Abstract
The aim of this study was to evaluate the correlation between lymphovascular invasion (LVI) and tumor size, histological grade, and the expression statuses of estrogen receptor (ER), progesterone receptor (PR), human epidermal growth factor receptor-2 (HER-2), Ki67, epidermal growth factor receptor (EGFR), vascular endothelial growth factor (VEGF), E-cadherin, and P53 in invasive breast cancer, then establish a prediction model of LVI based on the associated clinicopathological factors.
A total of 392 patients with primary invasive breast cancers were enrolled, and their paraffin-embedded tissues were manufactured into the tissue microarray. We evaluated the expression statuses of ER, PR, HER-2, Ki67, EGFR, VEGF, E-cadherin, and P53 based on immunohistochemistry, histological grade and LVI based on the hematoxylin and eosin stain, and tumor size.
The positivity of LVI was significantly higher in the patients with HER-2 positive expression, Ki67 high expression, and tumor size >2 cm by Chi-square test. HER-2, Ki67, and tumor size were risk factors of LVI by multivariate analysis. The areas under the receiver operating curve of HER-2, Ki67, tumor size, and the combination of the 3 clinicopathological factors were 0.614 [P = .001, 95% confidence interval (CI): 0.544–0.683], 0.596 (P = .006, 95% CI: 0.529–0.662), 0.575 (P = .03, 95% CI: 0.510–0.641), and 0.670 (P < .001, 95% CI: 0.607–0.734), respectively.
HER-2 positive expression, Ki67 high expression, and tumor size >2 cm were risk factors of LVI, whereas the power of the prediction model of LVI based on the 3 clinicopathological factors in invasive breast cancer was low.
Keywords: clinicopathological factor, invasive breast cancer, lymphovascular invasion, model, predictor
1. Introduction
Lymphovascular invasion (LVI) is a very important predictor of poor prognosis, and the risk of local recurrence and distant metastasis in patients with LVI is significantly higher in breast cancer.[1–3] LVI is an important indication for postoperative chemotherapy and radiotherapy in breast cancer. Pathological diagnosis is the gold standard for the LVI. However, the detection of LVI is affected by many factors: the small biopsy tissue volume, the incomplete sampling of the resected tumor, neoadjuvant chemotherapy, and so on. These may lead to a missed LVI diagnosis. Because the application of neoadjuvant chemotherapy is more and more extensive in breast cancer and because core needle biopsy also increases, predicting LVI through clinical and pathological factors becomes very important, especially in prognostic evaluation and guiding treatment for early breast cancer.
The age of diagnosis, tumor size, histological grade, and statuses of estrogen receptor (ER), progesterone receptor (PR), human epidermal growth factor receptor-2 (HER-2), Ki67, epidermal growth factor receptor (EGFR), vascular endothelial growth factor (VEGF), E-cadherin, and P53 are very important prognostic indicators. There is a prognostication and treatment benefit tool and a prediction model of axillary lymph node positivity for early breast cancer. They incorporate almost all of the above indicators except LVI.[4,5] The associations between these indicators and LVI are currently reported in breast cancer, but the results are not consistent.[1,6–8] Furthermore, a prediction model of LVI with those clinicopathological factors has not been reported at present. If we can build an LVI prediction model, we will provide more valuable information on individualized treatment in breast cancer.
2. Materials and methods
2.1. Patients
The paraffin-embedded tissue samples were from 392 patients with primary invasive breast cancer who were diagnosed from 2010 to 2015 at Yuebei People's Hospital. The median age was 48 years old. The mean age was 48.8 ± 9.3 years old. All data were anonymized. The study was approved by the Institutional Review Board of Guangzhou Medical University. None of the enrolled patients underwent anticancer therapy before the extraction of the pathological specimens.
