Supplemental Digital Content is available in the text.
Abstract
Background:
Fluid is central to the resuscitation of critically ill children. However, many pay limited attention to continued fluid accumulation. Fluid overload (FO) is associated with significant morbidity and mortality. The Volume Status Awareness Program (VSAP) is a multi-phase quality improvement initiative aimed at reducing iatrogenic FO. For baseline data, the authors examined a retrospective cohort of patients admitted to the pediatric intensive care unit.
Methods:
Cohort included diuretic-naive patients admitted to the pediatric intensive care unit at a tertiary care children’s hospital in 2014. Furosemide-exposure was used to indicate provider-perceived FO. Variables included daily weight and total fluid (TF) orders, and their timing, frequency, and adherence. Implementation of VSAP phase 1 (bundle of interventions to promote consistent use of patient weights) occurred in June 2017.
Results:
Forty-nine patients met criteria. Five (10%) had daily weight orders, and 41 (84%) had TF orders—although 7 of these orders followed furosemide administration. Adherence to TF orders was good with 32 (78%) patients exceeding TF limits by < 10%. Thirty (63%) had > 5% FO by day 1, and 22 (51%) had > 10% cumulative FO by day 3. Following phase 1 of the VSAP, the frequency of daily weight orders increased from 6% to 88%.
Conclusions:
In our institution, use of fluid monitoring tools is both inconsistent and infrequent. Early data from the VSAP project suggests simple interventions can modify ordering and monitoring practice, but future improvement cycles are necessary to determine if these changes are successful in reducing iatrogenic FO
INTRODUCTION
Fluid resuscitation has long been central to the stabilization of critically ill patients in the acute setting.1 However, excessive fluid accumulation beyond the resuscitation phase—fluid overload (FO)—can be detrimental to the health of children.2–4 FO is associated with acute lung injury, acute kidney injury (AKI), a greater number of ICU days, and increased need for medical intervention, such as pharmacologic diuresis or renal replacement therapy.4–7 Most importantly, excessive FO is also associated with increased mortality in critically ill children.3,5,7–9
Despite evidence suggesting worse outcomes with excessive fluid accumulation, a standardized approach to fluid management remains elusive in critically ill children, as the transition from resuscitation to maintenance needs is often patient- and disease-specific. Fluid assessment in the pediatric intensive care unit (PICU) typically includes careful monitoring of clinical signs, strict recording of fluid intake and output, and measurement of patient weight at least once weekly. However, there are few other structures in place to augment recognition of FO and minimize associated complications.
The Volume Status Awareness Program (VSAP) is a multi-phase quality improvement initiative aimed at standardizing fluid management and minimizing the frequency and severity of iatrogenic FO. In this study, the authors identify baseline practices and key drivers of iatrogenic FO (Fig. 1) to highlight opportunities for improvement. Specifically, we characterize a retrospective cohort of children who received furosemide as a marker for provider-perceived FO. Although loop diuretics may be used to target urine production in oliguric renal failure or narcotic-induced oliguria, a more common indication is acute pharmacologic diuresis, thus isolating a heterogeneous cohort for whom FO was a concern. The authors hypothesized the following: (1) fluid management in these patients was unsystematic and widely varied; (2) simple fluid monitoring tools—such as daily weights or total fluid orders—were infrequently utilized. We additionally note results from the first phase of VSAP implementation.
Fig. 1.
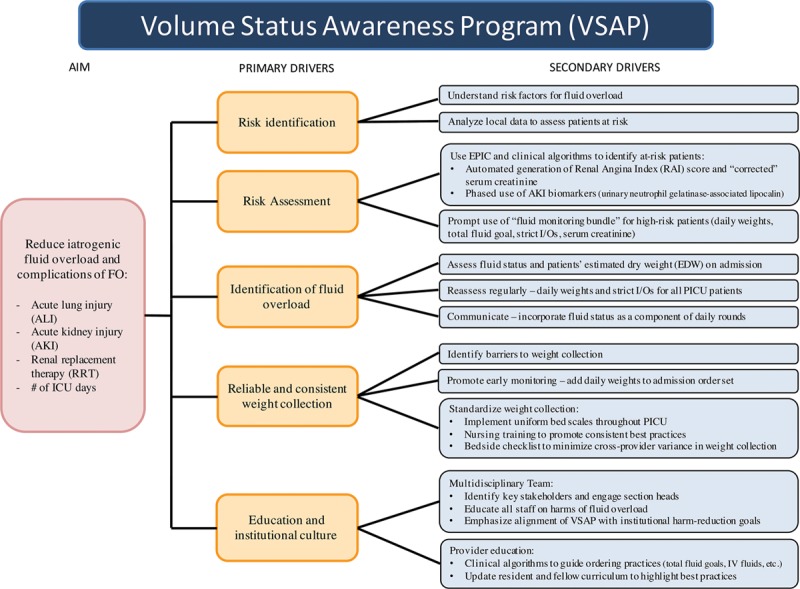
VSAP key driver diagram. IV; intravenous.
