Abstract
To explore the influence of the 75 g oral glucose tolerance test (OGTT) on pregnancy outcomes and to determine the risk factors for adverse outcomes among women with gestational diabetes mellitus (GDM).
This retrospective cohort study was conducted among women who had GDM and were treated between January 1, 2015 and December 31, 2017. The diagnostic criteria for GDM were proposed by the International Diabetes and Pregnancy Research Organization (IADPSG) in 2010. Women with GDM were stratified according to the number of abnormal OGTT values or the presence/absence of adverse pregnancy outcomes. Maternal characteristics, OGTT values, pregnancy outcomes, and the relationship between the latter 2 were analyzed.
In total, 3221 pregnant women with GDM were included. The incidence of adverse outcomes was affected by maternal age (28–37 years, in particular; odds ratio [OR], 1.403; 95% confidence interval [CI], 1.037–1.899; P = .028), days of pregnancy (OR, 0.904; 95% CI, 0.894–0.914; P < .001), gestational weight gain (OR, 1.018; 95% CI, 1.000–1.036;, P = .048), and age of menarche (OR, 0.925; 95% CI, 0.863–0.992; P = .029). Both fasting plasma glucose (FPG) and 2-h OGTT were positively correlated with adverse outcomes, of which FPG was more predictive (FPG: OR, 1.143; 95% CI, 1.007–1.297; P = .038; 2-h OGTT: OR, 1.074; 95% CI, 1.018–1.133; P = .009). Meanwhile, higher abnormal OGTT values were associated with significantly increased risks of antenatal insulin treatment, cesarean delivery, premature delivery, gestational hypertension, premature rupture of membranes, preeclampsia, macrosomia, neonatal asphyxia, and full term low weight infants.
OGTT values and the number of abnormal glucose are associated with various adverse pregnancy outcomes. Stratified management is recommended for pregnant women with GDM, especially those with fasting hyperglycemia and/or 3 abnormal OGTT values.
Keywords: adverse pregnancy outcomes, fasting plasma glucose, gestational diabetes mellitus, oral glucose tolerance test, risk factors
1. Introduction
Gestational diabetes mellitus (GDM) was defined as “hyperglycemia diagnosed in the second or third trimester of pregnancy that is not clearly overt diabetes” by the American Diabetes Association (ADA) in 2014.[1] GDM is associated with numerous pregnancy complications, such as gestational hypertension, cesarean delivery, macrosomia, and stillbirth.[2] Besides, an increasing body of evidence has showed that the longer-term health outcomes of both mother and child may be adversely affected by GDM.[3–5] For the mother, GDM is a risk factor for later development of type 2 diabetes;[3] and for the child, long-term exposure to intrauterine hyperglycemia increases the risks of obesity, diabetes, and other metabolic syndromes.[6]
In 2010, the International Association of Diabetes and Pregnancy Study Groups (IADPSG) proposed some new GDM diagnostic criteria[7] based on the result of the Hyperglycemia and Adverse Pregnancy Outcomes (HAPO) study.[8] The IADPSG criteria were published and modified by the Ministry of Health (MOH) in China in August, 2014,[9] which has proven appropriate for the Chinese population.[10] By the new criteria, GDM is diagnosed if one or more of the following criteria are met in the 75 g oral glucose tolerance test (OGTT): a fasting plasma glucose (FPG) level ≥ 5.1mmol/L, a 1-h plasma glucose (1-h OGTT) level ≥ 10.0 mmol/L, and / or a 2-h plasma glucose (2-h OGTT) level ≥ 8.5 mmol/L.[8] Giving all pregnant women a 75 g OGTT may facilitate early GDM detection and reduce adverse outcomes, as more women would receive treatment for hyperglycemia once detected.[11]
Nevertheless, reports have been limited with regard to the specific relationship between maternal blood glucose and obstetrical and neonatal outcomes; and thus it still remains largely unclear which parameter, FPG, 1-h OGTT, or 2-h OGTT, has the greatest impact on pregnancy outcomes.[12,13] Despite the fact that many studies[14–16] have identified risk factors of GDM, such as prepregnancy obesity, excessive gestational weight gain, and family history of diabetes, the risk factors of adverse pregnancy outcomes among GDM are yet to be explored. In addition, a previous study[17] found that pregnancy outcomes of GDM women may vary with the number of abnormal OGTT values. In this study, therefore, we sought to investigate the impact of each OGTT parameter defined by the new IADPSG criteria on adverse pregnancy outcomes and to determine the risk factors for adverse outcomes among women with GDM. We also aimed to analyze the relationship between the number of abnormal OGTT values and adverse pregnancy outcomes.
2. Methods
2.1. Participant selection
This retrospective cohort study included data of women who had GDM and were treated between January 1, 2015 and December 31, 2017 at West China Second University Hospital, Sichuan University, which is located in Western China. As one of the top-ranking maternity hospitals in China, West China Second University Hospital is responsible for a great amount of clinical work such as treatment, referral, and consultation for women and children with critical illnesses in Sichuan Province, and even across Southwestern China, and provides healthcare services to patients from different socioeconomic backgrounds with approximately 10,000 deliveries per year.
