Abstract
Purpose:
The prognostic value of tissue and serum osteopontin (OPN) in hepatocellular carcinoma (HCC) remain controversial. The aim of present meta-analysis was to evaluate the prognostic value of OPN in patients with HCC.
Methods:
Eligible studies were systematically searched by PubMed, EMBASE, and Google scholar. A meta-analysis of 12 studies included 2117 cases was performed to estimate the association between OPN level and overall survival (OS), disease-free survival (DFS) in HCC patients. Subgroup analyses were also performed in the meta-analysis.
Results:
The pooled data of studies showed that high OPN level was significantly associated with poor OS (hazard ratios [HR] 1.84; 95% confidence intervals [CI] 1.54–2.20; P = .000) and DFS (HR 1.67; 95% CI 1.40–1.98; P = .000) in HCC. Furthermore, in subgroup analysis, high tissue based OPN by immunohistochemistry detection and serum-based OPN by enzyme-linked immunosorbent assay (ELISA) detection were both significantly associated with OS (tissue: HR 1.88; 95% CI 1.53–2.31; P < .0001; serum: HR 2.38; 95% CI 1.58–3.59; P < .0001). Simultaneously, we also found that OPN expression was positively associated with stage (odds ratios [OR] 5.68; 95% CI 3.443–7.758), tumor size (Size≤5 cm vs >5 cm; OR 2.001; 95% CI1.036–3.867).
Conclusion:
The current evidence indicates that OPN could serve as a prognostic biomarker and a potential therapeutic target for HCC.
Keywords: hepatocellular carcinoma, meta-analysis, osteopontin, prognosis
1. Introduction
Hepatocellular carcinoma (HCC) is 1 of the most aggressive malignant neoplasms worldwide and the third causes of cancer-related mortality.[1] Though advances in diagnostic techniques and treatments could improve the prognosis of HCC patients, the patients prognosis remains very poor owing to high rate of recurrence.[2,3] So it is urgent to identify novel biological markers that could predict the prognosis and metastatic recurrence of patients with HCC.
Osteopontin (OPN) is a multifunctional phosphoprotein, which could be expressed in different cell types, Including T lymphocytes, macrophages and osteoclasts.[4–6] Recent studies have demonstrated that OPN plays a crucial role in tumorigenesis and metastasis.[7,8] Earlier studies have shown that increased OPN levels are linked to poor prognosis of HCC,[9,10] and promote HCC metastasis.[11–13] Moreover, OPN overexpression has been detected in different solid tumors, including lung cancer,[14] breast cancer,[15,16] gastric cancer,[17] HCC,[18] colorectal cancer[19] and so on. It is suggested that OPN overexpression can predict the prognosis of carcinomas. However, the relationship between OPN expression and prognostic outcomes in HCC patients were controversial and inconclusive. Therefore, we conducted this meta-analysis to systematically and comprehensively evaluate the prognostic value of OPN in HCC.
In this study, we systematically reviewed studies to explore the prognostic significance of OPN for overall survival (OS) and disease-free survival (DFS) in HCC patients. And the correlation between OPN expression and clinicopathological features was also discussed.
2. Material and methods
2.1. Identification and selection of studies
Studies were identified by searching PubMed, EMBASE and Google Scholar databases covering all papers published update to October 31, 2017. The search strategy was used the following terms: (OPN or SPP1 [MESH] or OPN or sialoprotein 1 [TEXT WORD]) AND (carcinoma, hepatocellular [MESH] or HCC [TEXT WORD]). Studies in English were eligible for inclusion. Additionally, the appropriate references of the identified studies were also checked to identify additional suitable articles. Once studies were found with overlapping data published by the same research center, only the paper with most complete data was included. This project was approved by the Institutional Review Board of Shengli Oilfield Central Hospital.
2.2. Inclusion and exclusion criteria
Eligible studies in this meta-analysis should meet the following criteria:
-
(1)
evaluated the relationship between OPN expression and survival outcomes of HCC;
-
(2)
reported explicit methods for the measurement of OPN expression in the primary HCC;
-
(3)
Examined the association of OPN expression in HCC patients with survival, recurrences, and a series of clinicopathological parameters;
-
(4)
Provided the hazard ratios (HR) or odds ratios (OR) with the 95% confidence intervals (CI) or sufficient data to calculate them;
-
(5)
have a maximum follow-up time exceeding 3 years;
-
(6)
editorial letters, comments or conference abstracts, reviews, and case reports were excluded.
2.3. Qualitative assessment
The Newcastle–Ottawa Scale (NOS) was used to assess the quality of included studies.[20] The score was based on subject selection, comparability of subject, clinical outcome in the NOS. NOS score of 0 to 9 was used to indicate the quality of studies, and a score ≥ 6 denoted a high quality.