2.2. Tumor characteristics and molecular analyses
The samples were fixed with 10% neutral buffered formalin and embedded in paraffin. To analyze the pathological factors, immunohistochemistry (IHC) staining was retrospectively performed on the tissue microarray (TMA) constructed by 2 trained pathologists. There were 24 tissue cores per block, and each core was 0.6 mm in diameter. The most representative and well-preserved tumor areas in the donor tissue blocks were marked by the pathologists according to the corresponding hematoxylin and eosin (H&E)-stained slides. The tissue volume in TMA was smaller compared with the conventional paraffin section. To reduce the false negatives and positives, 3 duplicate cores were taken from each patient sample, with 1 core serving as the negative control and 1 core as the positive control in each block. We evaluated the age of diagnosis, tumor size, histological grade, LVI, and statuses of ER, PR, HER-2, Ki67, EGFR, VEGF, E-cadherin, and P53.
2.3. Immunohistochemistry
Each TMA block was cut into 2.5-μm thick sections that were stained with the monoclonal antibodies ER, PR, HER-2, Ki67, EGFR, VEGF, E-cadherin, and P53. All the antibodies were ready-to-use from OriGene. A positive control was taken from a breast cancer tissue sample with positive IHC staining results, and a negative control was taken from a paraffin-embedded breast cancer tissue sample that had not been submitted for incubation with primary antibody. The alkaline phosphatase-anti-alkaline phosphatase assay was adopted. IHC was performed according to the ASCO/CAP guidelines, and the results were analyzed using 200× microscopic fields by 1 independent pathologist and were then independently reviewed by another pathologist according to the following criteria: the positivity of ER and PR were defined as nuclear staining of >1% of the tumor cells, and P53 was defined as nuclear staining of ≥10% of the tumor cells. HER-2 status was assessed using the IHC method according to the criteria set by DAKO, with scores of 0 and 1+ regarded as negative; 2+ regarded as equivocal, leading to further testing using in situ hybridization, and 3+ was regarded as positive. High expression for Ki67 was defined as nuclear staining of ≥14% of tumor cells. Positivity for E-cadherin and EGFR was defined as membranous staining of ≥50% and ≥10% of the tumor cells, respectively. Positivity for VEGF was defined as cytoplasmic staining of ≥25% of the tumor cells.
2.4. Definition of LVI
LVI was assessed on H&E-stained sections of the original cancer tissues. LVI was defined as the presence of cancer cells within a definite endothelial-lined space (lymphatic or blood vessel), for which there was a specified distance between the tumor foci and LVI (Figure 1).
Figure 1.
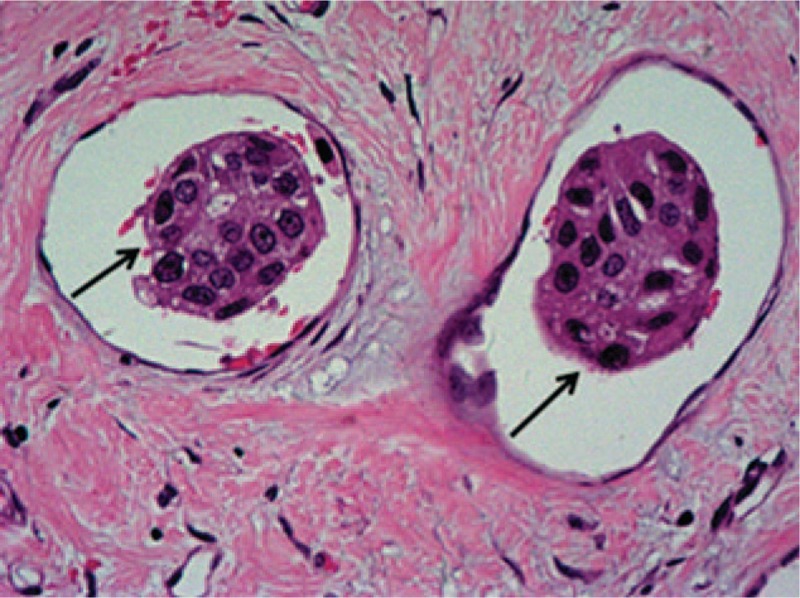
Lymphovascular invasion (LVI; single arrows) in invasive breast cancer tissue section stained with hematoxylin and eosin (H&E; magnification ×200).
