Abstract
The integrase strand transfer inhibitor (INSTI) class of antiretroviral therapy (ART) may result in faster time to virologic suppression compared with regimens that contain protease inhibitors (PIs) or non-nucleoside reverse transcriptase inhibitors (NNRTIs). However, differences in time to achieve virologic suppression are not well-defined in routine clinical settings with contemporary antiretroviral agents.
Study was a retrospective single-center study of treatment-naïve human immunodeficiency virus (HIV) patients initiating ART between 2013 and 2016. Among patients on different ART regimen types, we compared rates of and median time to virologic suppression [viral load (VL) <50 copies/mL].
A total of 155 patients—45 (29%) female and 110 (71%) male—met study inclusion criteria. Median age was 42 years (interquartile range 31–52), and median baseline CD4 count was 288 cells/μL and VL was 60,000 copies/mL. Seventy-one (46%) initiated an INSTI-based regimen, 58 (37%) were on NNRTI-based regimens, and 26 (17%) on PI-based regimens. In total, 112 (72%) patients achieved virologic suppression at 12 months. Patients on INSTI-based regimens were more likely to achieve virologic suppression by 3, 6, and 12 months (P < .01), and had lower median time to suppression (60 vs 137 days on NNRTI-based regimens and 147 days on PI-based regimens, P < .01).
Patients on INSTI-based ART regimens in a real-world setting experienced higher rates of virologic suppression and shorter time from ART initiation to virologic suppression. For HIV patients on INSTI-based ART regimens, virologic failure should be suspected in those with VLs >50 copies/mL before the current recommendation of 48 weeks.
Keywords: human immunodeficiency virus/acquired immunodeficiency syndrome, integrase inhibitor, nucleoside (tide) reverse transcriptase inhibitors, protease inhibitor, virologic failure
1. Introduction
In the United States and worldwide, more human immunodeficiency virus (HIV)-infected individuals are taking combination antiretroviral therapy (ART) than ever before.[1] This has led to improved outcomes for patients including extended life expectancy compared to historical observations.[2] There are now 7 classes of antiretroviral drugs available and approved by the United States Federal Drug Administration for the treatment of HIV-1 infection, providing multiple treatment options for individuals who are newly infected or diagnosed.[3]
The antiretroviral agents approved for treatment of HIV-1 infection differ widely in their mechanism of action, pharmacokinetic properties, drug interaction potentials, side effect profiles, and dosing frequency. All of these are important considerations for providers initiating therapy for treatment-naïve HIV-infected individuals. A principal goal of HIV therapy is to achieve rapid suppression of the HIV virus, which allows restoration of the immune system to protect against acquired immunodeficiency syndrome–associated opportunistic infections and also decreases the time of infectivity thereby preventing disease transmission from infected individuals.[4,5] Thus, one important measure of the efficacy of a chosen ART regimen is the time to full virologic suppression.
Virologic failure is defined in US Department of Health and Human Services (DHHS) guidelines as failure to achieve or maintain full virologic suppression (<200 copies/mL) at 48 weeks after initiation of ART.[6] Virologic failure may occur as a result of various factors including poor medication adherence, development of drug resistance, and unrecognized drug-drug interactions that can affect ART pharmacokinetics and efficacy. Intensive virologic monitoring after treatment initiation allows for identification of individuals who are at risk of virologic failure and provides opportunities to address its potential causes.
Contemporary ART regimens for the treatment of HIV that include the integrase strand transfer inhibitor (INSTI) class of ART have demonstrated high efficacy, tolerability, and in initial clinical trials, have resulted in faster time to virologic suppression compared to that historically documented for ART regimens based on protease inhibitor (PI) and non-nucleoside reverse transcriptase inhibitor (NNRTI) classes.[7,8] However, these differences are not well-defined in routine clinical settings.
Therefore, our study aimed to describe the time from treatment initiation to full virologic suppression among HIV-infected treatment-naïve individuals in a real world setting and compare regimens by base ART class.
2. Methods
2.1. Study aims
We performed a retrospective single-center chart review of antiretroviral-naïve patients with HIV who initiated ART at any Yale-New Haven Hospital (YNHH) System site from January 1, 2013 to December 31, 2016. The aim of the study was to compare rates of and time to virologic suppression among HIV-infected patients initiating INSTI-, PI-, and NNRTI-based ART regimens in a routine clinical setting.
