Abstract
Background:
Xingnaojing injection (XNJ) sharpen the mind and induce consciousness and are widely used in acute phases of intracerebral hemorrhage (ICH). Naloxone hydrochloride injection (NX) performs equally well and replace the effects of morphine-like substances to promote conscious awareness. The applications of XNJ combined with NX for ICH show some advantages compared with NX applied individually. The aim of this systematic review is to evaluate the effectiveness and safety of XNJ combined with NX for ICH.
Methods:
Comprehensive searches were conducted in 8 medical databases (PubMed, Cochrane Library, Web of Science, Embase, CNKI, VIP, CBM and Wanfang database) from inceptions to October 2017 for randomized controlled trials (RCTs) that compared the applications of XNJ and NX with NX applied individually in ICH. Literature screening, assessing risk of bias and data extraction were conducted by 2 reviewers independently. According to the Cochrane Collaboration's RevMan5.3 software to perform the data analysis.
Results:
32 RCTs (3068 cases) were selected and the quality of studies were low. All trials compared XNJ and NX with NX applied individually. The overall meta-analysis results showed that XNJ combined with NX have significant effect on clinical efficacy (OR 3.78, 95% CI: 3.03–4.73; P < .00001), GCS score (MD 3.86, 95% CI: 3.46–4.25; P < .00001), coma duration (MD −5.59, 95% CI: −6.96 to −4.22; P < .00001), NIHSS score (MD −6.24, 95% CI: -8.05 to −4.42; P < .00001), Barthel Index score (MD 14.12, 95% CI: 6.7–21.54; P < .0002), cerebral hematoma volume (MD -6.05, 95% CI: −6.85 to −5.24; P < .00001) than NX applied individually. Adverse events reported in 4 studies and included mild discomfort symptoms.
Conclusion:
The effectiveness and safety of XNJ combined with NX for ICH cannot be determined due to the low quality of literature, publication bias and heterogeneity. More rigorous RCTs are necessary to verify the role of XNJ combined with NX in the treatment of ICH.
Keywords: Intracerebral haemorrhage, naloxone, systematic review, xingnaojing
1. Introduction
Intracerebral haemorrhage (ICH) is characterized by brain parenchymal hemorrhage, with lesions locating in small intracerebral arteries and arterioles. ICH can be induced by hypertension, cerebral amyloid angiopathy, and other risk factors.[1] The primary pathological injuries are related to the occupied destruction of local brain tissue, [2] whereas the secondary injuries of perihematomal oedema and inflammatory may potentially cause further neurological deterioration. [3] ICH severity is variable due to the differences in hematoma volumes, bleeding sites and related risk factors. [4] Typically, the highest mortality of ICH accounts for 10% to 15% in stroke patients.[5] Notably, the mortality at acute stage remains as high as 30% to 50% in spite of the adopted comprehensive treatment, while the disability is up to 75%.[6] In developed countries, the incidence of ICH has been decreased thanks to the well-controlled blood pressure, [7] but it remains high in developing countries [8] (18%–24% in Japan[9] and Korea,[7] and 8%–15% in USA, UK, and Australia[10,11]). At the acute stage, the continuous expansion of hematoma is a predictor of illness deterioration.[12] Thus, rapid intervention is required to prevent the devastating neurological deficit.
At present, controlling blood pressure and surgical operation are the main interventions for ICH, so as to reduce the intracranial pressure (ICP).[13] Surgery is an important measure at the acute phase; however, many elderly patients cannot tolerate anaesthesia or operation.[14,15] In addition, the long-term application of dehydration drugs is associated with the risk of causing acute kidney injury (AKI). For instance, 2 retrospective studies published in 2014 and 2015 had estimated the incidence of mannitol-related AKI of 10.5 and 6.5%, respectively.[16,17] Notably, traditional Chinese medicine (TCM) has accumulated plenty of clinical experience in treating ICH. For instance, Xingnaojing injection (XNJ) was developed from the Angong Niuhuang Pill, with the main effects of detoxification, cooling blood and resuscitation.[5–8] XNJ has been approved by the China Food and Drug Administration (CFDA) and has achieved efficient therapeutic effect on stroke. [18] Moreover, clinical studies have demonstrated that XNJ exerts the protective effect through alleviating the consciousness disturbance severity, ameliorating neurological deficit symptom, and enhancing the activities of daily living (ADL).[19–22] Additionally, pharmacological study shows that XNJ plays a certain role in the central nervous system (CNS) through reducing blood-brain barrier (BBB) permeability, mitigating brain oedema and ameliorating microcirculation.[23] In addition, XNJ exerts its anti-inflammatory effect through inhibiting the secretion of inflammatory cytokines,[24–27] and plays its anti-autophagy role by repressing p53-DRAM signaling pathway.[28] Naloxone (NX), a competitive antagonist of the mu-opioid receptors, can promote sobriety, relieve respiratory depression and improve the respiratory and circulatory functions.[29] Therefore, the combination of XNJ with NX may be a good adjuvant therapy of ICH for improving the consciousness state, relieving the neurological deficit symptoms and restoring cognitive function.
