglycemia and urinary tract infections across EMPA and placebo group. Significant reduced risk of hyperglycemia was revealed in EMPA group vs placebo (risk ratio: 0.34, 95%confidence interval: 0.23–0.49, P < .00001), except in patients on background insulin therapy. However, increased risk of genital infection was noted across EMPA vs placebo (risk ratio: 2.59, 95% confidence interval: 1.80–3.71, P < .00001).
Conclusion:
Our evidence supports the application of EMPA in treatment of patients with T2DM who are obesity or at risk of weight gain.
Keywords: add-on therapy, empagliflozin, monotherapy, sodium glucose cotransporter 2 inhibitor, type 2 diabetes mellitus
1. Introduction
The sodium glucose cotransporter 2 (SGLT2) which is located in the proximal tubule of the kidney is responsible for more than 90% reabsorption of filtered glucose.[1] As a result of maladaptively increased expression of SGLT2 in patients with type 2 diabetes mellitus (T2DM), the capacity of the kidneys to reabsorb glucose is increased, which make the hyperglycemia further deteriorate.[1]
The kidney has emerged as a therapeutic target in the treatment of T2DM. Empagliflozin (EMPA) is an orally active, insulin independent, selective inhibitor of SGLT2. By blocking SGLT2, EMPA restrains glucose reabsorption, and finally leads to increased urinary glucose excretion and a reduction in fasting and postprandial plasma glucose. Current randomized controlled trials (RCTs) have demonstrated that EMPA improved glycemia control in patients with T2DM.[1–6] Meanwhile, the efficacy and tolerability of EMPA have also been further revealed in recent systemic reviews.[7–11]
However, Kohler et al[10,11] evaluated the safety and tolerability of EMPA in patients with T2DM according to pooled data from randomized clinical trials plus its extension studies, in which the effect of the same population would be doubled. Meanwhile, they did not further analysis on the efficacy aspect of EMPA. Devi et al meta-analyzed RCTs to assess the efficacy and safety of EMPA compared to placebo in T2DM without restriction to treatment duration.[7] As we know, to address meaningful changes inHbA1c, treatment duration should be no <12 weeks. While in this meta-analysis, treatment duration was 4 weeks in 2 of 14 included trials.[12,13] And they did not carry out further subgroup analysis the effect of EMPA as monotherapy and add-on therapy. Zhong et al only focused on examining the potential use of EMPA in combination with metformin as a therapeutic option for T2DM, in which EMPA as monotherapy and EMPA as add-on to other antidiabetes drugs was neglected.[8] Liakos et al evaluated the efficacy and safety of EMPA compared with placebo or other antidiabetes agents in patients with T2DM.[9]
To our knowledge, only by comparing the EMPA vs placebo or by comparing EMPA vs placebo as add-on to other oral antidiabetes drugs (OADs) in which the other antidiabetes therapy could be balanced across the treatment and control groups might fully answer the question whether EMPA is efficient in T2DM treatment. Thus, we carried out this meta-analysis to assess the efficiency and safety of EMPA (10 and 25 mg once daily) compared with placebo both as monotherapy and as add-on therapy to OAD in patients with T2DM.
2. Materials and methods
The present review was conducted in strict accordance with Handbook for systematic Reviews of Interventions Version 5.1.0.[14]
2.1. Literature search
First, we searched the Cochrane Central Register of Controlled Trials (CENTRAL), Web of Knowledge, and Pubmed databases without language restrictions (up to May 2018). Only the MeSH heading keyword of “empagliflozin (BI-10773)” was used so that all the possible studies would be systemically checked. Search outcome was limited to RCTs. Then, manual search was performed by cross-checking the reference list of selected articles. The literature search was last updated to ensure a comprehensive investigation. This is a meta-analysis which collected data from published papers. Thus, ethics approval was not necessary.
2.2. Criteria for considering studies for this review
All search items were evaluated for eligibility by 2 reviewers (YJZ and SLH). Studies were included in this review only when all of the following criteria were met:
-
1.
Types of studies: RCTs.
-
2.
Types of participants: Patients with T2DM were eligible for inclusion if they were both aged 18 years or older and drug-negative or patient with washout period for the other OAD between the screening and placebo run-in periods.
-
3.
Types of interventions: comparisons of EMPA vs placebo, or EMPA vs placebo as add-on to other antidiabetes therapy.
-
4.
The main outcomes of interest was change from baseline in hemoglobin A1c (HbA1c), proportion of patients with HbA1c ≥7.0% at baseline who reached HbA1c <7.0% at last follow-up; changes from baseline in fasting plasma glucose (PFG); changes from baseline in body weight, proportion of patients with >5.0% reduction in body weight; change from baseline in systolic and diastolic blood pressures (SBP and DBP), percentage of patients with uncontrolled blood pressure (SBP ≥ 130 mm Hg or DBP ≥ 80 mm Hg) at baseline who had controlled blood pressure (SBP < 130 mm Hg or DBP < 80 mm Hg); incidence of urinary and genital tract infections, and morbidity of hypoglycemia and hyperglycemia.
To address meaningful changes in HbA1c, we only included trials with treatment duration more than 12 weeks. Disagreements in study selection between 2 reviewers were resolved by discussion. If no consensus could be reached, a third reviewer was consulted (XFS).
2.3. Assessment of risk of bias
Cochrane recommended “risk of bias table” was used for quality assessment by 2 investigators (YJZ and SLH). Each eligible study was graded for risk of bias (low, high, unclear) in 6 domains (namely, random sequence generation, allocation concealment, blinding of patient and personnel, blinding of outcome assessment, incomplete outcome data, and selective reporting risk). Disagreement was resolved by consultation with a third reviewer (XFS).
2.4. Data extraction
Data were extracted independently by 2 investigators (YJZ and SLH). Then a double-check procedure was performed to make sure the accuracy of the data extracted. At last, a manager inputted the extracted data into a spreadsheet. The following information was subtracted from the study: first author name, publishing year, study design, clinical trial registered number, sample volume, baseline demographic characteristic of patients, and main outcomes of interest.
2.5. Statistical analysis
We calculated the weighted mean differences (WMDs) for continuous outcomes and the risk ratio (RR) for the dichotomous data, along with the 95% confidence intervals (CIs).
Prior to analyzing the data, heterogeneity was assessed by Cochran Q test and quantified by I2 test. A fixed-effect model was used when the effects were assumed to be homogenous (P > .05 or I2 < 50%). However, given that the magnitude of EMPA might vary depending on duration of follow-up, background therapy, and participant clinical settings, we assumed the presence of heterogeneity and used random-effects model in all subsequent analyses.
As for studies with multiple intervention groups, we selected the most relevant pair of interventions while exclude the others. As for multiple studies reported on the same patient population, only the published report with the largest sample size was included. If studies had more than 1 EMPA dosage group, we first combined the data from all EMPA groups to create a single pair-wise comparison to evaluate the effect of EMPA vs placebo both as monotherapy and add-on therapy following the method recommended by Cochrane Handbook 5.1.0.