Singleton pregnant women diagnosed as GDM were eligible to be included in this study unless they met one or more of the following exclusion criteria: multiple pregnancies, pregestational diabetes, pre-existing systemic diseases that may affect GDM, or lack of OGTT or delivery data. All information on women with GDM was recorded in the Hospital Information System (HIS), and was selected and extracted from the HIS by 2researchers independently.
2.2. Diagnostic criteria for GDM
GDM was diagnosed when at least one abnormal plasma glucose value was determined as ≥ 5.1mmol/L (fasting), ≥ 10.0 mmol/L (1 hour), and / or ≥ 8.5 mmol/L (2 hours) at 24 to 28 weeks of gestation for all women not previously found to have overt diabetes or GDM during any earlier 75 g OGTT test in their current pregnancy.[7] For women who had more than one 75 g OGTT test performed during their pregnancy, the latest OGTT results were recorded if the earlier 75 g OGTT was diagnostic of GDM.
Based on the number of abnormal OGTT values, GDM women were stratified into 3 groups, that is, Group 1, Group 2, and Group 3, which consisted of pregnant women with 1, 2, or 3 abnormal OGTT values, respectively. In addition, GDM women were stratified by the presence/absence of adverse pregnancy outcomes into 2 groups.
2.3. Data collection
Maternal and pregnancy variables: age, nationality, gravidity, parity, days of pregnancy, gestational weight gain, history of abnormal pregnancy, family history of diabetes, age of first sexual contact, age of menarche, number of antenatal visits, FPG, 1-h OGTT, 2-h OGTT, and antenatal insulin treatment (AIT, administered when GDM women had a FPG ≥ 5.8 mmol/L, and a 2-h OGTT ≥ 6.7 mmol/L after dietary therapy).
Obstetric outcomes: cesarean delivery, postpartum hemorrhage, premature delivery, gestational hypertension, amniotic fluid pollution, intrahepatic cholestasis of pregnancy (ICP), premature rupture of membranes (PROM), placental abruption, preeclampsia, and polyhydramnios.
Neonatal variables: birth weight and Apgar score at 1 minute; and neonatal outcomes: macrosomia, fetal growth restriction, fetal distress in uterus, fetal malformations, neonatal asphyxia, stillbirth, full term low weight infants, protracted descent and small for gestational age (SGA).
Some of these variables (e.g., preeclampsia, macrosomia) were only reported as yes/no, while others as exact numerical values (gravida and birth weight). To examine associations between maternal glycemia and perinatal outcomes, each glucose measurement was considered as a continuous variable.
2.4. Statistical analysis
All data were analyzed using SPSS FOR WINDOWS version 21.0 (SPSS Inc, Chicago, IL). Mean ± standard deviation (SD) was reported for continuous variables, and number and percentage were reported for categorical variables. Student's t-test and Mann–Whitney U test were used to compare continuous variables while Pearson's chi-square test and Fisher's exact test were used to analyze categorical variables. A logistic model was fitted to estimate the odds ratio (OR) and its 95% confidence interval (CI). Binary logistic regression model was used to analyze the role of FPG, 1-h OGTT, and 2-h OGTT in predicting adverse pregnancy outcomes and identify the risk factors of adverse outcomes among women with GDM. P < .05 was considered statistically significant. However, when Pair-wise comparison of multiple independent sample rate was used, P < .017 was considered statistically significant.
2.5. Ethical approval
The study was approved by the Ethics Committee of West China Second University Hospital, Sichuan University (No. 2018027).
3. Results
3.1. Characteristics and outcomes of study participants
In total, 3702 pregnant women with GDM were included in the initial sample. After excluding 421 women without OGTT or delivery data and 60 women with pre-gestational or overt diabetes, 3221 women with GDM were eligible for our analysis. The study population consisted of 1,601 (49.7%) women with adverse pregnancy outcomes and 1,620 (50.3%) women without adverse pregnancy outcomes. Among them, 1876 (58.2%) had one abnormal OGTT value, 939 (29.2%) had 2abnormal OGTT values, and 406 (12.6%) had 3 abnormal OGTT values. The participants’ baseline characteristics and the incidence of obstetrical and neonatal outcomes are summarized (Table 1). The mean FPG, 1-h OGTT, and 2-h OGTT levels were 4.98 mmol/L, 10.03 mmol/L, and 8.89 mmol/L, respectively. The number of different adverse pregnancy outcomes because of hyperglycemia including FPG, 1-h OGTT, and 2-h OGTT are also presented (Fig. 1).
Table 1.
Characteristics and outcomes of study participants (n = 3221).