A number of randomized controlled trials (RCTs) and meta-analyses have reported XNJ-related treatments for ICH; however, the combination of XNJ with NX has not been reported yet. Besides, previous meta-analyses do not conduct the subgroup analysis according to the differences in efficacy evaluation criteria, dosages or treatment duration.[19,20,22] In addition, the evidence from these meta-analyses is insufficient owing to deficiency in methodological quality, small sample size and publication bias. Consequently, it is necessary to systematically evaluate the effectiveness and safety of XNJ combined with NX, so as to provide evidence for clinical applications.
2. Methods
2.1. Database and search strategy
To conduct literature searches and gather results, we strictly followed the guidelines recommended in the Preferred Reporting Items for Systematic Reviews and Meta-analysis (i.e., PRISMS statement).[30] We retrieved the databases using the combination of keywords and free-words (PubMed, Cochrane Library, Web of Science, Embase, CNKI, VIP, CBM, and Wanfang database). The search period was from inception to October 2017, which of the languages are Chinese and English.
PubMed search strategy:
-
(1)
cerebral haemorrhage OR intracerebral haemorrhage OR hemorrhagic stroke OR hypertensive intracerebral haemorrhage OR hemorrhagic apoplexy OR cerebral brain haemorrhage (in full text);
-
(2)
xingnaojing OR xingnaojing injection (in full text);
-
(3)
naloxone OR naloxone hydrobromide OR naloxon curamed OR naloxone hydrochloride dihydride OR naloxone hydrochloride (in full text);
-
(4)
clinical trial OR controlled clinical trial OR randomized controlled trial OR randomized clinical trial (in full text);
-
(5)
#1 AND #2 AND #3 AND #4.
2.2. Inclusion criteria
We used the following criteria to filter the retrieved results at the initial stage of the study searches:
-
(1)
The included studies were RCTs published in English or Chinese Journals;
-
(2)
Clinical symptoms and signs of patients were in line with diagnostic criteria,[31] referred to Chinese guidelines for cerebrovascular disease prevention and treatment,[32] or were diagnosed as acute intracerebral haemorrhage by computerized tomography (CT) or magnetic resonance imaging (MRI), or other recognized diagnostic criteria. The baseline data of patients included age, gender, bleeding volume, and the sites of bleeding were unrestricted;
-
(3)
The control groups were treated with NX (2∼4 mg, 1∼2 times a day) on the basis of conventional therapy to decrease intracranial pressure, prevent infection or symptomatic treatment. The treatment group was given XNJ (20 mg, 1∼2 times a day) on the foundation of the control group. Treatment periods were at least 10 days in duration.
-
(4)
The primary outcome measurement was the total effective rates (TER) evaluated by differences in means of change-from-baseline in the Glasgow Coma Scale (GCS), which the TER was defined as GCS score reached 3 points at least, awaken consciousness, patients of ICH cannot fully take care of themselves and need care assistance. The therapeutic evaluation criteria assess as follows:
-
(1)
clinical cure as GCS score reached 5 points, the nervous system functions recovery well and self-reliant completely;
-
(2)
markedly effective as GCS score reached 4 points, part of the intellectual and neurological damage and could take care of themselves basically;
-
(3)
effective as GCS score reached 3 points, awaken consciousness, patients of ICH cannot take care of themselves completely and need care assistance;
-
(4)
ineffective as GCS score reached 2 points, and patient of ICH is in a vegetative state, and
-
(5)
death as GCS score was 1 point. The total number of “cure, markedly effective, effective” were used to calculate total effective rate.
-
(1)
The second outcome included score changes on GCS, awake time, National Institute of Health Stroke Scale (NIHSS), Barthel Index (BI), Neurological Dysfunction Score (NDS), ADL, hematoma volume, adverse events, or other measurement indicators.