To assess whether the treatment effect of EMPA was modified by clinical variables, we performed subgroup analyses on the basis of the most common dosing regimens for EMPA (10 and 25 mg once daily) and concomitant therapy (monotherapy or add-on therapy).
Funnel plots were employed for detection of publication bias, in which the effect sizes (e.g., WMD) are plotted on the horizontal axis and its variance (e.g., the standard error of the intervention effect) on the vertical axis. Bias is revealed if the plots are asymmetrical about the pooled WMD.[14]
All statistical analyses were done with Review Manager 5.1.0 (Cochrane Collaboration, Oxford, UK). Results were regarded as statistically significant, if P < .05.
3. Result
3.1. Trial flow
The search strategy retrieved 390 citations (68 from Pubmed, 253 from ISI web of science, and 69 from CENTRAL). Subsequent scrutiny of the title and abstracts led to the exclusion of 312 articles either for they were irrelevant to the aim of this meta-analysis or for duplication. The full article was obtained for the remaining 37 publications. According to inclusion criteria, 6 articles were excluded for extension trials but with more than 30% participants lose to follow-up at least. Five RCTs were excluded for subgroup or post-hoc analysis of eligible studies. Seven articles were excluded for combined analysis of 2 or more eligible studies. Four articles were excluded for too short duration (<12 weeks) to address the changes in HbA1c. No eligible papers were further obtained from the bibliographies list of included studies. We last updated search strategy when submitted our manuscript. The study selection process and reasons for exclusions were explicitly described in Figure 1.
Figure 1.
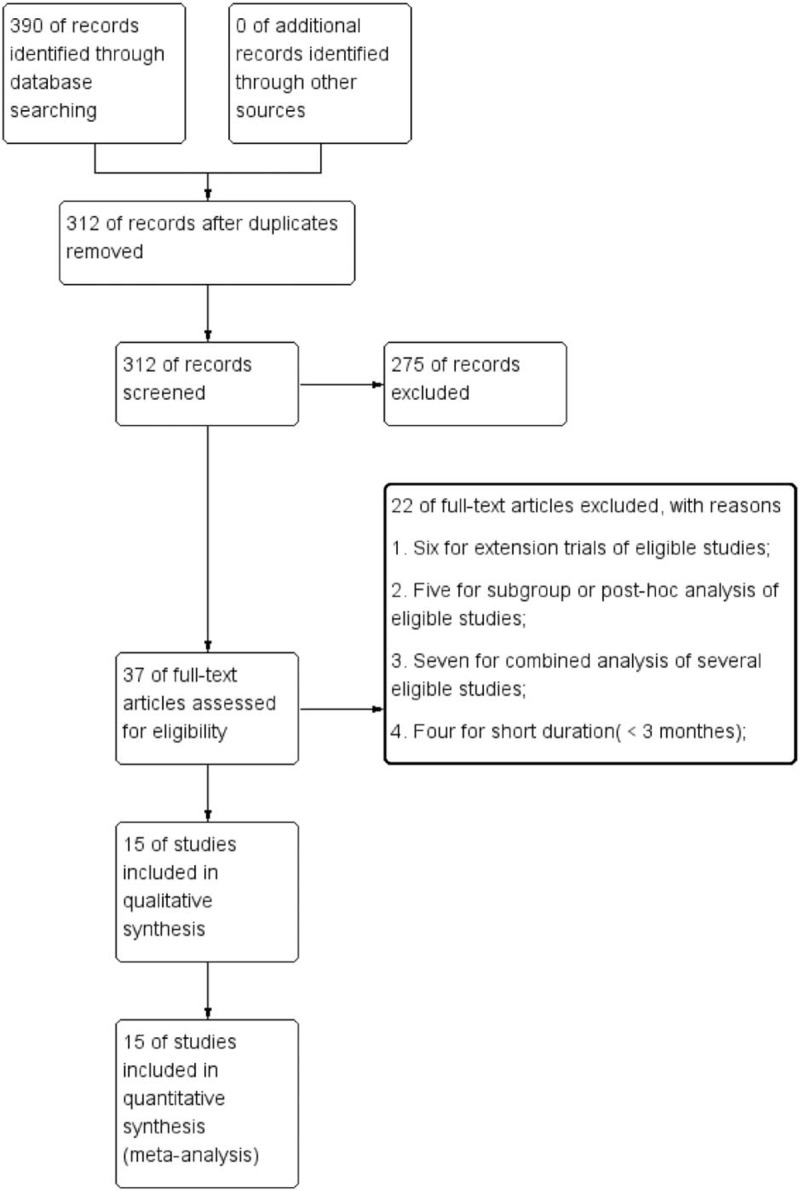
Flow diagram of the selected studies.
3.2. Study characteristics and quality
Finally, 15 RCTs were suitable for this meta-analysis.[2–6,13,15–23] A total of 7891 individuals were identified (5374 in EMPA group and 2517 in control group). There were 3 trials comparing EMPA vs placebo as monotherapy[13,17,18]; 3 trials comparing EMPA vs placebo as add-on to metformin[4,15,20]; 1 trial comparing EMPA vs placebo as add-on to metformin plus sulfonylurea[16]; 2 trials comparing EMPA vs placebo as add-on to metformin plus linagliptin[6,23]; 1 trial comparing EMPA vs placebo as add-on to pioglitazone or pioglitazone plus metformin[21]; 2 trials comparing EMPA vs placebo as add-on to insulin with or without OADs[3,19]; 1 trial comparing EMPA vs placebo as add-on to linagliptin[2]; 2 trials comparing EMPA vs placebo with other OADs unclear.[5,22] As for trials with multiple intervention groups, we excluded the group irrelevant to the aim of this meta-analysis. Characteristics of eligible studies are shown in Table 1. The result of risk of bias assessment is summarized in Figure 2.
Table 1.
Baseline characteristic of eligible trials.

Figure 2.

Risk of bias evaluation of eligible studies.
3.3. Efficacy outcome of EMPA
3.3.1. Glycemia efficacy
Fifteen studies with 7218 individuals (4859 in EMPA group and 2359 in control group) were available at the last follow-up which included change from baseline in HbA1c as outcome.[2–6,13,15–23] With the pooled WMD of −0.62 (95% CI: −0.67 to −0.57), it was demonstrated that EMPA was associated with significant decrease in HbA1c (P < .00001). Subgroup analysis according to concomitant therapy revealed that the direction of the effect was consistent for EMPA both as monotherapy and add-on therapy as seen in Table 2. Table 3 shows the result of the subgroup analysis according to EMPA dosage. With the pooled WMD value of −0.61 (95% CI: −0.66 to −0.55) and −0.63 (95% CI: −0.69 to −0.57), respectively, for EMPA dosage of 10 and 25 mg, it was indicated that HbA1c was significantly decreased after treatment with EMPA in comparison with placebo (P < .00001) (Table 2).
Table 2.
Subgroup analysis of efficacy effect sizes (e.g., change from baseline in HbA1c, proportion of patient with HbA1c >7% who had HbA1c <7% and change from baseline in FPG) according to concomitant therapy of EMPA on type 2 diabetes mellitus.