2.5. Statistical analyses
Statistical analysis was performed using SPSS version 20.0 (SPSS Inc, Chicago, IL). The correlations between LVI and several clinicopathological factors were evaluated by chi-square test. A logistic regression model was used to evaluate the multivariate analysis. P value of <.05 was considered statistically significant. The odds ratio (OR) and the 95% confidence interval (CI) were calculated for each variable.
3. Results
3.1. The characteristics of patients and tumors
The age of patients ranged from 24 to 80 years old (mean = 48.8 ± 9.3 years old, median = 48 years old). The clinicopathological characteristics of the 392 patients are shown in Table 1. The expression statuses of HER-2 and Ki67 detected by IHC are shown in Figure 2 A, B, C, D.
Table 1.
Summary of clinicopathological characteristics of 392 patients with breast cancer.
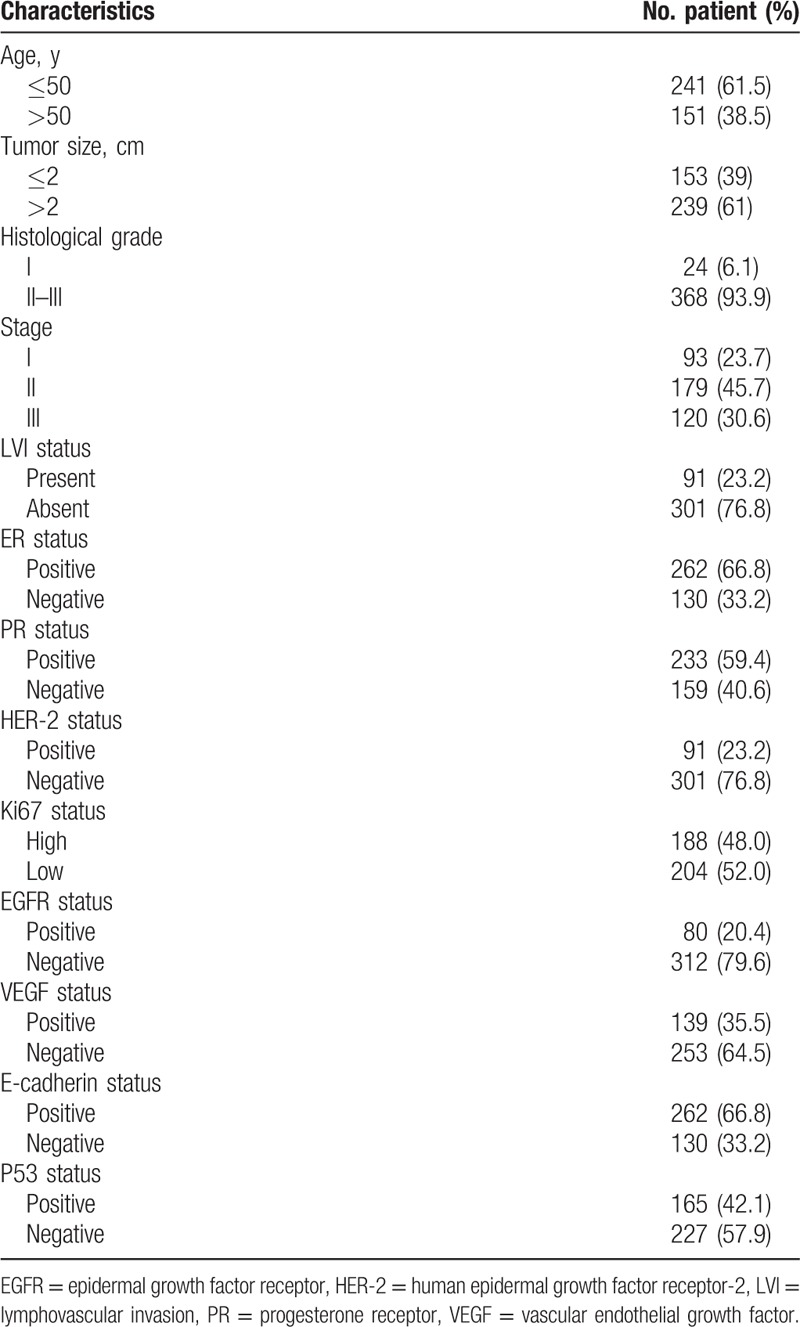
Figure 2.
