Abstract
Rationale: Patients of Hispanic origin with cystic fibrosis (CF) are the largest growing minority, representing 8.5% of patients with CF in the United States. No national survival analysis of this group has ever been undertaken.
Objectives: We aimed to determine whether Hispanic ethnicity within the CF population is associated with worse outcomes and whether any geographic differences exist.
Methods: Using U.S. Cystic Fibrosis Foundation Patient Registry data from 2010 to 2014, we performed a retrospective cohort analysis comparing survival rates between Hispanics and non-Hispanics using Kaplan-Meier and Cox regression analysis. A subject’s residence was categorized into geographic regions based on U.S. Census Bureau data: Northeast, Midwest, West, and South.
Measurements and Main Results: A total of 29,637 patients were included in the study; 2,493 identified themselves as Hispanic. Hispanics had a lower survival probability overall, with a mean age of death of 22.4 ± 9.9 years compared with non-Hispanics of 28.1 ± 10.0 years (P < 0.0001). Multivariate Cox proportional hazards modeling revealed that Hispanic patients with CF had a 1.27 times higher rate of death compared with non-Hispanics (95% confidence interval, 1.05–1.53) after adjusting for covariates including age, sex, genetic mutations, bacterial cultures, lung function, body mass index, use of CF respiratory therapies, low socioeconomic status, pancreatic enzyme use, and CF-related diabetes. When analyzed by region, Hispanics in the Midwest, Northeast, and West had shorter median survivals compared with non-Hispanics, which was not demonstrated in the South.
Conclusions: Patients with CF of Hispanic origin have a higher mortality rate than non-Hispanic patients with CF. This pattern was seen in the Midwest, Northeast, and West but not in the South.
Keywords: Hispanic, mortality, disparity, regional, cystic fibrosis
At a Glance Commentary
Scientific Knowledge on the Subject
Cystic fibrosis is predominantly a disease affecting white persons, although vulnerable subpopulations in the United States may exist based solely on ethnicity. There are no existing data on the mortality rate of Hispanic versus non-Hispanic patients in the United States.
What This Study Adds to the Field
This study shows for the first time that Hispanic patients with cystic fibrosis have a higher mortality rate than non-Hispanic patients. We further demonstrate that there are regional variations in mortality of the Hispanic population, which leads to many questions about the regional resources available for this population, details regarding socioeconomic and educational status and genetic variation in this population across the country.
Cystic fibrosis (CF) is an autosomal-recessive disease predominantly affecting white persons and involving multiple organ systems causing chronic lung and sinus disease, pancreatic insufficiency, gastrointestinal disorders, and male infertility. Despite being one of the most life-limiting inherited illnesses, advances in pulmonary and nutritional therapies in conjunction with multidisciplinary care at Cystic Fibrosis Foundation (CFF)-accredited centers have led to improved survival rates over the past three decades. Median predicted survival age has increased from 29 years during the period of 1986–1990 to greater than 47 years by 2016 (1). Furthermore, a recent study estimated that those born with CF in 2010 have projected median survival ages of 54–58 years old assuming a decreasing mortality rate seen from 2000 to 2010 (2).
Although CF is most prevalent in the white population, afflicting approximately 1 in 2,500 individuals and making up more than 90% the U.S. CF population, CF can be found in all races including African Americans (1 in 15,000), Hispanic Americans (1 in 8,000), and Asian Americans (1 in 35,000) (3–5). Of the 29,497 patients currently in the U.S. CFF patient registry (CFFPR) in 2016, a total of 8.5% are Hispanic and this percentage has steadily grown over the past 15 years (1), reflecting national population trends where Hispanics accounted for 18% of the nation’s population in 2016 and the second largest racial group behind white persons (6). The expected survival of all patients with CF continues to rise; however, certain subpopulations may still remain at risk for worse clinical outcomes. Hispanic patients with CF in California followed from 1991 to 2010 were found to have a 2.81 higher mortality rate than non-Hispanic patients with CF. This held true even after adjusting for socioeconomic status and clinical risk factors; yet no differences in access and use of CF center care were seen between these two groups (7). However, areas of Texas found no significant difference in outcomes of Hispanic versus non-Hispanic patients with CF (8). Given the contradictory data on Hispanic patients with CF in California versus Texas and existing data on geographic differences in the health of the Hispanic population in other respiratory diseases, such as asthma (9), we hypothesize that there are regional differences in outcomes of Hispanic versus non-Hispanic patients with CF.
Per reports from the Centers for Disease Control and Prevention, Hispanic death rate is 24% lower than white persons living in the United States (10) A “Hispanic paradox” has been described for the discrepancy seen in Hispanic subtypes and their burden of obstructive lung diseases in younger and older patients (9, 11). For example, Puerto Ricans have a higher prevalence of asthma and disproportionate burden of asthma attacks in the United States, whereas Mexican Americans have lower rates of chronic obstructive pulmonary disease and whether differences in among certain subtypes of Hispanic population have never been examined (9, 12–14). Based on U.S. Census data from 2010, Hispanic persons of Mexican origin are typically in western and southern states; those of South American origin live in southern states; and those of Puerto Rican, Dominican, and Cuban origin reside in the northeast (15). Given the geographic distribution and genetic diversity that exists within the U.S. Hispanic population, we sought to examine both national and regional differences in clinical outcomes between Hispanic and non-Hispanic patients with CF.
Methods
CFFPR is a national registry including nearly all patients seen at more than 110 CFF-accredited care centers in the United States. CFF distributes annual patient data questionnaires to be completed by clinic personnel at each individual center. This questionnaire contains information on basic demographic, diagnostic, clinical, and outcome data that is then entered into the CFFPR, which has been maintaining data since 1986. Historically, the registry has accurately reported 92%–97% of all CF deaths reported in the U.S. Vital Statistics (16). Hispanic origin and residing state location are parenterally or self-reported on these questionnaires. The University of Texas Southwestern Medical Center institutional review board approved the study protocol (#STU 022015–002).