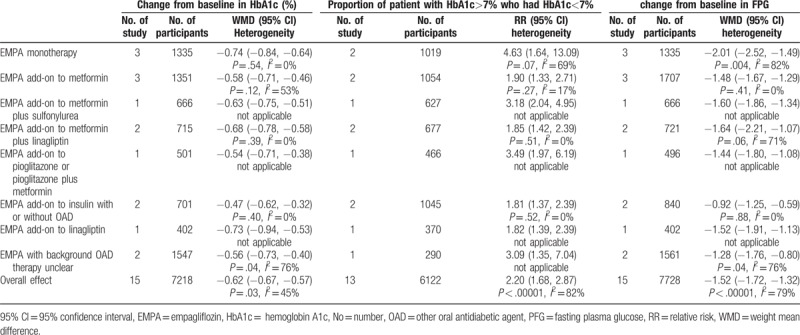
Table 3.
Subgroup analysis of efficacy outcome according to EMPA dosage on type 2 diabetes mellitus.
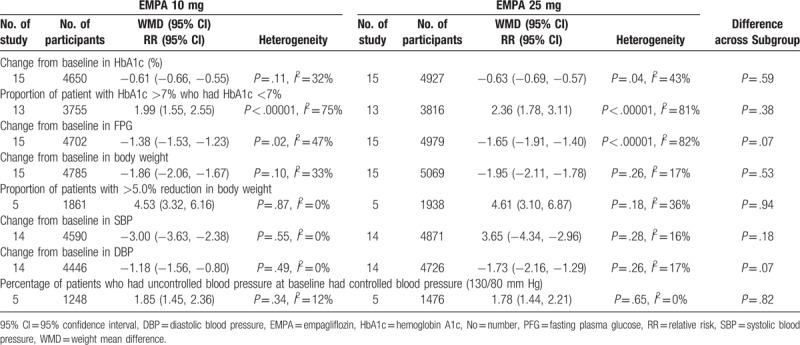
Consistently, of 13 trials reporting HbA1c as dichotomous data (n = 6122), patient with HbA1c ≥7% who had HbA1c <7% was noted in 1523 out of 4299 individuals in EMPA group (35.4%) and 315 out of 1823 in control group (17.3%).[2–4,6,15–23] A significantly higher proportion of patient achieved HbA1c <7.0% in the EMPA groups than in the placebo group (RR 2.20, 95% CI: 1.68–2.87, P < .00001). The direction of the effect was consistent for subgroup analysis based on the concomitant therapy for EMPA both as monotherapy and add-on therapy as seen in Table 2. Subgroup analysis according EMPA dosage demonstrated that both 10 and 25 mg EMPA were associated with high proportion of patient with HbA1c >7% who had HbA1c <7% (Table 3).
Fifteen 15 studies with 7728 individual (5345 in EMPA group and 2383 in control group) were available at the last follow-up which included change from baseline in FPG as outcome.[2–6,13,15–23] With the pooled WMD of −1.52 (95% CI: −1.72 to −1.32), it was demonstrated that EMPA was associated with a significant decrease in FPG (P < .00001). Subgroup analysis according to concomitant therapy revealed that EMPA both as monotherapy and add-on therapy could significantly decrease the FPG. The direction of the effect was consistent as seen in Table 2. Table 3 shows the result of the subgroup analysis according to EMPA dosage. With the pooled WMD value of −1.38 (95% CI: −1.53 to −1.23) and −1.65 (95% CI: −1.91 to −1.40), it was indicated that significant reductions in FPG were related with both for EMPA 10 and 25 mg EMPA (P < .00001).
3.3.2. Body weight
Data on change from baseline in body weight were available for 7847 individual (5409 in EMPA group and 2438 in control group) in 15 studies.[2–6,13,15–23] With the pooled WMD of −1.91 (95% CI: −2.07 to −1.75), it was demonstrated that EMPA was associated with a significant decrease in body weight (P < .00001). Subgroup analysis according to concomitant therapy revealed that EMPA could significantly decrease the body weight. The direction of the effect was consistent as seen in Table 4. Table 3 shows the result of the subgroup analysis according to EMPA dosage. With the pooled WMD value of −1.86 (95% CI: −2.06 to −1.67) and −1.95 (95% CI: −2.11 to −1.78) respectively for dosage of 10 and 25 mg, it was indicated that body weight was significantly decreased after treatment with EMPA vs placebo (P < .00001).
Table 4.
Subgroup analysis of efficacy effect sizes (e.g., change from baseline in body weight, and proportion of patients with >5.0% reduction in body weight) according to concomitant therapy of EMPA on type 2 diabetes mellitus.
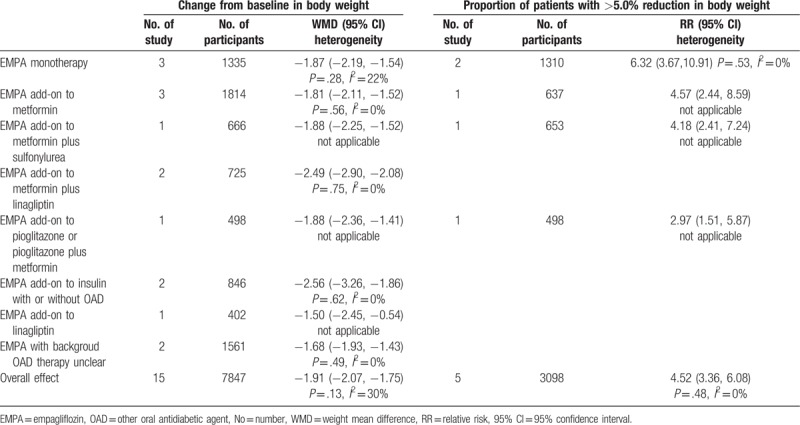
Consistently, of 5 trials reporting body weight as dichotomous data (n = 2999), patient with >5.0% reduction in body weight was noted in 501 out of 2078 individuals in EMPA group (24.1%) and 45 out of 921 in control group (4.9%).[16–18,20,21] A high proportion was noted in EMPA group (RR 4.52, 95% CI: 3.66–6.08, P < .00001). The direction of the effect was consistent for subgroup analysis based on the concomitant therapy as seen in Table 4. Subgroup analysis according EMPA dosage demonstrated that both 10 and 25 mg EMPA were associated with high proportion of patient with >5.0% reduction in body weight (Table 3).