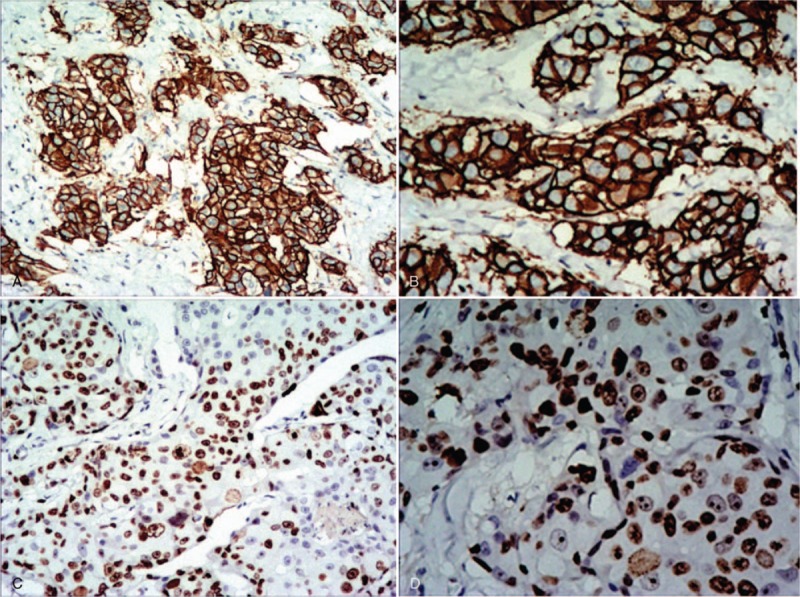
The expression of human epidermal growth factor receptor-2 (HER-2) and Ki67 in invasive breast cancer detected by immunohistochemistry (IHC). A, B, The expression of HER-2 is positive in invasive breast cancer, and the cell membrane is brown and continuous. A magnification ×100, B magnification ×200. C, D, The expression of Ki67 is high in invasive breast cancer, and the nucleus is brown, tumor cell positivity 70%. C Magnification ×100. D Magnification ×200.
3.2. Comparison of present LVI rates among different clinicopathological factors
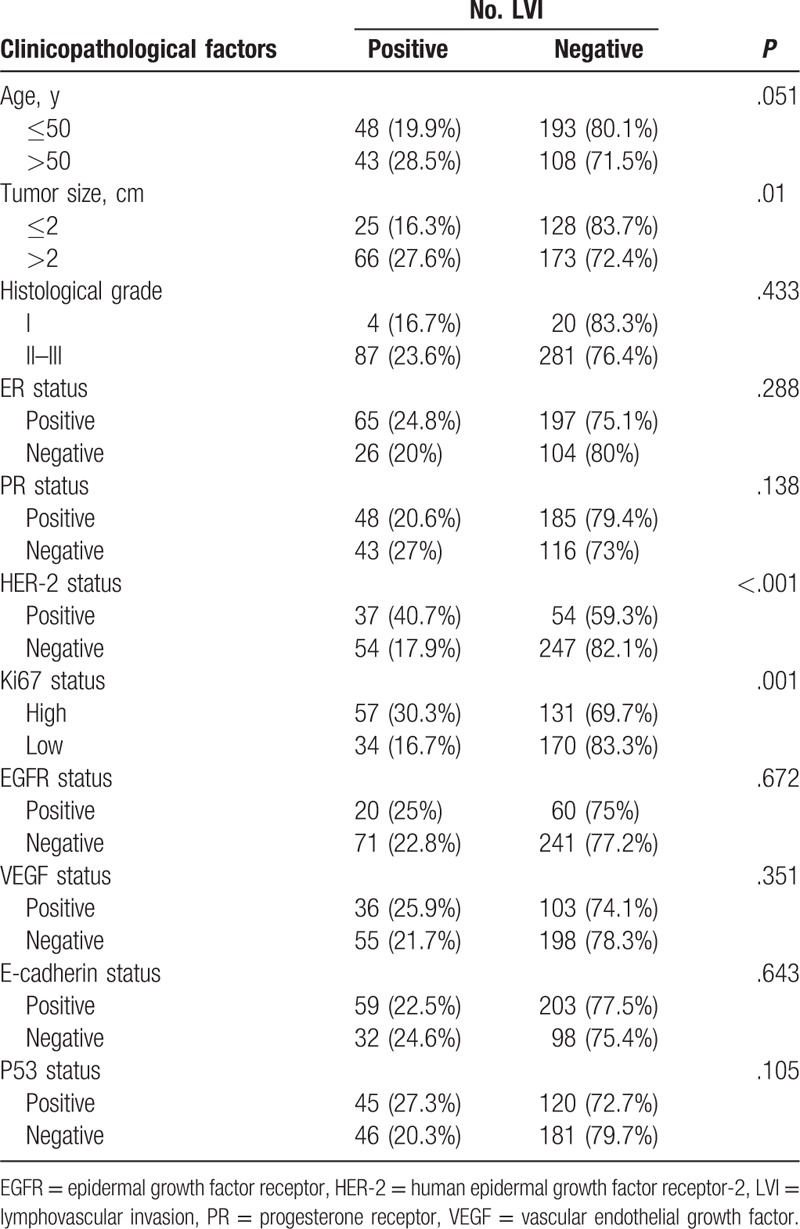
A total of 392 female patients with invasive breast cancer were included in this analysis. The positivity of LVI was significantly high in patients with HER-2 positive expression (χ2 = 20.233, P < .001), Ki67 high expression (χ2 = 10.230, P = .001), and