2.4. Data extraction
The studies information of this meta-analysis were retrieved by the reporting checklists of Preferred Reporting Items for Systematic Reviews and Meta-analysis (PRISMA) guidelines.[21] Data extracted from the studies included the following items:
-
(1)
publication information: first author's last name, year of publication;
-
(2)
patients’ characteristic information: study population and regions, patients’ number, follow-up time;
-
(3)
OPN information: method, cut-off level, and relationship between OPN and OS, DFS or clinicopathological features; and
-
(4)
HR, 95% CI for survival analysis. If the relevant data were not reported (NR), items were treated as “NR (not reported)”. If without the HR and 95% CI in articles, Kaplan–Meier curve was available, data were extracted from graphical survival plot and HR was estimated.[22]
2.5. Statistical analysis
Included studies were divided into 3 groups for analysis: those with data regarding OS, DFS, and clinicopathological parameters. OR and 95% CI were used to measure the association of OPN expression with clinical parameters, while the HR and 95% CI were used for the meta-analysis of survival rates. An OR > 1 indicated higher probability for later stage, lager tumor size or the presence of vascular invasion in the group with elevated OPN expression. While a combined HR > 1 implies a poor outcome for OPN overexpression in HCC patients. Heterogeneity of pooled results was conducted using I-squared statistic. If the P < .10 or I2 > 50%, we considered heterogeneity as significant heterogeneity, and then a random effect model was used. Otherwise, a fixed-effects model was used. subgroup analysis was evaluated the differences between HRs in included studies with different detection methods, treatments. Sensitivity analysis was performed to assess the reliability and validity of the meta-analysis. Both Begg funnel plot and Egger test were used to examine the publication bias. And we conducted a trim and fill analysis,[23] which yields an effect adjusted for funnel plot asymmetry. Meta-analysis was conducted with STATA version 12.0 (STATA Corporation, College Station, TX). All P values were 2 sides and P < .05 was considered statistically significant.
3. Results
3.1. Studies selection and characteristics of studies
A total of 462 articles were initially identified via the search criteria delineated above. After screening of the title and abstract,29 articles were selected for detailed evaluation. Subsequently, 7 studies were excluded after full assessment due to lacking relevant survival data. Another 22 studies were careful identified.[9,10,13,24–42] Of the 22 studies, 11 articles[9,10,13,27,29,31,36–40] were reported by the same research group, and the patients were partly overlapping in this studies. To avoid duplicate counting, only 3 studies[10,27,31] with complete data were included. Another 3 studies[24,41,42] were also from the same study center, however, only the most complete 1 was selected.[24] Finally, 12 studies[10,24–28,30–35] were included in our meta-analysis (Fig. 1).
Figure 1.
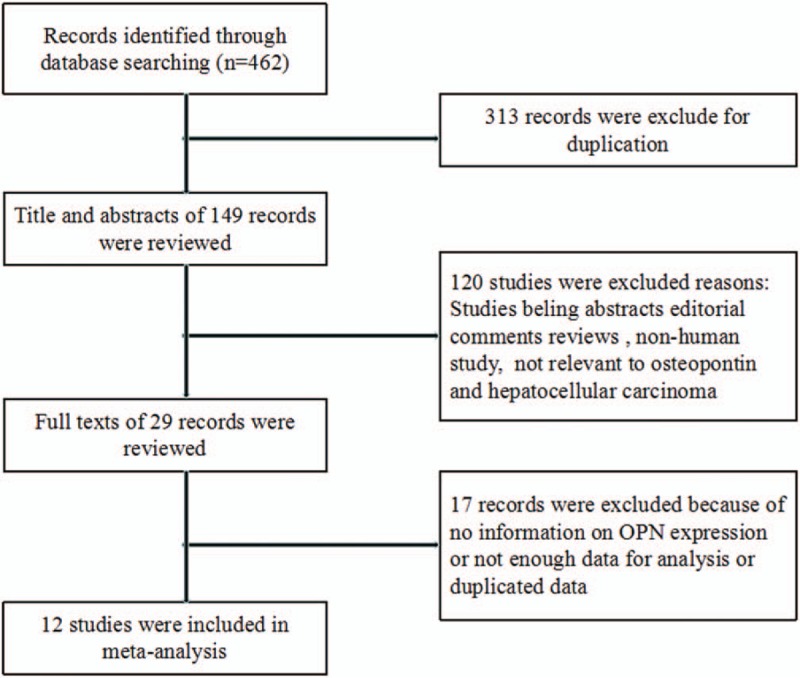
Flow chart of the selection of the studies in the meta-analysis.