2.2. Eligibility criteria
Included in the study were all patients with diagnosed HIV infection who were ART naïve and initiated on a typical ART regimen consisting of an NNRTI, PI, or INSTI in combination with 2 nucleoside/nucleotide reverse transcriptase inhibitors. Patients who did not have both baseline (at treatment initiation) and subsequent viral load (VL) values available (at least 1 VL measurement within 3, 6, and between 6 and 12 months of ART initiation), who had provider-documented nonadherence to their prescribed ART medication upon chart review, who were started on an atypical antiretroviral regimen (i.e., not meeting criteria for typical regimen described above, for example, a regimen containing both a PI and NNRTI together), and who switched regimens during the study period were excluded.
2.3. Patient selection and data collection
With the help of YNHH's Joint Data Analytics Team, HIV-infected patients who were newly prescribed any antiretroviral medication within the study period were identified in the electronic medical record system. A subsequent chart review was conducted to confirm eligibility criteria and collect data on patient demographics, comorbidities, HIV-related clinical factors including presence of opportunistic infection(s) (as defined by DHHS guidelines),[9] CD4+ T-lymphocyte count, and HIV RNA VL measurements up to 1 year after treatment initiation. Our laboratory uses COBAS Ampliprep/COBAS Taqman, version 2.0, Linear range: 20 to 10,000,000 copies/mL (Roche Diagnostics, Indianapolis, IN) for VL estimation and flow cytometry for CD4 counts.
2.4. Data analysis
HIV VLs at baseline and closest to 3-, 6-, 9-, and 12-month time points after ART initiation were recorded and entered into a database for the study analysis. Virologic suppression was defined as a VL <50 copies/mL on HIV-RNA quantitative assay, according to currently accepted clinical practice. Analysis was also performed on time to VL <200 copies/mL, which is the cutoff specified in current DHHS guidelines.[9] Time to virologic suppression was calculated as period (in days) from initiation of ART to achievement of virologic suppression.
We compared rates of achievement of virologic suppression among patients on INSTI-, NNRTI-, and PI-based regimens at 3-, 6-, and 12-month time points using Chi-square test. We also compared time to virologic suppression using independent samples median testing and Kaplan-Meier analysis. We assessed for variables that were associated with virologic suppression at 12 weeks using Cox regression analysis. Statistical significance was set at P value <.05. Statistics were performed using IBM SPSS software version 24.0.
2.5. Study approval
The study was approved by Yale University human investigations committee.
3. Results
3.1. Eligibility screen
Of 1388 screened for eligibility, 193 were found to be ART naïve and had at least 2 VL tests on record in the post-ART initiation period. Of these, 6 patients were initiated on a nontraditional ART regimen and 32 were documented to be nonadherent to therapy and were not included. Therefore, 155 patients were included in the analysis.
3.2. Demographics
In total, 155 patients—45 (29.0%) females and 110 (71%) males—met study inclusion criteria. The relative proportion of men in the INSTI group (83%) was higher than that of NNRTI (66%) and PI-based (50%) ART groups. Forty-three (28%) were white, 73 (47%) were black, 32 (21%) Hispanic, and 7 (5%) of other ethnicity/race. Median age at ART initiation was 42 years [interquartile range (IQR) 31–52] and median body mass index (BMI) was 26 (IQR 23–30) with no difference between the different ART groups (Table 1).
Table 1.
Patient demographics, clinical characteristics, and time to viral suppression.

3.3. HIV status and comorbidities
Before ART initiation, median CD4 was 288 cells/μL and median VL was 60,100 copies/mL (4.8 log10 copies/mL). Thirteen (8%) patients had an opportunistic infection diagnosed at time of ART initiation. Seventy-one (46%) initiated an INSTI-based ART regimen (Table 1), of which 56 initiated a dolutegravir-based regimen, 12 initiated a raltegravir-based regimen, and 3 initiated an elvitegravir-based regimen. Fifty-eight (37%) initiated an NNRTI-based regimen, of which 37 initiated an efavirenz-based regimen and 21 initiated a rilpivirine-based regimen. Twenty-six (17%) initiated a PI-based regimen, of which 15 initiated an atazanavir-based regimen, 5 initiated darunavir, and 6 initiated lopinavir/ritonavir. Patients on INSTI-based regimens had higher median pre-ART initiation VL (109,000 copies/mL) compared with the PI (55,000 copies/mL) and NNRTI (56,000 copies/mL) regimens (P = .03).
