To the Editor:
AAT (alpha-1 antitrypsin) deficiency (AATD) is a hereditary disorder characterized by low circulating levels of AAT and development of chronic obstructive pulmonary disease (1). The decreased levels of AAT in the lung lead to a protease–antiprotease imbalance and unopposed NE (neutrophil elastase) activity. Infusion of plasma-purified AAT protein (“augmentation therapy”) has proven therapeutic benefit in AATD (2, 3). However, plasma-purified AAT is not a limitless resource, and alternative treatments for AATD are necessary. Null mutations of AAT include nonsense and frameshift mutations, resulting in a premature termination codon (PTC) in the mRNA coding region. Various read-through compounds including ataluren (PTC124) have been shown to suppress disease-causing PTCs in mammalian cells (4), and clinical studies in patients with Duchenne muscular dystrophy (5) and cystic fibrosis showed initial promise (6). Here, we extend these concepts to AATD. Some of the results of this study have been previously reported in the form of an abstract (7).
Methodology
Patient demographics and AAT studies
The Q0bolton mutation was diagnosed by sequencing all coding exons (II–V) of the AAT gene (SERPINA1, RefSeq: NG_008290). Healthy donor (MM) and patient-specific induced pluripotent stem cell (iPSC)-derived hepatic cells were generated as previously described (8). AAT was isolated from plasma by use of Alpha-1 Antitrypsin Select Resin and chromatographed by fast protein liquid chromatography (GE Healthcare Life Sciences).
Statistical analysis
Data sets were analyzed for statistical significance using GraphPad Prism 5.0 software package (GraphPad Software), with significance determined at P < 0.05. For data sets with three or more paired groups, a repeated measures ANOVA was employed, followed by a post hoc Bonferroni multiple comparison test. Results are expressed as mean ± SEM.
Results
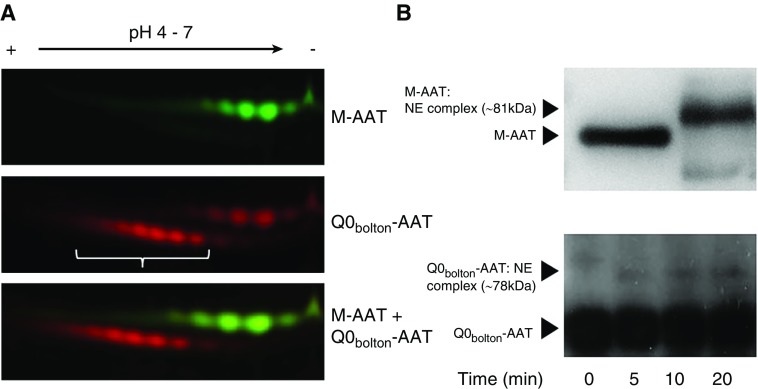
A 46-year-old never-smoker presented with dyspnea, obstructive lung disease (FEV1, 59% predicted; FEV1/FVC, 55%; DlCO, 56% predicted), and chest computed tomography scan showing bi-basal pan-acinar emphysema. Plasma AAT was undetectable by routine nephelometry. After sequencing of all coding exons (II–V) of the SERPINA1 gene, the patient was identified as homozygous for the Q0bolton mutation, with two PTCs at amino acid 373 and 374 on exon V. Molecular dynamics simulations of Q0bolton-AAT suggested sufficient interactions to stabilize native-like protein structure, should it form during the early steps of protein folding. As a consequence, we investigated the potential presence of Q0bolton-AAT protein in the patient’s plasma by chromatography and two-dimensional fluorescence difference gel electrophoresis and successfully identified Q0bolton-AAT as a truncated protein of a molecular mass of 49 kD (Figure 1A).
Figure 1.
Premature stop codon in the SERPINA1 gene encodes a truncated functional AAT (alpha-1 antitrypsin) protein. (A) Plasma-purified healthy donor (M)-AAT and patient Q0bolton-AAT protein were CyDye fluorescently labeled before two-dimensional gel analyses, using linear pH 4–7 immobilized pH gradient strips. Cy5-labeled Q0bolton-AAT (red and indicated by white bracket) demonstrated multiple glycoforms of a lower molecular mass and negative charge compared with Cy3-labeled M-AAT (green). (B) Formation of AAT:NE (neutrophil elastase) inhibitory complexes. M-AAT (top) was incubated with NE at a AAT:NE molar ratio of 1:1 for 10 minutes. Reactions were subjected to Western blot analysis employing a goat anti-human AAT antibody for AAT or AAT:NE complexes. M-AAT incubated with NE resulted in the formation of an 81-kD AAT:NE complex, with no free AAT (52 kD) remaining. Q0bolton-AAT incubated with NE for 0, 5, 10, or 20 minutes resulted in the formation of a 78-kD AAT:NE complex. Experiments illustrated are representative of three separate experiments.
