To the Editor:
Intermittent hypoxia (IH) is a highly prevalent occurrence in a large variety of respiratory diseases and has been associated with a wide array of morbidities. Moreover, recent experimental and clinical studies have revealed a potential relationship between obstructive sleep apnea (OSA) and cancer incidence and mortality (1). Indeed, IH mimicking OSA increases tumor malignant properties in melanoma and lung adenocarcinoma in vitro and in in vivo murine models (2, 3), where IH-induced immune deregulation appears to play a pivotal role in IH-induced malignancy enhancement (3, 4). However, there are still several important open questions regarding the relationship between OSA and cancer, and more particularly how aging affects IH–tumor interactions. The translational relevance of age as a potential modulator of IH-facilitated malignant properties is quite obvious considering that the prevalence of cancer and OSA increases with increasing patient age, and that similarly to IH, aging downregulates the immune system (3–5). In contrast, we should also point out that the association between OSA incidence and adverse outcomes and cancer is more prominent in younger patients (<55 yr) than in older patients (6, 7), such that the effect of advanced age is unclear. Here, we report our findings from a murine model of OSA in which we tested the hypothesis that aging would reduce the protumoral effect of IH both in vivo and in vitro, and that the aging effect would be linked to altered immune responses.
After approval by the Ethics Committee of the University of Barcelona, 45 female C57Bl/6j mice (young: n = 24, 2 mo old; old: n = 21, 20 mo old) were studied. The animals were preexposed to either IH or room air (RA) for 6 hours/day for 10 days as previously described (8) and then subcutaneously injected with 105 Lewis lung carcinoma (LLC1) cells (American Type Culture Collection) in the right flank while continuing their corresponding exposures (8). Four weeks later, the mice were killed and tumors were excised and weighed. A portion of each tumor was digested to evaluate the abundance of tumor-associated macrophages (TAMs) and regulatory T lymphocytes by flow cytometry (FACS Canto II, BD Biosciences), followed by analysis with FlowJo software (Tree Star) as previously described (3, 4). In addition, TAMs were isolated from each tumor with magnetic beads coupled to anti-CD11b antibody (StemCell Technologies), and the effect of TAMs on the proliferative rate of naive LLC1 cells in culture was also assessed (3). All results are shown as mean ± SE. The effects of aging (young vs. old) and treatment (RA vs. IH) were assessed by two-way ANOVA.
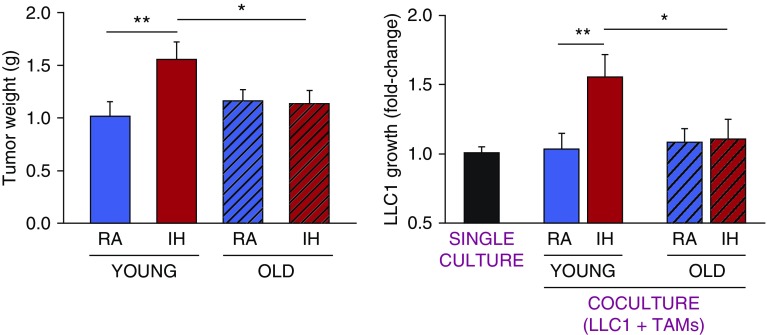
Consistent with previous findings (2–4, 9), young mice exposed to IH exhibited an ∼55% increase (P = 0.007) in tumor growth relative to RA-exposed mice. However, these changes were absent in aged mice (Figure 1, left), a finding that concurs with epidemiological evidence pointing to a prominent and significant association between OSA and cancer aggressiveness exclusively among younger patients with OSA (6, 7). We also found that chronic exposures to IH induced an ∼85% increase in macrophage infiltration in the tumors of young mice (from 7.1 × 106 ± 1.1 × 106 TAMs in RA to 13.0 × 106 ± 1.7 × 106 TAMs in IH; P = 0.015), but not in aged mice (from 5.4 × 106 ± 0.8 × 106 TAMs in RA to 6.8 × 106 ± 1.2 × 106 TAMs in IH; P = 0.288). Furthermore, the capability of TAMs to increase tumor proliferation in vitro was enhanced only in TAMs isolated from tumors of young mice exposed to IH (Figure 1, right). The reduced recruitment of TAMs to the tumors of old mice under IH conditions suggests that aged animals may not be as able as young mice to develop a depot of inflammatory cells with peritumoral adipose tissues (4). Moreover, it is likely that the lack of changes in the proliferative properties of naive tumor cells when placed in coculture with TAMs isolated from aged mice exposed to IH reflects the tumor microenvironment’s inability to shift the polarity of TAMs to a more protumoral phenotype in aged mice, as was previously reported for young mice (3, 4). Of note, the relative abundance of regulatory T lymphocytes (expressed as the ratio to total lymphocyte [CD3+ cells] counts) increased (P = 0.042) within the tumors of young and old mice exposed to IH (0.68% ± 0.17% vs. 0.43% ± 0.10%, respectively) compared with RA-exposed mice (0.37% ± 0.08% vs. 0.18% ± 0.06%, respectively), but were significantly reduced by aging (P = 0.012). These lymphocytes are potent immunosuppressive cells and have been associated with poor prognosis in many solid tumors.
Figure 1.
(Left) Tumor weight assessed in young and old mice exposed to either intermittent hypoxia (IH) or room air (RA). (Right) Proliferation of lung adenocarcinoma cells in single culture or cocultured with tumor-associated macrophages (TAMs) from young or old mice exposed to either IH or RA. Data are presented as mean ± SE. *P < 0.05 and **P < 0.01.
