Supplemental Digital Content is available in the text.
Abstract
Introduction:
As survival has improved in the Neonatal Intensive Care Unit (NICU), there has been a 10-fold increase in the proportion of infants requiring a tracheostomy. At our institution, we observed a wide variation in the duration of opioid use posttracheostomy from 6 to 148 days. We aimed to decrease the duration of opioid exposure in postoperative tracheostomy patients in the NICU from a baseline average of 24 days to 7 days by December 31, 2017.
Methods:
We established a multidisciplinary team to develop change ideas to implement in 3 Plan-Do-Study-Act cycles that focused on enhanced care plan standardization and communication in patient care rounds with subsequent documentation in the medical record and the timely addition of dexmedetomidine to the postoperative care plan.
Results:
Baseline population was from October 2014 to December 2016. The mean posttracheostomy opioid duration was 24.6 days (range, 6–148 days); neuromuscular blockade was 2.89 days (range, 0–9 days), and benzodiazepine exposure was 20.9 days (range, 1–114 days). Following our interventions, the mean duration of posttracheostomy opioid duration was 5.4 days (range, 4–21 days); neuromuscular blockade was 3.14 days (range, 1–5 days), benzodiazepine duration was 8.88 days (range, 4–25 days), and dexmedetomidine was 4.6 days (range, 0–32 days).
Conclusions:
We utilized quality improvement methodology to standardize posttracheostomy management and demonstrate that we could significantly reduce the duration of opioid and benzodiazepine use after tracheostomy with the timely addition of dexmedetomidine, a structured written daily care plan, and clarification of roles and responsibilities.
INTRODUCTION
As neonatal practices and survival have improved, the proportion of infants requiring a tracheostomy has increased 10-fold from 1997 to 2005.1 Infants in the Neonatal Intensive Care Unit (NICU) that require a tracheostomy tube placement have a myriad of airway and pulmonary conditions.2 Following tracheostomy, the most common complications during the immediate postoperative period include accidental decannulation, tube obstruction, and wound problems.3,4 To limit these complications, some organizations and institutions have created guidelines and protocols that address postoperative care including the initial tracheostomy tube change, wound care, and management of emergencies and complications.3,5 Several national and international organizations have emphasized the importance of effective postoperative pain management in neonates.6–8
Emerging reports of adverse effects of opiates and GABA agonists such as benzodiazepines in patients during a time of rapid brain growth generates a sense of urgency to minimize drug exposure when possible to this vulnerable patient population.9 In animal studies, morphine use has resulted in microglial apoptosis, decreased myelination, decreased motor activity, and impaired learning ability.10 Patients require sedation during the first few days posttracheostomy to avoid large motor movements of the head, neck, and trunk. They may also require neuromuscular blockade in addition to adequate sedation to prevent movements that may impair wound healing of the ostomy site. Dexmedetomidine is a potent alpha-2 agonist that has hypnotic, anxiolytic, sedative, sympatholytic, and analgesic properties and provides a rapid onset of action.11,12 In animal models, dexmedetomidine has demonstrated neuroprotection via antiapoptotic effects, reduced N-methyl-D-aspartate receptor activation, and reduction of calcium influx and glutamate scavenger abilities. These protective drug properties are effective against the neurotoxic effects of anesthetics.13–15
Although dexmedetomidine is less studied in infants than opiates and GABA agonists, its pharmacologic properties make it promising for use as a bridge to provide sedation and allow weaning of opioids to minimize or eliminate the risk of opioid withdrawal.12,16 In pediatric surgical populations, intraoperative administration of dexmedetomidine reduced postoperative pain intensity, reduced use of opioids, and reduced agitation or delirium.17 It would seem appropriate to optimize the use of analgesics and sedatives to achieve an intended sedation goal with the least amount of drug necessary for the shortest period.