The study design is a retrospective cohort analysis using longitudinal data from the CFFPR to compare survival rates between Hispanic and non-Hispanic patients with CF. We included all patients with CF with data entered in the CFFPR from 2010 to 2014. Patients were censored at the time of lung transplant or last visit date. Individuals were excluded if they did not self-report whether they are of Hispanic origin, if they received a transplant before the study period, or if their age of diagnosis was less than 0. Patients older than age 50 at the start of the study were also excluded because prior research has noted that more than 50% of the patients in this age demographic did not have their deaths reported in the CFFPR (17). The primary outcome of interest was survival, which was defined as the length of time between date of birth and date of death as has been used for previous CF survival analysis (18, 19). Secondary outcomes included yearly CF exacerbations because this is commonly a marker for worse outcomes (20).
Subjects’ residing state was labeled into one of four main regions based on U.S. Census Bureau data: Northeast, Midwest, South, and West (15). If subjects in the registry lived in more than one state during the study period of 2010–2014, the state they lived in the longest was used. If equal time was spent in each state, then the last reported state was used.
Demographic data were evaluated using Student’s t test for continuous variables and chi-square for categorical variables. Survival analysis was completed using the Kaplan-Meier estimation of the proportion of subjects surviving at any point during follow-up and the log-rank statistic to assess statistically significant differences between the survival curves. Stepwise Cox regression analysis was conducted to investigate significant differences in survival between Hispanic and non-Hispanic patients with CF after controlling for the effects of confounding factors. Covariates were selected based on known clinical risk factors that affect survival in the CF population (18, 20). These variables were identified a priori from medical literature review and include age of birth, female sex, CF transmembrane conductance regulator genotype (F508del homozygous, F508del heterozygous, other, or missing) (18), or pancreatic enzyme use. CF-related diabetes mellitus or impaired glucose tolerance was defined per published consensus guidelines (21). Culture information was presented as positive if ever infected with Pseudomonas aeruginosa, Burkholderia complex, or methicillin-resistant Staphylococcus aureus (MRSA). These organisms were chosen based on data supporting their impact on mortality in CF (19, 22, 23). Public health insurance was noted to be yes if subject was ever on public health insurance during the study period (18–20). No health insurance was also documented as yes if ever without health insurance of any kind. Medications used including dornase alpha, hypertonic saline, and ivacaftor for those eligible were also defined as yes if ever used. Number of outpatient visits was defined per year. Percent-predicted FEV1 is displayed as mean value for those greater than or equal to 6 years of age at entry into the cohort for the univariate and multivariate analysis and is broken down by age groups for the demographics. Body mass index (BMI) at entry into the cohort was used and was defined as underweight, adequate weight, and overweight. For subjects 2–19, they were defined as underweight if BMI percentile was less than or equal to 12, adequate weight if BMI percentile was 13–84, and overweight if BMI was greater than or equal to 85. For subjects over 19, they were defined as underweight if BMI was less than 18.5 kg/m2, adequate weight if between 18.5–24.9 kg/m2, or overweight if greater than or equal to 25.kg/m2 (18). Use of dornase alpha, hypertonic saline, or ivacaftor were reported as yes if ever used in the 5-year study period. No variables were analyzed as time dependent based on the definitions used. Secondary endpoint of exacerbation rate was analyzed using Poisson regression.
Patients with milder clinical phenotypes have longer survival times. Therefore, differences in proportions of patients with milder CF disease in the Hispanic and non-Hispanic would bias survival estimates. To address this potential ascertainment bias, we conducted an additional subgroup analysis only including subjects diagnosed at less than 2 years of age or with pancreatic insufficiency. The multivariable Cox proportional hazards model was repeated as described above.
All statistical data were analyzed using SAS version 9.4 (SAS Institute).
Results
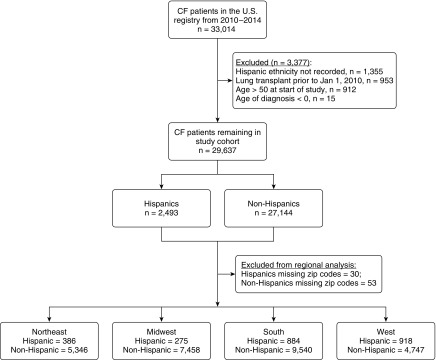
A total of 33,014 individuals with CF were registered in the CFFPR from 2010 to 2014. Of these, we excluded patients if Hispanic ethnicity was unrecorded (n = 1,355; 4.1%), if they underwent lung transplant before 2010 (n = 953; 2.9%), if they were older than age 50 at the start of the study (n = 912; 2.8%), and if their age at diagnosis was less than 0 (n = 15; 0.001%) (Figure 1). A total of 29,637 subjects with CF comprised the study cohort with 8.4% reporting Hispanic ethnicity. Baseline characteristics between Hispanics and non-Hispanics are listed in Table 1. Hispanic patients compared with their non-Hispanic counterparts were overall younger in this study cohort, younger at time of CF diagnosis, and younger at the time of death. There was no difference in sex distribution. Hispanics had a lower prevalence of F508del homozygous mutations and lower mean sweat tests had less culture positivity for MRSA, P. aeruginosa and Burkholderia complex, and were on less pancreatic enzymes (a surrogate for pancreatic insufficiency). Hispanic patients with CF also had lower rates of CF-related diabetes. Importantly, they had higher use of public health insurance and no insurance, but no difference in number of outpatient visits to their CF center per year. In addition, similar usage of dornase alpha, hypertonic saline, and ivacaftor was seen between the two groups. Lung function among different age groups showed lower percent-predicted FEV1 in all age groups outside of the group older than 35 years of age, which was similar. Notably, 896 patients had undergone lung transplantation during the study period. Fifty Hispanic subjects (2.0%) underwent lung transplant, and 846 non-Hispanic subjects (3.1%) underwent lung transplant (P = 0.002). Interestingly, the average age of transplant in the Hispanic subjects was 22.0 ± 8.1 years versus 30.1 ± 9.2 in the non-Hispanics subjects (P < 0.0001). One hundred and twenty-three Hispanic subjects were lost to follow-up (4.93%), whereas 1,211 non-Hispanic subjects were lost to follow up (4.46%; P = 0.392). Lost to follow-up was defined as subjects who were not censored for death or lung transplant and who had no data in the last 2 years of the study period as per definition of prior groups (18).