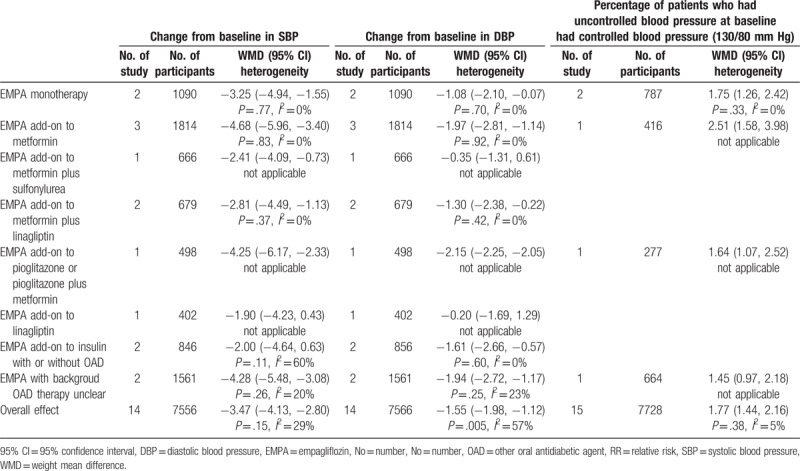
3.3.3. Blood pressure
Fourteen eligible studies were available at last follow-up which included change from baseline in blood pressure as outcome.[2–6,15–23] With the pooled WMD of −3.47 (95% CI: −4.13 to −2.80) and −1.55 (95% CI: −1.98 to −1.12), it was demonstrated that EMPA was associated with a significant decrease in both SBP and DBP (P < .00001). However, only EMPA monotherapy and EMPA as add-onto metformin, metformin plus linagliptin, pioglitazone or pioglitazone plus metformin were effective in both SBP and DBP control according to subgroup analysis (Table 5). Meanwhile, it was revealed that patient with uncontrolled blood pressure at baseline who received EMPA monotherapy and EMPA as add-on to metformin, pioglitazone or pioglitazone plus metformin achieved a high proportion of blood control. The outcome was explicitly expressed in Table 5. Table 3 shows the result of the subgroup analysis according to EMPA dosage. And it was indicated that both SBP and DBP was significantly decreased after treatment with EMPA in comparison to placebo.
Table 5.
Subgroup analysis of efficacy effect sizes (e.g., change from baseline in SBP, change from baseline in DBP and percentage of patients who had uncontrolled blood pressure at baseline had controlled blood pressure (130/80 mm Hg)) according to concomitant therapy of EMPA on type 2 diabetes mellitus.
3.4. Side-effect
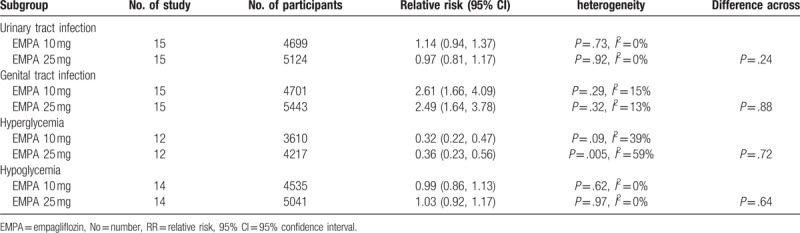
Data on urinary tract infection (UTI) was available for 7972 participants (5469 in EMPA group and 2053 in the control group) in 15 studies.[2,3,5,6,13,15–23] UTI was reported in 469 patients in the EMPA group (8.58%) and 211 patients in the placebo group (10.28%). No significant difference was revealed both in overall and subgroup analysis according to concomitant therapy (Fig. 3). Subgroup analysis according to EMPA dosage demonstrated no difference either (Table 6).
Figure 3.
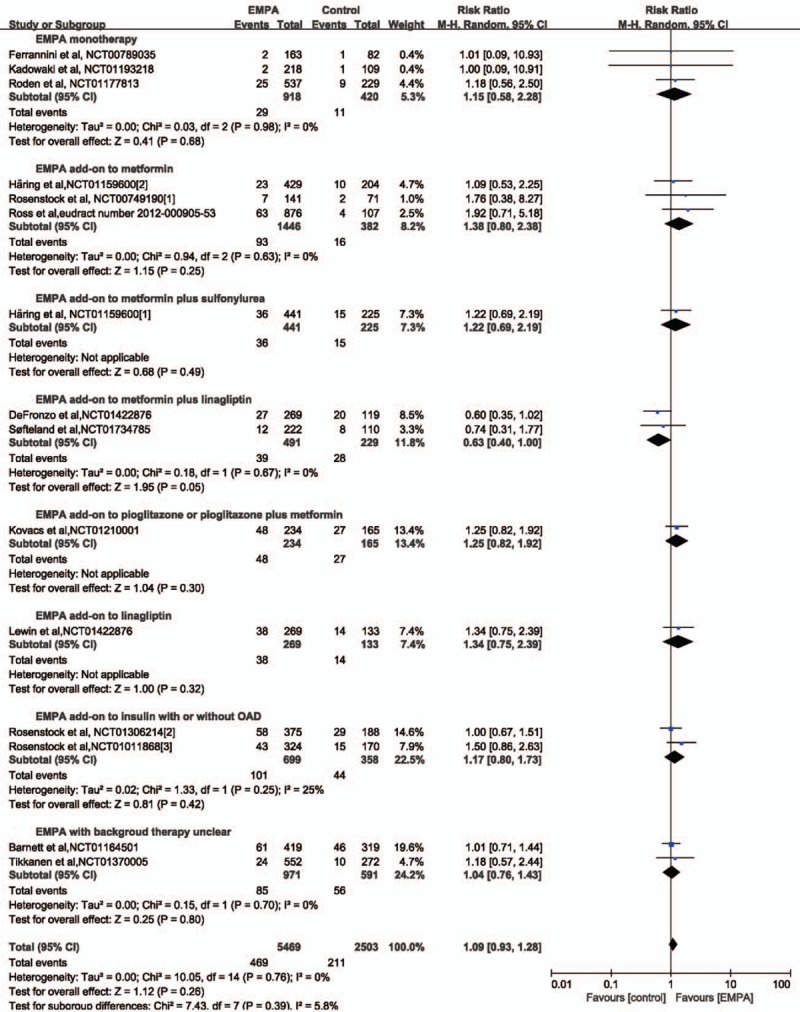
Forest plot: relative risks on urinary tract infection (UTI) of empagliflozin (EMPA) on type 2 diabetes mellitus.
Table 6.
Safety outcome of EMPA on type 2 diabetes mellitus.
Fifteen studies with a total of 7972 patients (5469 in EMPA group and 2053 in the control group) provided genital infection data for meta-analysis.[2,3,5,6,13,15–23] Genital infection was reported in 236 patients in the EMPA group (4.3%) and 33 patients in the placebo group (1.6%). A higher incidence of genital infection was revealed after EMPA treatment (RR 2.59, 95% CI: 1.80–3.71, P < .00001). Subgroup analysis according to concomitant therapy revealed that only EMPA monotherapy, EMPA as add-on to pioglitazone or pioglitazone plus metformin and EMPA as add-on to insulin with or without OAD was associated with higher morbidity of genital infection as seen in Figure 4. Subgroup analysis according to EMPA dosage demonstrated that both 10 and 25 mg EMPA were associated high morbidity of genital infection (Table 6).
Figure 4.