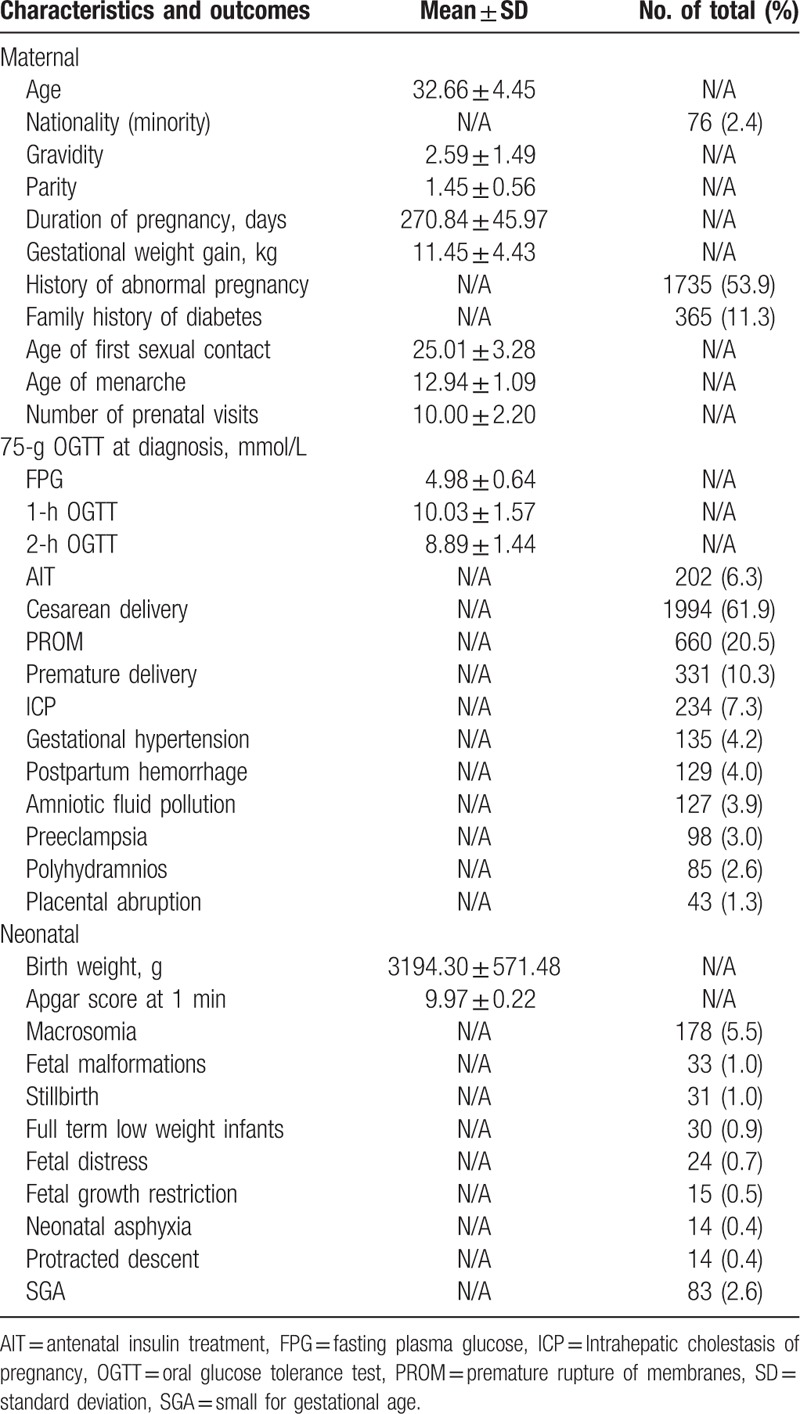
Figure 1.
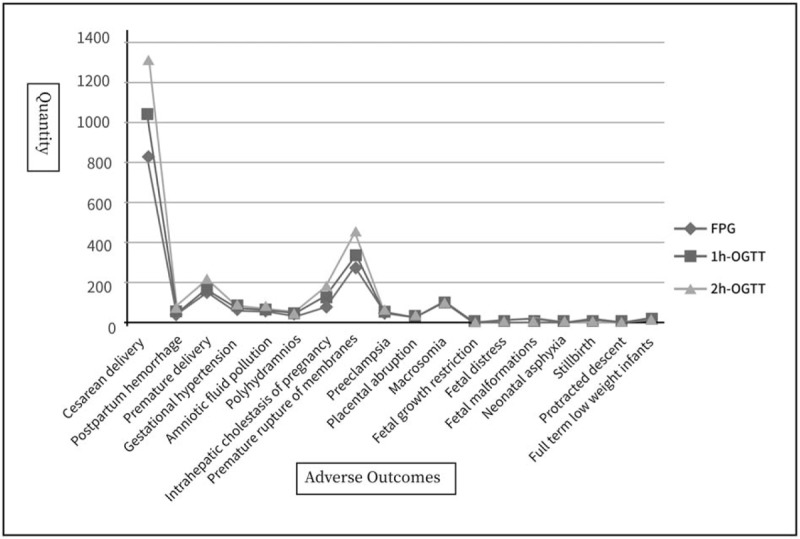
The number of different adverse pregnancy outcomes on hyperglycemia including FPG, 1-h, and 2-h OGTT. 1-h plasma glucose = 1-h OGTT, 2-h plasma glucose = 2-h OGTT, FPG = fasting plasma glucose, OGTT = oral glucose tolerance test.