Figure 1.
Flowchart of study population. A flowchart of individuals with cystic fibrosis (CF) registered in the Cystic Fibrosis Foundation patient registry from 2010 to 2014 is displayed. A total of 33,014 individuals with CF were entered. Of these, 1,355 patients were excluded because Hispanic ethnicity was not recorded, 953 were excluded because they underwent lung transplantation before 2010, a total of 912 were excluded for age greater than 50 at the start of the study period, and 15 if age of diagnosis was less than 0. A total of 29,637 subjects with CF comprised the study cohort with 2,493 Hispanic patients (8.41% of the population).
Table 1.
Baseline Characteristics of Study Cohort
| Characteristics | Hispanic (n = 2,493) | Non-Hispanic (n = 27,144) | P Value | Standardized Differences |
|---|---|---|---|---|
| Age at entry into the cohort, mean (SD), yr | 11.6 (10.2) | 17.0 (12.3) | <0.0001 | 0.447 |
| Age at diagnosis, mean (SD), yr | 2.8 (5.9) | 3.1 (7.0) | 0.005 | 0.052 |
| Age at death, mean (SD), yr | 22.4 (9.9) | 28.1 (10.0) | <0.0001 | 0.562 |
| Deaths, n (%) | 122 (4.9) | 1,588 (5.9) | 0.050 | 0.030 |
| Age of lung transplantation, mean (SD), yr | 22.0 (8.1) | 30.1 (9.2) | <0.0001 | 0.894 |
| Underwent lung transplantation, n (%) | 50 (2.0) | 846 (3.1) | 0.002 | 0.050 |
| Female sex, n (%) | 1,190 (47.7) | 13,027 (48.0) | 0.805 | 0.003 |
| ΔF508 genotype | <0.0001 | |||
| ΔF508 homozygous, n (%) | 629 (25.2) | 12,732 (46.9) | 0.198 | |
| ΔF508 heterozygous, n (%) | 1,038 (41.6) | 10,333 (38.1) | 0.049 | |
| No ΔF508, n (%) | 719 (28.9) | 3,250 (11.9) | 0.141 | |
| Unknown allele or alleles, n (%) | 107 (4.3) | 829 (3.1) | Ref | |
| Sweat test value, mmol/L, mean (SD) | 90.9 (25.2) | 96.6 (22.0) | <0.0001 | 0.255 |
| CF-related diabetes status | <0.0001 | |||
| CF-related diabetes mellitus present, n (%) | 208 (8.4) | 3,817 (14.1) | Ref | |
| Impaired glucose tolerance, n (%) | 130 (5.2) | 1,528 (5.6) | 0.149 | |
| No CF-related diabetes mellitus, n (%) | 1,977 (85.4) | 20,606 (79.4) | 0.131 | |
| Pancreatic enzyme use | <0.0001 | |||
| Not using pancreatic enzymes, n (%) | 382 (16.7) | 3,496 (13.6) | 0.606 | |
| Using pancreatic enzymes, n (%) | 1,908 (83.3) | 22,200 (86.4) | Ref | |
| Methicillin-resistant Staphylococcus aureus, n (%) | 391 (17.5) | 6,104 (24.6) | <0.0001 | 0.123 |
| Pseudomonas aeruginosa, n (%) | 990 (44.2) | 11,977 (48.2) | 0.0003 | 0.056 |
| Burkholderia complex, n (%) | 31 (1.4) | 613 (2.5) | 0.001 | 0.056 |
| ppFEV1, mean (SD) | 78.2 (25.1) | 77.6 (25.6) | 0.358 | 0.025 |
| ppFEV1, age 6–12, mean ± SD (n) | 90.0 ± 20.0 (476) | 96.3 ± 17.0 (3,978) | <0.0001 | 0.366 |
| ppFEV1, age 13–18, mean ± SD (n) | 84.7 ± 23.5 (421) | 88.5 ± 20.5 (4,525) | 0.001 | 0.184 |
| ppFEV1, age 19–25, mean ± SD (n) | 66.9 ± 22.0 (304) | 72.1 ± 23.4 (4,672) | 0.0001 | 0.225 |
| ppFEV1, age 26–35, mean ± SD (n) | 57.0 ± 21.8 (157) | 63.7 ± 23.9 (3,925) | 0.0005 | 0.283 |
| ppFEV1, age >35, mean ± SD (n) | 56.8 ± 22.7 (71) | 58.7 ± 23.4 (2,326) | 0.504 | 0.081 |
| On public health insurance, n (%) | 1,543 (61.9) | 11,612 (42.8) | <0.0001 | 0.276 |
| No insurance, n (%) | 48 (2.07) | 398 (1.53) | 0.046 | 0.029 |
| Number of outpatient visits per year, mean (SD) | 4.6 (2.7) | 4.6 (2.9) | 0.790 | 0.006 |
| Prescribed dornase alfa, n (%) | 1,615 (71.0) | 18,358 (71.9) | 0.329 | 0.015 |
| Prescribed hypertonic saline, n (%) | 932 (40.9) | 10,944 (42.8) | 0.075 | 0.027 |
| Prescribed VX770, n (% of eligible) | 2 (6.25) | 44 (3.64) | 0.334 | 0.085 |
Definition of abbreviations: CF = cystic fibrosis; ppFEV1 = percent predicted FEV1; Ref = reference.