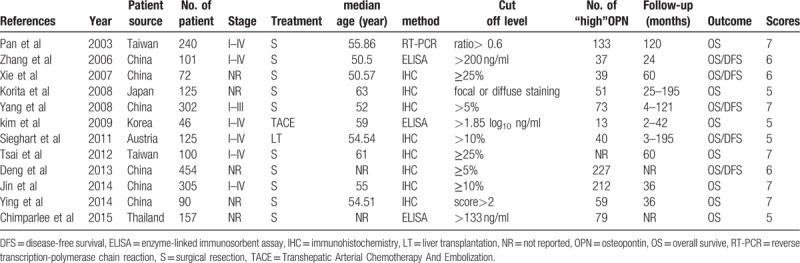
The basic characteristics of the 12 studies are summarized in Table 1. A total of 2117 patients from China, Taiwan, Korea, Austria, Thailand and Japan were enrolled with sample number ranging from 46 to 454. Regarding OPN detection in HCC, immunohistochemistry (IHC) was used in 8 studies,[25–27,30–34] another 3 studies[10,28,35] used enzyme-linked immunosorbent assay (ELISA) to measure serum-based OPN expression. For HCC treatment, surgical resection as initial treatment was reported in 10 studies,[10,24–27,31–35] and liver transplantation (LT) and transhepatic arterial chem otherapy (TACE) reported in 1 study respectively.[30,28] OS was presented in all studies, however, DFS was only reported in 5 studies.[10,25,27,30–31] Meanwhile, All studies had the follow-up times exceeding 3 years, with 7 studies more than 5 years. The NOS scores ranged from 5 to 8 with a mean of 6.1.
Table 1.
Characteristics of studies for association between OPN and HCC.
3.2. OPN expression and clinicopathological parameters
To investigate the association between OPN expression and clinicopathological features, we conducted the meta-analysis. Five studies reported the association of tumor size >5 cm with OPN expression in HCC. Pooled data from all 4 studies showed high OPN expression was significantly associated with tumor size >5 cm (OR 2.001; 95% CI 1.036–3.867; P = .039). Two studies reported the association of the TNM stage with OPN expression. The combined data showed that increased OPN expression was significantly correlated to TNM stage (OR 5.680; 95% CI 3.443–7.758; P = .000). On the contrary, OPN overexpression was not found to be associated with age (OR 1.120; 95% CI 0.867–1.446; P = .386), Gender (OR 0.928, 95% CI 0.700–1.230; P = .604), HBsAg (OR 1.106; 95% CI 0.434–2.820; P = .833), Child-Pugh grade (OR 2.793; 95% CI 0.869–8.978; P = .085), AFP (OR 1.766; 95% CI 0.987–3.160; P = .055), Liver Cirrhosis (OR 0.941; 95% CI 0.9410.653–1.355; P = .742), Vascular invasion (OR 2.56; 95% CI 0.92–7.12; P = .073), or tumor grade (OR 1.196; 95% CI 0.611–2.336; P = .601). These results suggested that HCC with overexpressed OPN exhibited aggressive biological behaviors (Table 2).
Table 2.
Meta-analysis for the association of increased OPN expression and clinicopathological features of HCC.

3.3. OPN expression and OS in HCC
Overall, all 12 studies, including 2117 patients, had a relationship between OPN expression and OS in HCC; and 10 presented reported data on OS and OPN expression in HCC initially treated by surgical resection. Heterogeneity among studies was statistically significant (P = .061, I2 = 43.3%), so a random-effects model was used. Pooled data suggested that high OPN expression was significantly correlated with reduce OS (HR 1.84; 95% CI 1.52–2.22; P = .000) (Table 3, Fig. 2A). Sensitivity analysis was performed and the overall trend was not changed by removing the studies 1 by 1 in Figure 2B. We also performed subgroup analysis for method and treatment. subgroup analysis suggested that the detection method, either ELISA or IHC, of OPN expression had significantly influence for OS (ELISA: HR 2.38; 95% CI 1.58–3.59; P = .000; IHC: HR 1.88; 95% CI 1.53–2.31; P = .000), without significant heterogeneity in the data. For treatment, our results indicated that the overall HR estimate of surgical resection group for OS was 1.90 (95% CI 1.54–2.33; P = .000) (Table 3).
Table 3.
Main meta-analysis results.
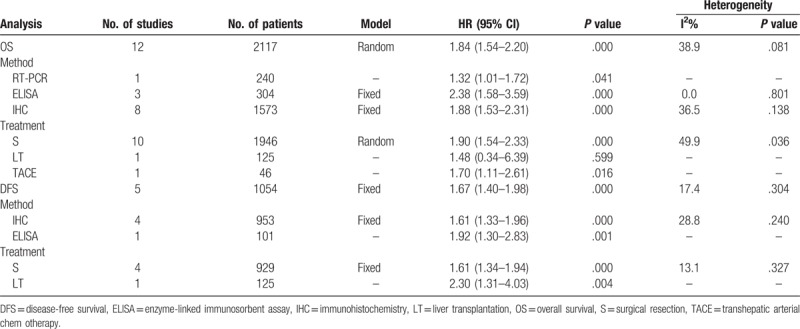
Figure 2.