3.2. Characteristics of GDM women with or without adverse pregnancy outcomes
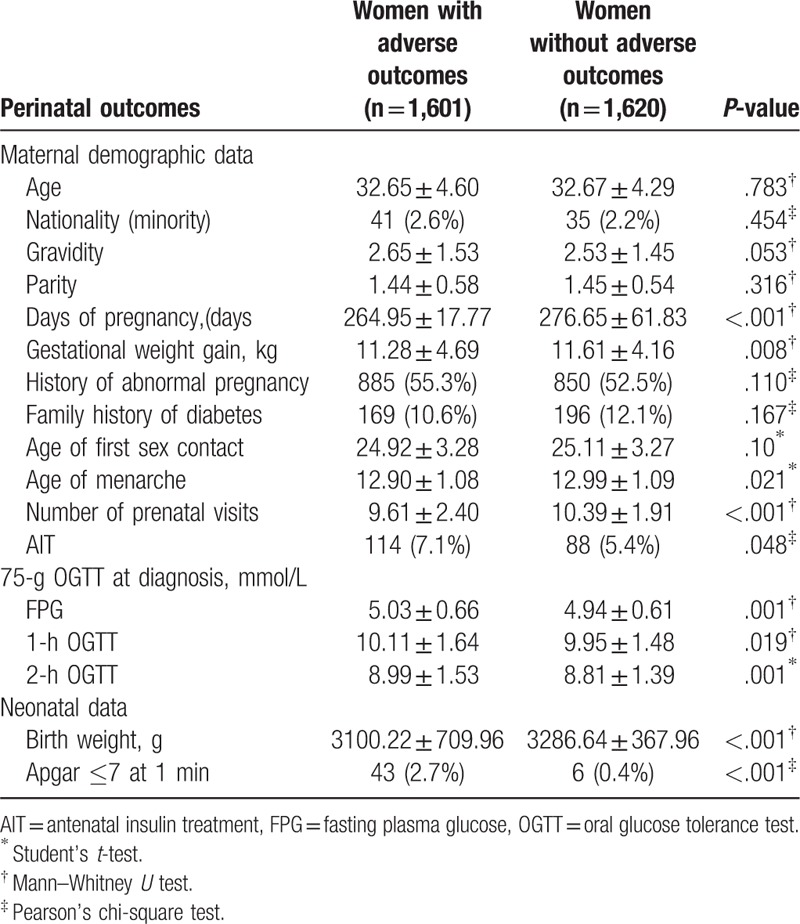
In our study, GDM women were stratified by the presence/absence of adverse pregnancy outcomes. Women with adverse outcomes had fewer days of pregnancy, smaller gestational weight gain, smaller number of prenatal visits, younger age of menarche, smaller number of AIT, and lower neonatal birth weight than those without adverse outcomes; and the differences were all statistically significant. The FPG, 1-h OGTT, and 2-h OGTT values and the incidence of an Apgar score at 1 minute ≤ 7 were significantly higher in women with adverse outcomes than those without. No significant differences were found in terms of age, nationality, gravidity, parity, history of abnormal pregnancy, family history of diabetes, and age of first sexual contact (Table 2).
Table 2.
Characteristics of women having gestational diabetes mellitus with or without adverse outcomes.
3.3. Relationship between glucose levels and adverse outcomes
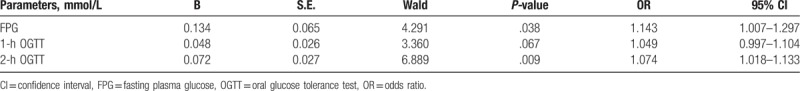
Because a collinearity problem was identified among FPG, 1-h OGTT, and 2-h OGTT, logistic regression analysis was performed for each variable, respectively. After adjusting for age, gravidity, days of pregnancy, gestational weight pain, history of abnormal pregnancy, family history of diabetes, and the number of antenatal visits, both FPG and 2-h OGTT were significant predictors for adverse outcomes among women with GDM (Table 3). Nevertheless, FPG had a stronger association with adverse pregnancy outcomes than 2-h OGTT did (FPG: OR, 1.143; 95% CI, 1.007–1.297; P = .038 vs 2-h OGTT: OR, 1.074, 95% CI, 1.018–1.133, P = .009).
Table 3.
Logistic regression analysis after controlling for potential confounders of predictors for adverse outcomes.
3.4. Risk factors of adverse outcomes among women with GDM
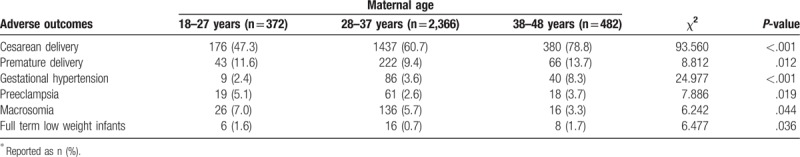
According to the logistic regression analysis, the incidence of adverse pregnancy outcomes was affected by maternal age, especially during 28–37 years (OR, 1.403; 95% CI, 1.037–1.899; P = .028), days of pregnancy (OR, 0.904; 95% CI, 0.894–0.914; P ≤ .001), gestational weight gain (OR, 1.018; 95% CI, 1.000–1.036; P = .048), and age of menarche (OR, 0.925; 95% CI, 0.863–0.992; P = .029) after adjusting for nationality, gravidity, parity, history of abnormal pregnancy, family history of diabetes, and age of first sexual contact (Table 4). We further analyzed the difference in adverse pregnancy outcomes among different age groups and found a significant difference in cesarean delivery (P ≤ .001), premature delivery (P = .012), gestational hypertension (P ≤ .001), pre-eclampsia (P = .019), macrosomia (P = .044), and full term low weight infants (P = .036). Meanwhile, the age of the woman was positively associated with the rate of cesarean delivery and gestational hypertension, and negatively associated with the rate of macrosomia, which was consistent with Fu's study.[18] (Table 5).
Table 4.
Risk factors of adverse outcomes.
