Abstract
Our previously studies indicated that inflammatory responses are involved in the hematoma expansion (HE) after intracranial hemorrhage (ICH) ictus. Here, we aim to evaluate the correlations among the ratio of neutrophil to lymphocyte ratio (NLR), HE, and island sign in patients with ICH.
Patients with spontaneous ICH were retrospectively included. Clinical characteristics, imaging features, and laboratory parameters were obtained. Multivariable analysis was performed to evaluate the association of NLR with HE or island sign. Receiver-operator analysis was also used to estimate their predictive abilities for HE and its imaging features.
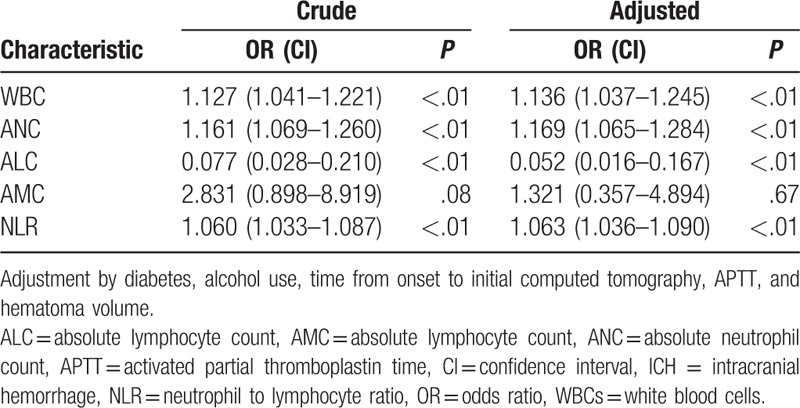
A total of 279 patients were enrolled in present study, and 78 patients had early hematoma growth, while 43 of them exhibited island sign. Elevation of both leukocyte (odds ratio [OR] 1.136, 95% confidence interval [CI] 1.037–1.245, P < .01) and neutrophil absolute numbers (OR 1.169, 95% CI 1.065–1.284, P < .01), as well as reduction of lymphocyte counts (OR 0.052, 95% CI 0.016–0.167, P < .01) were strongly associated with the existence of island sign. Moreover, despite the predictive ability of NLR on the existence of island sign (OR 1.063, 95% CI 1.036–1.090, P < .01), it also showed the best predictive accuracy (sensitivity 76.74%, specificity 79.66%, positive predictive value 40.70%, negative predictive value 94.90%, area under the curve 0.817) by comparing with peripheral leukocyte counts.
The NLR could be used as an independently marker for reflecting the island sign in patients with ICH. Our findings indicated that systemic inflammatory responses might be involved in the pathologic process of active bleeding in cerebral.
Keywords: hematoma expansion, inflammation, intracranial hemorrhage, island sign, neutrophil to lymphocyte
1. Introduction
Spontaneous intracranial hemorrhage (ICH) is one of the most severe diseases thus lead to high ratio of mortality and morbidity in patients.[1,2] It is well-documented that inflammatory responses[3] and early hematoma growth[4–7] play important roles in the pathologic processes of ICH. However, the relationship between inflammation and hematoma expansion (HE) remains controversial due to the inconsistent results that observed in previous studies.[8,9] Recently, neutrophil to lymphocyte ratio (NLR) is employed as a novel inflammatory marker to independently predict the prognosis of patients with ICH.[8] By comparing with the absolute counts of both neutrophil count (ANC) and lymphocyte count (ALC), NLR exhibited the best predictive value for functional outcome in patients with ICH. Here, we aimed to evaluate the potential association of NLR with HE, as well as island sign, which was recently identified as an excellent imaging predictor for HE in patients with ICH.