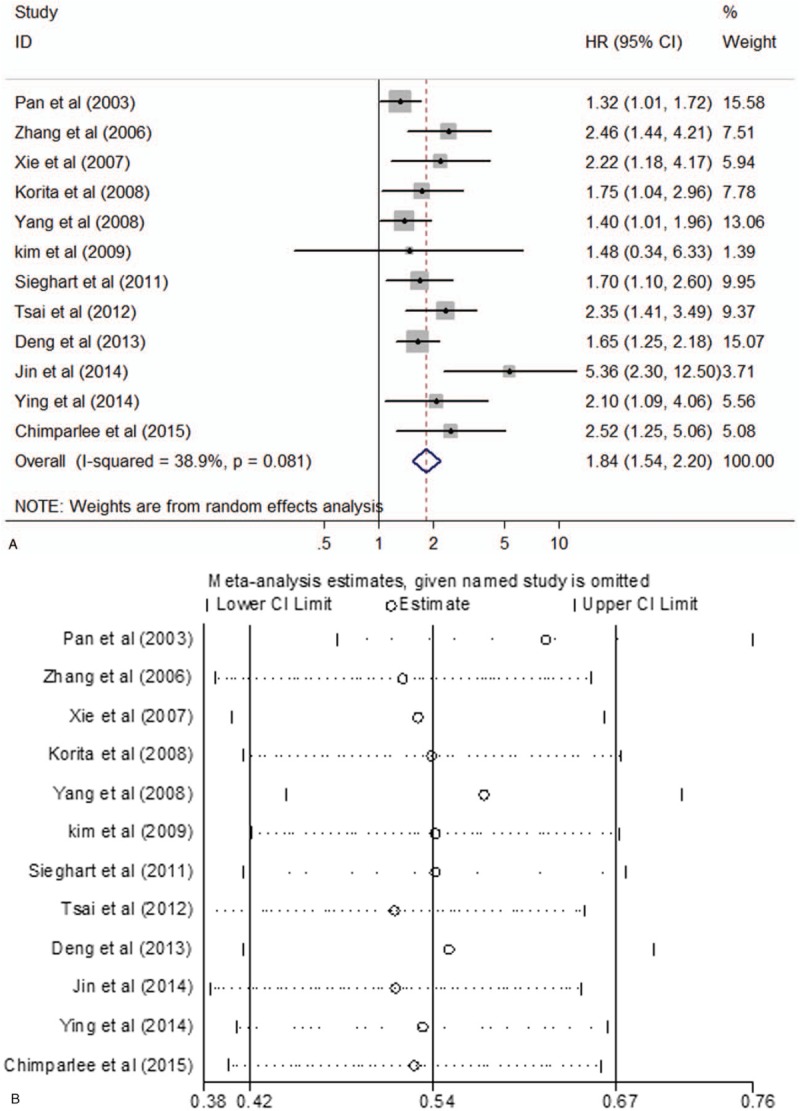
(A) Forest plot for the relationships between OPN expression and OS. (B) Sensitivity analysis for meta-analysis of OPN. OPN = osteopontin, OS = overall survival.
3.4. OPN expression and DFS in HCC
Five studies comprising a total 1054 patients reported results on OPN expression and DFS in HCC. There was no significant heterogeneity (P = .304, I2 = 17.4%) among them, so a fixed-effect model was used. Pooled data from these studies suggested that increased OPN expression was significantly correlated with poor DFS (HR 1.67; 95% CI 1.40–1.98; P = .000), indicating that high OPN expression was an indicator of disease recurrence in HCC patients (Table 3 and Fig. 3). Meanwhile, subgroup analysis by method and treatment indicated a significant association in IHC (HR 1.61; 95% CI 1.33–1.96; P = .000), surgical resection (HR 1.61; 95% CI 1.34–1.94; P = .000) with DFS, without significant heterogeneity in the data (Table 3).
Figure 3.
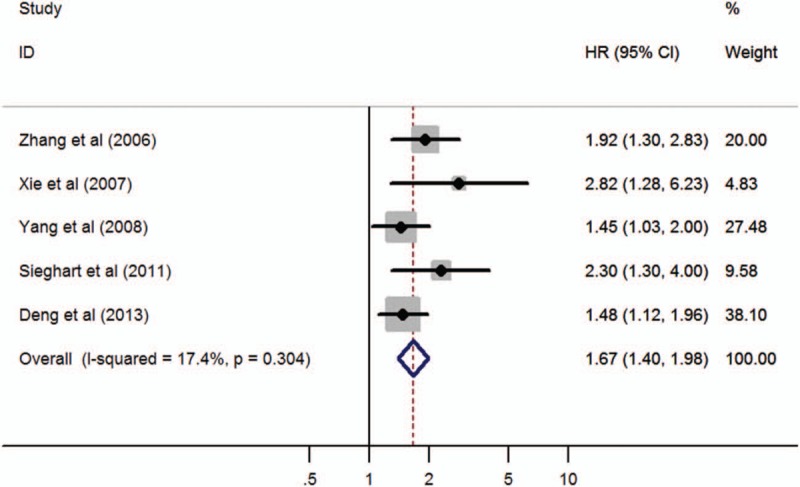
Forest plot of hazard ratio for the association of OPN overexpression and DFS. DFS = disease-free survival, OPN = osteopontin.