tumor size >2 cm (χ2 = 6.653, P = .01). LVI did not show significant associations with the expression status of ER, PR, EGFR, VEGF, E-cadherin, P53, the age of diagnosis, and histological grade (Table 2).
Table 2.
Association between lymphovascular invasion and other clinicopathological factors in invasive breast cancer.
3.3. Multivariate logistic regression analyses of clinicopathological factors associated with LVI
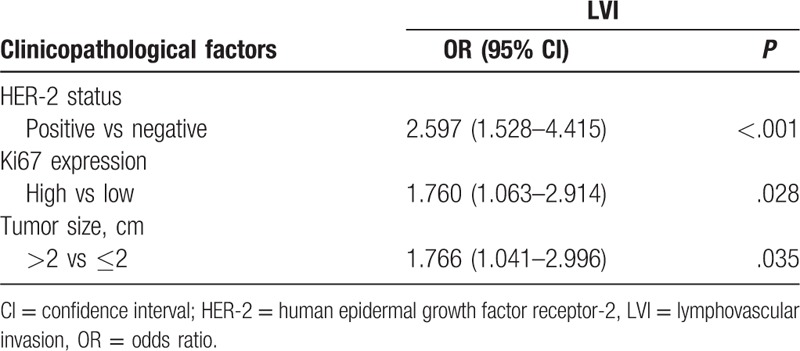
A multivariate logistic regression analysis confirmed a significant association between HER-2 positive expression (OR = 2.597, 95% CI: 1.528–4.418, P < .001), Ki67 high expression (OR = 1.760, 95% CI: 1.063–2.914, P = .028), tumor size >2 cm (OR = 1.766, 95% CI: 1.041–2.996, P = .035), and LVI (Table 3).
Table 3.
Multivariate logistic regression analysis of lymphovascular invasion with clinicopathological factors.