2. Methods
2.1. Patient selection
All the cases of patients with ICH that admitted in West China Hospital between September 2014 and October 2016 were retrospectively reviewed. Inclusion criteria were as follow: patients were diagnosed as ICH by computed tomography (CT); laboratory tests were performed within 24 hours after disease onset; initial CT scans were performed within 6 hours after admission and followed up CT scans were performed within 24 hours after initial CT; the age of all patients were older than 18 years. While exclusion criteria includes: secondary ICH were caused by trauma, brain tumor, aneurysm, or arteriovenous malformation; any types of infection happened within 2 weeks; followed up CT scans were unavailable to perform; patients with systemic diseases, including neoplasm, chronic obstructive pulmonary disease, autoimmune disease, chronic heart disease, uremia, and severe renal dysfunction; immunosuppressant or anticoagulant treatments were performed; hematoma evacuation were performed before follow-up CT; patients with stroke history within 6 months.
This study was approved by Biomedical Ethic Committee of West China Hospital of Sichuan University and performed in accordance with relevant guidelines and regulations of Sichuan University. All patients’ guardians have signed their informed consent.
2.2. Clinical data
The following demographic and clinical characteristics were retrospectively collected: age, gender, admission blood pressure, smoking and alcohol use, history of hypertension or diabetes, family history of ICH, cerebral infarction or aneurysm. Admission blood examination parameters were also obtained, including white blood cells (WBCs), ANC, ALC, absolute monocyte count (AMC), admission platelet count, prothrombin time (PT), activated partial thromboplastin time (APTT), and international normalized ratio (INR). NLR was calculated according to the ANC divided by the ALC.
2.3. Imaging data
Two neuroradiologists were blinded to independently evaluate all the CT scans of patients. Any disagreement between them would be discussed and reached a consensus. All radiologic results were collected from head CT data, including hematoma location, hematoma size, island sign, presence of subarachnoid hemorrhage or intraventricular hemorrhage, cerebral infarction, and acute hydrocephalus. Hematoma volume was estimated by ABC/2 method that described in our previously studies.[10] In brief, A represents the longest diameter of the hematoma of patients, whereas B is the diameter perpendicular to A. And C represents the CT section numbers of hemorrhage multiplied the scanning thickness. HE was determined by 33% or more increasing of hematoma volume from the results of followed up CT, or 12.5 mL or more volumes of hematoma growth.[11] The island sign was identified by using the method that showed in previously study.[12] Generally, more than 3 separately small hematomas were found to around the major hematoma or more than 4 small hematomas were found to around the major hematoma.
2.4. Statistical analysis
Clinical data, laboratorial parameters, and imaging characteristics were compared between patients with ICH with or without HE. Results were shown as mean ± standard deviation, or median with interquartile range (IQR) and compared with independent t test or Mann–Whitney U test for normal distribution and non-normal distribution respectively. Chi-squared test or Fisher exact test were used for analyzing categorical data, which presented as frequency and percentage. The variables identified that have statistically significant contribute to the outcome (P < .15) in univariate analysis were then included into multivariable logistic regression analysis to evaluate association of NLR and HE as well as island sign. Receiver-operator analysis was applied to estimate the predictive ability of NLR for HE and island sign. The variables were considered statistically significant if P < .05. All the above-mentioned analyses were performed using SPSS 23.0.
3. Results
A total of 279 cases of patients with ICH, including 207 males and 72 females were enrolled in currently study (Fig. 1). The average age was 56.59 ± 11.95 years ranging from 31 to 89 years. The interval time from symptom onset to perform the initial CT scan was 3.80 ± 2.05 hours. The baseline of hematoma volume was 28.13 ± 16.71 mL. The total number of patients with HE is 78, whereas 75 of them were caused by supratentorial hemorrhage over 259 patients (29.0%), and 3 of patients with HE (15.0%) were due to infratentorial hemorrhage (P = .18). All the detail information and baseline features of included patients with or without HE are shown in Table 1. In addition, island sign was found in 43 patients (15.4%). The κ value for interobserver reliability of island sign was 93.5%, which suggested an excellent interobserver agreement between 2 neuroradiologists. By comparing with patients with ICH without HE, more island sign could be observed in patients with HE (44.9%). The association between clinical characteristics and island sign was also analyzed (Table 2).
Figure 1.