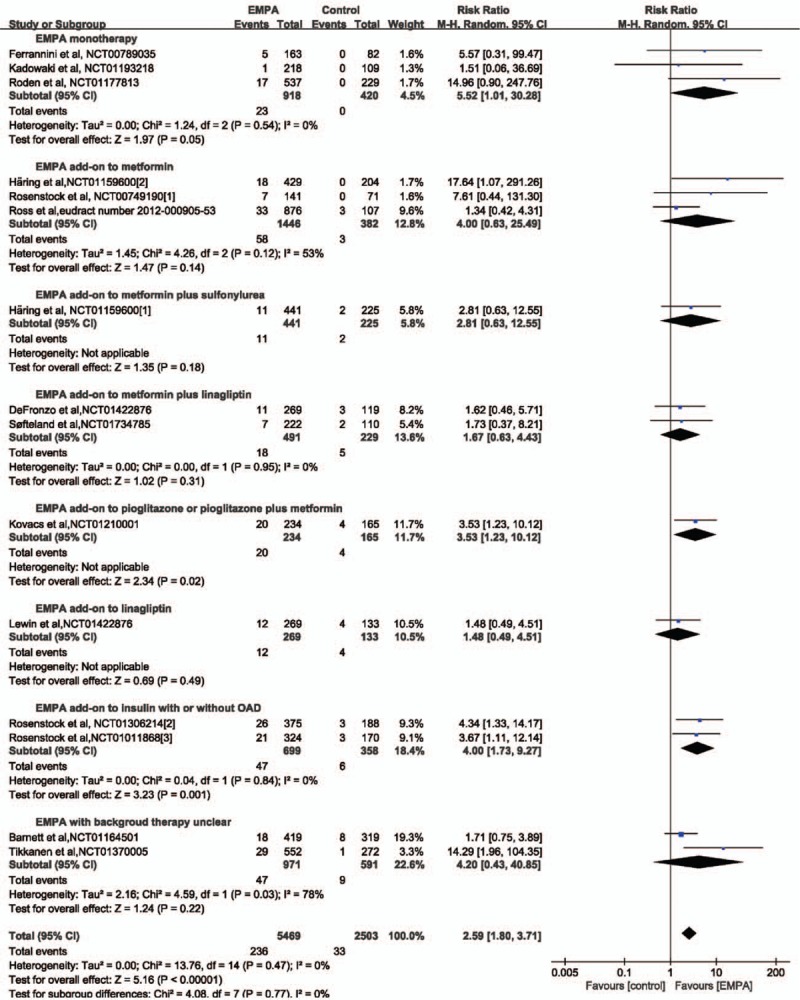
Forest plot: relative risks on genital infection of empagliflozin (EMPA) on type 2 diabetes mellitus.
Data on hyperglycemia were available for 5838 participants (3823 in EMPA group and 2015 in the control group) in 12 studies.[2,3,6,13,15–17,19–23] Hyperglycemia was reported in 146 patients in the EMPA group (3.82%) and 218 patients in the placebo group (10.82%). A significantly lower incidence of hyperglycemia (64%) was revealed after EMPA treatment (RR 0.34, 95% CI: 0.23–0.49, P < .00001). The direction of the effect was consistent for subgroup analysis based on the concomitant therapy, except for EMPA as added on-to insulin with or without other antidiabetes therapy as seen in Figure 5. Subgroup analysis according to EMPA dosage demonstrated that both 10 and 25 mg EMPA were associated lower morbidity of hyperglycemia (Table 6).
Figure 5.
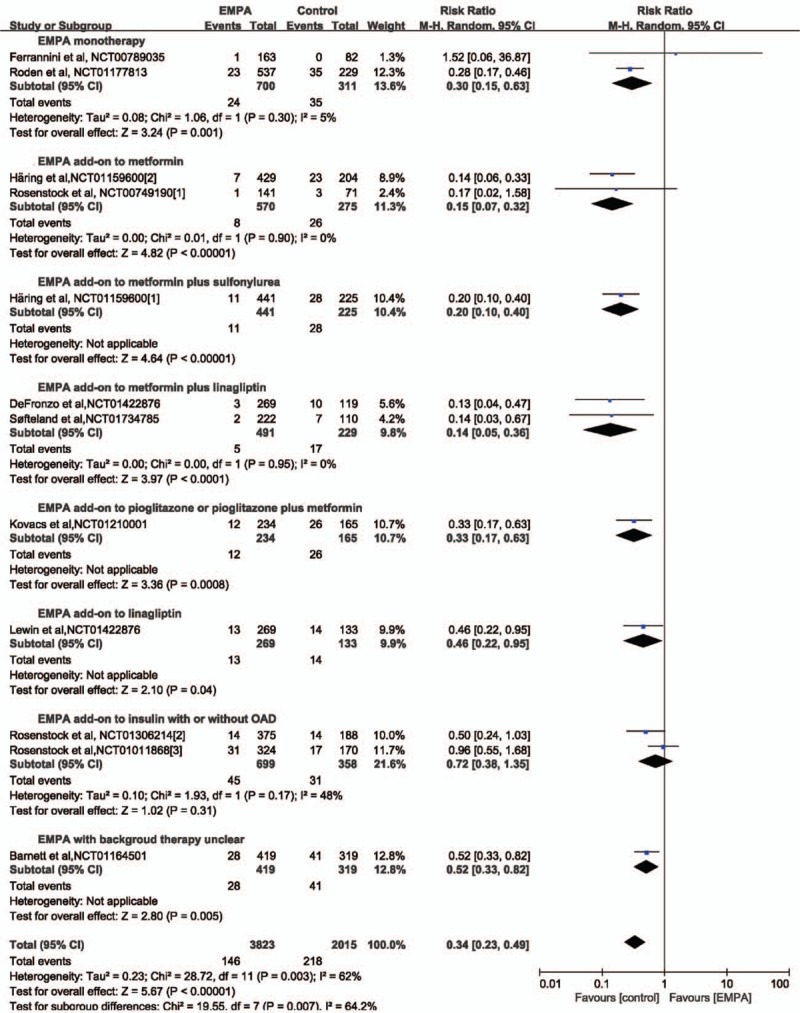
Forest plot: relative risks on hyperglycemia of empagliflozin (EMPA) on type 2 diabetes mellitus.
Fourteen studies with a total of 7727 patients (5306 in EMPA group and 2421 in the control group) provided hypoglycemia data for meta-analysis.[2–6,15–23] Hypoglycemia was reported in 563 patients in the EMPA group (10.6%) and 302 patients in the placebo group (12.5%). No significant difference was revealed both in overall and subgroup analysis according to concomitant therapy (Fig. 6). Subgroup analysis according to EMPA dosage demonstrated no difference either (Table 6).
Figure 6.