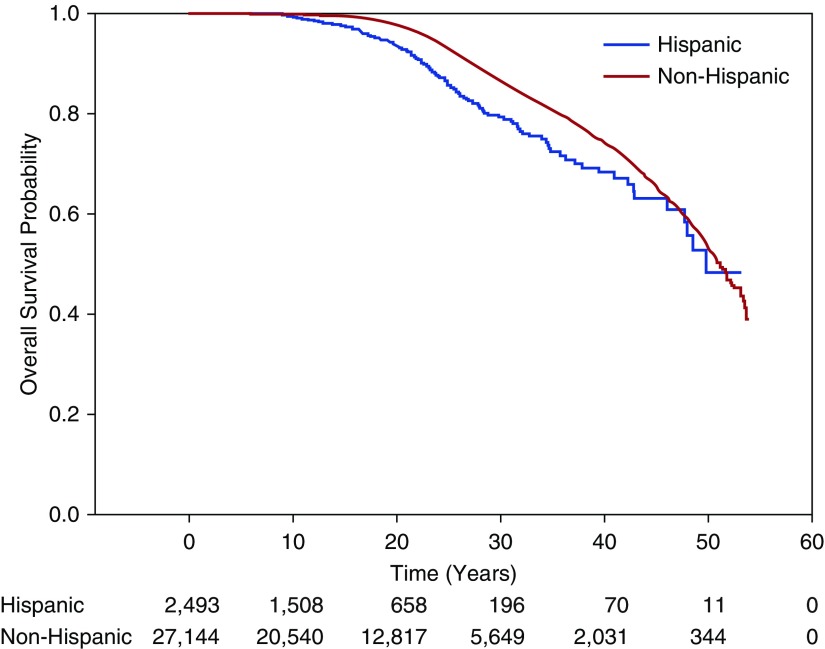
A total of 1,710 patient deaths occurred, 122 among the Hispanics (4.9%) and 1,588 among the non-Hispanics (5.9%). The median estimated survival was 51.1 years (interquartile range [IQR], 50.1–51.9) for the total population with a median estimated survival age of 49.7 years (IQR, 47.7–nonestimable) for Hispanic patients and 51.2 years (IQR, 50.2–51.9) for non-Hispanic patients (P < 0.0001)) (Figure 2).
Figure 2.
Kaplan-Meier estimates of survival according to Hispanic ethnicity. Displayed are the Kaplan-Meier survival estimates for total U.S. Hispanic versus non-Hispanic population taken from the Cystic Fibrosis Foundation patient registry. The median estimated survival was 49.8 years (interquartile range, 47.7–nonestimable) for Hispanic cohort versus 51.2 years (interquartile range, 50.2–51.9) for non-Hispanic patients (P < 0.0001). Displayed below the graph are the numbers of subjects remaining under observation at each time point in each cohort.
The unadjusted and adjusted hazard ratios (HRs) for the subjects are presented in Table 2. The unadjusted HR for Hispanic patients with CF compared with non-Hispanics was significantly higher (HR, 1.67; 95% confidence interval [CI], 1.43–1.97). After adjusting for all listed covariates, the HR for Hispanic patients with CF was 1.27 (95% CI, 1.05–1.53). In this study population, missing data were seen in culture results of MRSA, P. aeruginosa, and Burkholderia complex (8.5%); pancreatic enzyme use (5.6%); percent-predicted FEV1 (29.7%); BMI (15.5%); treatment with hypertonic saline and dornase alpha (6.2%); cystic fibrosis–related diabetes (CFRD) status (4.6%); outpatient visits (4.9%); and no insurance (4.7%). There was no missing data for variables including date of birth, sex, pulmonary exacerbations, ΔF508 genotyping, and public health insurance.
Table 2.
Hazard Ratios of Mortality Calculated from Cox Regression Modeling
| Covariate | Observations (n) | Unadjusted HR (95% CI) | P Value | Adjusted HR (95% CI) | P Value |
|---|---|---|---|---|---|
| Hispanic ethnicity | 29,637 | 1.67 (1.43–1.97) | <0.0001 | 1.27 (1.05–1.53) | 0.015 |
| Date of birth | 29,637 | <0.0001 | <0.0001 | ||
| 1961–1970 | 8,644.67 (5,059.72–14,769.67) | 136,276.60 (67,998.40–273,113.98) | |||
| 1971–1980 | 796.53 (561.31–1130.32) | 4,345.57 (2,758.13–6,846.68) | |||
| 1981–1990 | 123.51 (90.37–168.80) | 308.65 (205.51–463.54) | |||
| 1991–2000 | 9.67 (7.52–12.43) | 16.11 (11.66–22.27) | |||
| ≥2001–2010 | Ref | Ref | |||
| Female sex | 29,637 | 1.34 (1.24–1.45) | <0.0001 | 1.25 (1.14–1.38) | <0.0001 |
| ΔF508 genotype | 29,637 | <0.0001 | <0.0001 | ||
| ΔF508 homozygous | 0.68 (0.59–0.79) | 0.61 (0.50–0.74) | |||
| ΔF508 heterozygous | 0.44 (0.37–0.51) | 0.56 (0.46–0.68) | |||
| No ΔF508 | 0.34 (0.28–0.41) | 0.54 (0.42–0.68) | |||
| Unknown genotype | Ref | Ref | |||
| Sweat value | 25,593 | 1.01 (1.01–1.01) | <0.0001 | 1.00 (0.99–1.00) | 0.718 |
| On pancreatic enzymes | 27,986 | 3.50 (2.98–4.12) | <0.0001 | 1.25 (1.02–1.53) | 0.032 |
| ppFEV1 | 20,855 | 0.95 (0.95–0.96) | <0.0001 | 0.95 (0.94–0.95) | <0.0001 |
| BMI | 25,056 | <0.0001 | 0.010 | ||
| Underweight | Ref | Ref | |||
| Adequate weight | 0.40 (0.37–0.44) | 1.02 (0.91–1.15) | |||
| Overweight | 0.13 (0.11–0.15) | 0.78 (0.63–0.95) | |||
| Diabetes | 28,266 | <0.0001 | <0.0001 | ||
| CFRD present | 2.11 (1.93–2.30) | 1.29 (1.17–1.43) | |||
| Impaired glucose tolerance | 1.74 (1.56–2.06) | 1.24 (1.06–1.45) | |||
| No CFRD | Ref | Ref | |||
| MRSA | 27,101 | 1.84 (1.68–2.01) | <0.0001 | 1.22 (1.10–1.35) | <0.0001 |
| Pseudomonas aeruginosa | 27,101 | 1.73 (1.55–1.93) | <0.0001 | 1.08 (0.95–1.23) | 0.222 |
| Burkholderia complex | 27,101 | 1.83 (1.54–2.17) | <0.0001 | 1.36 (1.13–1.64) | 0.001 |
| Public health insurance | 29,637 | 2.60 (2.40–2.82) | <0.0001 | 1.19 (1.07–1.32) | 0.0009 |
| No insurance | 28,266 | 1.79 (1.23–2.60) | 0.0022 | 1.30 (0.76–2.22) | 0.332 |
| Outpatient visits | 28,187 | 1.11 (1.10–1.12) | <0.0001 | 1.02 (1.01–1.03) | 0.003 |
| Rate of pulmonary exacerbations | 29,637 | 1.64 (1.61–1.67) | <0.0001 | 1.32 (1.28–1.36) | <0.0001 |
| Use of hypertonic saline | 27,802 | 0.69 (0.63–0.76) | <0.0001 | 0.92 (0.83–1.01) | 0.077 |
| Use of dornase alpha | 27,802 | 0.32 (0.28–0.37) | <0.0001 | 0.909 (0.77–1.07) | 0.24 |
Definition of abbreviations: BMI = body mass index; CFRD = cystic fibrosis–related diabetes; CI = confidence interval; HR = hazard ratio; MRSA = methicillin-resistant Staphylococcus aureus; ppFEV1 = percent predicted FEV1; Ref = reference.