METHODS
Clinical Setting
Children’s Hospital Colorado (CHCO) is a free-standing children’s hospital and a level 1 trauma center affiliated with the University of Colorado School of Medicine. The CHCO PICU is a 32-bed unit that admits approximately 2,600 patients annually from a broad catchment area that includes the Denver metro area and greater Rocky Mountain region. CHCO also has separate neonatal and cardiac intensive care units that provide specialized intensive care. These units and their patients were excluded from this analysis.
Study Design
We conducted a retrospective chart review of all patients admitted to the PICU at this institution from January 1, 2014, to December 31, 2014. Patients were identified using Informatics for Integration Biology and the Bedside (I2B2, Partners HealthCare System, Boston, Mass.) using the reference date, age range, and receipt of furosemide. Manual chart review was then performed to identify eligible patients from the larger I2B2 query. Baseline VSAP data reflect a post hoc analysis of a study designed to assess furosemide-responsiveness for predicting AKI. Patients were included if they received intravenous or oral furosemide during the first 7 days of their PICU course and were excluded for the following reasons: (1) known chronic kidney disease; (2) history of renal transplant; (3) any prior exposure to furosemide on current hospital admission (ie, were not furosemide-naive); (4) received furosemide dose > 20 mg; or (5) received an additional dose of furosemide within 7 days of the tracked dose. Figure 2 summarizes the construct of patients included in the analysis.
Fig. 2.
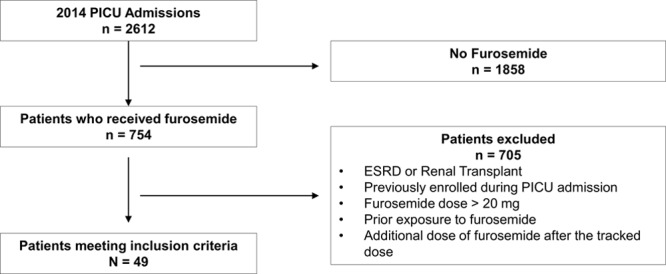
Construct of patient cohort. Summary of the construct of patients included in the analysis. ESRD, end-stage renal disease.
Neither heart failure nor electrolyte derangements were criteria for exclusion, though patients with primary cardiac disease were admitted to the cardiac intensive care unit and not included in this analysis. In addition to patient demographics, the authors evaluated the following clinical variables during patient admission to the PICU: total fluid orders, weight orders, recorded fluid intake, and output for up to 7 days following PICU admission.
Study Definitions
Hospital admission weights are used for baseline fluid status determination. Daily fluid intake was considered the sum of all intravenous and enteral fluids, including blood products, intravenous hydration, medications, and nutritional support. Weighed stools, “urine/stool mix,” and all outputs of known quantity are included. Instances of fluid output of unknown volume were rare and excluded. Though an important consideration in evaluating fluid needs, insensible losses were disregarded in this analysis due to an inability to retrospectively evaluate each clinical situation or obtain reliable estimates across varied conditions. Positive fluid accumulation (%FO) is normalized for patient body weight using the following formula9,10:
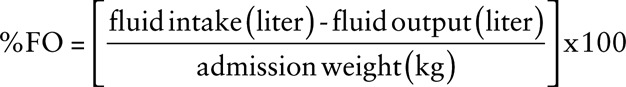 |
FO is further delineated by the rate and degree of fluid accumulation. Time point zero is defined as the time of admission to the PICU with “day 1” defined as the 24 hours following admission. Daily FO is defined as the fluid accumulation recorded each day, expressed as a percentage of patient weight (%FO, above). Cumulative FO is defined as the summative fluid accumulation since time of admission, again calculated as %FO. As such, cumulative and daily FO were equivalent by definition on day 1 of admission. The endpoint was discharge from the PICU.