Nationwide Children’s Hospital NICU, a Level IV referral nursery in Columbus, Ohio consisting of 114 beds within a free-standing children’s hospital, is capable of caring for the most medically complex infants. The pain assessment scale used in our NICU is the Neonatal Pain, Agitation and Sedation Scale.18 From our local experience, patients who require tracheostomy have the potential for severe pain postoperatively. Our center has utilized consensus guidelines for 10 years to assist with optimizing postoperative analgesia and minimizing the incidence of abstinence.
PROBLEM
In our NICU, the duration of postoperative opioid use in infants that required tracheostomy tube placement varied from 6 to 148 days.
Irrespective of the etiology for tracheostomy, these infants have complex medical needs, thereby providing opportunities to optimize care, including standardization of postoperative care. This variability in sedative duration, and the recent Food and Drug Administration warning to minimize the duration of exposure to sedatives when possible suggested there was a potential opportunity for improvement.
AIM
We aimed to decrease the duration of opioid exposure in postoperative tracheostomy patients in the NICU from a baseline average of 24 days to 7 days by December 31, 2017.
DESIGN
Methods
We established a multidisciplinary quality improvement (QI) team, consisting of neonatologists, ear, nose, and throat (ENT) surgeons, an advanced practice nurse, a respiratory therapist, a wound team representative, a parent advisor, and a clinical pharmacist. The study was classified as QI and thus did not require full review by the Institutional Review Board at Nationwide Children’s Hospital.
Figure 1 shows the key driver diagram with the identified key drivers and our planned interventions. Our team utilized brainstorming, process mapping, careful dive into patient charts and discussions with the frontline clinical team members to identify our drivers. Patients who undergo tracheostomy tube placement in our NICU receive a continuous infusion of morphine and midazolam after tube placement that is titrated to effect until the date of the first tracheostomy tube change. After the tracheostomy tube change both drugs are weaned as tolerated. In the pre-QI phase, baseline data collected included the duration of therapy in days, of opioids, benzodiazepines, and neuromuscular blockers after the tracheostomy placement. We also reviewed medical progress notes to determine if the patient care team documented the plan for the duration of paralysis and date of first tracheostomy tube change. These data indicated opportunities for improvement in communication and documentation among medical professionals regarding the patient care plan. Postoperative wound care protocol was not changed during this process.19
Fig. 1.
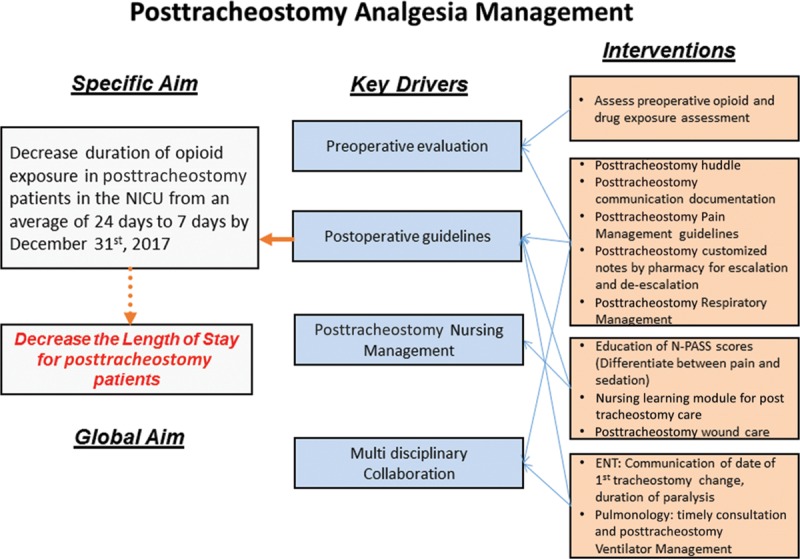
Key Driver Diagram: depicts the specific aim, global aim, the key drivers, and the interventions that impact posttracheostomy management.
The ENT surgeon in collaboration with the neonatologist determined the duration of postoperative paralysis and the date of tracheostomy tube change. This QI team developed change ideas to improve communication with ENT.