To address any ascertainment bias patients with milder disease would have on the analysis, we ran an additional sensitivity analysis only including subjects diagnosed at less than 2 years of age and with pancreatic insufficiency. We found 23,507 subjects who met these criteria, of which 1,949 were Hispanic and 21,558 were non-Hispanic. Using the same Cox proportional analysis as mentioned previously, this cohort was evaluated and found the adjusted HR for Hispanic race to be 1.24 (95% CI, 1.03–1.49; P = 0.023). This is very similar to the 1.27 HR found with our primary analysis (P = 0.999). Finally, despite the notable difference in survival, the secondary endpoint of mean CF exacerbation rates was not statistically different between Hispanics (0.69 exacerbations/yr) and non-Hispanics (0.67 exacerbations/yr) (P = 0.4561).
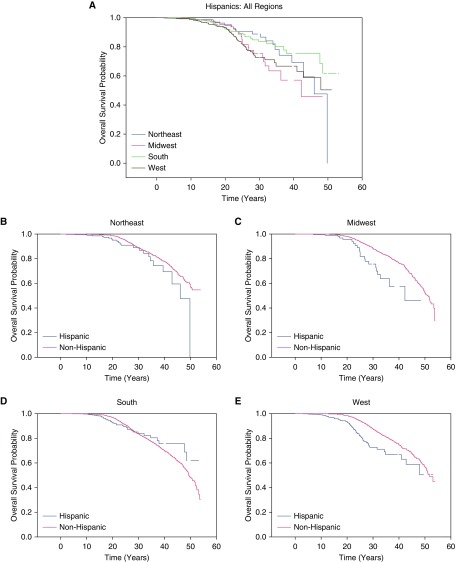
We further evaluated the outcomes in Hispanics relative to non-Hispanics by classifying patients into one of four geographic regions they reside in within the United States (15). The distribution of Hispanic patients differed with Hispanics making up 6.73% of the population in the Northeast, 3.54% of the population in the Midwest, 8.49% of the population in the South, and 16.20% of the population and in the West. In the Northeast, Midwest, and West regions, Hispanics demonstrated a shorter median age of survival than non-Hispanics: Northeast Hispanics (46.0 yr; IQR, 42.9–49.8) to Northeast non-Hispanics (>50.7) (P = 0.01) (Figure 3B), Midwest Hispanics (42.2 yr; IQR, 32.8–nonestimable) to Midwest non-Hispanics (50.8 yr; IQR, 49.4–52.3) (P = 0.0002) (Figure 3C), and Hispanics in the West (>42.8 yr) compared with non-Hispanics in the West (51.8 yr; IQR, 50.6–nonestimable) (P < 0.0001) (Figure 3E). In the South, however, Hispanics had a similar median age of survival (>48.5 yr) compared with non-Hispanics (49.4 yr; IQR, 48.4–51.4) (P = 0.88) (Figure 3D). When comparing Hispanic patients with CF in all four regions with one another, the Midwest region had the lowest median age of survival, whereas the Northeast and South had the highest median age of survival (P = 0.04) (Figure 3A). Survival analysis of the combined population of Hispanics and non-Hispanics by region can be found in the online supplement (see Figure E1).
Figure 3.
Kaplan-Meier estimates of regional Hispanic to non-Hispanic comparisons of survival. (A) When comparing Hispanic patients with cystic fibrosis in all four regions with one another, the Midwest region had the lowest median age of survival, whereas the Northeast and South had the highest median age of survival (P = 0.04). (B) In the Northeast, Hispanics had a lower median age of survival (46.0 yr; interquartile range [IQR], 42.9–49.8) than non-Hispanics (>50.7) (P = 0.01). (C) In the Midwest, Hispanics had a much lower median age of survival (42.2 yr; IQR, 32.8–nonestimable) compared with non-Hispanics (50.8 yr; IQR, 49.4–52.3) (P = 0.0002). (D) In the South, Hispanics had a similar median age of survival (>48.5 yr) compared with non-Hispanics (49.4 yr; IQR, 48.4–51.4) (P = 0.88). (E) In the West, Hispanics had a lower median predicted age of survival (>42.8 yr) compared with non-Hispanics (51.8 yr; IQR, 50.6–nonestimable) (P < 0.0001).