We assess adherence to total fluid orders by comparing the total fluid intake for a given patient to the “expected” fluids indicated by the limits of their total fluid orders. Deviation from total fluid orders is normalized to a daily fluid limit (ie, expected liters/day) to account for differences in duration of active orders. All patient weights recorded during the study period are noted with assessment of adherence to standard (weekly) or more frequent weight measurement orders. Orders are considered “placed on the day of admission” (DOA) if placed within 24 hours of arrival to the PICU to allow for variance in time to patient stabilization and finalization of admission orders.
VSAP: Phase 1 Intervention
The VSAP initiative encompasses basic fluid monitoring tools (daily weights, total fluid orders, strict intake/output) and more advanced metrics (electronic health record–generated renal angina index score,11 urinary AKI biomarkers, etc.) to assess for FO and its complications. In the first phase (June 2017), a task force identified barriers to the reliable collection of patient weights and then developed and implemented key drivers to increase the frequency and accuracy of patient weight collection. This bundle of interventions is elaborated upon in Figure 1.
The authors then assessed for changes in weight collection by tracking the following metrics for all patients admitted to the PICU from April to December 2017: (1) percentage of patients who receive a daily weight order upon admission and (2) percentage of patient-days with a weight measurement—with and without a daily weight order.
Statistical Analysis
The authors performed descriptive statistics. Normally distributed continuous variables are reported as mean with SD. Non-normally distributed variables are presented as median with interquartile range (IQR). Categorical variables are reported as number with percentage.
RESULTS
Forty-nine patients met inclusion criteria and are included in the analysis. There was a slight preponderance of females (n = 27, 55%) in the sample. The median age of the patients studied was 2.1 years (IQR, 0.7–8.1 years). The most common admission diagnosis was acute respiratory failure, occurring in 25 (51%) patients, with sepsis (n = 15, 31%) and surgical/trauma (n = 16, 33%) being the next most common. The median Pediatric Risk of Mortality scores (PRISM III) was 8 (IQR, 2–13). Thirty patients (61%) received mechanical ventilation on day of furosemide administration. The median duration of mechanical ventilation was 5 days (IQR, 3–10 days), with a median PICU length of stay of 7 days (IQR, 4–11 days). Three patients required renal replacement therapy, and 1 patient required extracorporeal membrane oxygenation (ECMO). The median furosemide dose was 0.5 mg/kg (IQR, 0.3−0.8 mg/kg). Although we did consider the use of other diuretic classes before furosemide administration, no patients received any. The indication for furosemide administration could not be determined from the medical record. The authors summarize demographic, admission, and fluid characteristics in the attached table (Supplemental Digital Content, available at http://links.lww.com/PQ9/A44).
Of the 49 patients included, 41 (84%) had an active total fluid order while in the PICU, 23 (56%) of which had an active order on DOA. In contrast, only 5 (10%) had an active daily weight order, and only 2 of these (40%) were active on DOA. Seven patients (17%) with a total fluid order during the study period had it placed after receiving furosemide, denoting a limitation of fluids after pharmacologic intervention.
Fluid Overload in the PICU
Thirty patients (63%) had greater than 5% daily FO on day 1 of admission, followed by 11 (23%) and 6 (14%) on day 2 and day 3, respectively. Similarly, 9 patients (19%) had greater than 10% daily FO on day 1 of admission compared with 2 patients (4%) on day 2. Thirty-five (71%) patients received furosemide by day 2, and 42 (86%) received furosemide by day 3. In contrast, the number of patients with a daily negative fluid balance increased with each DOA from 2 patients (4%) on day 1 to a maximum of 21 patients (68%) on day 5.
Twenty-two patients (51%) had greater than 10% cumulative FO by day 3, as compared with 10 patients (42%) on day 7. The percentage of patients with a net negative fluid balance gradually increased from day 1 to day 7 (4–21%) as the number of observed patients decreased. The degree of daily and cumulative FO decreased with subsequent days of hospitalization as summarized in Figure 3.