3.4. Virologic suppression rates
In total 112 (72%) achieved virologic suppression <50 copies/mL within 1 year of initiating ART; 139 (90%) achieved DHHS goal of <200 copies/mL. The median time to virologic suppression was 105 days (IQR 50–164). Patients on INSTI-based regimens were more likely to achieve virologic suppression at 3, 6, and 12 months, and had a lower median time to suppression of 60 days compared to 137 days on NNRTI regimens and 147 days on PI regimens (P < .01; Table 1). On Kaplan-Meier analysis, time-to-virologic suppression was significantly lower in INSTI-based regimens than PI- or NNRTI-based regimens (P < .01; Fig. 1). On multivariate analysis, although age at ART initiation, race/ethnicity, and BMI were not significantly associated with virologic suppression at 12 weeks, regimen type (INSTI vs PI vs NNRTI) was (P < .001). No incidences of immune reconstitution inflammatory syndrome occurred in this cohort.
Figure 1.
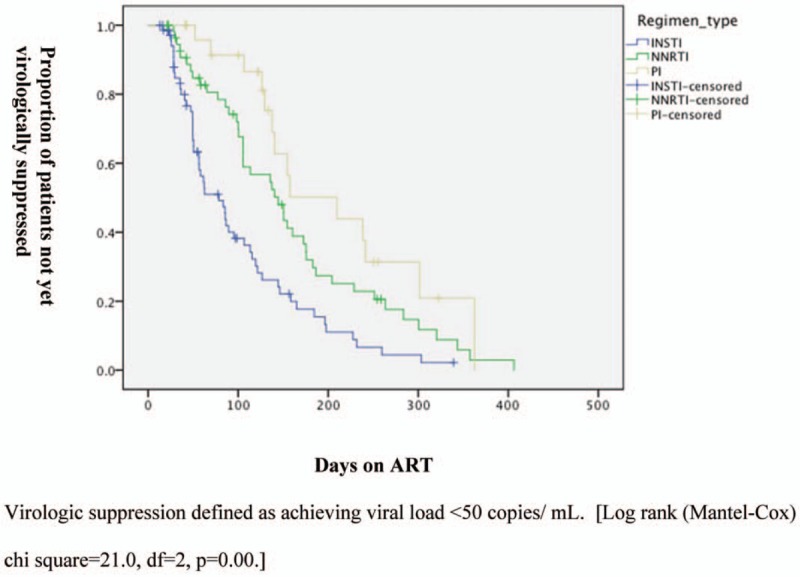
Kaplan Meier analysis of time from ART initiation to virologic suppression. The proportion of patients on antiretroviral therapy (ART) who had not yet achieved virologic suppression declined faster in the integrase inhibitor (INSTI) group compared with the protease inhibitor (PI) and non-nucleoside reverse transcriptase inhibitor (NNRTI) group.
4. Discussion
INSTI-based regimens are now the recommended and preferred first-line ART for the treatment of HIV-1 infection in ART-naïve patients due to their favorable side effect profile, limited drug-drug interactions, and virologic potency.[10] Accordingly, among our cohort, INSTI-based regimens were the most frequently initiated ART regimen class.
In this real world analysis, patients on INSTI-based ART regimens experienced higher rates of virologic suppression at 3, 6, and 12 months, and shorter median time from ART initiation to virologic suppression compared to patients on other ARV regimens. Furthermore, in our cohort, the observation that patients taking INSTI-based regimens had higher initial VLs, makes this finding even more significant. Our findings are consistent with previous studies that have similarly documented faster time to virologic suppression on INSTI-based ART regimens compared with NNRTI- and PI-based regimens.[10,11] Indeed, in clinical trials, majority of patients on INSTI-based ART achieve full virologic suppression at 12 to 16 weeks of therapy.[12–14] This occurrence may attributable to where INSTIs act in the viral life cycle and how they affects viral decay dynamics preintegration particularly among different T-cell populations.[15,16] Models show that INSTI-based regimens may decrease the slope of and lengthen the first phase (rapid phase) of virologic decay, potentially explaining the faster virologic suppression observed in our and other studies.[16–18]
For individuals who have achieved full virologic suppression, the first evidence of HIV treatment failure, preceding immunologic decline and the development of opportunistic infections, is virologic rebound. Therefore, routine VL monitoring may allow for earlier detection of treatment failure. Current DHHS guidelines define virologic failure as inability to achieve or maintain an HIV VL < 200 copies/mL, typically expected after 24 to 48 weeks on ART,[6] but our findings suggest that patients on INSTI-based ART regimens should be evaluated for treatment failure if they have not achieved virologic suppression as early as 12 weeks after ART initiation. This has important implications for clinical practice, particularly in situations in which patients would particularly benefit from shorter time to virologic suppression, for example, in pregnancy and among serodifferent couples where transmission is a concern.