The main function of AAT is to act as an antiprotease; thus, ensuing experiments investigated the ability of patient-purified Q0bolton-AAT to inhibit NE. The Western blot in Figure 1B (top) depicts the AAT complex that occurs when M-AAT (52 kD) inactivates NE (29 kD) at a 1:1 ratio, with no free AAT remaining. Although less effective than M-AAT, an increase in Q0bolton-AAT-NE binding, indicated by an increase in the intensity of an immuno-band of approximately 78 kD, during the 20-minute time course was observed (Figure 1B, lower).
In subsequent experiments, we evaluated read-through compounds for their ability to overcome the PTC in the SERPINA1 gene in the Q0bolton-AAT, leading to translation of the full-length AAT protein. iPSC-derived hepatic cells from this individual were treated with ataluren (62.5 μg/ml) for 48 hours. For Q0bolton-iPSC-hepatic cells, a significant 10-fold increase in AAT mRNA expression levels was observed compared with untreated cells (P < 0.05). Moreover, a significant 2-fold increase in the concentration of secreted Q0bolton-AAT protein was recorded with ataluren treatment (P < 0.001; Figure 2A). By Western blot analyses, it was demonstrated that the reduced molecular mass of Q0bolton-AAT (49 kD) compared with M-AAT (52 kD) persisted (Figure 2B).
Figure 2.
Increased healthy donor (M)-AAT (alpha-1 antitrypsin) and Q0bolton-AAT production by donor-derived induced pluripotent stem cell (iPSC)-hepatic cells in response to ataluren. (A) AAT gene and protein expression, by MM healthy control and Q0bolton patient-derived iPSC-hepatic cells in response to ataluren (At; 62.5 μg/ml) for 48 hours. Q0bolton-AAT mRNA expression (P < 0.05), and levels of secreted M-AAT (P < 0.01) and Q0bolton-AAT (P < 0.001) protein measured by ELISA, were significantly increased in the presence of At compared with untreated (Un) cells (ANOVA, three independent experiments). (B) Western blot analyses of extracellular secreted AAT from MM healthy control and Q0bolton patient-derived iPSC-hepatic cells treated with ataluren. The electrophoretic mobility of 52-kD M-AAT and 49 kD truncated Q0bolton AAT was observed. Whole-cell lysates were prepared, and Western blots for actin demonstrated equal protein loading supporting the use of equal cell numbers per reaction (representative blots of three separate experiments).
Discussion
In this study, the discovery of the truncated size of plasma-purified Q0bolton-AAT protein of 49 kD is expected because of the position of the PTC on the mRNA sequence; however, the circulating retention time of Q0bolton-AAT protein and the degree to which it is distributed in the body would need further investigation. Indeed, the lower abundance of the circulating protein may indicate an attempt to eradicate the aberrant protein at a cellular level. This is the case with other null AATD proteins, including Q0hongkong (1), which is removed by endoplasmic reticulum–associated degradation. Moreover, for PTCs located more than 50 bp upstream of the most 3′ exon–exon junction of the mRNA, nonsense-mediated decay is thought to eradicate the transcript (9). As a consequence, in contrast to Q0hongkong, which is characterized by a complete absence of secreted protein, the Q0bolton-AAT protein we have identified may either represent protein that has escaped nonsense-mediated decay or is not targeted for destruction because of the terminal location of the PTC.
In the current study, the Q0bolton-AAT protein retained anti-NE capacity at a lower level than M-AAT but may provide lung tissue protection if present in sufficient quantity. We assessed the ability of ataluren (PTC124) to augment the levels of AAT protein. Ataluren has the ability to read through stop codons, with read-through highest in the genetic code UGA, followed by UAG and then UAA. The Q0bolton mutation results in UAA in the coding region, suggesting that ataluren may potentially trigger read-through and production of full-length protein. Intriguingly, in this study, ataluren enhanced protein levels of both M-AAT and Q0bolton-AAT in iPSC-derived hepatocytes, an effect most likely resulting from the stabilizing influence of ataluren on mRNA levels (10). Conversely, read-through was not apparent, as indicated by the persistence of truncated Q0bolton-AAT protein of 49 kD. As ataluren increased the secretion of AAT regardless of the two genotypes, it would be interesting to study whether the same for the much more common Z variant could occur; however, the pros and cons of increased plasma Z-AAT would need to be carefully studied.
In conclusion, this study demonstrates the potential use of ataluren to induce production and secretion of functional Q0bolton-AAT protein in vivo. By this approach, grouping AATD mutations according to theratype could provide new therapeutic options, although optimization to reach the necessary protective levels is required.
Acknowledgments
Acknowledgments
The authors are grateful to Prithvi Reddy Akepati and Derek C. Liberti, from the Center for Regenerative Medicine of Boston University and Boston Medical Center, Boston, Massachusetts, for their invaluable assistance. We are grateful to Oliver J. McElvaney, David A. Bergin, Vipatsorn Shutchaidat, and Tomás Carroll, RCSI, for their technical assistance and intellectual input into this study.
