Abstract
This study aims to explore the optimized digestive method of collagenase to nucleus pulposus (NP) cells by observing the digestive effects of type I and II collagenase in different concentrations to NP in degenerated intervetebral discs.
NP were collected from 18 human herniated intervertebral disc samples, and digested by type I and II collagenase, which were separated or combined in different concentrations. NP cells were counted using an inverted microscope, and the activities were determined by trypan blue staining at 4, 8, 16, and 24 hours after digestion. The growth of NP cells was also observed.
The amount of NP cells with combined collagenases was greater than that separated in an identical concentration. With the combined collagenases at 4 and 8 hours, the higher concentration, the greater the amount of NP cells became. The amount of cells in extremely low concentrations of collagenase increased after 16 and 24 hours, and its activities remained at a higher level.
The optimized digestion of extremely low concentrations of type I and II collagenase combined could save enzymes, was less harmful to NP cells, and was more adapted to separated and cultured NP cells.
Keywords: collagenase, nucleus pulposus cell, optimized digestion, separation and culture
1. Introduction
In clinic, lower back pain causes more disability worldwide than any other condition, and spinal disorders caused by intervertebral disc degeneration (IVDD) result in lower back pain in adults, which seriously affect their lives and health.[1–4]
IVDD is formed by the peripheral annulus fibrosus, nucleus pulposus (NP), and superior and inferior cartilage endplates. NP comprises mainly of proteoglycans, collagen type II, water, and NP cells, which are responsible for the homeostasis of the NP matrix.[5,6] Furthermore, NP is derived from mid-mesoderm notochordal structures, and has been reported to be affected by age-associated IVDD,[7–9] combined with decreased cellularity and water content, loss of proteoglycans in the extracellular matrix, and increased matrix stiffness.[10]
The enzymatic digestion method is a common method for the separation of NP cells, which has been reported by chain enzyme digestion.[11] However, trypsin and collagenase digestion of disc tissues are commonly used in experiments due to high cost. Collagenase belongs to the matrix metalloproteinase family, which can dissolve NP tissues at a certain pH and temperature.[12] However, under optimal conditions for giving full play to collagenase activity, digestive and NP cell damage is lesser, and is worth of further study.
In the present study, type I and II collagenase alone and in combination with digested NP cells were used, and these were digested in a good way to explore the different concentrations of NP cells under the influence of biological behavior, and observe the number of NP cells. Furthermore, for the effect of the growth of vitality, the concentration for the enzyme digestion method was optimized to explore more conducive approaches to the culture of NP cells.
2. Materials and methods
General information: Specimens were collected from 18 patients with lumbar disc herniation, who underwent resection of the intervertebral disc nucleus pulposus in our hospital from December 2Cer 2017. Among these patients, there were 10 males and 8 females, and the age of these patients ranged within 32 to 56 years old, with an average age of 37.8 years old. Furthermore, among these patients, 10 patients had herniation in L4-5, while 8 patients had herniation in L5-S1. Each patient provided a signed informed consent, which has been verified and approved by the Hospital Ethics Committee.
2.1. Experimental methods
Reagent: Trypsin was prepared with type I and II collagenase obtained from DMEM/F12 medium.
2.1.1. Experimental material
After surgical removal of NP tissues from the herniated lumbar disc, the specimen was immediately taken to the laboratory and immediately placed in saline at 4°C with a sterile console. Then, the specimen was rinsed with D-Hanks liquid at 4°C for 2 to 3 times to clear the blood. After the fibrous ring, cartilage and granulation tissues were removed in aseptic conditions, NP was separated by ophthalmic scissors. The NP tissue was separated into 1-mm3 tissue blocks and placed in a Petri dish containing D-Hanks solution.
2.1.2. Cell separation
The NP separation process was as follows: The sterile NP tissue fragments were placed in 3 times the volume of 0.25% trypsin solution, digested at room temperature for 20 minutes, and added with fetal bovine serum to stop the digestion. After centrifugation at 1000 rpm for 5 minutes, the supernatant was discarded, and the tissue was washed twice with DMEM/F12 medium.
2.1.3. According to the conditions of different groups.
Type I or II collagenase was separately added or both of the 2 were added for digestion, while 10% fetal bovine serum was also added.