3.4. Predictive power of the potential predictors identified based on ROC
Receiver operating curves (ROCs) corresponding to the multiple logistic model were applied to the data of 392 patients. The areas under the ROC for HER-2, Ki67, tumor size, and the combination of HER-2, Ki67, and tumor size were 0.614 (P = .001, 95% CI: 0.544–0.683), 0.596 (P = .006, 95% CI: 0.529–0.662), 0.575 (P = .03, 95% CI: 0.510–0.641), and 0.670 (P < .001, 95% CI: 0.607–0.734), respectively, which indicated the predictive power of the multivariate logistic regression model (Figure 3).
Figure 3.
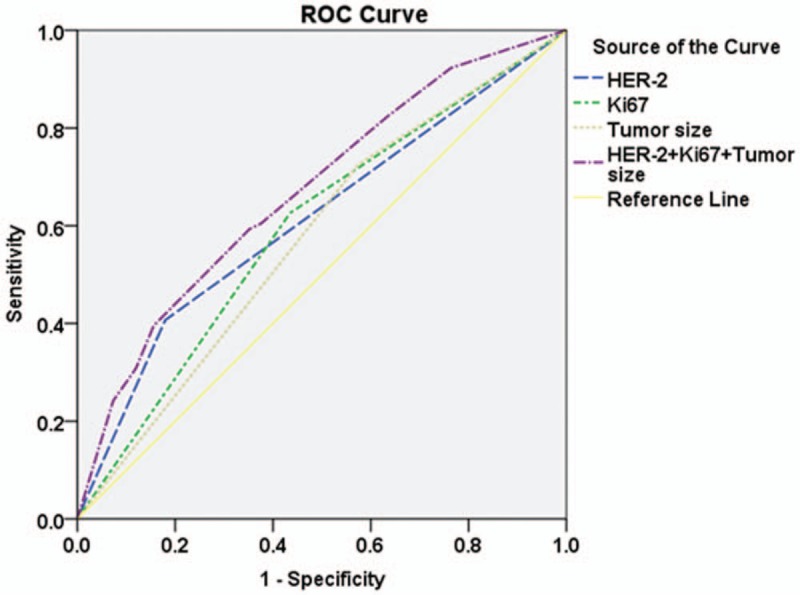
ROC corresponding to the multiple logistic model applied to the data of 392 patients. The areas under the ROC of human epidermal growth factor receptor-2 (HER-2), Ki67, tumor size, combination of HER-2, Ki67, and tumor size are 0.614 [P = .001, 95% confidence interval (CI): 0.544–0.683], 0.596 (P = .006, 95% CI: 0.529–0.662), 0.575 (P = .03, 95% CI: 0.510–0.641), and 0.670 (P < .001, 95% CI: 0.607–0.734), respectively. HER-2 = human epidermal growth factor receptor-2, ROC = receiver operating curve.
4. Discussion
Breast cancer is a highly heterogeneous malignant tumor, so the relations between the clinicopathological factors and LVI are varied. We found that HER-2, Ki67, and tumor size were statistically significantly associated with LVI, whereas the age of diagnosis, histological grade, and statuses of ER, PR, EGFR, VEGF, E-cadherin, and P53 were not related to LVI. However, Elkablawy et al[9] reported that Ki67 expression was not associated with LVI. Two possible factors existed: first, the race and region of the patients were not the same; second, the criterion of Ki67 high expression was different. The cut-off value that our study adopted was ≥14% according to the St. Gallen International Expert Consensus,[10] whereas the cut-off value that Elkablawy et al[9] adopted was ≥25%.
HER-2 is a cell surface receptor of the epidermal growth factor family and plays a role in the regulation of cell proliferation and differentiation. HER-2 positive expression can promote tumor growth and is an indication of target therapy in invasive breast cancer. This study found that the positive rate of LVI was significantly higher in patients with HER-2 positive expression than in patients with HER-2 negative expression (P < .001). A similar result was found by Ugras et al.[11] In the multivariate analysis, HER-2 was also a risk factor for LVI, and the area under the ROC was 0.614 (P = .001, 95% CI: 0.544–0.683).