In our study, patients on PI-based regimens were more likely to be women. This is most likely due to provider selection of a PI-based regimen for women of childbearing age, given the paucity of data on safety of INSTI use in pregnancy at the start of the study period and the concern for efavirenz-associated teratogenicity. In addition, it is plausible that patients suspected to be at higher risk of nonadherence were preferentially placed on PI-based regimens due to the higher barrier for resistance. In our cohort, women were more likely to have injection drug use (IDU) as their primary risk factor for acquiring HIV, and as IDU is a risk for nonadherence, this may explain both the preponderance of women and comparatively lower rates of virologic decline noted in the PI-based regimen group. However, we did exclude patients with documented poor adherence from the analysis to minimize its effect on study results. In addition, multivariate analysis did not find that sex was associated with virologic suppression at 12 weeks, although the study was not powered to detect differences stratified by sex.
Finally, we analyzed rates of viral suppression based on current DHHS guidelines using cutoff of HIV RNA <200 copies/mL as indicative of virologic suppression and the more contemporary definition of virologic suppression as <50 copies/mL as is currently accepted in clinical practice. Notably, there was a large difference in time to virologic suppression according to these 2 cutoffs. Guidelines should be updated to reflect current clinical practice and further studies should base outcome definitions on up-to-date practices.
Limitations of this study include small sample size, slight differences in clinical parameters between groups as it was a retrospective study, and nonuniform timing of VL measurements after ART initiation. Nonuniform frequency of VL monitoring may have skewed data, but this is expected in a real world study. Resistance profiles of patients were not analyzed as reasons for nonsuppression. We did not account for the impact of regimen tolerability that may have led patients to have poor adherence or change regimens, and thus were not analyzed in our data. Excluding patients with suspected nonadherence may limit generalizability to real-world clinic populations. We did not assess the impact of rapid virologic suppression on CD4 count recovery due to limited available data owing to differential practice of our clinic providers. Furthermore, we relied on chart documentation of medication adherence instead of more objective measurements such as pill count and pharmacy refill data, and changes in adherence would certainly impact VL decay. However, we believe that despite these limitations, these data contribute to understanding of ART-associated virologic decay patterns over time in a real-world clinical setting and is valuable to clinicians caring for patients living with HIV infection.
5. Conclusions
INSTI-based ART regimens resulted in faster time to virologic suppression compared to NNRTI- and PI-based regimens in this real-world cohort of HIV-infected treatment-naïve patients initiating ART. Patients on INSTI-based ART regimens should be evaluated for poor adherence and other causes of treatment failure if they have not achieved virologic suppression by 12 to 24 weeks, rather than 24 to 48 weeks as currently dictated by national guidelines.
Acknowledgments
The authors would like to thank the staff at the Nathan Smith Clinic, the Yale Joint Data Analytics team, and Yale Primary Care for their support in conducting this research.
Author contributions
Conceptualization: Karen Jacobson, Onyema Ogbuagu.
Data curation: Karen Jacobson.
Formal analysis: Karen Jacobson, Onyema Ogbuagu.
Investigation: Onyema Ogbuagu.
Methodology: Karen Jacobson, Onyema Ogbuagu.
Resources: Onyema Ogbuagu.
Software: Karen Jacobson, Onyema Ogbuagu.
Supervision: Onyema Ogbuagu.
Writing – original draft: Karen Jacobson, Onyema Ogbuagu.
Writing – review and editing: Karen Jacobson, Onyema Ogbuagu.
Onyema Ogbuagu orcid: 0000-0001-7342-2608.
Footnotes
Abbreviations: AIDS = acquired immunodeficiency syndrome, ART = antiretroviral therapy, BMI = body mass index, DHHS = Department of Health and Human Services, HIV = human immunodeficiency virus, IDU = injection drug use, INSTI = integrase strand transfer inhibitor, IQR = interquartile range, NNRTI = non-nucleoside reverse transcriptase inhibitor, PI = protease inhibitor, VL = viral load, YNHH = Yale-New Haven Hospital.
Conference presentation: Karen Jacobson K, Onyema Ogbuagu. Comparison of time to viral suppression among treatment-naive HIV-infected adults initiating combination antiretroviral therapy by antiretroviral regimen class. IDWeek2017/IDSA. October 4 to 8, 2017. San Diego. Abstract 1394.
Dr Ogbuagu is on speaker's Bureau for Gilead Sciences (HIV prevention). Dr Jacobson has no conflicts of interest to declare.