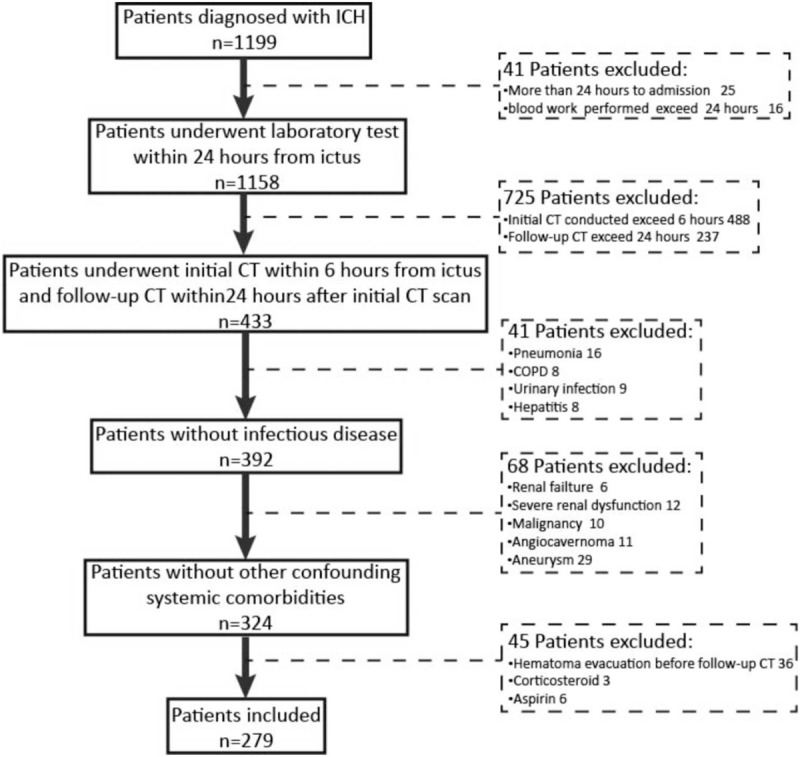
Flowchart of patient enrollment. COPD = chronic obstructive pulmonary disease.
Table 1.
Baseline clinical characteristics related to hematoma expansion in patients with ICH.
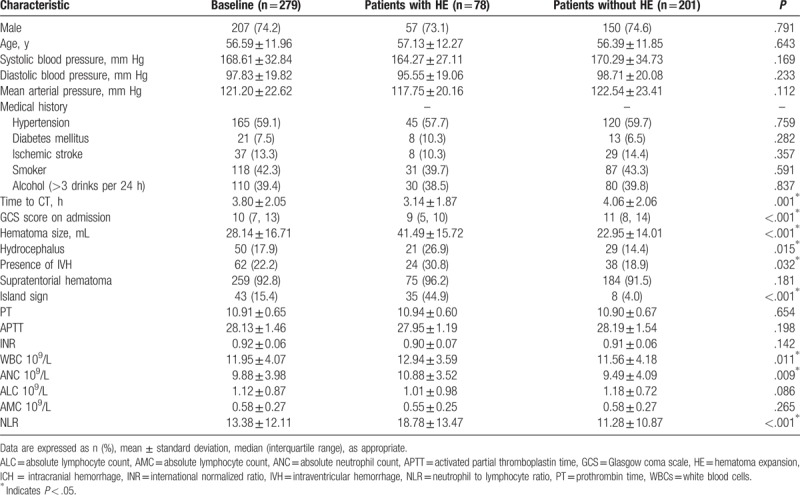
Table 2.
Clinical characteristics related to island sign in patients with ICH.