Ki67 is a nuclear protein that is associated with cellular proliferation. Ki67 high expression predicts quick tumor cell proliferation, resulting in a poor prognosis in breast cancer.[12,13] Furthermore, Ki67 was recommended as an index for differentiating luminal A from luminal B subtype in the St. Gallen conference.[10] Hence, Ki67 is now routinely examined by IHC. In our analysis, we found that the positivity of LVI was significantly higher in patients with Ki67 high expression than in patients with Ki67 low expression (P = .001). Similar results were found by Erdogan et al[14] and Yan et al.[15] In multivariate analysis, Ki67 was also a risk factor of LVI, and the area under the ROC was 0.596 (P = .006, 95% CI: 0.529–0.662).
Tumor size is a powerful predictor of local recurrence and systemic spread, and an important component of the tumor stage in invasive breast cancer.[16] It implies that patients with tumor size ≤2 cm have a better prognosis than those with tumors size >2 cm with equal factors. In our analysis, we found that the positive rate of LVI was significantly higher in patients with a tumor size >2 cm than in patients with a tumor size ≤2 cm (P = .01). The result was consistent with that of Gujam et al.[6] In multivariate analysis, the tumor size was a risk factor for LVI (OR, 1.766, 95% CI: 1.041–2.996, P = .035), and the area under the ROC was 0.575 (P = .03, 95% CI: 0.510–0.641).
Either Ki67 high expression or HER-2 positive expression can promote fast tumor growth. The larger tumor size may be due to fast tumor growth. The results implied that LVI was mainly attributed to rapid growth, not tumors encircling lymphovasculogenesis. Therefore, LVI was not correlated to ER, PR, EGFR, VEGF, E-cadherin, and P53.
The area under the ROC of the combination of HER-2, Ki67, and tumor size was 0.670 (P < .001, 95% CI: 0.607–0.734). The tumor with high expression of HER-2, Ki67, and size >2 cm should be more cautiously diagnosed as negative LVI because the probability was only approximately 37%. It may be necessary to check more fields under the microscope or sample further to confirm the result. The area was larger than that of each factor alone, but it was still <0.7. Hence, the accuracy of predicting LVI was low through evaluating tumor size and the expression statuses of HER-2 and Ki67, and the power of the LVI prediction model based on the 3 clinicopathological factors was low in invasive breast cancer. There are 2 main probable reasons that contributed to this: first, the heterogeneity of breast cancer is prominent, and it is very difficult to generalize it using the 3 clinicopathological factors; second, the proportion of tissue samples from core needle biopsy is 30.6%, which is too high. The representativeness of core needle biopsy is limited due to the small tissue volume. If the current result was attributed to the latter reason, all tissue samples should be confined to the whole resected tumor, and the tumor tissue should be sliced completely. In addition, there may be other factors involved in LVI. Therefore, further study should include many more clinicopathological factors.
Acknowledgments
The authors acknowledge the support of the pathology technologist (Caiyun Tan) who was involved in collection and Weiren Ding who revised the manuscript.
Author contributions
Conceptualization: Haibo Zhou.
Data curation: Sandi Shen, Guihua Wu, Gaofang Xiao, Richang Du.
Formal analysis: Sandi Shen, Guihua Wu.
Funding acquisition: Haibo Zhou.
Methodology: Sandi Shen, Guihua Wu, Gaofang Xiao, Haibo Zhou.
Project administration: Haibo Zhou.
Resources: Richang Du.
Software: Guihua Wu, Gaofang Xiao.
Supervision: Ningdong Hu, Xu Xia, Haibo Zhou.
Visualization: Gaofang Xiao.
Writing – original draft: Sandi Shen.
Writing – review and editing: Richang Du, Ningdong Hu, Xu Xia, Haibo Zhou.
Footnotes
Abbreviations: CI = confidence interval, EGFR = epidermal growth factor receptor, ER = estrogen receptor, H&E = hematoxylin and eosin, HER-2 = human epidermal growth factor receptor-2, IHC = immunohistochemistry, ISH = in situ hybridization, LVI = lymphovascular invasion, OR = odds ratio, PR = progesterone receptor, ROC = receiver operating curve, TMA = tissue microarray, VEGF = vascular endothelial growth factor.