The patient’s preoperative opioid and drug history exposure were also evaluated. The primary medical team collaborated with pharmacy both pre- and postoperatively to develop individualized posttracheostomy analgesia and sedation care plans along with guidelines for daily escalation or de-escalation of medications on each postoperative day as determined by patient need. This care plan was documented in the medical progress notes. We progressed through 3 Plan-Do-Study-Act (PDSA) cycles.
PDSA Cycle 1
The first step in our process change to improve care plan was to optimize communication. We called a team “Huddle” when the patient returned to the NICU from surgery. The huddle intended to facilitate handoff from the ENT service to the neonatology service. It was not uncommon for patients to return to the unit after hours, which resulted in an incomplete team available for the handoff. Collaboration with the ENT surgeons allowed subsequent tracheostomy procedures to be scheduled earlier in the day when more personnel were available. In addition, the ENT surgeon documented the minimum duration of paralysis and the anticipated date of tracheostomy tube change in the postoperative note. The ENT surgeon would evaluate the tracheostomy wound site each morning and write a note that typically occurred before the neonatology rounds, which allowed the medical team to make adjustments to the analgesia and sedation. The series of interventions, allowed the daily patient care team rounds to become the venue for care plan coordination and implementation. Postoperative analgesia and sedation plans were documented in the patient care chart the day before surgery. Medications were ordered from the pharmacy before the patient returning to the NICU from the operating room to prevent any delays in initiating postoperative medication plans.
PDSA Cycle 2
We identified that some patients required additional sedation following the discontinuation of the neuromuscular blocker. Before the intervention, our standard practice to maintain the desired level of sedation and prevent large motor movements was to increase the dosage of both morphine and midazolam. This dose increase would often impact our ability to wean these medications following the first tracheostomy tube change. When we could not attain the desired level of sedation to prevent large muscle movement, wound healing was adversely affected. The addition of dexmedetomidine (rationale for use available as Supplemental Digital Content 1 at http://links.lww.com/PQ9/A38) to the sedation regimen allowed rapid attainment of the desired level of sedation, appeared to avoid wound breakdown, and allowed reduction of the morphine dose before the first tracheostomy change and subsequent discontinuation on the day of tracheostomy tube change if the tracheostomy wound was healed. We implemented a structured postoperative daily analgesia and sedation guideline (available as Supplemental Digital Content 2 at http://links.lww.com/PQ9/A39) that included escalation and de-escalation options for the evening hours. The guideline included the addition of dexmedetomidine either the day before tracheostomy change or when the desired level of sedation could not be not easily attained with an opioid and benzodiazepine without paralysis. The sedation guideline was discussed in patient care rounds and individualized for each patient as needed. The team clinical pharmacist recorded the agreed upon detailed plan in the daily pharmacist note.
PDSA Cycle 3
Determination and documentation of the date of tracheostomy tube change by the ENT surgeons initially varied from 5 days to 9 days. Communication of the date and standardization of the time for first tracheostomy change postoperatively is important for determining the duration of sedation and the general care of the patient. This communication also determines the duration and weaning of opioids and thereby minimizes opioid withdrawal.
The ENT surgeon recorded the planned date of tracheostomy change and minimum duration of paralysis in the postoperative day 1 note. This documentation allowed effective and proactive individualized management of analgesia and sedation. Tracheostomy tube change was typically changed on postoperative day 5 if the wound healing was adequate and would not be delayed even on the weekend.
We also developed posttracheostomy nursing care learning modules and bedside tracheostomy cards to improve interdisciplinary communication. These cards specified the date of anticipated first tracheostomy change and the duration of neuromuscular blockade.
Analysis
We evaluated the impact of the interventions over time by statistical process control charts. Statistical process control uses statistical methods to differentiate common cause and special cause variations in a process, to assess the process capability, and to identify statistically significant variability due to special causes.20 Centerline represents the mean values; the upper control limit and lower control limit are set at 3 SDs from the mean. We used the unpaired 2-sample t test for means and Mann-Whitney test for the medians as applicable. P < 0.05 was considered significant.