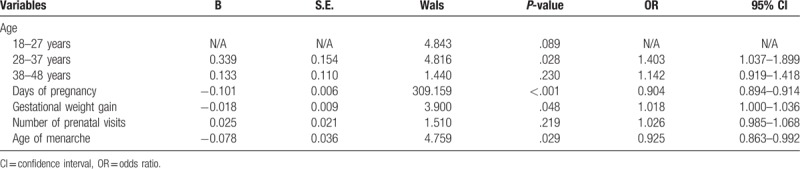
Table 5.
Difference in adverse pregnancy outcome among different age groups∗.
3.5. Relationship between the number of abnormal OGTT values and adverse outcomes
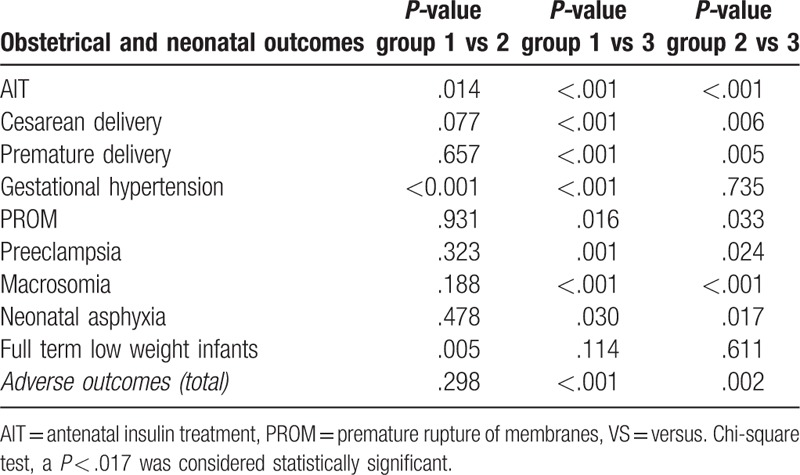
Based on the number of abnormal OGTT values, GDM women were stratified into 3 groups, that is, Group 1, Group 2, and Group 3, which consisted of pregnant women with 1, 2, or 3 abnormal OGTT values, respectively. Adverse outcomes among different groups were compared using Pearson's chi-square test (Table 6). When the results were statistically significant, pair-wise comparisons were performed and the level of significance was adjusted to 0.017 (α = 0.017) (Table 6). The incidences of gestational hypertension, full term low weight infants, and AIT were higher in Group 2 than those in Group 1; the incidences of cesarean deliveries, preterm births, gestational hypertension, premature rupture of membranes, preeclampsia, macrosomia, and AIT were higher in Group 3 than those in Group 1; and the incidences of cesarean deliveries, preterm births, macrosomia, and AIT were higher in Group 3 than those in Group 2. The overall incidence of adverse pregnancy outcomes increased with the number of abnormal OGTT values (47.7%, 49.7%, and 59.1% respectively). A larger number of abnormal OGTT values was associated with significantly increased risks of antenatal insulin treatment, cesarean delivery, premature delivery, gestational hypertension, premature rupture of membranes, preeclampsia, macrosomia, neonatal asphyxia, and full term low weight infants (Table 7).
Table 6.
Comparison of adverse outcomes in women having gestational diabetes mellitus stratified by the number of abnormal oral glucose tolerance test values.
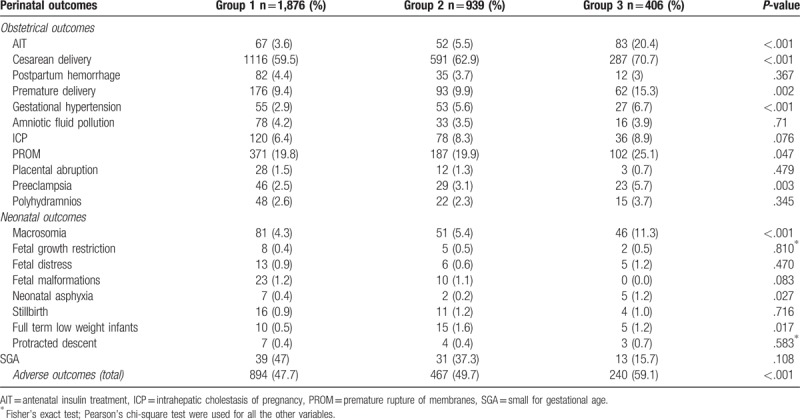
Table 7.
Pair-wise comparison of adverse outcomes in women having gestational diabetes mellitus stratified by the number of abnormal glucose values.
4. Discussion
GDM is one of the most common medical conditions associated with pregnancy. Both GDM mothers and babies have an increased risk of diabetes in their future lives. In 2011, based on the IADPSG guidelines, the ADA recommended for the first time that all pregnant women not known to have prior diabetes undergo a 75 g OGTT at 24 to 28 weeks of gestation.[19] Because both the cutoff value and the number of abnormal OGTT values to diagnose GDM were changed in the new IADPSG criteria, more pregnant women are considered as having GDM. Despite the expanded diagnosis, appropriate management of GDM is still a major challenge worldwide. Therefore, we attempted to investigate the precise relationship between OGTT values and adverse pregnancy outcomes, and to identify risk factors for adverse outcomes among women with GDM.