Fig. 3.
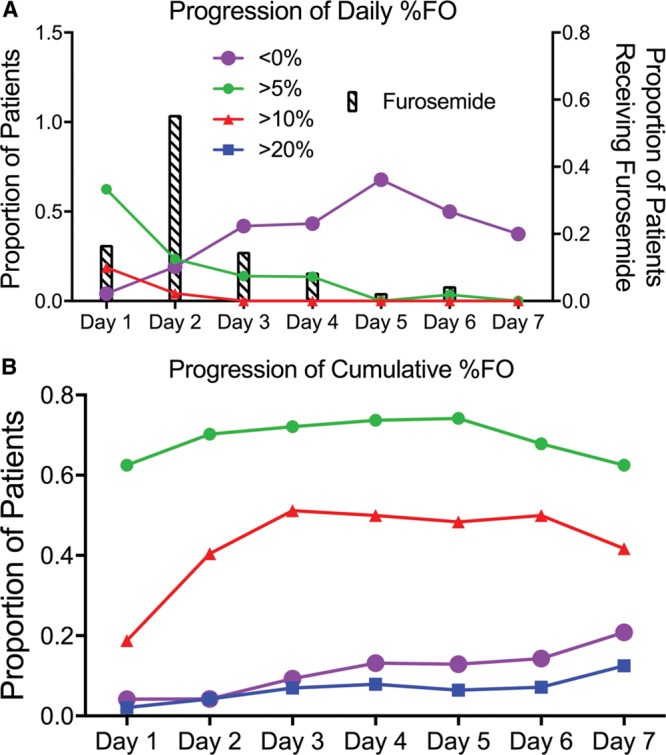
Line charts representing the progression of FO for the first 7 days of PICU admission. A, Progression of daily % FO, where the y axis is the proportion of patients with daily FO, and the x axis is the days of admission. “< 0%” is represented by open circles, “> 5%” is represented by gray circles, “> 10%” is represented by gray triangles, “> 20%” is represented by black squares; proportion of patients receiving furosemide each day is noted by bars. B, Progression of cumulative % FO, where the y axis represents the proportion of patients with cumulative FO, and the x axis represents the days since PICU admission.
Adherence to Total Fluid Order
Tight adherence to total fluid order occurred in 24 patients (59%), as defined by total daily fluids received within 5% of expected total fluids. An additional 8 patients (78% total) demonstrated daily deviation (%DD) within 10% of expected total fluids. The median deviation from the expected fluid administration was +3.5% (IQR, 1.4–7.2%), excluding a single outlier with a deviation of 560%. Figure 4 shows a scatterplot representing the %DD.
Fig. 4.
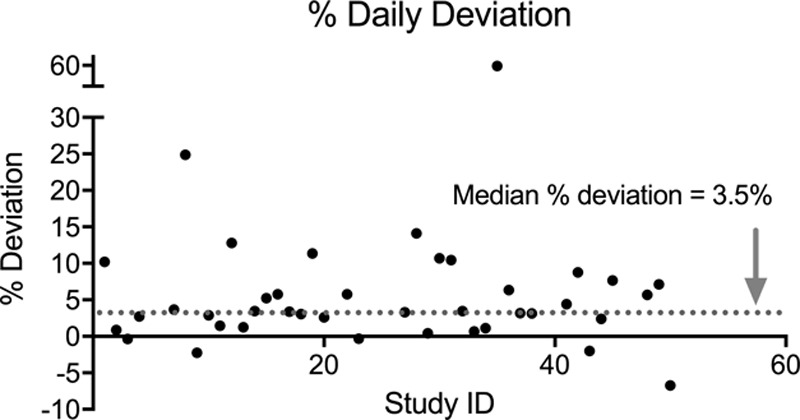
Percent daily deviation (%DD) of fluids administered based on the total fluid order. %DD is shown on the y axis with study ID on the x axis. The median %DD is demonstrated by the gray dotted line at 3.5%, and the outlier with the %DD of 560% is not included.
