Abstract
Rationale: Lung natural killer cells (NKs) kill a greater percentage of autologous lung parenchymal cells in chronic obstructive pulmonary disease (COPD) than in nonobstructed smokers. To become cytotoxic, NKs require priming, typically by dendritic cells (DCs), but whether priming occurs in the lungs in COPD is unknown.
Methods: We used lung tissue and in some cases peripheral blood from patients undergoing clinically indicated resections to determine in vitro killing of CD326+ lung epithelial cells by isolated lung CD56+ NKs. We also measured the cytotoxicity of unprimed blood NKs after preincubation with lung DCs. To investigate mechanisms of DC-mediated priming, we used murine models of COPD induced by cigarette smoke (CS) exposure or by polymeric immunoglobulin receptor (pIgR) deficiency, and blocked IL-15Rα (IL-15 receptor α subunit) trans-presentation by genetic and antibody approaches.
Results: Human lung NKs killed isolated autologous lung epithelial cells; cytotoxicity was increased (P = 0.0001) in COPD, relative to smokers without obstruction. Similarly, increased lung NK cytotoxicity compared with control subjects was observed in CS-exposed mice and pIgR−/− mice. Blood NKs both from smokers without obstruction and subjects with COPD showed minimal epithelial cell killing, but in COPD, preincubation with lung DCs increased cytotoxicity. NKs were primed by CS-exposed murine DCs in vitro and in vivo. Inhibiting IL-15Rα trans-presentation eliminated NK priming both by murine CS-exposed DCs and by lung DCs from subjects with COPD.
Conclusions: Heightened NK cytotoxicity against lung epithelial cells in COPD results primarily from lung DC–mediated priming via IL-15 trans-presentation on IL-15Rα. Future studies are required to test whether increased NK cytotoxicity contributes to COPD pathogenesis.
Keywords: human, chronic obstructive pulmonary disease, small-airway epithelial cells, cigarette smoke
At a Glance Commentary
Scientific Knowledge on the Subject
Natural killer cells (NKs) may contribute to lung tissue damage in chronic obstructive pulmonary disease (COPD) by inducing lung parenchymal cell apoptosis, but they are also essential to eliminate virally infected or malignant cells. Although previously thought to be innately competent to lyse targets, NKs are now believed to require priming, particularly by dendritic cells (DCs). However, whether lung DCs actually prime NKs in COPD, and if so, how, and whether lung epithelial cells are significant NK targets are all unknown.
What This Study Adds to the Field
We used human lung tissue and two murine models of COPD to demonstrate that lung NK cell cytotoxicity 1) targets lung epithelial cells; 2) depends more greatly on lung NK priming than on lung epithelial cell activation state; and 3) is significantly increased in COPD relative to smokers without airflow obstruction. Human and murine lung DCs increased the cytotoxicity of unprimed NKs by trans-presenting IL-15 bound to the IL-15 receptor α subunit. Inappropriate interactions between lung DCs and NKs in those at risk for COPD have the potential to contribute to COPD pathogenesis.
Chronic obstructive pulmonary disease (COPD), currently the fourth leading cause of death in the United States and third worldwide (1, 2), results from inhaled oxidants, including biomass fuel inhalation and cigarette smoke exposure. COPD is characterized by airway remodeling, mucus hypersecretion, parenchymal lung destruction, and progressively intensifying inflammatory cell infiltration (3, 4). Whether all of these infiltrating cell types contribute to COPD pathology, or instead if some are responding appropriately to microbial colonization (5), is unresolved.
Prominent among the leukocyte types infiltrating the lungs in COPD are natural killer cells (NKs), innate lymphocytes that rapidly target and kill abnormal cells. Regardless of smoking or COPD status, most NKs in normal human lung parenchyma display a differentiated CD56dimCD16+ phenotype associated with cytotoxicity (6, 7), which differs from the predominance of CD56bright NKs in other organs and within tumors. Mouse lung NKs show a similarly differentiated phenotype (8). In COPD, relative to smokers without COPD, NKs from sputum and alveolar fluid are more cytotoxic toward highly susceptible targets (9, 10). We have shown that CD56+ NKs, but not CD8+ or CD4+ cells, from human lung parenchyma can kill autologous lung parenchymal cells, and that this killing is increased in subjects with severe COPD compared with smokers without obstruction (6). In murine cigarette smoke (CS) exposure models, we and others have shown that lung NKs from CS-exposed mice are increased in numbers, display a more primed phenotype, and are more cytotoxic than in nonexposed mice (11–13). Because apoptosis of lung structural cells has been implicated in emphysema pathogenesis (14–16), defining the mechanisms driving NK cytotoxicity in COPD is a significant goal.
NKs require signals, termed priming, to transition from quiescence to readiness for cytotoxicity (17, 18). In vivo murine studies showed that dendritic cells (DCs) are essential for NK priming to viral and bacterial pathogens (19–21). DCs prime NKs either by cell–cell contact, via soluble mediators, or both, depending on the stimulus and location of their interaction. DC-produced cytokines known to activate NKs include type I interferons, IL-12, and IL-18 (11). IL-15 is a particularly important regulator of NK development, differentiation, homeostasis, and activation (22). In lymph nodes, IL-15 trans-presentation by CD11chigh DCs is necessary and sufficient to prime resting NKs (19). Human IL-15–derived DCs induce NK cytotoxicity toward both sensitive and resistant tumors (23). How cigarette smoke affects DC priming of NKs is unknown.
The goal of this study was to define whether and how lung DCs contribute to lung NK priming in COPD. Given the many similarities between mouse and human lung NKs, to address certain mechanistic questions we used two murine models. The first was CS exposure, which reproducibly induces many features of COPD, including pulmonary cellular infiltration, airway fibrosis, and emphysema (24). The second was the spontaneous pathology developing in mice lacking the polymeric immunoglobulin receptor (pIgR−/−) (25), which is necessary to transcytose secretory IgA into small airways. As they age, pIgR−/− mice develop progressive airway wall remodeling and emphysema (25). Our collective results show that lung epithelial cells are a major target of NK cytotoxicity in vitro, that killing is heightened in COPD, and that priming of NKs by DCs requires IL-15Rα (IL-15 receptor α subunit) trans-presentation. Some results have been previously reported as an abstract (26).
Methods
Ethics Statement
Studies and consent procedures were performed in accordance with the Declaration of Helsinki at the VA Ann Arbor Healthcare System and the University of Michigan Health System and were approved by the institutional review board at each site (FWA 00000348 and FWA 00004969, respectively). Written informed consent was obtained preoperatively.
Specimens and Patient Population
We recruited subjects undergoing clinically indicated resections for pulmonary nodules and collected only distal, nonneoplastic lung tissue lacking postobstructive changes as judged by a pathologist. All subjects (n = 49) underwent preoperative post-bronchodilator spirometry, prospectively collected medication history, and clinical evaluation by a pulmonologist. We categorized subjects on the basis of the 2001 classification of the Global Initiative for Chronic Obstructive Lung Disease (GOLD) (27). Subjects (n = 19) with a smoking history of at least 10 pack-years, an FEV1/FVC ratio greater than 0.70, normal spirometry, and no clinical diagnosis of COPD represent smoking control subjects. Subjects (n = 30) with a smoking history of at least 10 pack-years and an FEV1/FVC ratio less than 0.7 were considered to have COPD. Table 1 shows demographics, pulmonary function, and inhaled corticosteroid usage. We defined former smoking as having quit for 6 months. Some subjects also contributed peripheral blood 3–6 weeks before surgery.
Table 1.
Summary of Subject Demographics, Smoking History, and Spirometry
| Group | Smoker | COPD | P Value |
|---|---|---|---|
| Subjects, n | 19 | 30 | — |
| Sex: M, F | 16, 3 | 25, 5 | NS |
| Age, yr | 67 (7) | 65 (6) | NS |
| Smoking, pack-years | 55 (39) | 61 (28) | NS |
| Smoking status: active, former | 4, 15 | 14, 16 | NS |
| FEV1, % pred | 94 (13) | 77 (21) | 0.004 |
| DlCO, % pred | 94 (16) | 76 (21) | 0.002 |
| ICS usage: yes, no | 1, 18 | 10, 20 | 0.03 |
| Cancer diagnosis: NSCLC, other, no cancer | 14, 2, 0* | 24, 1, 4* | NS |
Definition of abbreviations: % pred = percentage of the predicted value; COPD = chronic obstructive pulmonary disease; ICS = inhaled corticosteroids; NS = not significant; NSCLC = non–small cell lung carcinoma.