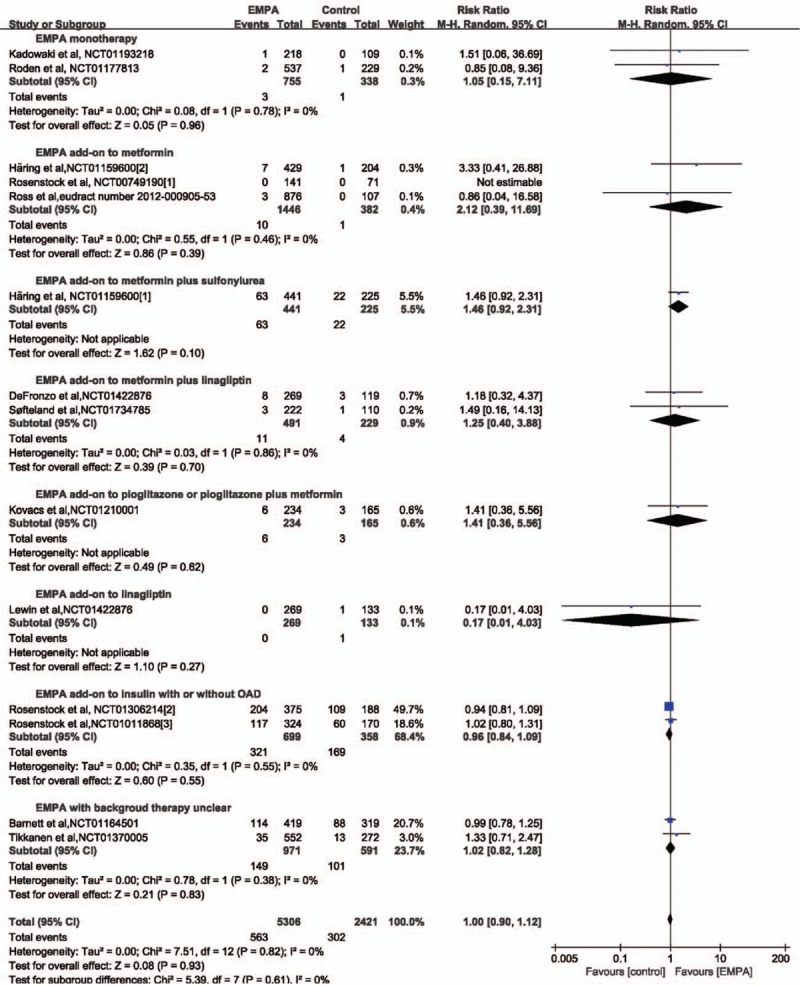
Forest plot: relative risks on hypoglycemia of empagliflozin (EMPA) on type 2 diabetes mellitus.
3.5. Publication bias
Publication bias statistics were determined by funnel plot. Figure 7 shows funnel plots of studies reporting WMD of change from baseline in HbA1c as a measure of treatment effect. The plot demonstrates asymmetry about the pooled effect which publication bias might exist.
Figure 7.
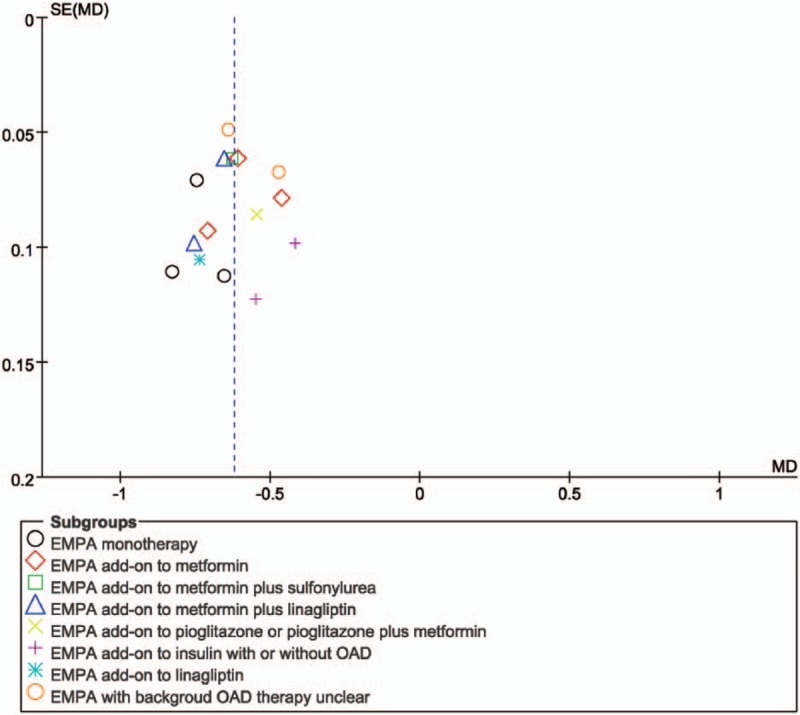
Funnel plot evaluating publication bias. EMPA = empagliflozin.
4. Discussion
This was the first meta-analysis to evaluate the efficacy and safety of EMPA monotherapy and all combination therapy schemes with comparison to placebo in T2DM mellitus simultaneously. By systematically reviewing and finally combining the published evidence, our meta-analysis showed that EMPA led to significant improvements in HbA1c and FPG in T2DM. Furthermore, EMPA treatment resulted in clinically relevant reductions in body weight, blood pressure, compared with placebo. EMPA both as monotherapy and as combination therapy (i.e., EMPA added to metformin, metformin plus sulfonylurea, metformin plus linagliptin, pioglitazone or pioglitazone plus metformin, and insulin with or without OAD can also effective improve glycemia control, bring on better weight loss and blood pressure control. Subgroup group analysis according to EMPA dosage demonstrated that both 10 and 25 mg EMPA were efficient and tolerable in T2DM.
In our study, a clinically and statistically significant reduction in HbA1c and FPG was revealed in treatment group which suggest sustained glycemia control of EMPA both as monotherapy and as add-on therapy (i.e., EMPA add-on to metformin, metformin plus sulfonylurea, metformin plus linagliptin, pioglitazone or pioglitazone plus metformin, linagliptin, and insulin with or without OAD). This is also well supported by a finding that a significant larger percentage of patients achieved glycemia target of HbA1c <7% compared to placebo. These findings are in line with other study, which found that treatment with EMPA resulted in similar reductions in HbA1c and FPG vs placebo in patients with T2DM.[6,11,24] The results further support the fact that SGLT2 inhibitors could lower glycemia by enhancing urinary glucose excretion. Due to reduction in insulin secretion and tissue glucose disposal, EMPA further corrected insulin resistance and restored β-cell function.[25]
In our study, significant decrease of body weight in patient with T2DM was observed in EMPA group both as monotherapy and as add-on therapy (i.e., EMPA add-on to metformin, metformin plus sulfonylurea, metformin plus linagliptin, pioglitazone or pioglitazone plus metformin, linagliptin, and insulin with or without OAD). Meanwhile, EMPA as monotherapy and as add-on to therapy (i.e., EMPA add-on to metformin, metformin plus sulfonylurea, metformin plus linagliptin, pioglitazone or pioglitazone plus metformin, and linagliptin) were effective in SBP control. EMPA as monotherapy and as add-on to therapy (i.e., EMPA add-on to metformin, metformin plus linagliptin, pioglitazone or pioglitazone plus metformin, and insulin with or without OAD) were effective in DBP control. As we all know, weight loss or avoiding weight gain is important to patients. The negative energy balance might be attributed to several reasons. Firstly, EMPA can inhibit SGLT2 which leads to caloric loss through urinary glucose excretion. Secondly, glycosuria results in osmotic diuresis which could also be accounted for the reduction in body weight and blood pressure.[25] Thirdly, clinical trials demonstrated that SGLT2 inhibitor can directly shift substrate utilization from carbohydrate to lipid which brings on fat loss in T2DM.[25,26]
As we all know, in the EMPA-REG OUTCOME trial, EMPA given in addition to standard of care was associated with significant reductions in 3-point major adverse cardiovascular (CV) events (3-point MACE: composite of CV death, nonfatal myocardial infarction, or nonfatal stroke),CV death, all-cause mortality, hospitalization for heart failure in patients with T2DM and established CV disease.[24,27] Although the mechanisms behind the observed effects are not yet understood, we believe that the beneficial effect of EMPA on CV risk might partly be explained by its body weight and blood pressure lowering properties in addition to glycemia control. And the potential for reduction in body weight is a notable feature of SGLT2 inhibitors. This superiority may make EMPA useful glycemia control agents either for patients with T2DM who are overweight and have problem in losing weight, or to combine with other antidiabetes therapies to mitigate any weight gain associated with improved glycemia control.