Footnotes
E.P.R acknowledges funding from the US Alpha-1 Foundation, and N.G.M received funding from the Medical Research Charities Group/Health Research Board Ireland. iPSC experiments were supported by the Alpha-1 Project, as well as National Institutes of Health grants R24HL123828 and U01TR001810. A.A.W. was supported by a Boston University School of Medicine Department of Medicine Pilot Grant and NIH grant R01DK101501.
Author Contributions: E.P.R., C.A.O’D., D.M.D., D.N.K., S.M.R., A.A.W., and N.G.M. contributed to study design; E.P.R., C.A.O’D., D.M.D., M.R.W., P.H., and M.A. performed experiments and analyzed and interpreted the data; and E.P.R. and N.G.M. wrote the manuscript.
Originally Published in Press as DOI: 10.1164/rccm.201802-0338LE on July 16, 2018
Author disclosures are available with the text of this letter at www.atsjournals.org.
References
- 1.Ferrarotti I, Carroll TP, Ottaviani S, Fra AM, O’Brien G, Molloy K, et al. Identification and characterisation of eight novel SERPINA1 null mutations. Orphanet J Rare Dis. 2014;26:172. doi: 10.1186/s13023-014-0172-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chapman KR, Burdon JG, Piitulainen E, Sandhaus RA, Seersholm N, Stocks JM, et al. RAPID Trial Study Group. Intravenous augmentation treatment and lung density in severe α1 antitrypsin deficiency (RAPID): a randomised, double-blind, placebo-controlled trial. Lancet. 2015;386:360–368. doi: 10.1016/S0140-6736(15)60860-1. [DOI] [PubMed] [Google Scholar]
- 3.McElvaney NG, Burdon J, Holmes M, Glanville A, Wark PA, Thompson PJ, et al. RAPID Extension Trial Group. Long-term efficacy and safety of α1 proteinase inhibitor treatment for emphysema caused by severe α1 antitrypsin deficiency: an open-label extension trial (RAPID-OLE) Lancet Respir Med. 2017;5:51–60. doi: 10.1016/S2213-2600(16)30430-1. [DOI] [PubMed] [Google Scholar]
- 4.Welch EM, Barton ER, Zhuo J, Tomizawa Y, Friesen WJ, Trifillis P, et al. PTC124 targets genetic disorders caused by nonsense mutations. Nature. 2007;447:87–91. doi: 10.1038/nature05756. [DOI] [PubMed] [Google Scholar]
- 5.McDonald CM, Campbell C, Torricelli RE, Finkel RS, Flanigan KM, Goemans N, et al. Clinical Evaluator Training Group; ACT DMD Study Group. Ataluren in patients with nonsense mutation Duchenne muscular dystrophy (ACT DMD): a multicentre, randomised, double-blind, placebo-controlled, phase 3 trial. Lancet. 2017;390:1489–1498. doi: 10.1016/S0140-6736(17)31611-2. [DOI] [PubMed] [Google Scholar]
- 6.Kerem E, Konstan MW, De Boeck K, Accurso FJ, Sermet-Gaudelus I, Wilschanski M, et al. Cystic Fibrosis Ataluren Study Group. Ataluren for the treatment of nonsense-mutation cystic fibrosis: a randomised, double-blind, placebo-controlled phase 3 trial. Lancet Respir Med. 2014;2:539–547. doi: 10.1016/S2213-2600(14)70100-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.O’Dwyer CA, McCarthy C, Saldova R, Carroll TP, Bergin DA, Henry M, et al. Targeting nonsense mutations for treatment of alpha-1 antitrypsin deficiency: implications for chronic obstructive pulmonary disease [abstract] Am J Respir Crit Care Med. 2015;191:A3639. [Google Scholar]
- 8.Wilson AA, Ying L, Liesa M, Segeritz CP, Mills JA, Shen SS, et al. Emergence of a stage-dependent human liver disease signature with directed differentiation of alpha-1 antitrypsin-deficient iPS cells. Stem Cell Reports. 2015;4:873–885. doi: 10.1016/j.stemcr.2015.02.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mort M, Ivanov D, Cooper DN, Chuzhanova NA. A meta-analysis of nonsense mutations causing human genetic disease. Hum Mutat. 2008;29:1037–1047. doi: 10.1002/humu.20763. [DOI] [PubMed] [Google Scholar]
- 10.Allamand V, Bidou L, Arakawa M, Floquet C, Shiozuka M, Paturneau-Jouas M, et al. Drug-induced readthrough of premature stop codons leads to the stabilization of laminin alpha2 chain mRNA in CMD myotubes. J Gene Med. 2008;10:217–224. doi: 10.1002/jgm.1140. [DOI] [PubMed] [Google Scholar]