Data are presented as mean (SD), except where otherwise noted.
Cancer diagnosis is missing for some individuals.
Animals
Male and female C57BL/6, B6.SJL, IL-15Rα−/− on a C57BL/6 background, and B6129SF2/J mice between 6 and 8 weeks of age were obtained from Jackson Laboratory. Mice were housed under specific pathogen–free conditions, regulated lighting, and ad libitum feeding at the VA Ann Arbor Healthcare System, which is fully accredited by the Association for Assessment and Accreditation of Laboratory Animal Care International. All experiments were approved by the Ann Arbor VA Subcommittee on Animal Studies. pIgR−/− mice were generated as previously described (25) and maintained at the Vanderbilt University Medical Center. All procedures involving pIgR−/− mice were approved by the Institutional Care and Use Committee of Vanderbilt University. Intact lung tissue of pIgR−/− mice was collected in medium and shipped on ice for next-day processing in Ann Arbor.
Murine Cigarette Smoke Exposure
We performed whole-body exposure of mice for 8 weeks as described in the online supplement.
Cell Isolation from Lung Tissue and Peripheral Blood
Human and murine lung samples were dispersed mechanically without enzyme treatments, producing single-cell suspensions of high viability and functional capacity (6, 28, 29). Cells were isolated with immunomagnetic beads, as described in the online supplement, to isolate lung NKs (human, CD56+; mouse, CD49b+), lung epithelial cells (CD326+ in both species), and lung DCs for immediate use in the cytotoxicity assay. We also isolated CD56+ NKs from the peripheral blood of some human subjects and cryopreserved them until their lung tissue was obtained.
NK Cytotoxicity Assay
We assayed specific cytotoxicity in a 4-hour flow cytometry–based assay based on detection of apoptosis, using annexin-V and 7-aminoactinomycin D (7-AAD), as described in the online supplement (6). When DCs and NKs were cocultured at a ratio of 1:1, they interacted in the absence of target cells for 16 hours. In some experiments, we added a 10-μg/ml concentration of anti-mouse IL-15R/IL-15 (clone GRW15PLZ; eBioscience) or a 0.5-μg/ml concentration of recombinant human IL-15Rα Fc chimera (R&D Systems).
DC Adoptive Transfer
Murine DCs were resuspended at 200,000 DCs in 20 μl of phosphate-buffered saline and administered intranasally to untreated congenic recipient mice under isoflurane sedation. After 48 hours, lungs and mediastinal lymph nodes were collected. We isolated NKs and epithelial cells from lung tissue to use in cytotoxicity assays. To verify DC transfer, lymph nodes and a portion of whole lung were stained with CD45.2 and CD45.1 antibodies.
Statistics
Statistical analyses were performed with GraphPad Prism 7.0 (GraphPad Software, Inc.) and SPSS (IBM Corporation) on a Macintosh Quad-Core Intel Xeon computer running OS X 10.12.6 (Apple). We used Mann-Whitney t tests to evaluate differences between two groups, and one-way ANOVA with Tukey’s multiple comparison test for three or more groups. A paired t test was used when comparisons were made using cell populations from the same sample. Correlations were tested by Spearman regression. A two-tailed P value less than 0.05 was considered to indicate significance.
Results
Lung NKs from Subjects with COPD Have an Increased Ability to Kill Autologous Lung Epithelial Cells
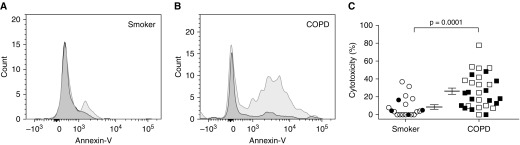
Although we have shown greater killing of autologous lung parenchymal cells by lung CD56+ cells from ever-smokers with COPD, relative to smokers without obstruction or never-smokers (6), those experiments did not test the target cell type. We extended those findings by showing that lung CD56+ cells kill lung cells that express the epithelial-specific homotypic cell adhesion molecule CD326 (EpCAM) (Figures 1A and 1B). Once again, lung CD56+ cells from subjects with COPD were significantly more cytotoxic than those from nonobstructed smokers, independent of current smoking status (Figure 1C). In a linear regression model (Table 2), CD56-mediated cytotoxicity against autologous epithelial cells positively correlated with GOLD spirometric stage, after controlling for age, sex, smoking status (current vs. former), and duration of smoking (pack-years). Subjects were categorized on the basis of the 2001 GOLD classification (27). Cytotoxicity did not correlate with FEV1% predicted (data not shown), perhaps attributable to the relatively mild COPD of this operative cohort (FEV1% predicted [mean ± SD], 77 ± 21%). Cytotoxicity also showed no correlation with age, sex, inhaled corticosteroid usage, pack-years of smoking, or DlCO % predicted; however, it did correlate inversely with FEV1/FVC (r = −0.351, P = 0.013; Spearman correlation).
Figure 1.
Autologous lung epithelial cells are killed more avidly by lung natural killer cells (NKs) from patients with chronic obstructive pulmonary disease (COPD) than from smokers without obstruction. From dispersed human lung tissue, we sequentially isolated CD56+ NKs and then CD326+ (epithelial cell adhesion molecule) cells, using magnetic beads. Epithelial cells were cultured either alone or with CD56+ cells, and then after 4 hours all cells were collected; stained with CD45, annexin-V, and 7-aminoactinomycin D; and analyzed by flow cytometry. Epithelial cells were identified as CD45− with high side scatter. Representative histograms are shown of annexin-V staining from (A) a smoker without COPD and (B) a subject with COPD, showing epithelial cells cultured alone (dark gray histograms) and epithelial cells cultured with NKs (light gray histograms). (C) The percent cytotoxicity (calculated as described in Methods) is shown for individual subjects: n = 19 smokers without obstruction and n = 30 subjects with COPD. Lines represent means ± SEM, offset to facilitate visualization. Open symbols, former smokers; solid symbols, active smokers. The Mann-Whitney t test was used to determine significance.
Table 2.
Demonstration by Linear Regression of Association between Cytotoxicity and GOLD Stage
| Model | β Coefficient | Significance |
|---|---|---|
| Dependent variable: GOLD stage | ||
| Independent variables | ||
| Age | −0.19 | 0.18 |
| Sex | 0.17 | 0.24 |
| Smoking status, former vs. current | 0.14 | 0.31 |
| Duration of smoking, pack-years | 0.16 | 0.24 |
| NK-mediated cytotoxicity | 0.40 | 0.007 |
Definition of abbreviations: GOLD = Global Initiative for Chronic Obstructive Lung Disease; NK = natural killer cell.
Because CD56 can be expressed by both NK cells and NK T cells, we separated lung CD56+ cells from n = 3 subjects with COPD into CD3+ and CD3− populations, which were assayed simultaneously against identical lung epithelial cell populations. Lung CD56+CD3− (NK) cells were significantly more cytotoxic than lung CD56+CD3+ (NK T) cells (13.6 ± 1.6 vs. 3.4 ± 2.4% killing, respectively; P < 0.006, paired t test). However, most resected lung specimens contained too few total CD56+ cells to permit routine separation into CD3+ and CD3− populations. We also evaluated phenotypic markers, including CD3, CD56, CD16, and CD69 expression, on lung samples from a subset of the subjects (n = 6 smokers without obstruction; n = 15 subjects with COPD). We determined the percentage of CD56+CD3− NK cells and CD56+CD3+ NK T cells in the unfractionated lung specimen. These percentages neither differed between subject groups, in agreement with our previous results (6), nor correlated with cytotoxicity toward autologous epithelial cells (data not shown). Accordingly, as it is unlikely that isolation with anti-CD56 immunomagnetic beads would alter these ratios in the experiments in which we did not separate on the basis of CD3 expression, we refer to the cytotoxic CD56+ cells in these experiments as lung NK cells.
Using flow cytometry, we confirmed our previous finding (6) that CD3− lung NK were predominantly CD56+ CD16+ rather than CD56+CD16− (12.3 ± 9.6% vs. 2.0 ± 1.2% of lymphocytes) and that this predominance did not differ between smokers without versus with COPD (data not shown). We further confirmed that the majority CD56+ CD16+ lung NK subset (the terminology used in our previous article) are CD56dim relative to the minority CD56brightCD16− NKs, based on significant differences in mean fluorescence intensity for CD56 staining (730 ± 567 vs. 1,994 ± 1,411 arbitrary units; P = 0.0005 by Mann-Whitney t test). There was no difference in the percentage of NKs expressing CD69 between subject groups (4.2 ± 3.3% in smokers without obstruction vs. 7.0 ± 8.3% in subjects with COPD). These results are similar to a study that also found human lung NKs to be predominantly CD69− (30).