Table 3 shows baseline demographics of the Hispanic population categorized by the four regions. Mean age, mean age at diagnosis, female sex, lung transplant, FEV1, public health insurance, and outpatient visits did not statistically differ among all four regions for Hispanic patients with CF. Northeast Hispanic patients with CF had less ΔF508 homozygous and heterozygous mutations (P = 0.015), more Burkholderia cepacia (P = 0.03), but similar rates of pancreatic enzyme use, CFRD, and MRSA and P. aeruginosa culture positivity compared with Midwest Hispanic patients with CF. Hispanic patients with CF residing in the South compared with the Midwest had fewer females (P = 0.03) and higher rates of pancreatic enzyme use (P = 0.003) but similar rates of ΔF508 mutations (P = 0.29), CFRD (P = 0.52), MRSA (P = 0.29), P. aeruginosa (P = 0.35), and Burkholderia (P = 0.21).
Table 3.
Baseline Demographics of Hispanic Patients with CF Compared by Region
| Northeast (n = 386) | Midwest (n = 274) | South (n = 884) | West (n = 917) | All P Value | |
|---|---|---|---|---|---|
| Age at entry into cohort, mean (SD), yr | 12.26 (10.80) | 11.16 (10.58) | 11.67 (10.23) | 11.29 (9.69) | 0.39* |
| Age at diagnosis, mean (SD), yr | 2.97 (6.23) | 2.49 (5.83) | 2.81 (6.14) | 2.72 (5.69) | 0.77* |
| Age at death, mean (SD), yr | 27.51 (13.30) | 25.75 (6.55) | 21.69 (10.80) | 20.80 (8.68) | 0.05* |
| Deaths, n (%) | 16 (4.15) | 14 (5.11) | 30 (3.39) | 61 (6.65) | 0.01 |
| Underwent lung transplantation, n (%) | 8 (2.07) | 9 (3.28) | 19 (2.15) | 13 (1.42) | 0.26 |
| Female sex, n (%) | 188 (48.70) | 147 (53.65) | 409 (46.27) | 431 (47.00) | 0.18 |
| ΔF508 genotype | |||||
| ΔF508 homozygous, n (%) | 90 (23.32) | 76 (27.73) | 244 (27.60) | 210 (22.90) | <0.0001 |
| ΔF508 heterozygous, n (%) | 131 (33.94) | 116 (42.34) | 404 (45.70) | 377 (41.11) | |
| Other, n (%) | 142 (36.79) | 75 (27.37) | 200 (22.62) | 293 (31.95) | |
| CF-related diabetes status | |||||
| CF-related diabetes present, n (%) | 31 (8.03) | 24 (8.76) | 85 (9.62) | 65 (7.09) | 0.002 |
| Impaired glucose tolerance, n (%) | 10 (2.59) | 9 (3.28) | 41 (4.64) | 69 (7.52) | |
| On pancreatic enzymes, n (%) | 276 (71.50) | 199 (72.6) | 701 (79.30) | 712 (77.64) | 0.0005 |
| Methicillin-resistant Staphylococcus aureus, n (%) | 59 (25.11) | 44 (28.76) | 169 (33.33) | 118 (20.92) | <0.0001 |
| Pseudomonas aeruginosa, n (%) | 126 (36.10) | 108 (42.86) | 368 (46.23) | 377 (45.92) | 0.01 |
| Burkholderia complex, n (%) | 10 (2.87) | 1 (0.40) | 13 (1.63) | 7 (0.85) | 0.03† |
| ppFEV1, mean (SD) | 77.85 (25.83) | 79.44 (27.63) | 78.23 (24.61) | 78.27 (24.79) | 0.95* |
| On public health insurance, n (%) | 260 (67.36) | 174 (63.50) | 527 (59.62) | 576 (62.81) | 0.07† |
| Number of outpatient visits per year, mean (SD) | 4.687 ± 2.858 | 4.972 ± 2.687 | 4.702 ± 2.793 | 4.482 ± 2.456 | 0.06* |
| Prescribed dornase alfa, n (%) | 213 (60.34) | 176 (69.84) | 593 (73.76) | 620 (73.46) | <0.0001 |
| Prescribed hypertonic saline, n (%) | 125 (35.41) | 91 (36.11) | 342 (42.54) | 363 (43.01) | 0.03 |
Definition of abbreviations: CF = cystic fibrosis; ppFEV1 = percent predicted FEV1.
ANOVA.
Fisher exact test; otherwise by chi-square test.
Discussion
Although the median predicted survival has improved for all patients with CF over the past three decades, our results confirm that patients with CF of Hispanic origin have a worse overall survival than non-Hispanic patients with CF when evaluating a contemporary CF population. The unadjusted impact of Hispanic ethnicity on survival revealed a 1.4-year shorter life span for those of Hispanic ethnicity than those of non-Hispanic ethnicity. Even after adjusting for important clinical variables known to impact mortality, Hispanic patients with CF had a 1.27 higher risk of death than non-Hispanics in those younger than age 50 at the study onset. We censored patients at the time of transplant for purposes of this survival analysis. However, we found that the Hispanic patients that did undergo lung transplantation were younger overall than the non-Hispanic patients who were transplanted, supporting another metric of increased disease severity in the Hispanic population. Our study also underscores the importance that the Hispanic population in the United States is not homogenous with notable regional differences, which may affect mortality in these subgroups. Among all four regions of the United States, Hispanic patients with CF had the worst median age of survival in the Midwest, yet similar rates of culture positivity, mean age at diagnosis, female sex, lung transplant, public health insurance, and outpatient visits compared with their Hispanic counterparts in the Northeast, South, and West regions.
One possible explanation for the lower median survival of Hispanics in the Midwest may be because of the overall smaller population of Hispanics in this region (n = 275), thereby potentially affecting the composition of providers at these CFF centers in regards to language translational services and cultural sensitivity. The current construction of the CFFPR questionnaire remains patient-centered, but questions pertaining to each center including the make-up of their care providers and language and cultural competencies have yet to be examined. To fully address health disparities seen in this subpopulation, understanding any discrepancies that may exist at the care center level is of utmost importance. Disparities in health and access to care are further highlighted by recent reports that compare CF outcomes among patients living in the United States with those living in Canada, where the outcomes were higher (18). Notably, they did not break down race in this study other than white versus not.