In this retrospective cohort study, we found that maternal gestational weight gain and neonatal birth weight were lower in women with adverse outcomes than those without. This may be partially because once diagnosed as GDM, these women usually went on an overly strict diet due to ignorance of GDM treatment alternatives and a common fear of insulin. Consequently, they may have had inadequate energy intake, negative body weight growth, and other undesirable outcomes that were not conducive to fetal growth and development and may have increased risk of adverse pregnancy outcomes.[16]
In clinical practice, the main goal of GDM treatment is to control glucose metabolism and reduce adverse pregnancy outcomes. It has been demonstrated that optimal glycemic control improves pregnancy outcomes,[20] while poor glycemic control may significantly increase both maternal and fetal risks of adverse outcomes,[21] which is consistent with our finding that more frequent use of antenatal insulin was observed in GDM women with adverse outcomes. Prior studies have reported that 10.8% to 52.8% of GDM patients required AIT to achieve good glycemic control.[22–25] By contrast, the rate of AIT use in this study was only 6.3%, which may be related to the strict eligibility criteria of participants that excluded many other GDM women who might need AIT, such as multiple pregnancies or those with other systemic diseases.
In our study, maternal age, days of pregnancy, age of menarche, and gestational weight gain were identified as risk factors for adverse outcomes, with maternal age exerting the most significant effect. Older women with GDM are more likely to have adverse pregnancy outcomes associated with decreased egg quality, embryonic chromosomal mutations, and embryo implantation issues caused by an injured uterus.[18] In addition, due to the previous cesarean section history, and pathological and psychological factors, the rate of cesarean section in older women are also higher.[26] Because the first 3 variables are not easy to modify, ensuring optimal weight before conception and preventing excessive weight gain during pregnancy are 2important measures that may help prevent negative outcomes. In addition, medical nutrition therapy (MNT) has also proved very effective. Pregnant women who are diagnosed to have GDM should receive MNT as early as possible to reduce the burden of the pancreas, improve the sensitivity of target tissues to insulin, and enhance their binding with insulin to maintain blood glucose at normal levels. Meanwhile, addressing the physiological needs of pregnant women and securing the normal growth and development of the fetus are equally important.[27–29] Shi et al found that reasonable and effective MNT could control and stabilize body weight gain each week from diagnosis to delivery and reduce the use of insulin during pregnancy.[16,30] Thus, for women diagnosed as GMD, pregnancy nutrition monitoring and personalized nutrition therapy should be provided by the hospital, while extensive nutrition and health education should be offered by the community.
Still, uncertainty lingers regarding whether blood glucose levels at 3 time points of OGTT are sufficient for predicting increased risks of adverse perinatal outcomes. Previous studies suggested that FPG had a high predictive value for large-for-gestational age (LGA) infants and macrosomia.[31,32] A Danish study involving pregnant women with mild glucose intolerance but without GDM found a linear association, after adjustment for confounders, between maternal 2-h OGTT and cesarean delivery, spontaneous preterm delivery, shoulder dystocia, and macrosomia.[33] A recent systematic review revealed that fasting glucose level had a stronger association with LGA than post-load one.[34] Nevertheless, we found that both FPG and 2-h OGTT values had positive associations with adverse outcomes, of which FPG was more predictive. As a result, for GDM patients, fasting hyperglycemia may be more likely to cause adverse pregnancy outcomes.
It is assumed that the number of abnormal OGTT values may help identify different degrees of maternal/fetal risks. Compared with GDM women having only one hyperglycemic value, those with 2 or more elevated glucose values may have a more severe disruption in glucose metabolic balance and insulin sensitivity. Consistent with previous reports,[35,36] our study found similar trends between the number of abnormal OGTT values and the frequencies of adverse outcomes, such as cesarean delivery, premature delivery and macrosomia. Women with 2 or 3 abnormal OGTT values required more frequent AIT than those with only one abnormal value. A larger number of abnormal OGTT values were associated with higher odds of adverse pregnancy outcomes. For GDM women with 3 abnormal OGTT values, fasting and 2-h hyperglycemia in particular, stricter glucose control including diet regulation, exercise, and administration of insulin may be taken into account during pregnancy.
Our study has several strengths. First, most previous studies focus on the high risk factors for GDM, whereas our study was aimed at risk factors for pregnancy outcomes. Second, we controlled for a range of potential confounding factors in our analysis, allowing us to assess the independent effect of maternal hyperglycemia on pregnancy outcomes. More importantly, this is one of the very few studies in Western China to investigate the risk factors for adverse pregnancy outcomes among women with GDM and to determine the relationship between OGTT values and pregnancy outcomes. Despite these strengths, this study has some limitations though. First, body mass index (BMI) is an important indicator of nutritional status, and a high pre-pregnant BMI is a risk factor for GDM.[37] Because of the lack of accurate prepregnancy weight and other missing data, we were unable to analyze the influence of BMI on adverse outcomes. Second, due to the lack of specific blood glucose values, we could not analyze the relationship between each adverse pregnancy outcome and the specific values at the 3 time points of OGTT. Future multicenter studies are needed to determine the optimal threshold of FPG and 2-h OGTT for high-risk screening. In addition, as this is a single-center retrospective study, these results may be applicable to the Chinese population only.