Lung NKs, Not Lung Epithelial Cells, Drive the Increased Cytotoxicity in COPD
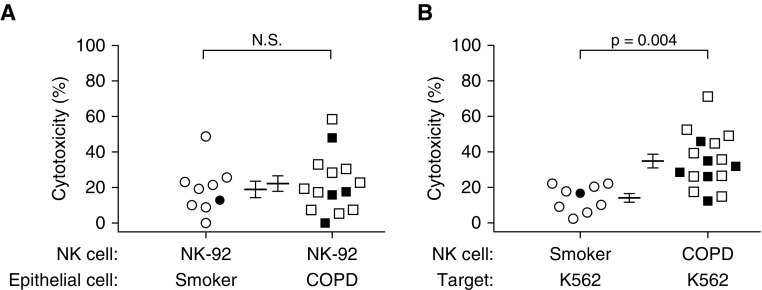
Because lung epithelial cells from subjects with COPD have increased expression of ligands for NK-activating receptors (6, 31), we simultaneously performed two types of mixing experiments to test whether cytotoxicity depended more greatly on epithelial cell ligand expression or on NK state. We cocultured human lung epithelial cells from smokers without obstruction or subjects with COPD with the same NK source, NK-92 cells; this cloned line lacks inhibitory killer-cell immunoglobulin-like receptors, and hence kills avidly (Figure 2A). In parallel, we cocultured lung NKs from the same subjects with K562 cells, a highly susceptible target because of their lack of MHC class I expression (Figure 2B). Results showed no difference between subject groups in epithelial cell killing by NK-92 cells (healthy smokers, 18.9 ± 4.6% vs. subjects with COPD, 22.3 ± 4.4%) (Figure 2A). In contrast, lung NKs from subjects with COPD had significantly greater cytotoxicity toward the K562 cells (34.8 ± 3.8% vs. 14.1 ± 2.5%; P = 0.004) (Figure 2B). By suggesting that the increased cytotoxicity in subjects with COPD was largely independent of epithelial cell ligands, these results led us to focus on lung NK priming.
Figure 2.
Lung natural killer cells (NKs), not lung epithelial cells, drive the increased cytotoxicity in chronic obstructive pulmonary disease (COPD). CD56+ NKs and CD326+ epithelial cells were isolated from human lung tissue. (A) Lung epithelial cells from smokers without obstruction (n = 9) or patients with COPD (n = 14) were cultured either alone or with NK-92 cells to determine the percent cytotoxicity; (B) K562 target cells were cultured either alone or with CD56+ cells from smokers without obstruction (n = 9) or from patients with COPD (n = 16) to determine the percent cytotoxicity. For both panels, lines represent means ± SEM, offset to facilitate visualization. Open symbols, former smokers; solid symbols, active smokers. The same subjects were used to generate the data in A and B, although we were unable to perform the experiment in A on two of the subjects with COPD. The Mann-Whitney t test was used to determine significance. N.S. = not significant.
CS Exposure in Mice Increases NK Cytotoxicity against Lung Epithelial Cells
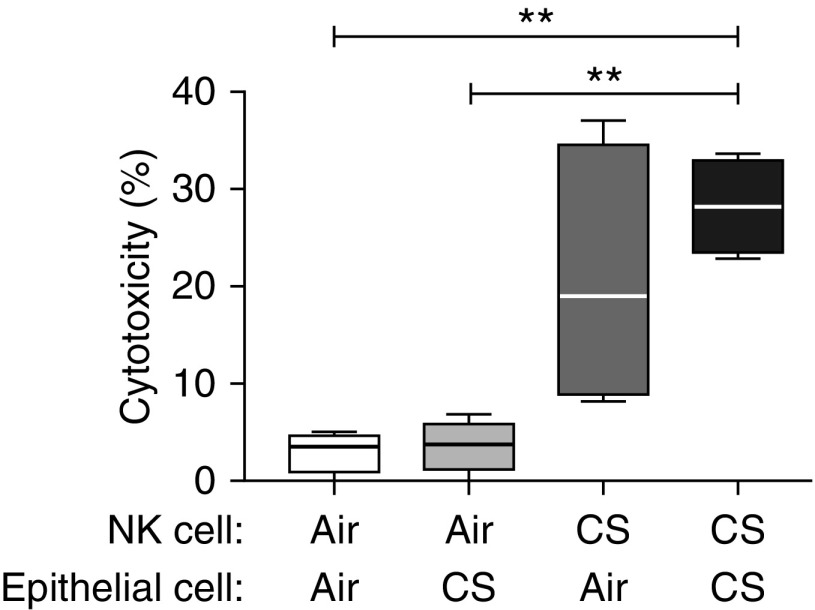
To address mechanistic questions, we used a murine model of CS exposure (13). We isolated CD49b+ (DX5+) NKs and CD326+ epithelial cells from the lungs of CS-exposed or air-exposed mice and cocultured them in various combinations (Figure 3). In all experiments, epithelial cells were also cultured by themselves to determine baseline viability, and we used the same flow cytometry–based cytotoxicity assay as for human samples. NKs from air-exposed mice showed minimal killing, regardless of epithelial cell source (Figure 3). In contrast, NKs from CS-exposed mice exhibited considerable cytotoxicity toward epithelial cells from either air- or CS-exposed mice, although only the latter target reached statistical significance. We performed these experiments using both male and female mice, without difference in results between sexes (data not shown). These data support our human data, collectively suggesting that epithelial factors are important and necessary, but insufficient, to induce NK-mediated killing. Further, these results support this model as relevant to investigate the molecular basis for increased lung NK cytotoxicity in human COPD.
Figure 3.
Lung natural killer cells (NKs) are more cytotoxic against epithelial cells in a murine model of cigarette smoke (CS) exposure. C57BL/6 mice were exposed to air or CS for 8 weeks, and then lung tissue was collected for the isolation of NKs (CD49b+) and CD326+ epithelial cells. Epithelial cells from air-exposed mice were cultured either alone or with NKs from air-exposed or CS-exposed mice. Likewise, epithelial cells from CS-exposed mice were cultured either alone or with NKs from air-exposed or CS-exposed mice. After 4 hours, cells were collected and stained for CD45, annexin-V, and 7-aminoactinomycin D for flow cytometry. Epithelial cells were identified as CD45− with high side scatter. The percent cytotoxicity is shown for four separate experiments (five mice per group per experiment). Box plots show the first and third quartiles plus the median, and the whiskers represent the minimum and maximum values. Repeated measures one-way ANOVA with Tukey’s multiple comparison test was used to determine significance. **P < 0.01.
Dendritic Cells Prime NKs to Become Cytotoxic after CS Exposure in Mice
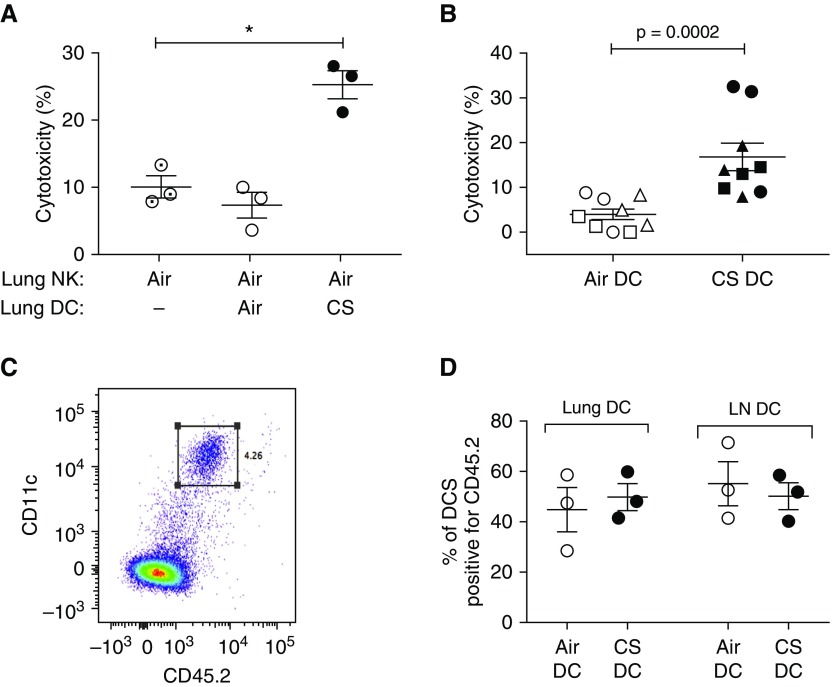
To determine whether lung DCs were essential to prime NKs in the context of CS, as previously shown for other stimuli (18, 19, 32), we isolated lung DCs from air- or CS-exposed mice and cocultured them overnight with lung NKs from air-exposed mice. For all conditions, we used epithelial cells pooled from the lungs of CS-exposed mice as targets. Coculture with DCs from air-exposed mice had no effect on cytotoxicity, but coculture with DCs from CS-exposed mice increased cytotoxicity approximately threefold (P = 0.0002) (Figure 4A). In the absence of NKs, DCs from air- or CS-exposed mice showed no epithelial cell cytotoxicity (data not shown).