This research received grants from the Medical Research and Innovation Fund of Qingyuan People's Hospital.
The authors of this work have nothing to disclose.
References
- [1].Rakha EA, Martin S, Lee AH, et al. The prognostic significance of lymphovascular invasion in invasive breast carcinoma. Cancer 2012;118:3670–80. [DOI] [PubMed] [Google Scholar]
- [2].Liu YL, Saraf A, Lee SM, et al. Lymphovascular invasion is an independent predictor of survival in breast cancer after neoadjuvant chemotherapy. Breast Cancer Res Treat 2016;157:555–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Tezuka K, Onoda N, Takashima T, et al. Prognostic significance of lymphovascular invasion diagnosed by lymphatic endothelium immunostaining in breast cancer patients. Oncol Rep 2007;17:997–1003. [PubMed] [Google Scholar]
- [4].Chung MJ, Lee JH, Kim SH, et al. Simple prediction model of axillary lymph node positivity after analyzing molecular and clinical factors in early breast cancer. Medicine (Baltimore) 2016;95:e3689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Wishart GC, Rakha E, Green A, et al. Inclusion of KI67 significantly improves performance of the PREDICT prognostication and prediction model for early breast cancer. BMC Cancer 2014;14:908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Gujam FJ, Going JJ, Mohammed ZM, et al. Immunohistochemical detection improves the prognostic value of lymphatic and blood vessel invasion in primary ductal breast cancer. BMC Cancer 2014;14:676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Lee JA, Bae JW, Woo SU, et al. D2-40, podoplanin, and CD31 as a prognostic predictor in invasive ductal carcinomas of the breast. J Breast Cancer 2011;14:104–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Shen SD, Zhong SZ, Wang CZ, et al. Correlation of lymphovascular invasion with clinicopathological factors in invasive breast cancer: a meta-analysis. Int J Clin Exp Med 2015;8:17789–95. [PMC free article] [PubMed] [Google Scholar]
- [9].Elkablawy MA, Albasri AM, Mohammed RA, et al. Ki67 expression in breast cancer. Correlation with prognostic markers and clinicopathological parameters in Saudi patients. Saudi Med J 2016;37:137–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Goldhirsch A, Winer EP, Coates AS, et al. Personalizing the treatment of women with early breast cancer: highlights of the St Gallen International Expert Consensus on the Primary Therapy of Early Breast Cancer 2013. Ann Oncol 2013;24:2206–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Ugras S, Stempel M, Patil S, et al. Estrogen receptor, progesterone receptor, and HER2 status predict lymphovascular invasion and lymph node involvement. Ann Surg Oncol 2014;21:3780–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Kirkpatrick CS, Lee JA, White E. Melanoma risk by age and socio-economic status. Int J Cancer 1990;46:1–4. [DOI] [PubMed] [Google Scholar]
- [13].Yerushalmi R, Woods R, Ravdin PM, et al. Ki67 in breast cancer: prognostic and predictive potential. Lancet Oncol 2010;11:174–83. [DOI] [PubMed] [Google Scholar]
- [14].Erdogan B, Turkmen E, Yalta TD, et al. Importance of Ki-67 in human epidermal growth factor receptor 2 positive breast cancer. J BUON 2015;20:730–6. [PubMed] [Google Scholar]
- [15].Yan J, Liu XL, Han LZ, et al. Relation between Ki-67, ER, PR, Her2/neu, p21, EGFR, and TOP II-alpha expression in invasive ductal breast cancer patients and correlations with prognosis. Asian Pac J Cancer Prev 2015;16:823–9. [DOI] [PubMed] [Google Scholar]
- [16].Giuliano AE, Edge SB, Hortobagyi GN. Eighth edition of the AJCC cancer staging manual: breast cancer. Ann Surg Oncol 2018;25:1783–5. [DOI] [PubMed] [Google Scholar]