RESULTS
Our baseline population was from October 2014 to December 2016. We included all patients who received tracheostomy tube placement in our NICU. We excluded patients with a tracheostomy tube at admission. The average corrected gestational age for our population at the time of tracheostomy was 51 weeks.
At baseline, the mean posttracheostomy opioid duration was 24.6 days (range, 6–148 days), and the neuromuscular blockade was 2.89 days (Range 0–9 days). The duration of benzodiazepine was 20.9 days (range, 0–114 days) (This measure includes the duration of midazolam infusion plus the time to wean off with enteral lorazepam/diazepam or the time to return to pretracheostomy lorazepam/diazepam dosing if the patient had been receiving before surgery) and dexmedetomidine was 4.6 days (range, 0–32 days).
Outcome Measure
Figure 2 shows the comparison of means of first tracheostomy change, benzodiazepine duration, and posttracheostomy neuromuscular blockade duration of the pre-QI phase in comparison to the QI phase. Following our interventions, the mean duration of first tracheostomy change decreased from 5.55 to 4.71 days (P < 0.01), the mean duration of benzodiazepine decreased to 8.88 days (range, 4–25 days) (P = 0.014) and mean neuromuscular blockade was 3.14 days (range, 1–5 days). Figure 3 shows the Individual control chart (i-Chart) with the date of tracheostomy on the x axis, the duration of opioid exposure on the y axis, and each diamond is an individual patient. There were a total of 14 patients in the QI phase. The mean number of days of exposure decreased significantly from 24 days before the onset of the project to 5.4 days after implementation of the interventions (P value = 0.0487; 2-tailed t test).
Fig. 2.

Bar graph displaying the comparison of the mean number of posttracheostomy days for the first tracheostomy change, benzodiazepine use, and neuromuscular blockade.
Fig. 3.

Annotated i-chart demonstrating the opioid usage posttracheostomy over time. The dotted lines represent the upper and lower control limits, and the center line is the mean.
Process Measure
The pharmacy and the primary team evaluated and documented preoperative opioid exposure for all patients. Pharmacy compliance in completing electronic notes to the team specifying an escalation and de-escalation plan was 100%. There was 1 patient in the QI period where the protocol was not followed. There was an escalation of the opioid rather than the sedative after tracheostomy change, which may have been due to multiple issues that included a breakdown of communication and the unavailability of a pharmacist to round with the primary team.
Balancing Measure
The revised analgesic guidelines were developed to ensure adequate sedation and pain control. As a balancing measure, we followed the wound site for pressure injury or skin breakdown that might increase if sedation and agitation control were not adequate. There have been no reports of wound site compromise following the revised guidelines. In our population, patients did not display hypotension after starting dexmedetomidine. Hence, dexmedetomidine was not discontinued in any patients secondary to hypotension.21
DISCUSSION
Improved communication between ENT and neonatology services expedited the change process. This change included moving surgery times from afternoon to morning to allow initiation of postoperative sedation during the daytime. Early determination of the date of first tracheostomy change, with the provision of adequate wound healing, and duration of paralysis not only minimized variation but also impacted our ability to wean morphine and midazolam in a window of opportunity that prevented abstinence in most cases. Implementation of a postoperative analgesia order set in the electronic medical records in addition to a written postoperative pain plan by the pharmacist facilitated the medications to be ordered and available at the bedside with lines primed before the patient returned from the operating room. Addition of dexmedetomidine, detailed daily progress notes by the pharmacist regarding the analgesia and sedation plans, including escalation and de-escalation options, permitted maintenance of the care plan throughout all patient care shifts. Development of the bedside posttracheostomy cards enhanced communication among all caregivers. Learning modules were developed for the nurses to improve the posttracheostomy care.