Overall, EMPA was well tolerated, with no major adverse events across treatment groups. Specially, owing to its insulin-independent mechanism of action, hypoglycemia was rarely reported in participant taking EMPA despite the reduction in FPG. And no statistically difference in hypoglycemia was revealed in either EMPA monotherapy or add-on therapy vs placebo. More importantly, a significantly lower incidence of hyperglycemia was revealed to be related to EMPA therapy both as monotherapy and add-on therapy when comparing with placebo group. However, in patients on background insulin therapy, no difference was documented across EMPA and control group which might be explained by the instinct of insulin. The incidence of UTIs was similar in EMPA vs placebo in this study, but there was a small increase in genital infections in the EMPA groups. This is consistent with other study, which shows higher proportion of genital infections, but similar proportion of UTIs with SGLT2 inhibitor vs placebo.[15] In our opinion, this could also be explained by the fact that EMPA can enhance urine and sugar excretion which finally washes out the urethral canal more efficiently. However, the scouring property could not be found in genital tract.
The results should be viewed with recognition of limitations inherent in this study. First, only studies evaluating the efficacy and safety of EMPA in T2DM were included in this meta-analysis outcome of which cannot be generalized to patients with T1DM, since the pathogenesis for these 2 types of diabetes mellitus is different. Similarly, we restricted our topic on EMPA; the outcome of which could not be extrapolated to other SGLT2 inhibitor, such as canagliflozin and dapagliflozin.
Secondly, although a broad review scope provides us with a larger sample size, we excluded extension trials if both studies reported the same patient population, since more than 30% of patients lost to follow-up.[28–30] Concomitantly, systemic review that included both studies reporting the same population would double the effect of the common population, and finally lower statistical power to detect a treatment effect.[10]
Thirdly, our search of ClinicalTrials.gov identified one additional eligible studies (NCT01649297) that have not yet been published. Meanwhile, we only included published RCTs. We employed funnel plot to evaluate whether publication bias. And the outcome showed that publication bias might exist. Clinician should understand result with caution.
5. Conclusion
In summary, it is demonstrated that EMPA therapy can improve glycemia, weight and blood pressure control, and was well tolerated except for increased genital infections in patients with T2DM. We recommend that EMPA should be offered to patients with T2DM, especially to patient who are overweight or at risk for body weight gain. As for combination therapy, we recommend EMPA firstly added to metformin, metformin plus sulfonylurea, metformin plus linagliptin, pioglitazone or pioglitazone plus metformin, insulin with or without OAD and linagliptin. However, further study directly comparing EMPA vs placebo both as monotherapy and add-on therapy to other antidiabetes drug is still warranted.
Author contributions
The authors meet criteria for authorship as recommended by the International Committee of Medical Journal Editors (ICMJE). Conceived and designed the experiments: SLH, YJZ. Performed the experiments: SLH, YJZ, XFS. Analyzed the data: SLH, YJZ. Wrote the paper: SLH and YJZ. Reviewed/edited the paper: SXW, HYW, XL, LC.
Data curation: Shi-Liang Han, Yun-Jing Zhang, Xi-Feng Sun.
Formal analysis: Shi-Liang Han, Yun-Jing Zhang.
Investigation: Shi-Liang Han, Yun-Jing Zhang.
Methodology: Shi-Liang Han, Yun-Jing Zhang.
Project administration: Shi-Liang Han, Hong-Yun Wang.
Resources: Shi-Liang Han.
Software: Shi-Liang Han, Yun-Jing Zhang.
Supervision: Xi-Feng Sun.
Writing – original draft: Shi-Liang Han, Yun-Jing Zhang, Hong-Yun Wang.
Writing – review & editing: Shi-Liang Han, Yun-Jing Zhang, Shu-Xiang Wang, Xiao Liu, Li Chen, Ling Xia.
Shi-Liang Han orcid: 0000-0001-7041-5808.
Footnotes
Abbreviations: CIs = confidence intervals, DBP = diastolic blood pressure, EMPA = empagliflozin, HbA1c = hemoglobin A1c, OAD = oral antidiabetic drug, PFG = fasting plasma glucose, RCT = randomized controlled trial, RR = risk ratio, SBP = systolic blood pressure, SGLT2 = sodium glucose cotransporter 2, T2DM = type 2 diabetes mellitus, UTI = urinary tract infection, WMDs = weighted mean differences.
YJZ and SLH authors contributed equally to this work.
The authors have no funding and conflicts of interest to disclose.