3.5. Publication bias
Publication bias was assessed by using Begg funnel plot and Egger test (Fig. 4). The results suggested evidence for publication bias in OS studies (Egger, P = .012). There was some evidence for publication bias. Meanwhie, we drew a funnel plot and conducted a trim and fill analysis (Fig. 4A). There was some evidence of asymmetry (5 studies trimmed) but the overall effect was strongly influenced by 5 studies, and the overall HR estimate showed that there was no effect of replacing missing studies (adjusted value HR 1.554; 95% CI 1.227–1.892). There was no significantly different (P = .090). While it did not reveal significant publication bias in DFS studies (Eegger, P = .630).
Figure 4.
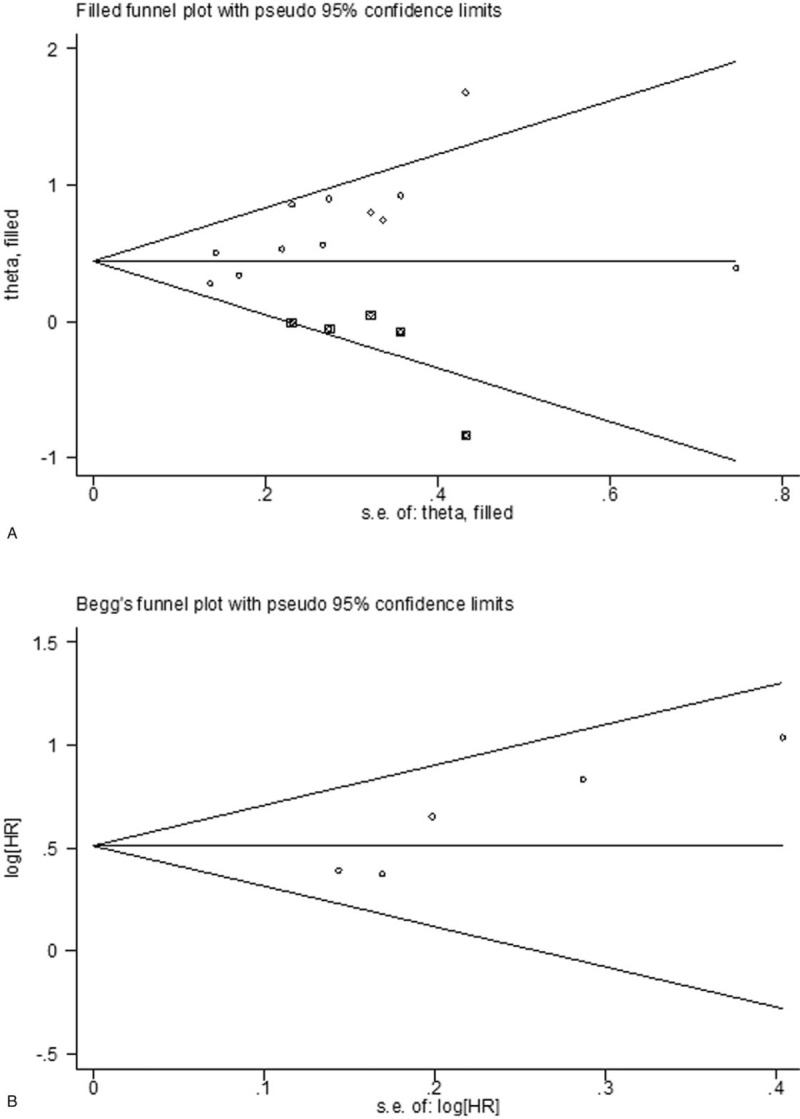
Funnel plots of publication bias in this meta-analysis. (A) OS by trim and fill analysis; (B) DFS by Begg funnel plot. DFS = disease-free survival, OS = overall survival.
4. Discussion
The patients with HCC usually have poor prognosis due to late diagnosis and high rate of recurrence. Therefore, it is of great significance to identify the clinical useful markers for predicting prognosis of HCC. In recent years, some studies have shown the potential clinical value of OPN to serve as a biomarker for prognosis and a potential therapeutic target in patients with HCC.[43,44] And the OPN overexpression could predict late-stage, high-grade, and early-recurrence of HCC.[24] So, a meta-analysis is needed to systematically evaluate the prognostic value of OPN in hepatocellular carcinoma.
The first meta-analysis investigating the relationship between OPN and hepatocellular carcinoma prognosis was reported by Zhang et al[45] Their results included 7 studies suggested that high OPN expression significantly predicted poor OS and DFS for HCC. Later, Cheng et al performed a meta-analysis on merely 4 studies and the evidence indicates that plasma OPN can act as an independent prognostic indicator for HCC patients.[46] In recent years, a number of studies have been carried out to analyze the protein expression profiles and prognostic significance of OPN in HCC, but the prognosis utilities of OPN are still controversial. Therefore, we performed a meta-analysis added 5 eligible studies than Zhang et al[45] in order to provide a quantitative reassessment. And we first performed the subgroup analysis to assess whether the pooled estimate of OS and DFS was different according to the different therapy methods and treatments. So, this meta-analysis may provide reliable and powerful statistics and draw a more conclusive result.