Given that Hispanic ethnicity is heavily confounded by socioeconomic status, prior research has tried to estimate socioeconomic status of patients with CF by median neighborhood income (7); however, this is subject to a high degree of measurement bias. Although the CFFPR does obtain data on family income, only 21.5% of subjects in our study cohort had completed this entry (data not shown; 9% Hispanic patients with CF were living <$30,000 annual income compared with 6% of non-Hispanic patients with CF; P < 0.0001), and thus we opted to not include this in our Cox regression modeling and instead use an established surrogate marker of socioeconomic status of ever being on public health insurance (24). The ethnic difference seen in our results may be influenced by unknown genetic phenotypes in the Hispanic subpopulation as has been previously reported (7) and environmental and social factors not taken into account by the CFFPR data collection, such as health literacy, treatment adherence, and even center-specific information of Spanish proficiency and cultural competency. In addition, the acculturation of patients with CF of Hispanic origin may be impacting outcomes. This is supported in the asthma population where Mexican Americans born in the United States have a higher risk of asthma than those born in Mexico (9). Other factors, such as the impact of differences in diet, stress, and other environmental variables, need to be considered.
Despite the clear association between Hispanic ethnicity and shorter survival in patients with CF demonstrated in this large cohort analysis, limitations of this study include those often found in epidemiologic studies of registry data. Sampling bias can occur because all patients in this study have been enrolled at CFF accredited-centers and provide consent into their inclusion in this database. Although more than 80% of the CF population in the United States are captured in this database (25), the results may not entirely be applicable for all patients with CF in the United States, particularly those not followed at CFF-accredited centers. Misclassification bias can also occur as patients or family members are identifying whether or not they are of Hispanic ethnicity and this may not account well for patients who were of mixed origin. Hispanic ethnicity is self-reported in the CFFPR and there has been research to suggest that the agreement between administrative ethnicity collection and self-report is higher for those that self-identify as white or African American versus those that self-identify as Hispanic, Asian, or Native American (26, 27).
In addition, we did not account for differences newborn screening may play on the Hispanic versus non-Hispanic population. It is quite possible that a larger proportion of the Hispanics were born outside of the United States and did not have CF diagnosed at birth. However, we ran our Kaplan-Meier analysis of survival using time from diagnosis to time to death in addition to age at death as shown in this analysis and found similar results with median time from diagnosis to death being 44.1 years (IQR, 41.5–48.2) for Hispanic patients and 46.1 years (IQR, 45.3–46.9) for non-Hispanic patients (P < 0.0001).
Finally, because more transplants were done in the non-Hispanic population than the Hispanic population and we censored at time of transplant, this could have biased the results. We, therefore, ran the Kaplan-Meier survival analysis including transplanted subjects and found the median survival in the Hispanic population to be greater than 48.0 years and greater than 53.5 years in the non-Hispanic population (P < 0.0001), which was similar to our findings but does demonstrate an amplified difference between the populations.
To conclude, in the United States, Hispanic patients with CF have a worse overall survival than non-Hispanic patients with CF. Moreover, regional differences in survival exist in the Hispanic CF population. Further insight is required to determine genetic and socioeconomic factors that may be playing a role and examining regional centers’ capabilities to providing improved health outcomes to this vulnerable subpopulation.
Acknowledgments
Acknowledgment
The authors thank the Cystic Fibrosis Foundation for providing the data for this study by way of use of the Cystic Fibrosis Foundation Patient Registry data.
Footnotes
Supported by the University of Texas Southwestern Medical Center CTSA (UL1 TR001105).
Author Contributions: J.R., G.S.S., R.J., and A.K. contributed to the design of the study, acquisition of data, analysis and interpretation of data results, and drafting and editing of the manuscript. C.A. and A.G. contributed to the statistical analyses performed in the study and editing of the manuscript.
This article has an online supplement, which is accessible from this issue's table of contents at www.atsjournals.org.
Originally Published in Press as DOI: 10.1164/rccm.201711-2357OC on May 9, 2018
Author disclosures are available with the text of this article at www.atsjournals.org.
References
- 1.Cystic Fibrosis Foundation. Bethesda, MD: Cystic Fibrosis Foundation; 2017. Cystic Fibrosis Foundation Patient Registry: 2016 annual data report. [accessed 2017 Apr]. Available from: https://www.cff.org/Research/Researcher-Resources/Patient-Registry/2016-Patient-Registry-Annual-Data-Report.pdf. [Google Scholar]
- 2.MacKenzie T, Gifford AH, Sabadosa KA, Quinton HB, Knapp EA, Goss CH, et al. Longevity of patients with cystic fibrosis in 2000 to 2010 and beyond: survival analysis of the Cystic Fibrosis Foundation patient registry. Ann Intern Med. 2014;161:233–241. doi: 10.7326/M13-0636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zvereff VV, Faruki H, Edwards M, Friedman KJ. Cystic fibrosis carrier screening in a North American population. Genet Med. 2014;16:539–546. doi: 10.1038/gim.2013.188. [DOI] [PubMed] [Google Scholar]