2.1.3.1. According to the different types of collagenase groups
To observe the separative effect of type I and II collagenase to NP cells, 0.25% trypsin digestion was conducted for 20 minutes, the tissues were replaced by type I and II collagenase alone or type I + type II collagenase, and continued the digestion. These were referred to as group I (0.2% type I collagenase), group II (0.2% type II collagenase), and group III (0.1% type I collagenase + 0.1% type II collagen Enzyme), respectively, to observe the digestive enzyme digestion and isolation effect of each group.
2.1.3.2. According to the different concentrations of collagenase groups
To explore the optimal concentration of digestive enzymes isolated in NP cells, 0.25% trypsin digestion was performed for 20 minutes, and this was replaced by a concentration of 0.1% (IIIa group, high concentration group), 0.05% (IIIb group, low concentration group), 0.01% (IIIc group, very low concentration group) of type II collagenase for codigestion.
2.2. Cell counting and cell viability assay
The count and viability of cells in groups I, II, III, IIIa, IIIb, and IIIc were determined at various time points after digestion at 37°C for 4, 8, 16, and 24 hours.
2.2.1. Cell counting method
Cells were count using a plate counter under a phase contrast microscope, and cell separation and growth were observed. The counting method was based on the "Principles and Techniques of In Vitro Culture." [13]
2.2.2. The determination of cell viability
In the cell separation process, trypan blue staining was performed to determine the cell viability. The steps were as follows: cells were placed in an equal volume of DMEM/F12 medium and 0.4% trypan blue staining, and observed with a plate counter microscope. The number of cells that were stained and alive was recorded, while blue dyed cells were dead. Cell viability was preliminarily obtained according to the percentage of the total number of cells not stained by the blue dye. Then, cell viability was calculated (the number of stained NP cells/the total number of high magnification NP cells × 100%).
2.3. Cultured NP cells
After digestion for 4 and 24 hours, NP cells were centrifuged at 1000 rpm for 5 minutes, the supernatant was discarded, DMEM/F12 medium was added, a sterile nylon filter with a pore size of 74 μm was used, counting was performed, and pressed at a density of 1 × 104/mL in a disposable flask containing 10% fetal bovine serum and DMEM/F12 medium. Each group was added with 10% fetal bovine serum during the collagenase digestion, cultured in an incubator at 37°C with 5% CO2, and changed every 3 days. Then, cells were trypsinized and passaged up to 80% confluency.
During the process of purification and culture of NP cultured in vitro, the fragments were digested with collagenase, added with DMEM/F12 culture medium, and inoculated into 3 culture dishes, respectively. After the first dish was inoculated for 5 minutes, and the medium was slightly aspirated. Furthermore, the second dish was inoculated according to the same procedures, Then the third dish was treated in the same way.
2.4. Statistical analysis
All data were presented as x standard deviation, and analyzed using SPSS 19.0 statistical software with 1-way analysis of variance for processing. The ∗ in the data sheet indicated that P < .05; #P < .01.
3. Results
3.1. Cell counting
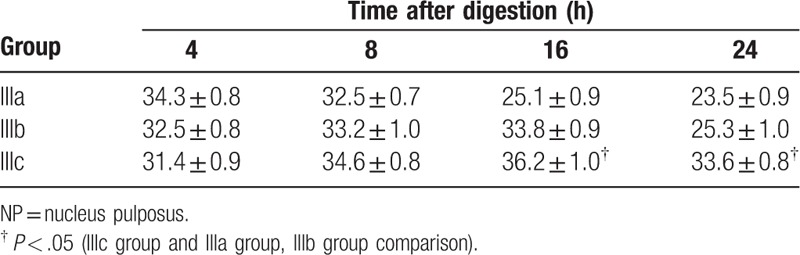
NP cell count was performed after digestion at 37°C for 4 hours, and the number of NP cells in each group was counted after 8, 16, and 24 hours (Table 1). Compared with the same collagenase concentration and digestion time, the number of cells in group III was greater than that in groups I and II. At the same digestion time point in group III, the number of cells were IIIa> IIIb> IIIc at 4 and 6 hours, and IIIc obviously increased, while IIIa and IIIb slightly increased at 16 hours after digestion. At 24 hours, the number of NP cells decreased in groups IIIa and IIIb, and cell viability was higher in IIIc than that in the other 2 groups (Table 2).