In conclusion, the incidence of adverse pregnancy outcomes may be affected by maternal age, days of pregnancy, gestational weight gain, and age of menarche. Both FPG and 2-h OGTT values are positively associated with adverse pregnancy outcomes, of which FPG is more predictive. Besides, a larger number of abnormal OGTT values is associated with a significant increase in perinatal adverse outcomes. Therefore, it is recommended that stratified management be provided for pregnant women with GDM, especially those with fasting hyperglycemia or 3 abnormal OGTT values.
Acknowledgments
The authors are grateful to Yanqiao Wu of West China Second University Hospital for making suggestions and evaluation on the statistical part of the article. The authors also thank Prof. Dongtao Lin of Sichuan University for copyediting the manuscript.
Author contributions
Conceptualization: Biru Luo, Juan Hu.
Data curation: Tingting Ding, Jie Xiang.
Formal analysis: Tingting Ding.
Methodology: Jie Xiang, Juan Hu.
Software: Jie Xiang.
Supervision: Biru Luo.
Writing – original draft: Tingting Ding, Jie Xiang.
Writing – review & editing: Tingting Ding, Biru Luo, Juan Hu.
Footnotes
Abbreviations: ADA = American Diabetes Association, AIT = Antenatal insulin treatment, BMI = body mass index, CI = confidence interval, FPG = fasting plasma glucose, GDM = gestational diabetes mellitus, HAPO = hyperglycemia and adverse pregnancy outcomes, HIS = hospital information system, IADPSG = International Diabetes and Pregnancy Research Organization, ICP = intrahepatic cholestasis of pregnancy, MOH = Ministry of Health, OGTT = oral glucose tolerance test, OR = odds ratio, PROM = premature rupture of membranes, SGA = small for gestational age.
TD and JX contributed equally to this work and share the first authorship. JH is the co-corresponding author.
The authors have no conflicts of interest to disclose.
References
- [1].American Diabetes Association. Diagnosis and classification of diabetes mellitus. Diabetes Care 2014;37suppl 1:S81–90. [DOI] [PubMed] [Google Scholar]
- [2].Sendag F, Terek MC, Itil IM, et al. Maternal and perinatal outcomes in women with gestational diabetes mellitus as compared to nondiabetic controls. J Reprod Med 2001;46:1057–62. [PubMed] [Google Scholar]
- [3].Bellamy L, Casas JP, Hingorani AD, et al. Type 2 diabetes mellitus after gestational diabetes: a systematic review and meta-analysis. Lancet 2009;373:1773–9. [DOI] [PubMed] [Google Scholar]
- [4].Lawlor DA, Fraser A, Lindsay RS, et al. Association of existing diabetes, gestational diabetes and glycosuria in pregnancy with macrosomia and offspring body mass index, waist and fat mass in later childhood: findings from a prospective pregnancy cohort. Diabetologia 2010;53:89–97. [DOI] [PubMed] [Google Scholar]
- [5].Tieu J, McPhee AJ, Crowther CA, et al. Screening for gestational diabetes mellitus based on different risk profiles and settings for improving maternal and infant health. Cochrane Database Syst Rev 2017;8:D7222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Peng S, Zhang J, Liu L, et al. Newborn meconium and urinary metabolome response to maternal gestational diabetes mellitus: a preliminary case-control study. J Proteome Res 2015;14:1799–809. [DOI] [PubMed] [Google Scholar]
- [7].Metzger BE, Gabbe SG, Persson B, et al. International association of diabetes and pregnancy study groups recommendations on the diagnosis and classification of hyperglycemia in pregnancy. Diabetes Care 2010;33:676–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Metzger BE, Lowe LP, Dyer AR, et al. Hyperglycemia and adverse pregnancy outcomes. N Engl J Med 2008;358:1991–2002. [DOI] [PubMed] [Google Scholar]
- [9].Wei YM, Yan J, Yang HX. Identification of severe gestational diabetes mellitus after new criteria used in China. J Perinatol 2016;36:90–4. [DOI] [PubMed] [Google Scholar]
- [10].Wei Y, Yang H, Zhu W, et al. International Association of Diabetes and Pregnancy Study Group criteria is suitable for gestational diabetes mellitus diagnosis: further evidence from China. Chinese Med J 2014;127:3553–6. [PubMed] [Google Scholar]
- [11].Farrar D, Simmonds M, Bryant M, et al. Risk factor screening to identify women requiring oral glucose tolerance testing to diagnose gestational diabetes: a systematic review and meta-analysis and analysis of two pregnancy cohorts. PLoS One 2017;12:e175288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Sesmilo G, Meler E, Perea V, et al. Maternal fasting glycemia and adverse pregnancy outcomes in a Mediterranean population. Acta Diabetol 2017;54:293–9. [DOI] [PubMed] [Google Scholar]
- [13].Ozgu-Erdinc AS, Iskender C, Uygur D, et al. One-hour versus two-hour postprandial blood glucose measurement in women with gestational diabetes mellitus: which is more predictive? Endocrine 2016;52:561–70. [DOI] [PubMed] [Google Scholar]
- [14].Reichelt AJ, Weinert LS, Mastella LS, et al. Clinical characteristics of women with gestational diabetes—comparison of two cohorts enrolled 20 years apart in southern Brazil. Sao Paulo Med J 2017;135:376–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Shi M, Liu ZL, Steinmann P, et al. Medical nutrition therapy for pregnant women with gestational diabetes mellitus—a retrospective cohort study. Taiwan J Obstet Gynecol 2016;55:666–71. [DOI] [PubMed] [Google Scholar]