References
- [1].Dutta A, Barker C, Kallarakal A. The HIV treatment gap: estimates of the financial resources needed versus available for scale-up of antiretroviral therapy in 97 countries from 2015 to 2020. PLoS Med 2015;12:e1001907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Survival of HIV-positive patients starting antiretroviral therapy between 1996 and 2013: a collaborative analysis of cohort studies. Lancet HIV 2017;4:e349–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Pau AK, George JM. Antiretroviral therapy: current drugs. Infect Dis Clin North Am 2014;28:371–402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Lundgren JD, Babiker AG, Gordin F, et al. Initiation of antiretroviral therapy in early asymptomatic HIV infection. N Engl J Med 2015;373:795–807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Cohen MS, Chen YQ, McCauley M, et al. Antiretroviral therapy for the prevention of HIV-1 transmission. N Engl J Med 2016;375:830–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Department of Health and Human Services Panel on Antiretroviral Guidelines for Adults and Adolescents. Guidelines for the Use of Antiretroviral Agents in Adults and Adolescents Living with HIV. Available at: http://www.aidsinfo.nih.gov/ContentFiles/AdultandAdolescentGL.pdf Accessed June 1, 2017. [Google Scholar]
- [7].Rockstroh JK, DeJesus E, Lennox JL, et al. Durable efficacy and safety of raltegravir versus efavirenz when combined with tenofovir/emtricitabine in treatment-naive HIV-1-infected patients: final 5-year results from STARTMRK. J Acquir Immune Defic Syndr 2013;63:77–85. [DOI] [PubMed] [Google Scholar]
- [8].Lee FJ, Amin J, Carr A. Efficacy of initial antiretroviral therapy for HIV-1 infection in adults: a systematic review and meta-analysis of 114 studies with up to 144 weeks’ follow-up. PLoS One 2014;9:e97482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Masur H, Brooks JT, Benson CA, et al. Prevention and treatment of opportunistic infections in HIV-infected adults and adolescents: updated guidelines from the Centers for Disease Control and Prevention, National Institutes of Health, and HIV Medicine Association of the Infectious Diseases Society of America. Clin Infect Dis 2014;58:1308–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Hoenigl M, Chaillon A, Moore DJ, et al. Rapid HIV viral load suppression in those initiating antiretroviral therapy at first visit after HIV diagnosis. Sci Rep 2016;6:32947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Lennox JL, DeJesus E, Lazzarin A, et al. Safety and efficacy of raltegravir-based versus efavirenz-based combination therapy in treatment-naive patients with HIV-1 infection: a multicentre, double-blind randomised controlled trial. Lancet 2009;374:796–806. [DOI] [PubMed] [Google Scholar]
- [12].Van Lunzen J, Maggiolo F, Arribas JR, et al. Once daily dolutegravir (S/GSK1349572) in combination therapy in antiretroviral-naive adults with HIV: planned interim 48 week results from SPRING-1, a dose-ranging, randomised, phase 2b trial. Lancet Infect Dis 2012;12:111–8. [DOI] [PubMed] [Google Scholar]
- [13].Raffi F, Rachlis A, Stellbrink HJ, et al. Once-daily dolutegravir versus raltegravir in antiretroviral-naive adults with HIV-1 infection: 48 week results from the randomised, double-blind, non-inferiority SPRING-2 study. Lancet 2013;381:735–43. [DOI] [PubMed] [Google Scholar]
- [14].DeJesus E, Rockstroh JK, Henry K, et al. Co-formulated elvitegravir, cobicistat, emtricitabine, and tenofovir disoproxil fumarate versus ritonavir-boosted atazanavir plus co-formulated emtricitabine and tenofovir disoproxil fumarate for initial treatment of HIV-1 infection: a randomised, double-blind, phase 3, non-inferiority trial. Lancet 2012;379:2429–38. [DOI] [PubMed] [Google Scholar]
- [15].Sedaghat AR, Dinoso JB, Shen L, et al. Decay dynamics of HIV-1 depend on the inhibited stages of the viral life cycle. Proc Natl Acad Sci U S A 2008;105:4832–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Cardozo EF, Andrade A, Mellors JW, et al. Treatment with integrase inhibitor suggests a new interpretation of HIV RNA decay curves that reveals a subset of cells with slow integration. PLoS Pathog 2017;13:e1006478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Andrade A, Rosenkranz SL, Cillo AR, et al. Three distinct phases of HIV-1 RNA decay in treatment-naive patients receiving raltegravir-based antiretroviral therapy: ACTG A5248. J Infect Dis 2013;208:884–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Gilmore JB, Kelleher AD, Cooper DA, et al. Explaining the determinants of first phase HIV decay dynamics through the effects of stage-dependent drug action. PLoS Comput Biol 2013;9:e1002971. [DOI] [PMC free article] [PubMed] [Google Scholar]