Table 1.
Number of NP cells after digestion of type I, type II collagenase alone or in combination at different points in time (×104/mL).
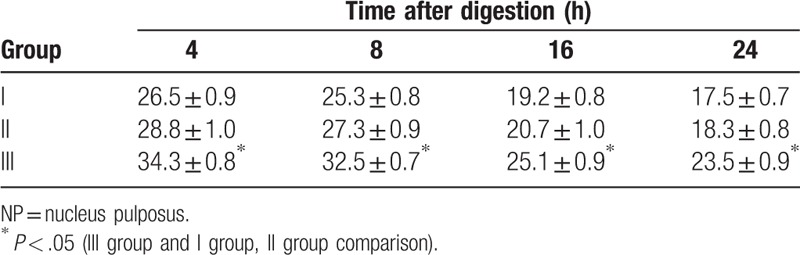
Table 2.
Number of NP cells after different concentrations of collagenase I + II digestion at different points in time (×104/mL).
3.2. Cell viability assay
There were no significant differences in the survival rate of NP cells between type I, type II and type I+type II collagenase after digestion (P > .05), as well as in type I+II collagenase combined with the digestion of the different concentration groups (IIIa, IIIb, and IIIc). Cell viability at each time point after digesting NP cells: Cell viability in the different isolation methods decreased to different extents at 24 hours after inoculation, when compared with inoculation. Among these, the decrease degree of cell survival rate in group IIIa was the largest. These results revealed that cytotoxicity was significantly higher in group IIIa than in groups IIIc and IIIb after 24 hours of digestion (P < .01) (Tables 3 and 4).
Table 3.
Rate of NP cell viability after collagenase type I, type II, and type I + type II collagenase digestion at different points in time (%).
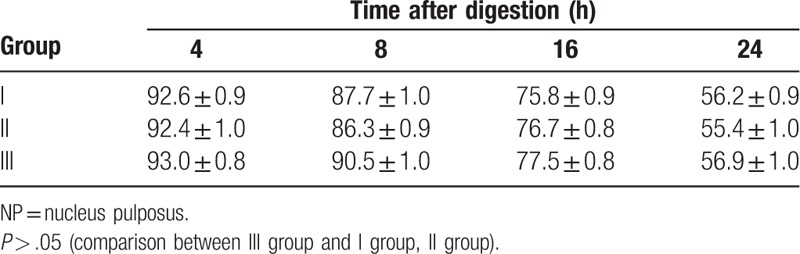
Table 4.
Rate of NP cell viability after combined digestion of collagenase type I and type II in different concentrations at different points in time (%).

3.3. Morphological observation
The IIIa and IIIb 2 groups had a relatively slow rate of attachment. After 72 hours, IIIc cells were more adherent, when compared with the IIIa and IIIb groups. Cells were polygonal or short spindled, had a clear outline, and the nucleus was round (Fig. 1). After 2 weeks, the number of adherent cells obviously increased, but the proliferation and proliferation of cells slowed down. Cells were passed on when they covered the bottle wall at approximately 24 days later. The subsequent passage of cells also needed approximately 6 days to adhere, and the growth was slower than that before passage, showing a relatively stable state of growth.
Figure 1.

Nucleus pulposus cells primary culture adherent growth.
4. Discussion
The NP of the intervertebral disc is derived from the notochord. This mainly refers to notochord cells and chondrocyte-like cells.[14] Embryos are mainly composed of notochord cells before 26 weeks. These chondrocyte-like cells are mainly observed in the NP of embryos and adults after 26 weeks, are mainly evolved from notochord cells, and are synthesized. The secretion of the extracellular macromolecular matrix maintains the normal physiological function of the intervertebral disc. In vivo, the NP cells of the intervertebral disc express aggrecan, type-II collagen 1, and SOX9.[15,16] Since adult NP cells are terminal cells, their proliferative capacity is weaker than that of ordinary cells, and the culture process is very strict. Furthermore, their morphology often changes in response to local mechanical changes.[17,18] The enzymatic digestion of the NP primary cell culture has an important impact, in addition to the media, pH, and other influencing factors.[19] A good growth environment was ensured to cells by using the experimental medium with F12/DMEM plus 10% fetal bovine serum, and the cell culture medium was changed every 3 days. Cells were spindle-shaped after passage, which reflects the trend of fibrosis.