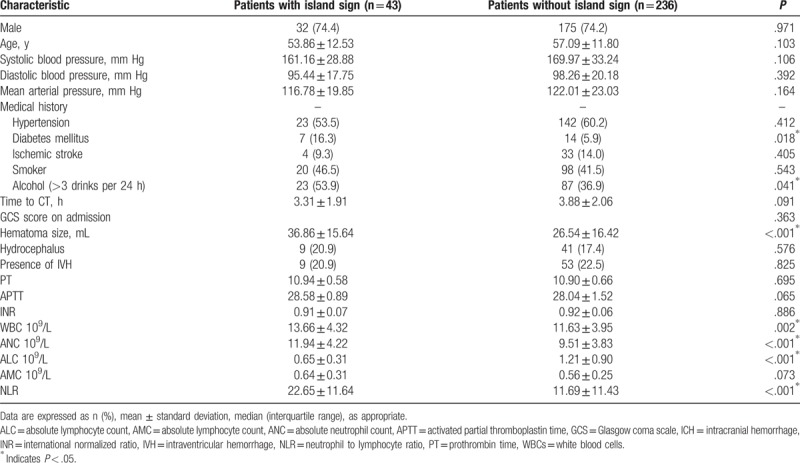
According to the blood tests and further analysis, higher WBC (with HE: without HE = 12.94 ± 3.59: 11.56 ± 4.18, P = .011), ANC (with HE: without HE = 10.88 ± 3.52: 9.49 ± 4.09, P = .009), as well as NLR (with HE: without HE = 18.78 ± 13.47: 11.28 ± 10.87, P < .001) showed in patients with HE (Table 1). Meanwhile, patients that possessed island sign had higher WBC (with island sign: without island sign = 13.66 ± 4.32: 11.63 ± 3.95, P = .002), ANC (with island sign: without island sign = 11.94 ± 4.22: 9.51 ± 3.83, P < .001), and NLR (with island sign: without island sign = 22.65 ± 11.64: 11.69 ± 11.43, P < .001), whereas lower ALC (with island sign: without island sign = 0.65 ± 0.31: 1.21 ± 0.90, P < .001) were also observed in patients with HE (Table 2). Based on the results from univariate analysis, we found that WBC, ANC and NLR was positively associated with HE and island sign, respectively (Tables 3 and 4). Furthermore, ALC but not AMC was strongly associated with island sign (Table 4). After optimization of potential confounding clinical variables, the multivariable analysis demonstrated that WBC (odds ratio [OR] 1.136, 95% confidence interval [CI] 1.037–1.245, P < .01), ANC (OR 1.169, 95% CI 1.065–1.284, P < .01) and NLR (OR 1.063, 95% CI 1.036–1.090, P < .01) could be used for predicting island sign but not HE (Tables 3 and 4). Simultaneously, our results also indicated that it is interesting that ALC (OR 0.052, 95% CI 0.016–0.167, P < .01) were negatively associated with island sign (Table 4).
Table 3.
Associations of laboratory values on admission with HE in patients with ICH.
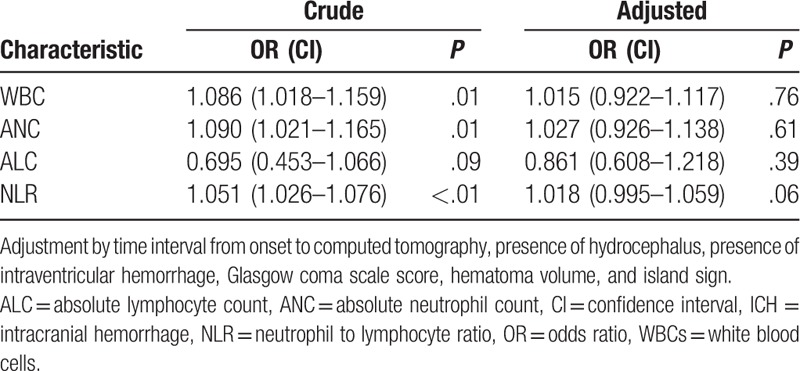
Table 4.
Associations of laboratory values on admission with island sign in patients with ICH.