- 4.Grody WW, Cutting GR, Watson MS. The cystic fibrosis mutation “arms race”: when less is more. Genet Med. 2007;9:739–744. doi: 10.1097/gim.0b013e318159a331. [DOI] [PubMed] [Google Scholar]
- 5.Adele Schneider NWH. Obstetric evidence based guidelines: genetic screening. London: Informa Healthcare; 2012. [Google Scholar]
- 6.Flores A. How the US Hispanic population is changing. Pew Research Center. 2017 [accessed 2017 Nov 20]. Available from: https://www.pewresearch.org/fact-tank/2017/09/18/how-the-u-s-hispanic-population-is-changing/
- 7.Buu MC, Sanders LM, Mayo JA, Milla CE, Wise PH. Assessing differences in mortality rates and risk factors between Hispanic and non-Hispanic patients with cystic fibrosis in California. Chest. 2016;149:380–389. doi: 10.1378/chest.14-2189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rho J, Ahn C, Gao A, Keller A, Jain R. Evaluating outcomes disparities in the Hispanic cystic fibrosis population: a need for a national analysis. Chest. 2016;150:751–753. doi: 10.1016/j.chest.2016.06.041. [DOI] [PubMed] [Google Scholar]
- 9.Rosser FJ, Forno E, Cooper PJ, Celedón JC. Asthma in Hispanics: an 8-year update. Am J Respir Crit Care Med. 2014;189:1316–1327. doi: 10.1164/rccm.201401-0186PP. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Centers for Disease Control and Prevention. Hispanic health. CDC Vital Signs. 2015 [accessed 2017 Oct 9]. Available from: https://www.cdc.gov/vitalsigns/hispanic-health/
- 11.Forno E, Celedón JC. Health disparities in asthma. Am J Respir Crit Care Med. 2012;185:1033–1035. doi: 10.1164/rccm.201202-0350ED. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Choudhry S, Burchard EG, Borrell LN, Tang H, Gomez I, Naqvi M, et al. Ancestry-environment interactions and asthma risk among Puerto Ricans. Am J Respir Crit Care Med. 2006;174:1088–1093. doi: 10.1164/rccm.200605-596OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bruse S, Sood A, Petersen H, Liu Y, Leng S, Celedón JC, et al. New Mexican Hispanic smokers have lower odds of chronic obstructive pulmonary disease and less decline in lung function than non-Hispanic whites. Am J Respir Crit Care Med. 2011;184:1254–1260. doi: 10.1164/rccm.201103-0568OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Homa DM, Mannino DM, Lara M. Asthma mortality in U.S. Hispanics of Mexican, Puerto Rican, and Cuban heritage, 1990-1995. Am J Respir Crit Care Med. 2000;161:504–509. doi: 10.1164/ajrccm.161.2.9906025. [DOI] [PubMed] [Google Scholar]
- 15.United States Census Bureau. The Hispanic population: 2010. 2010 Census Brief. United States Census Bureau; 2011 [accessed on 2017 Jun 10]. Available from: https://www.census.gov/prod/cen2010/briefs/c2010br-04.pdf.
- 16.FitzSimmons SC. The changing epidemiology of cystic fibrosis. J Pediatr. 1993;122:1–9. doi: 10.1016/s0022-3476(05)83478-x. [DOI] [PubMed] [Google Scholar]
- 17.Nick JA, Chacon CS, Brayshaw SJ, Jones MC, Barboa CM, St Clair CG, et al. Effects of gender and age at diagnosis on disease progression in long-term survivors of cystic fibrosis. Am J Respir Crit Care Med. 2010;182:614–626. doi: 10.1164/rccm.201001-0092OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Stephenson AL, Sykes J, Stanojevic S, Quon BS, Marshall BC, Petren K, et al. Survival comparison of patients with cystic fibrosis in Canada and the United States: a population-based cohort study. Ann Intern Med. 2017;166:537–546. doi: 10.7326/M16-0858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dasenbrook EC, Checkley W, Merlo CA, Konstan MW, Lechtzin N, Boyle MP. Association between respiratory tract methicillin-resistant Staphylococcus aureus and survival in cystic fibrosis. JAMA. 2010;303:2386–2392. doi: 10.1001/jama.2010.791. [DOI] [PubMed] [Google Scholar]
- 20.Liou TG, Adler FR, Fitzsimmons SC, Cahill BC, Hibbs JR, Marshall BC. Predictive 5-year survivorship model of cystic fibrosis. Am J Epidemiol. 2001;153:345–352. doi: 10.1093/aje/153.4.345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Moran A, Hardin D, Rodman D, Allen HF, Beall RJ, Borowitz D, et al. Diagnosis, screening and management of cystic fibrosis related diabetes mellitus: a consensus conference report. Diabetes Res Clin Pract. 1999;45:61–73. doi: 10.1016/s0168-8227(99)00058-3. [DOI] [PubMed] [Google Scholar]
- 22.Tablan OC, Chorba TL, Schidlow DV, White JW, Hardy KA, Gilligan PH, et al. Pseudomonas cepacia colonization in patients with cystic fibrosis: risk factors and clinical outcome. J Pediatr. 1985;107:382–387. doi: 10.1016/s0022-3476(85)80511-4. [DOI] [PubMed] [Google Scholar]
- 23.Emerson J, Rosenfeld M, McNamara S, Ramsey B, Gibson RL. Pseudomonas aeruginosa and other predictors of mortality and morbidity in young children with cystic fibrosis. Pediatr Pulmonol. 2002;34:91–100. doi: 10.1002/ppul.10127. [DOI] [PubMed] [Google Scholar]
- 24.Schechter MS, Shelton BJ, Margolis PA, Fitzsimmons SC. The association of socioeconomic status with outcomes in cystic fibrosis patients in the United States. Am J Respir Crit Care Med. 2001;163:1331–1337. doi: 10.1164/ajrccm.163.6.9912100. [DOI] [PubMed] [Google Scholar]
- 25.Knapp EA, Fink AK, Goss CH, Sewall A, Ostrenga J, Dowd C, et al. The Cystic Fibrosis Foundation Patient Registry: design and methods of a national observational disease registry. Ann Am Thorac Soc. 2016;13:1173–1179. doi: 10.1513/AnnalsATS.201511-781OC. [DOI] [PubMed] [Google Scholar]
- 26.Kressin NR, Chang BH, Hendricks A, Kazis LE. Agreement between administrative data and patients’ self-reports of race/ethnicity. Am J Public Health. 2003;93:1734–1739. doi: 10.2105/ajph.93.10.1734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gomez SL, Kelsey JL, Glaser SL, Lee MM, Sidney S. Inconsistencies between self-reported ethnicity and ethnicity recorded in a health maintenance organization. Ann Epidemiol. 2005;15:71–79. doi: 10.1016/j.annepidem.2004.03.002. [DOI] [PubMed] [Google Scholar]