Collagenase is extracted from Bacillus sp., can specifically hydrolyze the 3-dimensional helical structure of collagen, and can dissolve NP tissues under certain pH and temperature levels. This has been mainly used to digest bone, kidney, and other tissues. Fibroin tissue matrix components are mainly collagen, proteoglycan, elastin, and other ingredients. Since the crude enzyme contains a variety of proteases, it also digests cell surface proteins, which slightly damages cells.
From the experimental results, it can be observed that in the separation of NP cells, type I and type II collagenase digestion was stronger than type I and type II collagenase alone. The determination of cell viability revealed that cells that have just been isolated are highly viable, have the strongest activity after being cultivated for a period of time, and gradually weaken after subculture, which is in line with the cell growth cycle.
High concentrations of digestive enzymes have an impact on the number of cells and cell activity. The experiment confirms that very low concentrations of type I and II collagenase combined with the higher number and cell viability of NP cells after digestion for 16 hours, while the high and medium concentrations after collagenase digestion began to reduce the number of cells, significantly reduced vitality, may have induced cell damage due to high concentrations of digestive enzymes, and could even lead to cell death.
The time for cells of degenerated human intervertebral disc to attach to wall adherence time was 5 to 7 days or longer, the logarithmic growth phase was short, and the cell growth cycle was long, indicating that degenerative disc cell growth activity was low. After passage, NP cells grew relatively fast, and reached the first passage in approximately 24 days. However, when they reached the second passage, the intracellular vacuoles increased, and the secreted granules increased. Then, cells began to show signs of aging. Very low concentrations (0.01%) are more suitable for NP cells during the digestion with type I and type II collagenases. On one hand, collagenase has a weak digestive capacity at very low concentrations, which overcomes the effect of excessive enzyme activity on the activity of NP cells, and has a lower degree of damage to cells, which contributes to NP cell viability in subsequent cell cultures. On the other hand, the very low concentration of collagenase digestion method is simple, and omits the first digestion and other steps to remove digestive enzymes during centrifugation. Since collagenase potency is not affected by fetal bovine serum, similar to that of trypsin, this allows for the simultaneous collagenase digestion of NP tissues during the culture of cells in the medium containing fetal bovine serum, while extremely low concentrations of collagenase will be naturally consumed in the process of culture.
In the present experiment, the very low concentration collagenase digestion method was used to obtain high-purity and sufficient NP cells. Compared with the traditional collagenase digestion method, the new method has the advantages of simple operation, large yield of cells, and high purity. Very low concentrations of digestive enzymes can induce damage to NP cells to a lesser degree, are conducive to its survival, and maintain more active functions.
Acknowledgment
As the corresponding author, I am grateful to my supervisor, Professor Anmin Chen, who carefully checked and pointed out the mistakes of the draft. His constructive advice has guided me to complete the thesis.
Author contributions
Conceptualization: Li Liu, Bao-Qing Yu, Jian-Ming Huang.
Data curation: Li Liu, Bao-Qing Yu, Jian-Ming Huang.
Formal analysis: Long-Dian Gu, Da-Feng Xu.
Investigation: Li Liu, Bao-Qing Yu, Jian-Ming Huang.
Methodology: Long-Dian Gu, Da-Feng Xu.
Project administration: Xu Feng.
Resources: Li Liu, Bao-Qing Yu, Jian-Ming Huang.
Software: Long-Dian Gu, Da-Feng Xu.
Supervision: Xu Feng.
Writing – original draft: Xu Feng.
Writing – review & editing: Xu Feng.
Footnotes
Abbreviations: IVDD = intervertebral disc degeneration, NP = nucleus pulposus.
The authors have no conflicts of interest to disclose.