Adherence to Daily Weight Orders
All patients in the study had an order for at least once-weekly weight measurement, but only 4 (8%) critically ill children had a nonadmission weight within the 7 days preceding or following admission. Likewise, only 5 (10%) patients had an order for daily weights, and only one of these had an additional weight obtained. Weight measurement remained infrequent throughout, with only 2 patients in the study demonstrating 2 or more nonadmission weights.
VSAP Phase 1 Results
There were 1,137 admissions between April and December 2017 (median 118/mo; IQR, 113–134). The proportion of admissions who received a daily weight order in April 2017 was 5.9%. This proportion increased to 87.8% by December 2017 (Fig. 5A). Weights were measured more often in patients with a daily weight order (64.9% of patient-days in September with order present; 19.7% of patient-days without order), though the frequency of weight measurement declined for all patients in December (Fig. 5B).
Fig. 5.
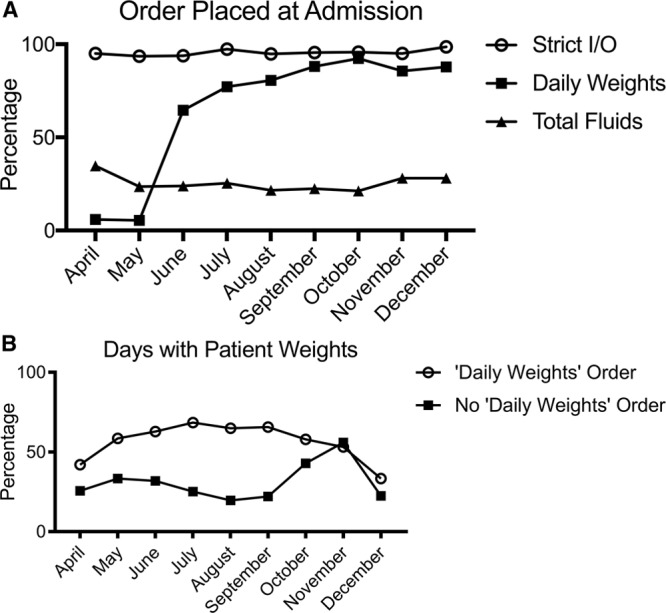
Run charts representing change in order practices following VSAP phase 1 interventions. A, Order placed at admission. The percentage of total patients admitted to the PICU that had a given order-type placed upon admission is shown on the y axis. Month of admission is shown on the x axis. “Strict I/O” is represented by open circles, “Daily Weights” is represented by black squares, “Total fluids” is represented by black triangles. B, Days with patient weights. The y axis shows the percentage of days a weight was measured out of the total patient-days for a given month. Month of admission to the PICU is shown on the x axis. Patient-days are delineated by presence or absence of a daily weight order with “Daily Weight order” represented by open circles and “No Daily Weight order” represented by black squares.
DISCUSSION
FO is associated with significant increases in morbidity and mortality in critically ill children.5,7,8,12 Improved fluid management practices have the potential to minimize these risks, decrease recovery times, reduce costs, and improve resource utilization. Medical therapies to reduce FO—such as furosemide—are associated with electrolyte derangements, ototoxicity, and nephrotoxicity. Renal replacement therapy requires placement of an indwelling catheter, which can increase the risk of infection and vessel thrombosis. Even if temporary, early FO is independently correlated with increased mortality,5 and volume overload more generally is associated with worse clinical outcomes. For those critically ill children who require continuous renal replacement therapy, the degree of cumulative FO at the initiation of therapy is further associated with decreased survival to discharge.3,7,9 These associations are especially worrisome when mechanical ventilation is common, as these patients may be at increased risk for FO (eg, closed respiratory circuits limit insensible losses; sedation impairs mobilization of extravascular fluids).
Characteristics of Baseline Practices
This retrospective analysis provides baseline data to inform the VSAP aim of reducing iatrogenic FO. Although the use of furosemide does not always indicate provider-perceived FO, its frequent use as an acute diuretic at this institution supports its selection as a proxy for retrospective identification of clinically significant FO. In the days preceding furosemide administration, many patients demonstrated greater than 5–10% FO per day, with half of all patients showing cumulative FO greater than 10% by day 3, further supporting this hypothesis.