References
- [1].DeFronzo RA, Davidson JA, Del Prato S. The role of the kidneys in glucose homeostasis: a new path towards normalizing glycaemia. Diabetes Obes Metab 2012;14:5–14. [DOI] [PubMed] [Google Scholar]
- [2].Lewin A, DeFronzo RA, Patel S, et al. Initial combination of empagliflozin and linagliptin in subjects with type 2 diabetes. Diabetes Care 2015;38:394–402. [DOI] [PubMed] [Google Scholar]
- [3].Rosenstock J, Jelaska A, Zeller C, et al. Impact of empagliflozin added on to basal insulin in type 2 diabetes inadequately controlled on basal insulin: a 78-week randomized, double-blind, placebo-controlled trial. Diabetes Obes Metab 2015;17:936–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Ross S, Thamer C, Cescutti J, et al. Efficacy and safety of empagliflozin twice daily versus once daily in patients with type 2 diabetes inadequately controlled on metformin: a 16-week, randomized, placebo-controlled trial. Diabetes Obes Metab 2015;17:699–702. [DOI] [PubMed] [Google Scholar]
- [5].Tikkanen I, Narko K, Zeller C, et al. Empagliflozin reduces blood pressure in patients with type 2 diabetes and hypertension. Diabetes Care 2015;38:420–8. [DOI] [PubMed] [Google Scholar]
- [6].Softeland E, Meier JJ, Vangen B, et al. Empagliflozin as add-on therapy in patients with type 2 diabetes inadequately controlled with linagliptin and metformin: a 24-week randomized, double-blind, parallel-group trial. Diabetes Care 2017;40:201–9. [DOI] [PubMed] [Google Scholar]
- [7].Devi R, Mali G, Chakraborty I, et al. Efficacy and safety of empagliflozin in type 2 diabetes mellitus: a meta-analysis of randomized controlled trials. Postgrad Med 2017;129:382–92. [DOI] [PubMed] [Google Scholar]
- [8].Zhong XY, Lai D, Ye Y, et al. Efficacy and safety of empagliflozin as add-on to metformin for type 2 diabetes: a systematic review and meta-analysis. Eur J Clin Pharmacol 2016;72:655–63. [DOI] [PubMed] [Google Scholar]
- [9].Liakos A, Karagiannis T, Athanasiadou E, et al. Efficacy and safety of empagliflozin for type 2 diabetes: a systematic review and meta-analysis. Diabetes Obes Metab 2014;16:984–93. [DOI] [PubMed] [Google Scholar]
- [10].Kohler S, Salsali A, Hantel S, et al. Safety and tolerability of empagliflozin in patients with type 2 diabetes. Clin Ther 2016;38:1299–313. [DOI] [PubMed] [Google Scholar]
- [11].Kohler S, Zeller C, Iliev H, et al. Safety and tolerability of empagliflozin in patients with type 2 diabetes: pooled analysis of phase I-III clinical trials. Adv Ther 2017;34:1707–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Heise T, Seewaldt-Becker E, Macha S, et al. Safety, tolerability, pharmacokinetics and pharmacodynamics following 4 weeks’ treatment with empagliflozin once daily in patients with type 2 diabetes. Diabetes Obes Metab 2013;15:613–21. [DOI] [PubMed] [Google Scholar]
- [13].Ferrannini E, Seman L, Seewaldt-Becker E, et al. A Phase IIb, randomized, placebo-controlled study of the SGLT2 inhibitor empagliflozin in patients with type 2 diabetes. Diabetes Obes Metab 2013;15:721–8. [DOI] [PubMed] [Google Scholar]
- [14].Cochrane Handbook for Systematic Reviews of Interventions. Online Kensaku 2014;35:154–5. [Google Scholar]
- [15].Rosenstock J, Seman LJ, Jelaska A, et al. Efficacy and safety of empagliflozin, a sodium glucose cotransporter 2 (SGLT2) inhibitor, as add-on to metformin in type 2 diabetes with mild hyperglycaemia. Diabetes Obes Metab 2013;15:1154–60. [DOI] [PubMed] [Google Scholar]
- [16].Haring HU, Merker L, Seewaldt-Becker E, et al. Empagliflozin as add-on to metformin plus sulfonylurea in patients with type 2 diabetes: a 24-week, randomized, double-blind, placebo-controlled trial. Diabetes Care 2013;36:3396–404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Roden M, Weng J, Eilbracht J, et al. Empagliflozin monotherapy with sitagliptin as an active comparator in patients with type 2 diabetes: a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Diabetes Endocrinol 2013;1:208–19. [DOI] [PubMed] [Google Scholar]
- [18].Kadowaki T, Haneda M, Inagaki N, et al. Empagliflozin monotherapy in Japanese patients with type 2 diabetes mellitus: a randomized, 12-week, double-blind, placebo-controlled, phase II trial. Adv Ther 2014;31:621–38. [DOI] [PubMed] [Google Scholar]
- [19].Rosenstock J, Jelaska A, Frappin G, et al. Improved glucose control with weight loss, lower insulin doses, and no increased hypoglycemia with empagliflozin added to titrated multiple daily injections of insulin in obese inadequately controlled type 2 diabetes. Diabetes Care 2014;37:1815–23. [DOI] [PubMed] [Google Scholar]
- [20].Haring HU, Merker L, Seewaldt-Becker E, et al. Empagliflozin as add-on to metformin in patients with type 2 diabetes: a 24-week, randomized, double-blind, placebo-controlled trial. Diabetes Care 2014;37:1650–9. [DOI] [PubMed] [Google Scholar]
- [21].Kovacs CS, Seshiah V, Swallow R, et al. Empagliflozin improves glycaemic and weight control as add-on therapy to pioglitazone or pioglitazone plus metformin in patients with type 2 diabetes: a 24-week, randomized, placebo-controlled trial. Diabetes Obes Metab 2014;16:147–58. [DOI] [PubMed] [Google Scholar]
- [22].Barnett AH, Mithal A, Manassie J, et al. Efficacy and safety of empagliflozin added to existing antidiabetes treatment in patients with type 2 diabetes and chronic kidney disease: a randomised, double-blind, placebo-controlled trial. Lancet Diabetes Endocrinol 2014;2:369–84. [DOI] [PubMed] [Google Scholar]
- [23].DeFronzo RA, Lewin A, Patel S, et al. Combination of empagliflozin and linagliptin as second-line therapy in subjects with type 2 diabetes inadequately controlled on metformin. Diabetes Care 2015;38:384–93. [DOI] [PubMed] [Google Scholar]
- [24].Zinman B, Wanner C, Lachin JM, et al. Empagliflozin, cardiovascular outcomes, and mortality in type 2 diabetes. N Engl J Med 2015;373:2117–28. [DOI] [PubMed] [Google Scholar]
- [25].Ferrannini E, Muscelli E, Frascerra S, et al. Metabolic response to sodium-glucose cotransporter 2 inhibition in type 2 diabetic patients. J Clin Invest 2014;124:499–508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Bolinder J, Ljunggren O, Kullberg J, et al. Effects of dapagliflozin on body weight, total fat mass, and regional adipose tissue distribution in patients with type 2 diabetes mellitus with inadequate glycemic control on metformin. J Clin Endocrinol Metab 2012;97:1020–31. [DOI] [PubMed] [Google Scholar]
- [27].Fischereder M, Schonermarck U. Empagliflozin, cardiovascular outcomes, and mortality in type 2 diabetes. N Engl J Med 2016;374:1092–3. [DOI] [PubMed] [Google Scholar]
- [28].Roden M, Merker L, Christiansen AV, et al. Safety, tolerability and effects on cardiometabolic risk factors of empagliflozin monotherapy in drug-naive patients with type 2 diabetes: a double-blind extension of a Phase III randomized controlled trial. Cardiovasc Diabetol 2015;14:154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Lewin AJ, Frias JP. Empagliflozin added to metformin and sulfonylurea therapy in patients with sub-optimally controlled type 2 diabetes mellitus. Expert Opin Pharmacother 2015;16:781–4. [DOI] [PubMed] [Google Scholar]
- [30].Haering HU, Merker L, Christiansen AV, et al. Empagliflozin as add-on to metformin plus sulphonylurea in patients with type 2 diabetes. Diabetes Res Clin Pract 2015;110:82–90. [DOI] [PubMed] [Google Scholar]