Following, the receiver operating characteristic analysis (ROC) was employed to compare the predictive abilities of relevant inflammatory predictors for island sign in patients with HE. The sensitivity, specificity, positive predictive value (PPV), negative predictive value (NPV), and area under the curve (AUC) of NLR for predicting island sign were 76.74%, 79.66%, 40.70%, 94.90%, and 0.817, respectively, the best predictive cut-off value was 14.53 (Fig. 2). The ROC curves and the areas under the curves (AUCs) of laboratory parameters for predicting island sign are also determined in our study, and results elucidated that NLR harbored the best predictive ability for island sign by comparing with other laboratory values (Fig. 2).
Figure 2.
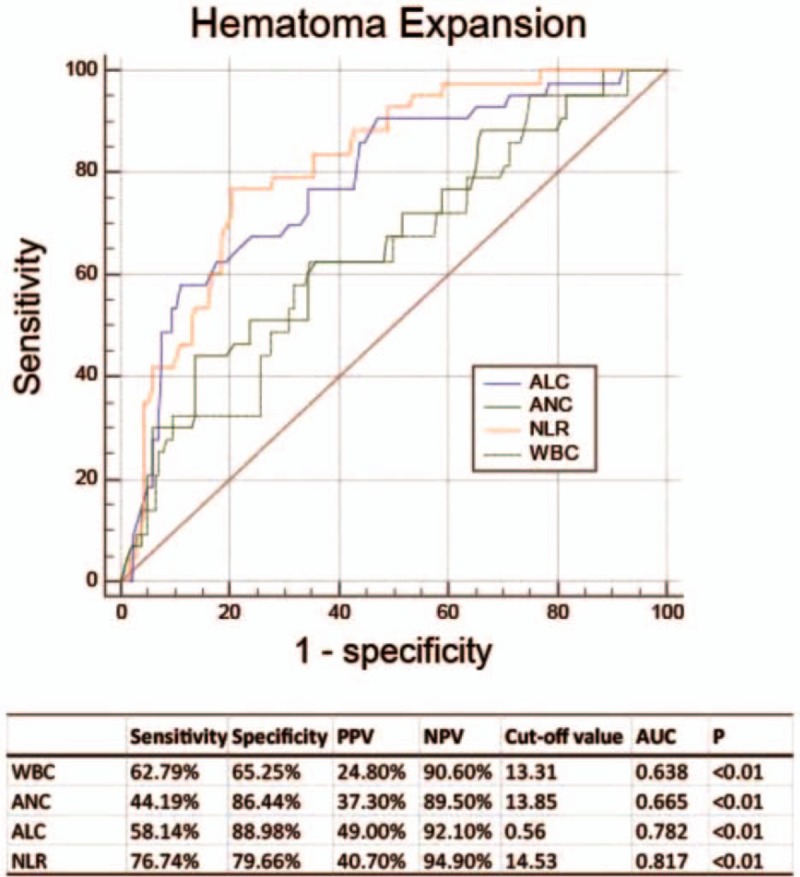
Receiver-operating characteristic curves of white blood count (WBC), absolute neutrophil count (ANC), absolute lymphocyte count (ALC), and neutrophil to lymphocyte ratio (NLR) with their corresponding areas under the curve (AUC) for predicting hematoma expansion. The best cut-off points were identified for WBC, ANC, and NLR with their sensitivity, specificity, positive predictive value (PPV) and negative predictive value (NPV).
4. Discussion
Based on the results, we concluded that elevated NLR could independently predict island sign but not HE. Moreover, increasing of both WBC and ANC are associated with the existence of island sign, while lower ANC could independently predict the existence of island sign. Over all the predicative parameters, NLR showed the best predictive ability for island sign. Taken together, we systematically demonstrated that NLR was associated with the outcome of patients with ICH with HE, and might be used as a valuable predictive marker for HE and the existence of island sign.