Figure 4.
Dendritic cells (DCs) from cigarette smoke (CS)–exposed mice prime natural killer cells (NKs) to become cytotoxic, both in vitro and in vivo. C57BL/6 mice were exposed either to air or CS for 8 weeks. Lung tissue was collected and dispersed for isolation of NKs (CD49b+), CD326+ epithelial cells, and pan-DCs. (A) Lung NKs from air-exposed mice were preincubated for 18 hours alone or with DCs from air- or CS-exposed mice, and then pooled lung epithelial cells from CS-exposed mice were added for an additional 4 hours. Cytotoxicity was then assayed. Data are from three separate experiments (each with five mice per group); a repeated measures one-way ANOVA with Tukey’s multiple comparison test was used to determine significance. (B) DCs from either air- or CS-exposed mice (CD45.2 congenic strain) were adoptively transferred (200,000 cells intranasal) into naive recipient mice (CD45.1 congenic strain). Recipient mice were killed 48 hours later and lungs were collected to isolate NKs and epithelial cells. Pooled epithelial cells from all mice were cultured either alone or with NKs from individual mice. After 4 hours, cells were collected and cytotoxicity was assayed. Data represent means ± SEM of three mice per group in each of three separate experiments, indicated by symbols. The Mann-Whitney t test was used to determine significance. (C) Representative flow staining of lung tissue from recipient mouse that received adoptive transfer of DCs. Cells that are double-positive for CD45.2 and CD11c are indicated by the square gate. (D) Lung tissue and mediastinal lymph nodes (LNs) were collected from recipient mice that received adoptive transfer of either air-exposed DCs or CS-exposed DCs. Antibodies against CD11c, MHC class II, CD11b, and CD103 were used to identify all lung and LN DCs. CD45.2 staining was used to determine the percentage of DCs that were positive for CD45.2, indicating they had been adoptively transferred from donor mice. There were no significant differences between groups, as determined by the Mann-Whitney t test. *P < 0.05.
We tested whether DCs could prime NKs in vivo, by adoptively transferring lung DCs from air- or CS-exposed mice of a congenic strain into naïve recipient C57BL/6 mice. After 48 hours, isolated lung NKs from individual recipient mice were tested for killing of pooled lung epithelial cells. Lung NKs from mice that received CS-exposed DCs were significantly more cytotoxic than NKs from mice that received air-exposed DCs (Figure 4B). To exclude possible difference between groups in lung retention or trafficking to lymph nodes, we analyzed the percentage of CD45.2+ donor DCs in lung tissue and mediastinal lymph nodes, and found no differences (Figures 4C and 4D).
Dendritic Cells Prime NK Cells in Mice That Develop a Spontaneous COPD Phenotype
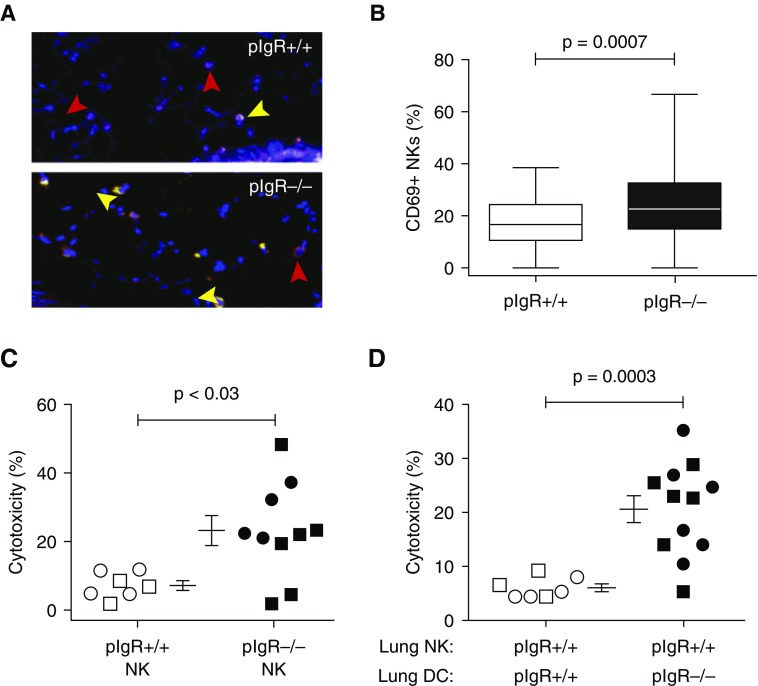
We next investigated whether DC-mediated priming of NKs contributes to the development of spontaneous, age-dependent small-airway remodeling and emphysema in pIgR−/− mice, which cannot translocate IgA to mucosal surfaces (25). Dual immunofluorescence staining for NCR1 (to identify NKs) and CD69 (a marker of priming) showed that percentages of double-positive NKs in the lungs of 6-month-old pIgR−/− mice were significantly increased (P = 0.0007) (Figures 5A and 5B). To determine functional relevance, we isolated lung NKs from pIgR+/+ and pIgR−/− mice, each at 2 and 6 months old, cocultured them with pooled, age-matched epithelial cells from pIgR+/+ mice, and analyzed for cytotoxicity. NKs from pIgR−/− mice showed significantly increased cytotoxicity at both ages (Figure 5C).
Figure 5.
Lung natural killer cells (NKs) from polymeric immunoglobulin receptor–deficient (pIgR−/−) mice have increased cytotoxicity against epithelial cells, which is driven by dendritic cell (DC)–mediated priming. (A) Paraffin-embedded lung tissue from 6-month-old pIgR+/+ and pIgR−/− mice was stained with anti-CD69 and anti-NCR1. Red arrowheads indicate NCR1 single-positive cells, and yellow arrowheads indicate NCR1 and CD69 double-positive cells. (B) The percentage of NKs coexpressing CD69 is shown. Data are pooled from six mice per group. (C) Lung tissue from 2- and 6-month-old pIgR+/+ and pIgR−/− mice was used to isolate NK (CD49b+) cells. CD326+ epithelial cells were isolated only from pIgR+/+ mice. Epithelial cells were cultured either alone or with pIgR+/+ NKs or pIgR−/− NKs. There was no difference in the percent cytotoxicity of the 2- and 6-month-old mice, and therefore these data have been combined (open symbols, 2-mo-old mice; solid symbols, 6-mo-old mice). (D) Lung NKs from pIgR+/+ mice were cocultured with lung DCs from either pIgR+/+ or pIgR−/− mice (2 and 6 mo old) for 18 hours. Epithelial cells from pIgR+/+ mice were added to the culture for an additional 4 hours, and then percent cytotoxicity was determined. For C and D, data are from two separate experiments, 5–10 total mice per group. The Mann-Whitney t test was used to determine significance.
To determine whether DC priming contributed to increased lung NK killing in this spontaneous COPD model, we cocultured lung NKs from pIgR+/+ mice with syngeneic lung DCs from either pIgR+/+ mice or pIgR−/− mice (both 2 and 6 mo old). Lung DCs from pIgR+/+ mice did not prime lung NKs at either age, but lung DCs from pIgR−/− mice of both ages led to increased cytotoxicity (Figure 5D). Lung DCs from pIgR−/− mice did not kill epithelial cells in the absence of NKs, nor did we observe sex differences in lung DC priming (data not shown).