There is limited literature regarding the pharmacological management of infants in the immediate posttracheostomy period. Multiple factors determine the success of postoperative opioid wean strategies. There is also significant heterogeneity in the etiology that necessitates tracheostomy placement. Several additional factors such as gestational age at the time of tracheostomy, duration of preoperative exposure to opioids and other sedatives, and steroid exposure before tracheostomy significantly influences the response to paralysis, the duration and success of postoperative opioid wean and wound healing. Although we have not addressed these factors yet, in this single-center QI initiative, we have highlighted that despite these limitations, multiple other factors that could still be addressed to effectively minimize the duration of opioid exposure and thereby decrease its neurotoxic effects.
The rapid onset of action of dexmedetomidine allowed the rapid attainment of sedative needs and allowed opioid to be weaned quickly in patients who had a stable and well-healed tracheostomy site. We do not recommend it’s routine use as further safety and efficacy studies in infants need to be done (see Supplemental Digital Content 2 available at http://links.lww.com/PQ9/A39). Daily rounds in the postoperative period with the pharmacist helped to improve compliance with the sedation and analgesia plan. Nonpharmacologic management options for comfort care need to be maximized whenever possible, and ventilation management strategies for this subpopulation of infants that may allow less use of sedation in general.
In this project, we did not intervene in patient selection for tracheostomy or evaluate the effects based on gestational age at the time of tracheostomy. There might also be further opportunities to focus on patients with intrinsic lung disease as compared with those with airway or neurological disorders as a unique population. Challenges of ventilator management on and off of paralysis and managing inflammatory response in patients with lung disease also affect the sedation needs, and, have not been addressed in this project.
CONCLUSIONS
We demonstrated that we could significantly reduce the duration of opioid use after tracheostomy from an average of 24 days to less than 7 days and benzodiazepine use from an average of 21 days to 8.9 days. Successful interventions in the postoperative period included improved communication between ENT and neonatology, standardizing the posttracheostomy care with regard to the first tracheostomy change, early identification of the duration of paralysis, standardizing nursing care, a structured written daily sedation plan by the pharmacist, and the timely addition of dexmedetomidine. Physician leadership was crucial in expediting the change cycles and coordinating physician roles and responsibilities for postoperative care plans. Quick identification of barriers and subsequent discussion of potential solutions on a regular basis allowed the process to continue as a fluid improvement project with positive forward momentum.
ACKNOWLEDGMENTS
The authors thank the multidisciplinary Tracheostomy team members for their contributions to the project.
DISCLOSURE
The authors have no financial interest to declare in relation to the content of this article.
Supplementary Material
Footnotes
Published online September 28, 2018
Supplemental digital content is available for this article. Clickable URL citations appear in the text.
To cite: Puthoff TD, Shah H, Slaughter JL, Bapat R. Reduction of Analgesia Duration after Tracheostomy in the Neonatal Intensive Care Unit: A Quality Initiative. Pediatr Qual Saf 2018;3:e106.