References
- [1].Katz JN. Lumbar disc disorders and low-back pain: socioeconomic factors and consequences. J Bone Joint Surg Am 2006;88:21–4. [DOI] [PubMed] [Google Scholar]
- [2].Hoy D, March L, Brooks P, et al. The global burden of low back pain: estimates from the Global Burden of Disease 2010 study. Ann Rheum Dis 2014;73:968–74. [DOI] [PubMed] [Google Scholar]
- [3].Daly C, Ghosh P, Jenkin G, et al. A Review of animal models of intervertebral disc degeneration: pathophysiology, regeneration, and translation to the clinic. Biomed Res Int 2016;2016:5952165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Lazebnik M, Singh M, Glatt P, et al. Biomimetic method for combining the nucleus pulposus and annulus fibrosus for intervertebral disc tissue engineering. J Tissue Eng Regen Med 2011;5:e179–187. [DOI] [PubMed] [Google Scholar]
- [5].Schubert AK, Smink JJ, Arp M, et al. Quality assessment of surgical disc samples discriminates human annulus fibrosus and nucleus pulposus on tissue and molecular level. Int J Mol Sci 2018;19:ii:E1761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Guo W, Zhang B, Li Y, et al. Gene expression profile identifies potential biomarkers for human intervertebral disc degeneration. Mol Med Rep 2017;16:8665–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Rodrigues-Pinto R, Richardson SM, Hoyland JA. An understanding of intervertebral disc development, maturation and cell phenotype provides clues to direct cell-based tissue regeneration therapies for disc degeneration. Eur Spine J 2014;23:1803–14. [DOI] [PubMed] [Google Scholar]
- [8].Liu Y, Fu S, Rahaman MN, et al. Native nucleus pulposus tissue matrix promotes notochordal differentiation of human induced pluripotent stem cells with potential for treating intervertebral disc degeneration. J Biomed Mater Res A 2015;103:1053–9. [DOI] [PubMed] [Google Scholar]
- [9].Richardson SM, Ludwinski FE, Gnanalingham KK, et al. Notochordal and nucleus pulposus marker expression is maintained by sub-populations of adult human nucleus pulposus cells through aging and degeneration. Sci Rep 2017;7:1501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Urban JP, Roberts S. Degeneration of the intervertebral disc. Arthritis Res Ther 2003;5:120–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Maeda S, Kokubun S. Changes with age in proteoglycan synthesis in cells cultured in vitro from the inner and outer rabbit annulus fibrosus. Responses to interleukin-1 and interleukin-1 receptor antagonist protein. Spine (Phila Pa 1976) 2000;25:166–9. [DOI] [PubMed] [Google Scholar]
- [12].Priyadarshani P, Li Y, Yang S, et al. Injectable hydrogel provides growth-permissive environment for human nucleus pulposus cells. J Biomed Mater Res A 2016;104:419–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Situ ZQ, Wu JZ. Cell Culture. 1st ed. Xi’an: World Publishing Corporation; 2001. [Google Scholar]
- [14].Risbud MV, Schaer TP, Shapiro IM. Toward an understanding of the role of notochordal cells in the adult intervertebral disc: from discord to accord. Dev Dyn 2010;239:2141–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Bridgen DT, Fearing BV, Jing L, et al. Regulation of human nucleus pulposus cells by peptide-coupled substrates. Acta Biomater 2017;55:100–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Mizuno H, Roy AK, Zaporojan V, et al. Biomechanical and biochemical characterization of composite tissue-engineered intervertebral discs. Biomaterials 2006;27:362–70. [DOI] [PubMed] [Google Scholar]
- [17].Roberts S, Evans H, Trivedi J, et al. Histology and pathology of the human intervertebral disc. J Bone Joint Surg Am 2006;88 suppl 2:10–4. [DOI] [PubMed] [Google Scholar]
- [18].Guterl CC, See EY, Blanquer SB, et al. Challenges and strategies in the repair of ruptured annulus fibrosus. Eur Cell Mater 2013;25:1–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Liu MC, Chen WH, Wu LC, et al. Establishment of a promising human nucleus pulposus cell line for intervertebral disc tissue engineering. Tissue Eng Part C Methods 2014;20:1–0. [DOI] [PubMed] [Google Scholar]