Accumulating evidences indicated that HE triggered inflammation accelerates brain injury after ICH in patients.[8,9,13,14] Although the relationship between leukocyte and prognosis in patients with ICH was well documented,[15,16] but the link between early HE and subsets of leucocytes was still controversial.[9,8,17,18] In our study, after strictly excluded the confounders, we could not find that WBC, ANC, and NLR act as biomarker in early HE, except an elevation of them were observed. However, WBC, ANC, and ALC were detected to have the strong predictive ability in island sign, which represented an efficient neuroimaging predictor for early hematoma enlargement. As one of the latest neuroradiologic features, island sign may reflect the active bleeding and is supposed to depict multifocal small bleeding hematomas around the main hematoma.[12] Due to this indirect evidence, we expect that all those biomarkers mentioned above exist potential predicting values of early HE in patients with ICH. However, the exact underlying mechanisms of the links between blood routine variables and HE remain elusive and need to further investigate.
The NLR represents a combined index that reflects systemic immune status. As a novel inflammatory marker, it was recently identified to independently predict the progression and outcome of many diseases in human patients.[19–22] Accumulating results from several studies reported that NLR was associated with hematoma size,[23] 30-day mortality,[24] 90-day mortality,[25] and poor outcome[8] in patients with IHC. However, whether NLR could be also used for predicting HE in patients with ICH remains unknown. Here, we revealed that NLR was associated with island sign and showed the best predictive value of this neuroradiologic feature by comparing with other laboratory parameters. But the irrelevance of NLR with hematoma growth in patients with ICH was also observed. The possible reasons of this phenomenon were as follow: firstly, elevating NLR could induce neurotoxicity thus activated the matrix metalloproteinases, which triggered the basal membrane components degradation, BBB breakdown, and brain edema and active bleeding.[26] Secondly, inflammatory cascades were activated by thrombin during HE and resulted the elevation of WBC count, C-reactive protein, and interleukin-6, which further promotes HE.[27,28] At last, cellular immune response was reported to damage coagulation function and microvascular integrity via inducing C-reactive protein (CRP) activation, which is a relatively chronic process.[13,29] Therefore, it is expected that systemic increasing of neutrophil correlated with participates the pathologic progression of active bleeding and contributes to secondary brain injury and poor clinical outcome of patients with ICH with ICH. But further researches need to be conducted to clarify the underlying details in patients with ICH.
There are still some limits in this study. Firstly, all our conclusions were obtained from retrospective study with relative small sample size. A significant proportion of patients were excluded due to unavailable imaging information or missing laboratory parameter from admission blood work which may lead to potential selection bias. Following, we involved a relatively small group of patients with subtentorial ICH, which might not represent the patients with brainstem hemorrhage and cerebellar hemorrhage. Thirdly, as the largest hospital in west of China, we always took the patients with worse clinical condition, which might also bias our results. Finally, ABC/2 method is less accurate than modern planimetric techniques to evaluate hematoma volume.
5. Conclusion
We demonstrated that NLR was an efficient marker to predict island sign in patients with ICH. Other than that significantly increasing of both WBC and ANC, as well as decreasing of ALC were correlated with HE in patients with ICH. Overall, our findings suggested that systemic cellular immunologic responses may be involved in the pathologic process of active bleeding.
Author contributions
Fan Zhang, Yang M, and Chuanyuan Tao conceived and coordinated the study, designed, performed and analyzed the experiments, wrote the paper. Chuanyuan Tao, Juan Qian, Sen Lin, Yuelong Wang and Fan Zhang carried out the data collection and data analysis. Chao You and Mu Yang revised the paper. All authors reviewed the results and approved the final version of the manuscript.
Conception and design: Zhang F, Yang M, Tao C.
Acquisition of data: Tao C, Zheng J, Qian J, Zhang F.
Analysis and interpretation of data: Zhang F, Yang M, Wang Y, Qian J, Zheng J, Lin S.
Drafting the article: Zhang F, Yang M, Qian J and Tao C.
Supervision: You C.
Review & editing: All the authors.
Data curation: Fan Zhang, Juan Qian, Yuelong Wang, Chao You.
Formal analysis: Fan Zhang, Sen Lin.
Investigation: Fan Zhang, Chuanyuan Tao.
Methodology: Fan Zhang, Juan Qian, Chuanyuan Tao, Yuelong Wang, Sen Lin, Chao You, Mu Yang.
Project administration: Fan Zhang, Sen Lin, Mu Yang.