Dendritic Cell Priming of NK Cells Is Mediated by Trans-Presentation of IL-15
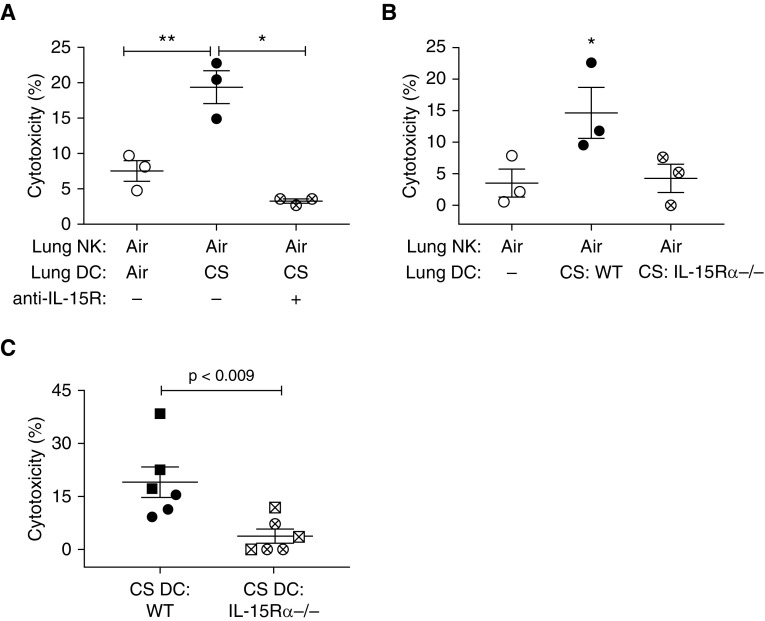
DC priming of NKs can occur via soluble factors (11) or cell-mediated contact (19). NKs separated from lung DCs from CS-exposed mice during overnight coculture in Transwell plates did not become primed (data not shown), implying the need for cell–cell contact. We cocultured lung NKs from air-exposed mice with lung DCs from CS-exposed mice, either alone or in the presence of anti–IL-15R/IL-15. Lung NKs cocultured with DCs from CS-exposed mice showed 3.5-fold greater cytotoxicity, as anticipated; importantly, such priming was completely abrogated by the addition of anti–IL-15R/IL-15 (Figure 6A). Anti–IL-15R/IL-15 had no effect on epithelial cells by themselves (data not shown).
Figure 6.
Blocking IL-15 trans-presentation prevents dendritic cell (DC)–mediated priming of natural killer cell (NK) cytotoxicity. (A) Lung NKs from air-exposed mice were cocultured for 18 hours with DCs from either air-exposed mice, cigarette smoke (CS)–exposed mice, or CS-exposed mice in the presence of anti-mouse IL-15R (IL-15 receptor)/IL-15. Epithelial cells from CS-exposed mice were added to the culture for an additional 4 hours, and then cytotoxicity was determined. (B) Lung NKs from air-exposed mice were cultured for 18 hours either by themselves, or with DCs from CS-exposed wild-type (WT) mice or CS-exposed IL-15Rα−/− mice. Lung epithelial cells pooled from CS-exposed WT mice were added for an additional 4 hours, and then cytotoxicity was determined. Data in A and B are from three separate experiments (five mice per group per experiment); repeated measures one-way ANOVA with Tukey’s multiple comparison test was used to determine significance. (C) DCs from either CS-exposed WT mice or CS-exposed IL-15Rα−/− mice were adoptively transferred via intranasal administration of 200,000 DCs into untreated recipient WT mice; 48 hours later, lungs were collected to isolate NKs and epithelial cells. Epithelial cells pooled from all mice were cultured alone or with NKs from individual mice for 4 hours, and then cytotoxicity was assayed. Lines represent means ± SEM of two independent experiments (indicated by symbols), each containing three recipient mice per group; significance was determined by the Mann-Whitney t test. *P < 0.05; **P < 0.01.
To confirm these findings, we exposed IL-15Rα−/− mice and control wild-type mice to air or CS for 8 weeks, and then tested the capacity of lung DCs to prime lung NKs from air-exposed wild-type mice in vitro. Lung DCs from air-exposed mice of both groups had no effect on NK cytotoxicity (data not shown), and as expected, lung DCs from CS-exposed wild-type mice primed NKs to become significantly more cytotoxic. However, DCs from CS-exposed IL-15Rα−/− mice did not prime wild-type lung NKs (Figure 6B). Adoptive transfer of lung DCs from CS-exposed wild-type and IL-15Rα−/− mice into naive wild-type mice led to a similar result. Although lung DCs from CS-exposed wild-type mice primed NKs in vivo, DCs from the IL-15Rα−/− mice showed a significantly decreased ability to do so (Figure 6C). Whether lung NK priming by IL-15 could be mediated in vivo by cell types other than DCs is unknown and not excluded by our data. NK priming via macrophage trans-presentation of IL-15 is supported by murine and human data from other organs (33, 34), making lung macrophages a potential focus of future studies.
Lung DCs from Subjects with COPD Increase NK Cytotoxicity via IL-15 Trans-Presentation
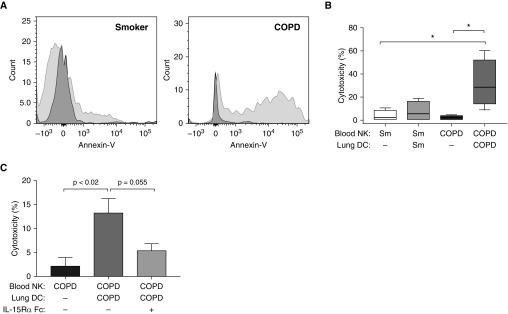
Finally, to test the relevance of our findings to human COPD, we investigated the ability of DCs from clinically indicated lung resections to prime the subjects’ own peripheral blood NKs, isolated before surgery and cryopreserved until lung tissue was obtained. There was no difference in the cytotoxicity of fresh versus cryopreserved blood CD56+ cells against K562 cells (data not shown). Cryopreserved blood CD56+ cells were resuscitated for 18 hours before overnight coculture alone or with freshly isolated lung DCs, and then autologous lung epithelial cells were added for an additional 4 hours and epithelial cell killing was assessed (Figure 7A). Unprimed blood CD56+ cells showed minimal cytotoxicity; in smokers without obstruction, coculture with autologous lung DCs led to an approximately twofold increase that did not attain significance (Figure 7B). In contrast, in subjects with COPD, coculture of blood CD56+ cells with autologous lung DCs induced a 14-fold increase in cytotoxicity (Figure 7B). DCs by themselves were unable to kill epithelial cells (data not shown). In subsequent experiments using only lung tissue and peripheral blood from subjects with COPD, the chimeric blocking reagent IL-15Rα Fc decreased cytotoxicity 2.5-fold (Figure 7C), supporting our murine data that IL-15 trans-presentation contributes to lung DC–mediated priming of NKs.
Figure 7.
Lung dendritic cells (DCs) from subjects with chronic obstructive pulmonary disease (COPD) increase natural killer cell (NK) cytotoxicity via IL-15Rα (IL-15 receptor α subunit) trans-presentation. Blood NKs from smokers without COPD (Sm) or subjects with COPD were cocultured either alone or with autologous lung DCs for 18 hours. Autologous lung epithelial cells were added for an additional 4 hours, and then cytotoxicity was measured via annexin and 7-aminoactinomycin D staining. (A) Representative annexin-V staining of gated CD326+ epithelial cells from a smoker without COPD (left) and subject with COPD (right). Dark gray histograms, coculture with blood NKs alone; light gray histograms, cocultured with NKs plus lung DCs. (B) Combined data of healthy smokers (n = 4) and subjects with COPD (n = 4), one-way ANOVA with Tukey’s multiple comparison test. *P < 0.05; no other comparisons are significant. (C) Blood NKs from subjects with COPD (n = 3) were cocultured for 18 hours alone, with autologous lung DCs, or with autologous lung DCs in the presence of IL-15Rα Fc chimera; autologous lung epithelial cells were added for an additional 4 hours, and then cytotoxicity was assayed. Repeated measures one-way ANOVA with Tukey’s multiple comparison test was used to determine significance.