A total of 12 studies with 2117 HCC patients were subjected to quantitative analysis. First of all, we observed that several clinical features were significantly associated with OPN expression. The results of pooled data suggested that high OPN expression significantly correlated with tumor size and TNM stage. In addition, we also analyzed the association of OPN expression with the Child-Pugh stage, AFP, and vascular invasion. An evident trend toward higher OPN expression with the higher Child-Pugh stage and AFP was identified. Taken together, the pooled data of this meta-analysis supported the suggestion that high OPN expression enhanced invasion and metastasis potential of HCC. Meanwhile, the association of OPN with survival of HCC patients was evaluated. The pooled data showed that high OPN expression predicted shorter OS and DFS. These results suggested that OPN is a novel prognostic marker and an indicator of invasiveness in HCC patients. Finally, the subgroup analysis was stratified by 3 different detection methods. And the subgroup analysis by surgical resection showed that OPN overexpression significantly predicted poorer OS and DFS (P = .000, both). Therefore, the pooled results in our study demonstrate that OPN might promote HCC invasion and metastasis, and can serve as a useful biomarker for HCC prognosis.
Some limitations of this meta-analysis should be discussed. First of all, in our meta-analysis, we found significantly heterogeneity among studies for OS (P = .081, I2 = 38.9%), so we used a random-effects model. We then performed a subgroup analysis on methods by RT-PCR, ELISA, IHC, which may influence the heterogeneity. Furthermore, the cutoff value and methods for levels of OPN expression varied in different studies. Different cutoff of OPN expression may impact on the accurate estimation of outcome for HCC, even may cause diametrically opposite results. These reasons may cause the heterogeneity of results. However, the statistical results by subgroup analysis and Sensitivity analysis seemed to be robust and convincing. Therefore, researchers should carry out large multicenter study to develop a definitive cut-off value and establish a method to classify OPN expression. Second, potential risk bias and language were a concern. Because studies with positive results were more likely to be published than negative ones. And we searched literatures written in English. However, the lack of these analyses may affect the reliability of results to some extent, and then reflected our publication bias evaluation. Moreover, the total sample size and the total number of included reports were relatively small. These also might partly influence the validity of our analysis. Third, when we evaluated the correlation between OPN expression and vascular invasion, tumor grade, tumor size, the heterogeneity was significant. However, we could not analysis the source of heterogeneity owing to the limited of studies. Finally, geographical bias was a concern; all patients of the included studies were from Asia, which the dominant risk factor of hepatocellular carcinom is hepatitis B virus infections. whereas hepatitis C virus-related or alcohol-related hepatocellular carcinom is the predominant tumor type in Western countries. The OPN expression and its function is still unclear in Western hepatocellular carcinom patients, because there is no data available till now.
In summary, current available evidence demonstrated the prognostic value of OPN in HCC patients. Our results indicated that OPN can serve as a novel prognostic marker in HCC patients. Due to the limitations of this study, larger and more well-designed studies are still required to clarify the prognostic value of OPN expression in HCC patients.
Author contributions
Conceptualization: Diwen Sun.
Data curation: Tingting Sun.
Formal analysis: Diwen Sun, Guoqiang Li.
Methodology: Peng Li, Qingao Bu.
Project administration: Peng Li.
Software: Guoqiang Li.
Writing – original draft: Tingting Sun.
Writing – review & editing: Peng Li.
Footnotes
Abbreviations: CI = confidence intervals, DFS = disease-free survival, ELISA = enzyme-linked immunosorbent assay, HCC = hepatocellular carcinoma, HR = hazard ratios, IHC = immunohistochemistry, NOS = Newcastle–Ottawa Scale, NR = not reported, OPN = osteopontin, OR = odds ratios, OS = overall survival.
The authors report no conflicts of interest.