Taken together, our findings suggest that IH potentiates tumor progression through changes in the immune system in young mice, and that such changes are conspicuously absent in aged mice. Given that we previously found that hypoxic severity (measured as blood oxygen desaturation) in response to obstructive apneas was remarkably similar in young and old rats (10), we postulated that the different immune responses in young and aged mice may be the consequence of reduced levels of reactive oxygen species generated in response to IH in older animals, as previously reported for rats exposed to obstructive apneas (10). A reduced oxidative stress level in older mice in response to IH could also help to explain our results, considering that IH-induced oxidative stress was recently suggested to play a pivotal role in lung adenocarcinoma aggressiveness (9). However, further characterization of metabolic rates, oxygen consumption, and tissue perfusion and oxygenation in tumors during exposure to IH in both young and old mice will allow us to better understand the differential effects caused by aging.
In summary, we show that chronological age emerges as an important factor to consider when studying the effects of IH in lung malignancies, and that the presence of a specific phenotypic presentation in younger ages may not necessarily become manifest later in life. As such, this study highlights the importance of employing experimental models that recapitulate with the utmost fidelity the pathophysiological conditions of the disease under study, in particular regarding the use of age-appropriate animals. Indeed, it is notable that most studies in animal models of chronic human diseases that primarily affect aged patients have been conducted in animals ranging in age from late adolescence to young adulthood—an issue that needs to be addressed moving forward.
Footnotes
I.A. is supported by SEPAR (595/2017). M.A.M.-G. is supported by the Spanish Ministry of Economy and Competitiveness–Instituto de Salud Carlos III (PI16/01772) and SEPAR (211/2012). R.F. is supported by the Spanish Ministry of Economy and Competitiveness (SAF2017-85574-R). D.N. is supported by the Spanish Ministry of Economy and Competitiveness (DPI2017-83721-P). P.N.N. is supported by the National Counsel of Technological and Scientific Development from Brazil-CNPq (207258/2014-7). D.G. is supported by NIH grant 1R01HL130984.
Author Contributions: I.A. and R.F. conceived the study. M.T., N.C., and P.N.N. performed the experiments. M.T., I.A., R.F., D.N., J.M.M., D.G., M.A.M.-G., and F.C.-R. contributed to data analysis and interpretation. M.T., I.A., R.F., and D.G. contributed to drafting the manuscript. All authors reviewed and approved the final version of the manuscript.
Originally Published in Press as DOI: 10.1164/rccm.201805-0892LE on July 17, 2018
Author disclosures are available with the text of this letter at www.atsjournals.org.
References
- 1.Martínez-García MÁ, Campos-Rodríguez F, Almendros I, Farré R. Relationship between sleep apnea and cancer. Arch Bronconeumol. 2015;51:456–461. doi: 10.1016/j.arbres.2015.02.002. [DOI] [PubMed] [Google Scholar]
- 2.Almendros I, Montserrat JM, Ramírez J, Torres M, Duran-Cantolla J, Navajas D, et al. Intermittent hypoxia enhances cancer progression in a mouse model of sleep apnoea. Eur Respir J. 2012;39:215–217. doi: 10.1183/09031936.00185110. [DOI] [PubMed] [Google Scholar]
- 3.Almendros I, Wang Y, Becker L, Lennon FE, Zheng J, Coats BR, et al. Intermittent hypoxia-induced changes in tumor-associated macrophages and tumor malignancy in a mouse model of sleep apnea. Am J Respir Crit Care Med. 2014;189:593–601. doi: 10.1164/rccm.201310-1830OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Almendros I, Gileles-Hillel A, Khalyfa A, Wang Y, Zhang SX, Carreras A, et al. Adipose tissue macrophage polarization by intermittent hypoxia in a mouse model of OSA: effect of tumor microenvironment. Cancer Lett. 2015;361:233–239. doi: 10.1016/j.canlet.2015.03.010. [DOI] [PubMed] [Google Scholar]
- 5.Montecino-Rodriguez E, Berent-Maoz B, Dorshkind K. Causes, consequences, and reversal of immune system aging. J Clin Invest. 2013;123:958–965. doi: 10.1172/JCI64096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Martínez-García MA, Campos-Rodriguez F, Durán-Cantolla J, de la Peña M, Masdeu MJ, González M, et al. Spanish Sleep Network. Obstructive sleep apnea is associated with cancer mortality in younger patients. Sleep Med. 2014;15:742–748. doi: 10.1016/j.sleep.2014.01.020. [DOI] [PubMed] [Google Scholar]
- 7.Campos-Rodriguez F, Martinez-Garcia MA, Martinez M, Duran-Cantolla J, Peña MdeL, Masdeu MJ, et al. Spanish Sleep Network. Association between obstructive sleep apnea and cancer incidence in a large multicenter Spanish cohort. Am J Respir Crit Care Med. 2013;187:99–105. doi: 10.1164/rccm.201209-1671OC. [DOI] [PubMed] [Google Scholar]
- 8.Campillo N, Torres M, Vilaseca A, Nonaka PN, Gozal D, Roca-Ferrer J, et al. Role of cyclooxygenase-2 on intermittent hypoxia-induced lung tumor malignancy in a mouse model of sleep apnea. Sci Rep. 2017;7:44693. doi: 10.1038/srep44693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Li L, Ren F, Qi C, Xu L, Fang Y, Liang M, et al. Intermittent hypoxia promotes melanoma lung metastasis via oxidative stress and inflammation responses in a mouse model of obstructive sleep apnea. Respir Res. 2018;19:28. doi: 10.1186/s12931-018-0727-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dalmases M, Torres M, Márquez-Kisinousky L, Almendros I, Planas AM, Embid C, et al. Brain tissue hypoxia and oxidative stress induced by obstructive apneas is different in young and aged rats. Sleep (Basel) 2014;37:1249–1256. doi: 10.5665/sleep.3848. [DOI] [PMC free article] [PubMed] [Google Scholar]