Discussion
We demonstrate that lung DCs prime NKs to kill autologous lung epithelial cells, as measured by an in vitro single-cell assay; that this process is increased in COPD relative to smokers without airflow obstruction, regardless of current smoking status; and that it is mediated largely by trans-presentation of IL-15 via DC-expressed IL-15Rα. These conclusions are supported by congruent results from experiments using human samples and two independent murine models that employed both inhibitory and genetic approaches. Our findings thus provide novel insights into pulmonary immune responses in COPD.
These results extend two publications on lung NKs and lung DCs in COPD. Suzuki and colleagues demonstrated enrichment of genes expressed by NKs and DCs (among other cell types) in helper T-cell type 1–associated expression profiles associated with emphysematous destruction (35). Using the ECLIPSE (Evaluation of COPD Longitudinally to Identify Predictive Surrogate End-points) and COPDGene (Genetic Epidemiology of COPD) cohorts, Reinhold and colleagues associated gene modules expressed in peripheral blood with COPD phenotypes, and also found overrepresentation of genes specific for NKs, DCs (and neutrophils) (36). In addition to providing a potential mechanistic explanation for these associations, our data imply that pathological NK–DC interactions are present when COPD is mild (by measures of airflow and gas exchange), although not necessarily while subjects are young. Increased appreciation that processes leading to overt COPD are evident in early adulthood argues for the importance of investigating lung NK–DC interactions in younger smokers.
Our findings superficially disagree with previous studies showing reduced function of lung NKs relative to peripheral blood NKs, which was attributed to suppression by pulmonary macrophages (30, 37, 38). However, these studies used lung NKs from subjects with normal pulmonary function and analyzed killing of highly susceptible target cell lines. Hence, one explanation for the differences is that COPD progression reduces the physiologically suppressive effect of the lung environment on lung NKs in the ways that exceed lung DC actions we demonstrate. A nonexclusive alternative is disparity between mechanisms of killing targets entirely lacking class I MHC molecules in previous studies and those targeting the complexly altered epithelial cell phenotypes that develop during progression to COPD. Chronic cigarette smoking profoundly perturbs small-airway epithelium, with loss of diversity, basal cell and goblet cell hyperplasia, mucus hypersecretion, and altered ciliated cell structure and function (39). Airway epithelial cells communicate with myeloid DCs, inducing upregulation of chemokines and other mediators to recruit NKs and additional proinflammatory cell types (40). A second point is that peripheral blood NKs from smokers were found to have decreased cytotoxic activity compared with those from control nonsmokers (41). Although we did not examine nonsmokers in our study, precluding direct comparisons, we found no differences between active and former smokers. Our focus on killing of autologous epithelial cells by lung NKs from either human ever-smokers or mice with features of COPD makes it likely that our results reflect ongoing immunological consequences in the lung, which may elude detection in peripheral blood.
Importantly, our pIgR−/− mouse results imply that DC-dependent priming leading to increased NK cytotoxicity is not unique to cigarette smoking. Instead, with the finding that loss of luminal secretory IgA is spatially associated with bacterial invasion and NF-κB expression in the small airways of human smokers (42), these data support loss of epithelial barrier function as a key tipping point in COPD pathogenesis toward an inflammatory process that is self-perpetuating even on removal of the inciting exposure. Long-term studies in these murine models are needed to provide evidence on whether enhanced NK cytotoxicity is not simply associated with but, rather, essential for COPD progression, and whether the propensity of the DC–NK axis to eliminate mildly CS-damaged epithelial cells can be safely dissociated from the crucial role of NK cells in antiviral and antitumor surveillance.
The literature on the effect of smoking status and COPD diagnosis on lung DCs is conflicting in both human subjects and animal models. Active smoking was associated with significantly reduced absolute numbers of DCs in both epithelial and subepithelial regions of proximal endobronchial biopsies (43). By contrast, using immunohistochemistry to stain for langerin+ DCs, Demedts and colleagues observed a significant increase in DC number in the epithelium and adventitial of small airways of patients with COPD (44). We demonstrated (28) increased expression of maturation markers (including CD80, CD83, and CD86) on human lung DCs from subjects with COPD, congruent with our current findings, but our previous study did not measure absolute DC numbers, and we did not examine those receptors in this study. The study by Demedts and colleagues and both of our studies used peripheral lung tissue overwhelmingly consisting of small airways, and comprised substantial fractions of ex-smokers because they were surgical cohorts. Hence, further study will be needed to distinguish the relative contributions of active smoking versus airway generation to airway DC numbers and the activation state.
Our mixing experiments, directly comparing killing of a standardized target by lung NKs of various subjects with killing of their lung epithelial cells by a single cloned NK cell line, favor the NKs themselves as the key variable explaining increased cytotoxicity in COPD. This finding was unexpected. We and others have shown that NK-activating ligands are upregulated on lung epithelial cells from patients with COPD and mice exposed to cigarette smoke (6, 31, 45). However, those studies focused on ligands specific to the NKG2D (natural killer group 2, member D) receptor. Activating receptors, including NKG2D, stimulate degranulation only in combination, but not alone (46). Although negative signals from inhibitory receptors tend to dominate over activing receptors, ligands for NK inhibitory receptors have not been studied in COPD or cigarette exposure models. Another possibility, untested in COPD, is that soluble ligands of NKG2D and other activating receptors saturate the NK cell surface, blocking membrane-bound ligands, as shown for tumors (47).
This is the first study, to our knowledge, to implicate IL-15 activation of NKs by lung ;DCs in humans or mice, although this action has been clearly documented in DCs from other murine organs (19, 48, 49). IL-15 plays an indispensable role in NK homeostasis (50). Elegant murine experiments showed that IL-15 does not act as a soluble cytokine, but instead must be presented by cell–cell contact, either bound to IL-15Rα or as a transmembrane molecule on the cell producing it; our IL-15Rα−/− results exclude the latter possibility in this system. Mature DCs are more capable than immature DCs of priming NKs to render them cytotoxic (51, 52), and we have shown that expression of receptors indicative of human lung DC maturity correlates significantly with spirometric severity (28).
Interestingly, data from the SPIROMICS (Subpopulations and Intermediate Outcome Measures in COPD Study) cohort identified lower baseline IL-15 blood levels as associated with reduced odds for exacerbation in one of two analyses (consistent exacerbators over 3 yr vs. those without exacerbations, n = 393, stepwise logistic regression) (53). However, in a single-center study of biomarkers of acute COPD exacerbations, employing a paired design that collected samples at baseline and during exacerbation before treatment, we found that IL-15 levels significantly increased during exacerbations (54). Given the different phases of disease activity between these two study designs, such results are not necessarily inconsistent. Rather, they might suggest a relative impairment of antiviral defenses in some patients with COPD, predisposing them to repeated exacerbations. Most importantly for the current results, the relevance of circulating IL-15 levels to focal trans-presentation of IL-15 by DCs within lung parenchyma is uncertain.
Our study has several limitations, notably the high percentage of cases having lung cancer, a consequence of the need for cells from human lungs removed for clinical indications. Because of the extensive preoperative evaluations now standard at our medical centers, few patients undergo resection of nonmalignant lesions. We did not test the ability of lung NKs in either species to kill other autologous cell types, an important area for future research. These results leave unanswered which DC subsets are responsible for priming, which we are currently investigating. We demonstrated that lung DCs in both species can prime NKs without participation by other cell types, but even our adoptive transfer experiments do not prove that this interaction occurs in the lungs, rather than in regional lymph nodes. Whether lung NK priming by IL-15 could be mediated in vivo by cell types other than DCs is unknown and not excluded by our data. NK priming via macrophage trans-presentation of IL-15 is supported by murine and human data from other organs (33, 34), making lung macrophages a potential focus of future studies.
In conclusion, we show that lung NKs in COPD are able to kill lung epithelial cells directly in response to IL-15–dependent priming by lung DCs. This process occurs in mild COPD and continues in ex-smokers, highlighting the importance of novel targeted therapeutic approaches to complement smoking cessation. We do not propose the IL-15 axis as such a target, due to the risk of increasing lung cancer in this high-risk group, but instead argue for increased investigation of the factors leading to inappropriate DC priming.
Acknowledgments
Acknowledgment
The authors thank Joanne Sonstein for assisting with laboratory procedures, and Deborah Postiff and Jacky Jaikisoon for help with tissue procurement.
Footnotes
Supported by Merit Review Awards I01 CX001553 (C.M.F.), I01 CX000911 (J.L.C.), and I01 BX002378 (T.S.B.) from the Department of Veterans Affairs; MedImmune, Ltd. (C.M.F. and J.L.C.); and K08HL138088 (B.W.R.) from the NHLBI. These investigations were also supported in part by the Tissue Procurement Core of the University of Michigan Comprehensive Cancer Center, grant P30 CA46952.