References
- [1].Venook AP, Papandreou C, Furuse J, et al. The incidence and epidemiology of hepatocellular carcinoma: a global and regional perspective. Oncologist 2010;15:5–13. [DOI] [PubMed] [Google Scholar]
- [2].Ferlay J, Shin HR, Bray F, et al. Estimates of worldwide burden of cancer in 2008: GLOBOCAN 2008. Int J Cancer 2010;127:2893–917. [DOI] [PubMed] [Google Scholar]
- [3].Tang ZY, Ye SL, Liu YK, et al. A decade's studies on metastasis of hepatocellular carcinoma. J Cancer Res Clin Oncol 2004;130:187–96. [DOI] [PubMed] [Google Scholar]
- [4].Bandopadhyay M, Bulbule A, Butti R, et al. Osteopontin as a therapeutic target for cancer. Expert Opin Ther Targets 2014;18:883–95. [DOI] [PubMed] [Google Scholar]
- [5].Ashkar S, Weber GF, Panoutsakopoulou V, et al. Eta-1 (osteopontin): an early component of type-1 (cell-mediated) immunity. Science 2000;287:860–4. [DOI] [PubMed] [Google Scholar]
- [6].Shin T. Osteopontin as a two-sided mediator in acute neuroinflammation in rat models. Acta Histochem 2012;114:749–54. [DOI] [PubMed] [Google Scholar]
- [7].Hahne JC, Meyer SR, Kranke P, et al. Studies on the role of osteopontin-1 in endometrial cancer cell lines. Strahlenther Onkol 2013;189:1040–8. [DOI] [PubMed] [Google Scholar]
- [8].Ramachandran S, Kwon KY, Shin SJ, et al. Regulatory role of osteopontin in malignant transformation of endometrial cancer. Mol Biol Rep 2013;40:3623–9. [DOI] [PubMed] [Google Scholar]
- [9].Sun J, Xu HM, Zhou HJ, et al. The prognostic significance of preoperative plasma levels of osteopontin in patients with TNM stage-I of hepatocellular carcinoma. J Cancer Res Clin Oncol 2010;136:1–7. [DOI] [PubMed] [Google Scholar]
- [10].Zhang H, Ye QH, Ren N, et al. The prognostic significance of preoperative plasma levels of osteopontin in patients with hepatocellular carcinoma. J Cancer Res Clin Oncol 2006;132:709–17. [DOI] [PubMed] [Google Scholar]
- [11].Ye QH, Qin LX, Forgues M, et al. Predicting hepatitis B virus-positive metastatic hepatocellular carcinomas using gene expression profiling and supervised machine learning. Nat Med 2003;9:416–23. [DOI] [PubMed] [Google Scholar]
- [12].Sun BS, Dong QZ, Ye QH, et al. Lentiviral-mediated miRNA against osteopontin suppresses tumor growth and metastasis of human hepatocellular carcinoma. Hepatology 2008;48:1834–42. [DOI] [PubMed] [Google Scholar]
- [13].Xue YH, Zhang XF, Dong QZ, et al. Thrombin is a therapeutic target for metastatic osteopontin-positive hepatocellular carcinoma. Hepatology 2010;52:2012–22. [DOI] [PubMed] [Google Scholar]
- [14].Yu TT, Han ZG, Shan L, et al. Expression of osteopontin in non-small cell lung cancer and correlative relation with microvascular density. Asian Pac J Cancer Prev 2014;15:29–32. [DOI] [PubMed] [Google Scholar]
- [15].Li NY, Weber CE, Mi Z, et al. Osteopontin up-regulates critical epithelial-mesenchymal transition transcription factors to induce an aggressive breast cancer phenotype. J Am Coll Surg 2013;217:17–26. [DOI] [PubMed] [Google Scholar]
- [16].Bramwell VH, Tuck AB, Chapman JA, et al. Assessment of osteopontin in early breast cancer: correlative study in a randomised clinical trial. Breast Cancer Res 2014;16:R8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Kim JY, Bae BN, Kim KS, et al. Osteopontin, CD44, and NFkappaB expression in gastric adenocarcinoma. Cancer Res Treat 2009;41:29–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Shang S, Plymoth A, Ge S, et al. Identification of osteopontin as a novel marker for early hepatocellular carcinoma. Hepatology 2012;55:483–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Huang J, Pan C, Hu H, et al. Osteopontin-enhanced hepatic metastasis of colorectal cancer cells. PloS One 2012;7:e47901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Stang A. Critical evaluation of the Newcastle–Ottawa scale for the assessment of the quality of nonrandomized studies in meta-analyses. Eur J Epidemiol 2010;25:603–5. [DOI] [PubMed] [Google Scholar]
- [21].Liberati A, Altman DG, Tetzlaff J, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate healthcare interventions: explanation and elaboration. BMJ 2009;339:b2700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Tierney JF, Stewart LA, Ghersi D, et al. Practical methods for incorporating summary time-to-event data into meta-analysis. Trials 2007;8:16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Duval SJ, Tweedie RL. Trim and fill: a simple funnel-plot based method of accounting for publication bias in meta-analysis. Biometrics 2000;56:455–63. [DOI] [PubMed] [Google Scholar]
- [24].Pan HW, Ou YH, Peng SY, et al. Overexpression of osteopontin is associated with intrahepatic metastasis, early recurrence, and poorer prognosis of surgically resected hepatocellular carcinoma. Cancer 2003;98:119–27. [DOI] [PubMed] [Google Scholar]
- [25].Xie H, Song J, Du R, et al. Prognostic significance of osteopontin in hepatitis B virus-related hepatocellular carcinoma. Dig Liver Dis 2007;39:167–72. [DOI] [PubMed] [Google Scholar]