- [16].Ngala RA, Fondjo LA, Gmagna P, et al. Placental peptides metabolism and maternal factors as predictors of risk of gestational diabetes in pregnant women. A case-control study. PLoS One 2017;12:e181613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Wang CS, Wei YM, Yang HX. Analysis of the effects of gestational diabetes mellitus based on abnormal blood glucose on pregnancy outcomes. Zhonghua Fu Chan Ke Za Zhi 2013;48:899–902. [PubMed] [Google Scholar]
- [18].Fu J, Qu Y, Ji F, et al. A retrospective cohort survey of problems related to second childbirths during the 2-child policy period in Jiangbei District of Ningbo City in China. Medicine 2018;97:18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Basevi V, Di Mario S, Morciano C, et al. Comment on: American Diabetes Association. Standards of medical care in diabetes—2011. Diabetes Care 2011;34suppl 1:S11–61. Diabetes Care 2011;34:e53-e54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Song Y, Yang H. Clinical use of continuous glucose monitoring system in gestational diabetes mellitus and type 2 diabetes complicated with pregnancy. Zhonghua Fu Chan Ke Za Zhi 2014;49:579–83. [PubMed] [Google Scholar]
- [21].Garrison EA, Jagasia S. Inpatient management of women with gestational and pregestational diabetes in pregnancy. Curr Diab Rep 2014;14:457. [DOI] [PubMed] [Google Scholar]
- [22].Meshel S, Schejter E, Harel T, et al. Can we predict the need for pharmacological treatment according to demographic and clinical characteristics in gestational diabetes? J Matern Fetal Neonatal Med 2016;29:2062–6. [DOI] [PubMed] [Google Scholar]
- [23].Crowther CA, Hiller JE, Moss JR, et al. Effect of treatment of gestational diabetes mellitus on pregnancy outcomes. N Engl J Med 2005;352:2477–86. [DOI] [PubMed] [Google Scholar]
- [24].Sapienza AD, Francisco RP, Trindade TC, et al. Factors predicting the need for insulin therapy in patients with gestational diabetes mellitus. Diabetes Res Clin Pract 2010;88:81–6. [DOI] [PubMed] [Google Scholar]
- [25].Bakiner O, Bozkirli E, Ozsahin K, et al. Risk factors that can predict antenatal insulin need in gestational diabetes. J Clin Med Res 2013;5:381–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Xie QG, Balasch J, Gratacos E. Indications for cesarean section in 5706 cases since implementation of two-child policy. J Tongji Univ (Med Sci) 2017;38:90–3. [Google Scholar]
- [27].Forbes S, Godsland IF, Taylor-Robinson SD, et al. A history of previous gestational diabetes mellitus is associated with adverse changes in insulin secretion and VLDL metabolism independently of increased intrahepatocellular lipid. Diabetologia 2013;56:2021–33. [DOI] [PubMed] [Google Scholar]
- [28].Seghieri G, Tesi F, Anichini R, et al. Influence of gestational diabetes on the long-term control of glucose tolerance. Diabetologia 2007;50:2234–8. [DOI] [PubMed] [Google Scholar]
- [29].Perucchini D, Fischer U, Spinas GA, et al. Using fasting plasma glucose concentrations to screen for gestational diabetes mellitus: prospective population based study. BMJ 1999;319:812–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Russo LM, Nobles C, Ertel KA, et al. Physical activity interventions in pregnancy and risk of gestational diabetes mellitus: a systematic review and meta-analysis. Obstet Gynecol 2015;125:576–82. [DOI] [PubMed] [Google Scholar]
- [31].Black MH, Sacks DA, Xiang AH, et al. Clinical outcomes of pregnancies complicated by mild gestational diabetes mellitus differ by combinations of abnormal oral glucose tolerance test values. Diabetes Care 2010;33:2524–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Legardeur H, Girard G, Journy N, et al. Factors predictive of macrosomia in pregnancies with a positive oral glucose challenge test: importance of fasting plasma glucose. Diabetes Metab 2014;40:43–8. [DOI] [PubMed] [Google Scholar]
- [33].Jensen DM, Korsholm L, Ovesen P, et al. Adverse pregnancy outcome in women with mild glucose intolerance: is there a clinically meaningful threshold value for glucose? Acta Obstet Gynecol Scand 2008;87:59–62. [DOI] [PubMed] [Google Scholar]
- [34].Farrar D, Simmonds M, Bryant M, et al. Hyperglycaemia and risk of adverse perinatal outcomes: systematic review and meta-analysis. BMJ 2016;354:i4694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Ikenoue S, Miyakoshi K, Saisho Y, et al. Clinical impact of women with gestational diabetes mellitus by the new consensus criteria: two year experience in a single institution in Japan. Endocr J 2014;61:353–8. [DOI] [PubMed] [Google Scholar]
- [36].Feng H, Zhu WW, Yang HX, et al. Relationship between oral glucose tolerance test characteristics and adverse pregnancy outcomes among women with gestational diabetes mellitus. Chin Med J (Engl) 2017;130:1012–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Yeung EH, Hu FB, Solomon CG, et al. Life-course weight characteristics and the risk of gestational diabetes. Diabetologia 2010;53:668–78. [DOI] [PMC free article] [PubMed] [Google Scholar]