Software: Yuelong Wang.
Supervision: Chao You, Mu Yang.
Writing – original draft: Fan Zhang, Chuanyuan Tao, Chao You.
Writing – review & editing: Juan Qian, Mu Yang.
Footnotes
Abbreviations: ALC = absolute lymphocyte count, AMC = absolute monocyte count, ANC = absolute neutrophil count, APTT = activated partial thromboplastin time, AUC = area under the curve, CI = confidence interval, CT = computed tomography, HE = hematoma expansion, ICH = intracranial hemorrhage, INR = international normalized ratio, IQR = interquartile range, IVH = intraventricular hemorrhage, NLR = neutrophil to lymphocyte ratio, OR = odds ratio, PT = prothrombin time, ROC = receiver operating curve, SAH = subarachnoid hemorrhage, sICH = spontaneous intracranial hemorrhage, WBC = white blood count.
FZ is supported by Sichuan University postdoctoral grant (2017SCU12048) and China postdoctoral science foundation grant (2018M633373) and Sichuan Health and Family Planning Commission grant (18PJ425). MY is Catherine Bushnell fellow (2015). This study was also supported by Sichuan province science and technology grant (2015SZ0051) and West China hospital academic excellence grant (2016102).
The authors have no conflicts of interest to disclose.
References
- [1].Qureshi AI, Mendelow AD, Hanley DF. Intracerebral haemorrhage. Lancet 2009;373:1632–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Qureshi AI, Tuhrim S, Broderick JP, et al. Spontaneous intracerebral hemorrhage. N Engl J Med 2001;344:1450–60. [DOI] [PubMed] [Google Scholar]
- [3].Aronowski J, Zhao X. Molecular pathophysiology of cerebral hemorrhage: secondary brain injury. Stroke 2011;42:1781–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Brouwers HB, Chang Y, Falcone GJ, et al. Predicting hematoma expansion after primary intracerebral hemorrhage. JAMA Neurol 2014;71:158–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Chan S, Conell C, Veerina KT, et al. Prediction of intracerebral haemorrhage expansion with clinical, laboratory, pharmacologic, and noncontrast radiographic variables. Int J Stroke 2015;10:1057–61. [DOI] [PubMed] [Google Scholar]
- [6].Chen S, Zhao B, Wang W, et al. Predictors of hematoma expansion predictors after intracerebral hemorrhage. Oncotarget 2017;8:89348–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Yu Z, Zheng J, Ali H, et al. Significance of satellite sign and spot sign in predicting hematoma expansion in spontaneous intracerebral hemorrhage. Clin Neurol Neurosurg 2017;162:67–71. [DOI] [PubMed] [Google Scholar]
- [8].Lattanzi S, Cagnetti C, Provinciali L, et al. Neutrophil-to-lymphocyte ratio predicts the outcome of acute intracerebral hemorrhage. Stroke 2016;47:1654–7. [DOI] [PubMed] [Google Scholar]
- [9].Silva Y, Leira R, Tejada J, et al. Molecular signatures of vascular injury are associated with early growth of intracerebral hemorrhage. Stroke 2005;36:86–91. [DOI] [PubMed] [Google Scholar]
- [10].Kothari RU, Brott T, Broderick JP, et al. The ABCs of measuring intracerebral hemorrhage volumes. Stroke 1996;27:1304–5. [DOI] [PubMed] [Google Scholar]
- [11].Davis SM, Broderick J, Hennerici M, et al. Hematoma growth is a determinant of mortality and poor outcome after intracerebral hemorrhage. Neurology 2006;66:1175–81. [DOI] [PubMed] [Google Scholar]
- [12].Li Q, Liu QJ, Yang WS, et al. Island sign: an imaging predictor for early hematoma expansion and poor outcome in patients with intracerebral hemorrhage. Stroke 2017;48:3019–25. [DOI] [PubMed] [Google Scholar]