REFERENCES
- 1.Lee JH, Smith PB, Quek MB, et al. Risk factors and in-hospital outcomes following tracheostomy in infants. J Pediatr. 2016;173:39–44 e31.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Allen J, Zwerdling R, Ehrenkranz R, et al. ; American Thoracic Society. Statement on the care of the child with chronic lung disease of infancy and childhood. Am J Respir Crit Care Med. 2003;168:356–396.. [DOI] [PubMed] [Google Scholar]
- 3.Lippert D, Hoffman MR, Dang P, et al. Care of pediatric tracheostomy in the immediate postoperative period and timing of first tube change. Int J Pediatr Otorhinolaryngol. 2014;78:2281–2285.. [DOI] [PubMed] [Google Scholar]
- 4.Corbett HJ, Mann KS, Mitra I, et al. Tracheostomy—a 10-year experience from a UK pediatric surgical center. J Pediatr Surg. 2007;42:1251–1254.. [DOI] [PubMed] [Google Scholar]
- 5.Mitchell RB, Hussey HM, Setzen G, et al. Clinical consensus statement: tracheostomy care. Otolaryngol Head Neck Surg. 2013;148:6–20.. [DOI] [PubMed] [Google Scholar]
- 6.Walker SM. Neonatal pain. Paediatr Anaesth. 2014;24:39–48.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Anand KS; and the International Evidence-Based Group for Neonatal P. Consensus statement for the prevention and management of pain in the newborn. Arch Pediatr Adolesc Med. 2001;155:173–180.. [DOI] [PubMed] [Google Scholar]
- 8.Lim Y, Godambe S. Prevention and management of procedural pain in the neonate: an update, American Academy of Pediatrics, 2016. Arch Dis Childhood Educ Pract. 2017:edpract2016311066. [DOI] [PubMed] [Google Scholar]
- 9.Food U, Administration D. FDA drug safety communication: FDA review results in new warnings about using general anesthetics and sedation drugs in young children and pregnant women. Internet Document. 2016;14. [Google Scholar]
- 10.Attarian S, Tran LC, Moore A, et al. The neurodevelopmental impact of neonatal morphine administration. Brain Sci. 2014;4:321–334.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.O’Mara K, Gal P, Wimmer J, et al. Dexmedetomidine versus standard therapy with fentanyl for sedation in mechanically ventilated premature neonates. J Pediatr Pharmacol Ther. 2012;17:252–262.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Schwartz LI, Twite M, Gulack B, et al. The perioperative use of dexmedetomidine in pediatric patients with congenital heart disease: an analysis from the Congenital Cardiac Anesthesia Society-Society of Thoracic Surgeons Congenital Heart Disease Database. Anesth Analg. 2016;123:715–721.. [DOI] [PubMed] [Google Scholar]
- 13.Ma D, Rajakumaraswamy N, Maze M. α2-Adrenoceptor agonists: shedding light on neuroprotection? Brit Med Bulletin. 2005;71:77–92.. [DOI] [PubMed] [Google Scholar]
- 14.Sanders RD, Xu J, Shu Y, et al. Dexmedetomidine attenuates isoflurane-induced neurocognitive impairment in neonatal rats. Anesthesiology. 2009;110:1077–1085.. [DOI] [PubMed] [Google Scholar]
- 15.Andropoulos DB. Effect of anesthesia on the developing brain: infant and fetus. Fetal Diagn Ther. 2018;43:1–11.. [DOI] [PubMed] [Google Scholar]
- 16.Su F, Hammer GB. Dexmedetomidine: pediatric pharmacology, clinical uses and safety. Expert Opin Drug Saf. 2011;10:55–66.. [DOI] [PubMed] [Google Scholar]
- 17.Zhu A, Benzon HA, Anderson TA. Evidence for the efficacy of systemic opioid-sparing analgesics in pediatric surgical populations: a systematic review. Anesth Analg. 2017;125:1569–1587.. [DOI] [PubMed] [Google Scholar]
- 18.Hummel P, Puchalski M, Creech SD, et al. N-PASS: neonatal pain agitation and sedation scale—reliability and validity. 2003Paper presented at: Poster presented at: the Pediatric Academic Societies annual meeting. [Google Scholar]
- 19.McEvoy TP, Seim NB, Aljasser A, et al. Prevention of post-operative pediatric tracheotomy wounds: a multidisciplinary team approach. Int J Pediatr Otorhinolaryngol. 2017;97:235–239.. [DOI] [PubMed] [Google Scholar]
- 20.Provost LP, Murray S. The Health Care Data Guide: Learning from Data for Improvement. 2011San Francisco, CA:John Wiley & Sons. [Google Scholar]
- 21.Lam F, Bhutta AT, Tobias JD, et al. Hemodynamic effects of dexmedetomidine in critically ill neonates and infants with heart disease. Pediatr Cardiol. 2012;33:1069–1077.. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