Author Contributions: D.K.F., J.F., J.L.C., and C.M.F. designed the experiments and interpreted data; V.R.S., H.A., M.R.K., and B.W.R. performed experiments; L.M. assisted with tissue collection and dissemination of participant data; B.W.R., V.V.P., and T.S.B. provided specimens and assisted with data analysis and interpretation; all authors participated in manuscript revisions and approved the final version.
This article has an online supplement, which is accessible from this issue’s table of contents at www.atsjournals.org.
Originally Published in Press as DOI: 10.1164/rccm.201712-2513OC on April 20, 2018
Author disclosures are available with the text of this article at www.atsjournals.org.
References
- 1.Kochanek KD, Murphy S, Xu J, Arias E. Mortality in the United States, 2016. NCHS Data Brief. 2017;293:1–8. [PubMed] [Google Scholar]
- 2.GBD 2015 Tobacco Collaborators. Smoking prevalence and attributable disease burden in 195 countries and territories, 1990-2015: a systematic analysis from the Global Burden of Disease Study 2015. Lancet. 2017;389:1885–1906. doi: 10.1016/S0140-6736(17)30819-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hogg JC, Chu F, Utokaparch S, Woods R, Elliott WM, Buzatu L, et al. The nature of small-airway obstruction in chronic obstructive pulmonary disease. N Engl J Med. 2004;350:2645–2653. doi: 10.1056/NEJMoa032158. [DOI] [PubMed] [Google Scholar]
- 4.Curtis JL, Freeman CM, Hogg JC. The immunopathogenesis of chronic obstructive pulmonary disease: insights from recent research. Proc Am Thorac Soc. 2007;4:512–521. doi: 10.1513/pats.200701-002FM. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Curtis JL, Freeman CM, Huffnagle GB. “B” for bad, beneficial, or both? Lung lymphoid neogenesis in chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2015;192:648–651. doi: 10.1164/rccm.201506-1230ED. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Freeman CM, Stolberg VR, Crudgington S, Martinez FJ, Han MK, Chensue SW, et al. Human CD56+ cytotoxic lung lymphocytes kill autologous lung cells in chronic obstructive pulmonary disease. PLoS One. 2014;9:e103840. doi: 10.1371/journal.pone.0103840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tomasello E, Yessaad N, Gregoire E, Hudspeth K, Luci C, Mavilio D, et al. Mapping of NKp46+ cells in healthy human lymphoid and non-lymphoid tissues. Front Immunol. 2012;3:344. doi: 10.3389/fimmu.2012.00344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Björkström NK, Ljunggren HG, Michaëlsson J. Emerging insights into natural killer cells in human peripheral tissues. Nat Rev Immunol. 2016;16:310–320. doi: 10.1038/nri.2016.34. [DOI] [PubMed] [Google Scholar]
- 9.Hodge G, Mukaro V, Holmes M, Reynolds PN, Hodge S. Enhanced cytotoxic function of natural killer and natural killer T–like cells associated with decreased CD94 (Kp43) in the chronic obstructive pulmonary disease airway. Respirology. 2013;18:369–376. doi: 10.1111/j.1440-1843.2012.02287.x. [DOI] [PubMed] [Google Scholar]
- 10.Urbanowicz RA, Lamb JR, Todd I, Corne JM, Fairclough LC. Enhanced effector function of cytotoxic cells in the induced sputum of COPD patients. Respir Res. 2010;11:76. doi: 10.1186/1465-9921-11-76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Motz GT, Eppert BL, Wortham BW, Amos-Kroohs RM, Flury JL, Wesselkamper SC, et al. Chronic cigarette smoke exposure primes NK cell activation in a mouse model of chronic obstructive pulmonary disease. J Immunol. 2010;184:4460–4469. doi: 10.4049/jimmunol.0903654. [DOI] [PubMed] [Google Scholar]
- 12.Wortham BW, Eppert BL, Motz GT, Flury JL, Orozco-Levi M, Hoebe K, et al. NKG2D mediates NK cell hyperresponsiveness and influenza-induced pathologies in a mouse model of chronic obstructive pulmonary disease. J Immunol. 2012;188:4468–4475. doi: 10.4049/jimmunol.1102643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Stolberg VR, Martin B, Mancuso P, Olszewski MA, Freeman CM, Curtis JL, et al. Role of CC chemokine receptor 4 in natural killer cell activation during acute cigarette smoke exposure. Am J Pathol. 2014;184:454–463. doi: 10.1016/j.ajpath.2013.10.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Demedts IK, Demoor T, Bracke KR, Joos GF, Brusselle GG. Role of apoptosis in the pathogenesis of COPD and pulmonary emphysema. Respir Res. 2006;7:53. doi: 10.1186/1465-9921-7-53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Imai K, Mercer BA, Schulman LL, Sonett JR, D’Armiento JM. Correlation of lung surface area to apoptosis and proliferation in human emphysema. Eur Respir J. 2005;25:250–258. doi: 10.1183/09031936.05.00023704. [DOI] [PubMed] [Google Scholar]
- 16.Segura-Valdez L, Pardo A, Gaxiola M, Uhal BD, Becerril C, Selman M. Upregulation of gelatinases A and B, collagenases 1 and 2, and increased parenchymal cell death in COPD. Chest. 2000;117:684–694. doi: 10.1378/chest.117.3.684. [DOI] [PubMed] [Google Scholar]
- 17.Kerdiles Y, Ugolini S, Vivier E. T cell regulation of natural killer cells. J Exp Med. 2013;210:1065–1068. doi: 10.1084/jem.20130960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Long EO. Ready for prime time: NK cell priming by dendritic cells. Immunity. 2007;26:385–387. doi: 10.1016/j.immuni.2007.04.001. [DOI] [PubMed] [Google Scholar]
- 19.Lucas M, Schachterle W, Oberle K, Aichele P, Diefenbach A. Dendritic cells prime natural killer cells by trans-presenting interleukin 15. Immunity. 2007;26:503–517. doi: 10.1016/j.immuni.2007.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Schleicher U, Liese J, Knippertz I, Kurzmann C, Hesse A, Heit A, et al. NK cell activation in visceral leishmaniasis requires TLR9, myeloid DCs, and IL-12, but is independent of plasmacytoid DCs. J Exp Med. 2007;204:893–906. doi: 10.1084/jem.20061293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kassim SH, Rajasagi NK, Ritz BW, Pruett SB, Gardner EM, Chervenak R, et al. Dendritic cells are required for optimal activation of natural killer functions following primary infection with herpes simplex virus type 1. J Virol. 2009;83:3175–3186. doi: 10.1128/JVI.01907-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Huntington ND. The unconventional expression of IL-15 and its role in NK cell homeostasis. Immunol Cell Biol. 2014;92:210–213. doi: 10.1038/icb.2014.1. [DOI] [PubMed] [Google Scholar]
- 23.Anguille S, Van Acker HH, Van den Bergh J, Willemen Y, Goossens H, Van Tendeloo VF, et al. Interleukin-15 dendritic cells harness NK cell cytotoxic effector function in a contact- and IL-15–dependent manner. PLoS One. 2015;10:e0123340. doi: 10.1371/journal.pone.0123340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ghorani V, Boskabady MH, Khazdair MR, Kianmeher M. Experimental animal models for COPD: a methodological review. Tob Induc Dis. 2017;15:25. doi: 10.1186/s12971-017-0130-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Richmond BW, Brucker RM, Han W, Du RH, Zhang Y, Cheng DS, et al. Airway bacteria drive a progressive COPD-like phenotype in mice with polymeric immunoglobulin receptor deficiency. Nat Commun. 2016;7:11240. doi: 10.1038/ncomms11240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Stolberg VR, McCloskey L, Kady MR, Ferguson J, Sandig H, Finch DK, et al. Lung dendritic cells prime natural killer cells to become cytotoxic in both COPD patients and cigarette smoke–exposed mice [abstract] Am J Respir Crit Care Med. 2017;195:A7354. [Google Scholar]
- 27.Pauwels RA, Buist AS, Calverley PMA, Jenkins CR, Hurd SS GOLD Scientific Committee. Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease: NHLBI/WHO Global Initiative for Chronic Obstructive Lung Disease (GOLD) Workshop summary. Am J Respir Crit Care Med. 2001;163:1256–1276. doi: 10.1164/ajrccm.163.5.2101039. [DOI] [PubMed] [Google Scholar]