- [13].Kuhlmann CR, Librizzi L, Closhen D, et al. Mechanisms of C-reactive protein-induced blood-brain barrier disruption. Stroke 2009;40:1458–66. [DOI] [PubMed] [Google Scholar]
- [14].Lee KR, Colon GP, Betz AL, et al. Edema from intracerebral hemorrhage: the role of thrombin. J Neurosurg 1996;84:91–6. [DOI] [PubMed] [Google Scholar]
- [15].Leira R, Davalos A, Silva Y, et al. Early neurologic deterioration in intracerebral hemorrhage: predictors and associated factors. Neurology 2004;63:461–7. [DOI] [PubMed] [Google Scholar]
- [16].Sun W, Peacock A, Becker J, et al. Correlation of leukocytosis with early neurological deterioration following supratentorial intracerebral hemorrhage. J Clin Neurosci 2012;19:1096–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Suzuki S, Kelley RE, Dandapani BK, et al. Acute leukocyte and temperature response in hypertensive intracerebral hemorrhage. Stroke 1995;26:1020–3. [DOI] [PubMed] [Google Scholar]
- [18].Morotti A, Phuah CL, Anderson CD, et al. Leukocyte count and intracerebral hemorrhage expansion. Stroke 2016;47:1473–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Crumley AB, McMillan DC, McKernan M, et al. Evaluation of an inflammation-based prognostic score in patients with inoperable gastro-oesophageal cancer. Br J Cancer 2006;94:637–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Xue P, Kanai M, Mori Y, et al. Neutrophil-to-lymphocyte ratio for predicting palliative chemotherapy outcomes in advanced pancreatic cancer patients. Cancer Med 2014;3:406–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Tokgoz S, Keskin S, Kayrak M, et al. Is neutrophil/lymphocyte ratio predict to short-term mortality in acute cerebral infarct independently from infarct volume? J Stroke Cerebrovasc Dis 2014;23:2163–8. [DOI] [PubMed] [Google Scholar]
- [22].Brooks SD, Spears C, Cummings C, et al. Admission neutrophil-lymphocyte ratio predicts 90 day outcome after endovascular stroke therapy. J Neurointerv Surg 2014;6:578–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Giede-Jeppe A, Bobinger T, Gerner ST, et al. Neutrophil-to-lymphocyte ratio is an independent predictor for in-hospital mortality in spontaneous intracerebral hemorrhage. Cerebrovasc Dis 2017;44:26–34. [DOI] [PubMed] [Google Scholar]
- [24].Wang F, Hu S, Ding Y, et al. Neutrophil-to-lymphocyte ratio and 30-day mortality in patients with acute intracerebral hemorrhage. J Stroke Cerebrovasc Dis 2016;25:182–7. [DOI] [PubMed] [Google Scholar]
- [25].Tao C, Wang J, Hu X, et al. Clinical value of neutrophil to lymphocyte and platelet to lymphocyte ratio after aneurysmal subarachnoid hemorrhage. Neurocrit Care 2017;26:393–401. [DOI] [PubMed] [Google Scholar]
- [26].Nguyen HX, O’Barr TJ, Anderson AJ. Polymorphonuclear leukocytes promote neurotoxicity through release of matrix metalloproteinases, reactive oxygen species, and TNF-alpha. J Neurochem 2007;102:900–12. [DOI] [PubMed] [Google Scholar]
- [27].Mayer SA, Lignelli A, Fink ME, et al. Perilesional blood flow and edema formation in acute intracerebral hemorrhage: a SPECT study. Stroke 1998;29:1791–8. [DOI] [PubMed] [Google Scholar]
- [28].Xi G, Wagner KR, Keep RF, et al. Role of blood clot formation on early edema development after experimental intracerebral hemorrhage. Stroke 1998;29:2580–6. [DOI] [PubMed] [Google Scholar]
- [29].Roberts CJ, Birkenmeier TM, McQuillan JJ, et al. Transforming growth factor beta stimulates the expression of fibronectin and of both subunits of the human fibronectin receptor by cultured human lung fibroblasts. J Biol Chem 1988;263:4586–92. [PubMed] [Google Scholar]