Although total fluid orders were prevalent, only half were placed or active upon admission to the PICU. Further, the use of total fluid orders after intervention with furosemide suggests an attempt to mitigate and prevent further harm from newly recognized FO. These trends in timing and frequency of order initiation highlight opportunities for improved management at the outset of patient care. When a total fluid order was in place, there was low variance around stated fluid goals, suggesting reasonable compliance with total fluid limits. Given consistent levels of adherence across varied disease processes and fluid needs, the potential for improvement in this setting is large.
Closely trended patient weights are a more elegant and practical approach to tracking fluctuations in fluid balance. Selewski et al10 have shown that weight-based assessments of volume status are well-correlated with daily fluid flowsheets over short periods of time and can be sensitive indicators of early fluid positivity. Unfortunately, there was a noted infrequency of weight assessment throughout this cohort, irrespective of the presence of weight measurement orders. Even the department standard of at least once weekly weights was noted in only 8% of observed patients. Irrespective of the cause, this finding revealed a tremendous divide between our reported standard of care and actual practice.
VSAP: Promising Process Measures
In June 2017, the first phase of VSAP went into effect with a focus on regular tracking of patient weight. Interventions included new PICU fluid monitoring standards, automated prompts to assist in identifying and modifying care for high-risk patients, and system-wide engagement of key stakeholders as detailed in Figure 1. Early data suggest providers are now utilizing daily weight orders with greater frequency. Although order adherence has also improved, patients with daily weight orders still only have recorded weights for ~2/3 of their inpatient stay. Monthly data also suggest new challenges, highlighting a broad decline in measurement practices for all patients aligning with the start of the “respiratory season” when the census at our institution tends to climb. Additional work is needed to better characterize contributors to incomplete adherence over time.
Though this analysis focused on patients admitted to the PICU, the risk of volume-associated complications is not restricted to the critical care setting. Future iterations of VSAP will incorporate a screening component to promote close monitoring of all children whose fluid status is in question at the time of admission and will streamline initiation of aggressive fluid monitoring for those who sustain AKI or other complications sensitive to FO. Beyond improved management of patients at risk for FO, improved tracking of FO will allow for automated correction of serum creatinine for patient volume status, assisting providers with a more accurate picture of renal injury. Further standardization of the timing of furosemide administration based on a threshold %FO may also be beneficial in reducing practice variation and alleviate worsening FO.
Limitations
There are numerous limitations to this study. The assumption that patient admission weights were comparable with their “dry weight” is problematic in a retrospective analysis. At the time of this study, there was no requirement for providers to document fluid status upon admission, and internal institutional review suggests underreporting of FO and dehydration. Excessive and incomplete fluid-resuscitation before admission have divergent effects on the appearance of FO, and patients unstable on admission may have an estimated weight continued upon arrival.
Even dedicated staff cannot provide a perfect accounting of fluid losses. Unmeasured or unquantified output and insensible losses artificially bias assessment toward FO and are not accounted for in this analysis. Methods to estimate insensible losses could be applied but would require broad assumptions in a retrospective analysis of a heterogeneous patient population. Delays in documentation (“catch-up charting”), communication of order changes, and typographical errors in orders or charting may also misrepresent the timing of fluid administration. Furthermore, assessment of cumulative FO by fluid balance measurements alone may be misleading as this aggregates the limitations above and may overestimate peak overload.
Variability in charting practices also yields some uncertainty in retrospectively ascertaining the “intent” of fluid administration. For example, a patient with clinical FO may also be intravascularly deplete, forcing providers to balance the risks and benefits of additional resuscitation. Inferences from chart review may imply evolving FO, even as providers are appropriately prioritizing tissue perfusion. This scenario confounds the association between FO and poor outcomes, highlighting the value of trending weights and delineating periods of resuscitation and steady-state. We also acknowledge that the median PICU length of stay (7 days) may limit adherence to daily weights. Further implementation of weight measurements following PICU transfer may facilitate improved awareness of fluid balance and need for additional diuretic therapy.
This study only includes patients who received a single dose of furosemide, excluding those with repeat, prior or high doses (> 20 mg). These exclusions reflect the aims of our source dataset (a study assessing furosemide-responsiveness) and likely select for a lower risk population. Similarly, furosemide-exposure may not always correlate with clinical assessment of FO, and the trends noted here may reflect circumstances specific to furosemide-exposure rather than FO more broadly. Finally, this was a single-center study, and our results may reflect individual institutional practice and approach to fluid balance rather than broader trends in critical care.