- 28.Freeman CM, Martinez FJ, Han MK, Ames TM, Chensue SW, Todt JC, et al. Lung dendritic cell expression of maturation molecules increases with worsening chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2009;180:1179–1188. doi: 10.1164/rccm.200904-0552OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Freeman CM, Han MK, Martinez FJ, Murray S, Liu LX, Chensue SW, et al. Cytotoxic potential of lung CD8+ T cells increases with chronic obstructive pulmonary disease severity and with in vitro stimulation by IL-18 or IL-15. J Immunol. 2010;184:6504–6513. doi: 10.4049/jimmunol.1000006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Marquardt N, Kekäläinen E, Chen P, Kvedaraite E, Wilson JN, Ivarsson MA, et al. Human lung natural killer cells are predominantly comprised of highly differentiated hypofunctional CD69−CD56dim cells. J Allergy Clin Immunol. 2017;139:1321–1330.e4. doi: 10.1016/j.jaci.2016.07.043. [DOI] [PubMed] [Google Scholar]
- 31.Borchers MT, Wesselkamper SC, Curull V, Ramirez-Sarmiento A, Sánchez-Font A, Garcia-Aymerich J, et al. Sustained CTL activation by murine pulmonary epithelial cells promotes the development of COPD-like disease. J Clin Invest. 2009;119:636–649. doi: 10.1172/JCI34462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Moretta A. Natural killer cells and dendritic cells: rendezvous in abused tissues. Nat Rev Immunol. 2002;2:957–964. doi: 10.1038/nri956. [DOI] [PubMed] [Google Scholar]
- 33.Mortier E, Advincula R, Kim L, Chmura S, Barrera J, Reizis B, et al. Macrophage- and dendritic-cell–derived interleukin-15 receptor α supports homeostasis of distinct CD8+ T cell subsets. Immunity. 2009;31:811–822. doi: 10.1016/j.immuni.2009.09.017. [DOI] [PubMed] [Google Scholar]
- 34.Mattiola I, Pesant M, Tentorio PF, Molgora M, Marcenaro E, Lugli E, et al. Priming of human resting NK cells by autologous M1 macrophages via the engagement of IL-1β, IFN-β, and IL-15 pathways. J Immunol. 2015;195:2818–2828. doi: 10.4049/jimmunol.1500325. [DOI] [PubMed] [Google Scholar]
- 35.Suzuki M, Sze MA, Campbell JD, Brothers JF, II, Lenburg ME, McDonough JE, et al. The cellular and molecular determinants of emphysematous destruction in COPD. Sci Rep. 2017;7:9562. doi: 10.1038/s41598-017-10126-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Reinhold D, Morrow JD, Jacobson S, Hu J, Ringel B, Seibold MA, et al. Meta-analysis of peripheral blood gene expression modules for COPD phenotypes. PLoS One. 2017;12:e0185682. doi: 10.1371/journal.pone.0185682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Robinson BW, Pinkston P, Crystal RG. Natural killer cells are present in the normal human lung but are functionally impotent. J Clin Invest. 1984;74:942–950. doi: 10.1172/JCI111513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Weissman DN, deShazo RD, Banks DE. Modulation of natural killer cell function by human alveolar macrophages. J Allergy Clin Immunol. 1986;78:571–577. doi: 10.1016/0091-6749(86)90073-4. [DOI] [PubMed] [Google Scholar]
- 39.Shaykhiev R, Zuo WL, Chao I, Fukui T, Witover B, Brekman A, et al. EGF shifts human airway basal cell fate toward a smoking-associated airway epithelial phenotype. Proc Natl Acad Sci USA. 2013;110:12102–12107. doi: 10.1073/pnas.1303058110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Agrawal S, Srivastava R, Rahmatpanah F, Madiraju C, BenMohamed L, Agrawal A. Airway epithelial cells enhance the immunogenicity of human myeloid dendritic cells under steady state. Clin Exp Immunol. 2017;189:279–289. doi: 10.1111/cei.12983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Mian MF, Lauzon NM, Stämpfli MR, Mossman KL, Ashkar AA. Impairment of human NK cell cytotoxic activity and cytokine release by cigarette smoke. J Leukoc Biol. 2008;83:774–784. doi: 10.1189/jlb.0707481. [DOI] [PubMed] [Google Scholar]
- 42.Polosukhin VV, Cates JM, Lawson WE, Zaynagetdinov R, Milstone AP, Massion PP, et al. Bronchial secretory immunoglobulin a deficiency correlates with airway inflammation and progression of chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2011;184:317–327. doi: 10.1164/rccm.201010-1629OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Rogers AV, Adelroth E, Hattotuwa K, Dewar A, Jeffery PK. Bronchial mucosal dendritic cells in smokers and ex-smokers with COPD: an electron microscopic study. Thorax. 2008;63:108–114. doi: 10.1136/thx.2007.078253. [DOI] [PubMed] [Google Scholar]
- 44.Demedts IK, Bracke KR, Van Pottelberge G, Testelmans D, Verleden GM, Vermassen FE, et al. Accumulation of dendritic cells and increased CCL20 levels in the airways of patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2007;175:998–1005. doi: 10.1164/rccm.200608-1113OC. [DOI] [PubMed] [Google Scholar]
- 45.Borchers MT, Harris NL, Wesselkamper SC, Vitucci M, Cosman D. NKG2D ligands are expressed on stressed human airway epithelial cells. Am J Physiol Lung Cell Mol Physiol. 2006;291:L222–L231. doi: 10.1152/ajplung.00327.2005. [DOI] [PubMed] [Google Scholar]
- 46.Long EO, Kim HS, Liu D, Peterson ME, Rajagopalan S. Controlling natural killer cell responses: integration of signals for activation and inhibition. Annu Rev Immunol. 2013;31:227–258. doi: 10.1146/annurev-immunol-020711-075005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Marcus A, Gowen BG, Thompson TW, Iannello A, Ardolino M, Deng W, et al. Recognition of tumors by the innate immune system and natural killer cells. Adv Immunol. 2014;122:91–128. doi: 10.1016/B978-0-12-800267-4.00003-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Koka R, Burkett P, Chien M, Chai S, Boone DL, Ma A. Cutting edge: murine dendritic cells require IL-15R α to prime NK cells. J Immunol. 2004;173:3594–3598. doi: 10.4049/jimmunol.173.6.3594. [DOI] [PubMed] [Google Scholar]
- 49.Mortier E, Woo T, Advincula R, Gozalo S, Ma A. IL-15Rα chaperones IL-15 to stable dendritic cell membrane complexes that activate NK cells via trans presentation. J Exp Med. 2008;205:1213–1225. doi: 10.1084/jem.20071913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Luu TT, Ganesan S, Wagner AK, Sarhan D, Meinke S, Garbi N, et al. Independent control of natural killer cell responsiveness and homeostasis at steady-state by CD11c+ dendritic cells. Sci Rep. 2016;6:37996. doi: 10.1038/srep37996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Alli RS, Khar A. Interleukin-12 secreted by mature dendritic cells mediates activation of NK cell function. FEBS Lett. 2004;559:71–76. doi: 10.1016/S0014-5793(04)00026-2. [DOI] [PubMed] [Google Scholar]
- 52.Gerosa F, Baldani-Guerra B, Nisii C, Marchesini V, Carra G, Trinchieri G. Reciprocal activating interaction between natural killer cells and dendritic cells. J Exp Med. 2002;195:327–333. doi: 10.1084/jem.20010938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Han MK, Quibrera PM, Carretta EE, Barr RG, Bleecker ER, Bowler RP, et al. SPIROMICS Investigators. Frequency of exacerbations in patients with chronic obstructive pulmonary disease: an analysis of the SPIROMICS cohort. Lancet Respir Med. 2017;5:619–626. doi: 10.1016/S2213-2600(17)30207-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Freeman CM, Martinez CH, Todt JC, Martinez FJ, Han MK, Thompson DL, et al. Acute exacerbations of chronic obstructive pulmonary disease are associated with decreased CD4+ & CD8+ T cells and increased growth & differentiation factor-15 (GDF-15) in peripheral blood. Respir Res. 2015;16:94. doi: 10.1186/s12931-015-0251-1. [DOI] [PMC free article] [PubMed] [Google Scholar]