- [26].Korita PV, Wakai T, Shirai Y, et al. Overexpression of osteopontin independently correlates with vascular invasion and poor prognosis in patients with hepatocellular carcinoma. Hum Pathol 2008;39:1777–83. [DOI] [PubMed] [Google Scholar]
- [27].Yang GH, Fan J, Xu Y, et al. Osteopontin combined with CD44, a novel prognostic biomarker for patients with hepatocellular carcinima undergoing curative resection. Oncologist 2008;13:1155–65. [DOI] [PubMed] [Google Scholar]
- [28].Kim SH, Chung YH, Yang SH, et al. Prognostic value of serum osteopontin in hepatocellular carcinoma patients treated with transarterial chemoembolization. Korean J Hepatol 2009;15:320–30. [DOI] [PubMed] [Google Scholar]
- [29].Huang H, Zhang XF, Zhou HJ, et al. Expression and prognostic significance of osteopontin and caspase-3 in hepatocellular carcinoma patients after curative resection. Cancer Sci 2010;101:1314–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Sieghart W, Wang X, Schmid K, et al. Osteopontin expression predicts overall survival after liver transplantation for hepatocellular carcinoma in patients beyond the Milan criteria. J Hepatol 2011;54:89–97. [DOI] [PubMed] [Google Scholar]
- [31].Tsai WC, Tsai WC, Lee HS, et al. Association between Osteopontin and EGFR expression with clinicopathological parameters in hepatocellular carcinoma. Chin J Physiol 2012;55:412–20. [DOI] [PubMed] [Google Scholar]
- [32].Deng B, Zhang XF, Zhu XC, et al. Correlation and prognostic value of osteopontin and Bcl-2 in hepatocellular carcinoma patients after curative resection. Oncol Rep 2013;30:2795–803. [DOI] [PubMed] [Google Scholar]
- [33].Jin Y, Chen JN, Feng ZY, et al. OPN and αvβ3 expression are predictors of disease severity and worse prognosis in hepatocellular carcinoma. PLoS One 2014;9:e87930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Ying X, Zhao Y, Wang JL, et al. Serum anti-osteopontin autoantibody as a novel diagnostic and prognostic biomarker in patients with hepatocellular carcinoma. Oncol Rep 2014;32:1550–6. [DOI] [PubMed] [Google Scholar]
- [35].Chimparlee N, Chuaypen N, Khlaiphuengsin A, et al. Diagnostic and prognostic roles of serum osteopontin and osteopontin promoter polymorphisms in hepatitis b-related hepatocellular carcinoma. Asian Pac J Cancer Prev 2015;16:7211–7. [DOI] [PubMed] [Google Scholar]
- [36].Chen RX, Xia YH, Cui JF, et al. Osteopontin, a single marker for predicting the prognosis of patients with tumor-node-metastasis stage I hepatpcellular carcinoma after surgical resection. J Gastroenterol Hepatol 2010;25:1435–42. [DOI] [PubMed] [Google Scholar]
- [37].Zhou C, Zhou HJ, Zhang XF, et al. Postoperative serum osteopontin level is a novel monitor for treatment response and tumor recurrence after resection of hepatitis B-related hepatocellular carcinoma. Ann Surg Oncol 2013;20:929–37. [DOI] [PubMed] [Google Scholar]
- [38].Zhu W, Guo L, Zhang B, et al. Combination of osteopontin with peritumoral infiltrating macrophages is associated with poor prognosis of early-stage hepatocellular carcinoma after curative resection. Ann Surg Oncol 2014;21:1304–13. [DOI] [PubMed] [Google Scholar]
- [39].Dong QZ, Zhang XF, Zhao Y, et al. Osteopontin promoter polymorphisms at locus -443 significantly affect the metastasis and prognosis of human hepatocellular carcinoma. Hepatology 2013;57:1024–34. [DOI] [PubMed] [Google Scholar]
- [40].Xue TC, Zou JH, Chen RX, et al. Spatial localization of the JAG1/Notch1/osteopontin cascade modulates extrahepatic metastasis in hepatocellular carcinoma. Int J Oncol 2014;45:1883–90. [DOI] [PubMed] [Google Scholar]
- [41].Peng SY, Ou YH, Chen WJ, et al. Aberrant expressions of annexin A10 short isoform, osteopontin and alpha-fetoprotein at chromosome 4q cooperatively contribute to progression andpoor prognosis of hepatocellular carcinoma. Int J Oncol 2005;26:1053–61. [PubMed] [Google Scholar]
- [42].Yuan RH, Jeng YM, Chen HL, et al. Stathmin overexpression cooperates with p53 mutation and osteopontin overexpression, and is associated with tumour progression, early recurrence, and poor prognosis in hepatocellular carcinoma. J Pathol 2006;209:549–58. [DOI] [PubMed] [Google Scholar]
- [43].Coppola D, Szabo M, Boulware D, et al. Correlation of osteopontin protein expression and pathological stage across a wide variety of tumor histologies. Clin Cancer Res 2004;10:184–90. [DOI] [PubMed] [Google Scholar]
- [44].Rittling SR, Chambers AF. Role of osteopontin in tumour progression. Br J Cancer 2004;90:1877–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Zhang CH, Xu GL, Jia WD, et al. Prognostic significance of osteopontin in hepatocellular carcinoma: a meta-analysis. Int J Cancer 2012;130:2685–92. [DOI] [PubMed] [Google Scholar]
- [46].Cheng J, Wang W, Sun C, et al. Meta-analysis of the prognostic and diagnostic significance of serum/plasma osteopontin in hepatocellular carcinoma. J Clin Gastroenterol 2014;48:806–14. [DOI] [PubMed] [Google Scholar]