CONCLUDING SUMMARY
This study demonstrates that total fluid and daily weight orders are infrequently placed in critically ill patients. When orders are present, there is often incomplete adherence. Both these factors may lead to iatrogenic FO. Implementation of a phased quality improvement project, beginning with the placement of daily weight orders has resulted in improved compliance. Assessment of whether this practice change reduces iatrogenic FO has yet to be determined. Further assessment of quality metrics, including balancing measures, is necessary to determine if the metrics in this study will lead to improved patient outcomes.
DISCLOSURE
The authors have no financial interest to declare in relation to the content of this article.
Supplementary Material
Footnotes
Published online October 10, 2018.
Supplemental digital content is available for this article. Clickable URL citations appear in the text.
To cite: Ahearn MA, Soranno DE, Stidham T, Lusk J, Gist KM. Adherence to Daily Weights and Total Fluid Orders in the Pediatric Intensive Care Unit: A Retrospective Descriptive Study. Pediatr Qual Saf 2018;3:e110.
REFERENCES
- 1.Brierley J, Carcillo JA, Choong K, et al. Clinical practice parameters for hemodynamic support of pediatric and neonatal septic shock: 2007 update from the American College of Critical Care Medicine. Crit Care Med. 2009;37:666–688.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Arikan AA, Zappitelli M, Goldstein SL, et al. Fluid overload is associated with impaired oxygenation and morbidity in critically ill children. Pediatr Crit Care Med. 2012;13:253–258.. [DOI] [PubMed] [Google Scholar]
- 3.Goldstein SL, Somers MJ, Baum MA, et al. Pediatric patients with multi-organ dysfunction syndrome receiving continuous renal replacement therapy. Kidney Int. 2005;67:653–658.. [DOI] [PubMed] [Google Scholar]
- 4.Goldstein S, Bagshaw S, Cecconi M, et al. ; ADQI XII Investigators Group. Pharmacological management of fluid overload. Br J Anaesth. 2014;113:756–763.. [DOI] [PubMed] [Google Scholar]
- 5.Li Y, Wang J, Bai Z, et al. Early fluid overload is associated with acute kidney injury and PICU mortality in critically ill children. Eur J Pediatr. 2016;175:39–48.. [DOI] [PubMed] [Google Scholar]
- 6.Sinitsky L, Walls D, Nadel S, et al. Fluid overload at 48 hours is associated with respiratory morbidity but not mortality in a general PICU: retrospective cohort study. Pediatr Crit Care Med. 2015;16:205–209.. [DOI] [PubMed] [Google Scholar]
- 7.Sutherland SM, Zappitelli M, Alexander SR, et al. Fluid overload and mortality in children receiving continuous renal replacement therapy: the prospective pediatric continuous renal replacement therapy registry. Am J Kidney Dis. 2010;55:316–325.. [DOI] [PubMed] [Google Scholar]
- 8.Acheampong A, Vincent JL. A positive fluid balance is an independent prognostic factor in patients with sepsis. Crit Care. 2015;19:251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Goldstein SL, Currier H, Graf C, et al. Outcome in children receiving continuous venovenous hemofiltration. Pediatrics. 2001;107:1309–1312.. [DOI] [PubMed] [Google Scholar]
- 10.Selewski DT, Cornell TT, Lombel RM, et al. Weight-based determination of fluid overload status and mortality in pediatric intensive care unit patients requiring continuous renal replacement therapy. Intensive Care Med. 2011;37:1166–1173.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Basu RK, Zappitelli M, Brunner L, et al. Derivation and validation of the renal angina index to improve the prediction of acute kidney injury in critically ill children. Kidney Int. 2014;85:659–667.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Valentine SL, Sapru A, Higgerson RA, et al. ; Pediatric Acute Lung Injury and Sepsis Investigator’s (PALISI) Network; Acute Respiratory Distress Syndrome Clinical Research Network (ARDSNet). Fluid balance in critically ill children with acute lung injury. Crit Care Med. 2012;40:2883–2889.. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