2.3. Exclusion criteria
The following situations were excluded:
-
(1)
Repeatedly published studies, case reports or reviews;
-
(2)
Intervention measures in the control group where TCM preparations;
-
(3)
Patients with cerebral haemorrhage were in a recovery period.
2.4. Study selection and management
The data extraction table was formulated and extracted by 2 researchers (Y-MX and X-CW). Extract content includes the title, author, date of publication, literature sources, sample sizes, baseline data of subjects (age, gender, hematoma volume and state of consciousness), diagnostic criteria, interventions, treatment period, outcomes and the adverse events. In the case of disagreements on data extraction after cross-checking, researchers (Y-MX and X-CW) discussed and reached a consensus with the senior author (W-XL). One researcher (Y-MX) contacted the corresponding author via e-mail or telephone to confirm and request information when literature data and outcomes were uncertain.
2.5. Assessment of quality of evidence in included studies
The RCTs included in this meta-analysis were evaluated on methodological quality according to the Cochrane Handbook for Systematic Reviews of Intervention, which mainly include 7 components: random sequence generation, allocation concealment, blinding of participants and personnel, blinding of outcome assessment, incomplete outcome data, selective reporting and other bias. Quality assessments were carried out independently by the researchers (Y-MX, X-CW), and the resolution was sought from the senior author (W-XL) when the discrepancy occurred.
2.6. Data analysis
Statistical analyses were performed using Review Manager [Computer program] Version 5.3. (RevMan5.3, Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2014). The model of random-effects or fixed-effects was applied to gather the effect estimates. The odds ratio (OR) was used to analyze the dichotomous data, and the mean difference (MD) was used for the analysis of continuous data, both comprising 95% confidence intervals (CI). The heterogeneity between the studies was examined by the χ2 test. The random-effects model was applied to the meta-analysis with significant heterogeneity (P < .1 and I2 > 50%). Simultaneously, the application of subgroup analysis and sensitivity analysis to explore the sources of heterogeneity. Homogeneity studies were performed using a fixed-effects model for meta-analysis. When the number of studies was more than 10, a funnel analysis was used to determine whether there was publication bias. In addition, Egger's test detected the publication bias when the study numbers were less than 10 or fewer cases in each study. If the data provided by the clinical trial was not sufficient for meta-analysis, descriptive analysis was performed only.
3. Results
3.1. Characteristics of the included studies
A total of 1708 articles were retrieved from the electronic databases (including CNKI = 397, Wan Fang database = 437, VIP = 442, and CBM = 432), among which, 32 [33–64] were included through scanning the titles, abstracts and full texts. The basic characteristics of all studies (n = 3,068; including experimental group = 1537 and control group = 1531) were generalized in Figure 1 . The flow diagrams of systematic review and meta-analysis were shown in Figure 2. At the same time, foreign studies, conference papers and other databases were excluded. In addition, 32 RCTs [33–64] were conducted in parallel design, with the test sites locating in China. As could be observed, the studies were published in Chinese journals from 2010 to 2016, and the age of patients [33–64] ranged from 54 [58] to 68[40] years. Besides, the volume of cerebral hematoma ranged from 10 [37,56] to 71[37,56] millilitres, and the GCS score was stated to range from 5.34 [62] to 13.55.[40] Moreover, NX (2∼4 mg, for 1∼2 times a day) and routine pharmacotherapy were utilized in both the treatment and control groups, while XNJ (20 mg, for 1∼2 times a day) was used in the treatment groups individually, with the course of treatment spanning from 10 to 15 days. 20 trials had evaluated the GCS score,[33,36,40–45,47,50–55,57,60–62,64] 19 had assessed the cerebral hematoma volume[33,40–47,50–52,55,57,60–64] 13 had examined the awake time, [33,36,40,45–47,50,51,53,55,60–62] 30 had investigated the total effective rate (TER),[34–41,43–64] 8 had studied the NIHSS score,[35,38,39,48,56–58,62] 3 had evaluated the Barthel Index (BI),[38,42,56] 1 had evaluated the clinical neurological deficit score (NDS) [37] and 3 had studied the ADL.[38,42,56] Additionally, adverse events were reported in 4 trials [34,39,55,57], with the common discomfort symptoms of nausea, vomiting, skin itching, dizziness, palpitations and irritability. No studies had carried out follow-up observations after the trials.
Figure 1.
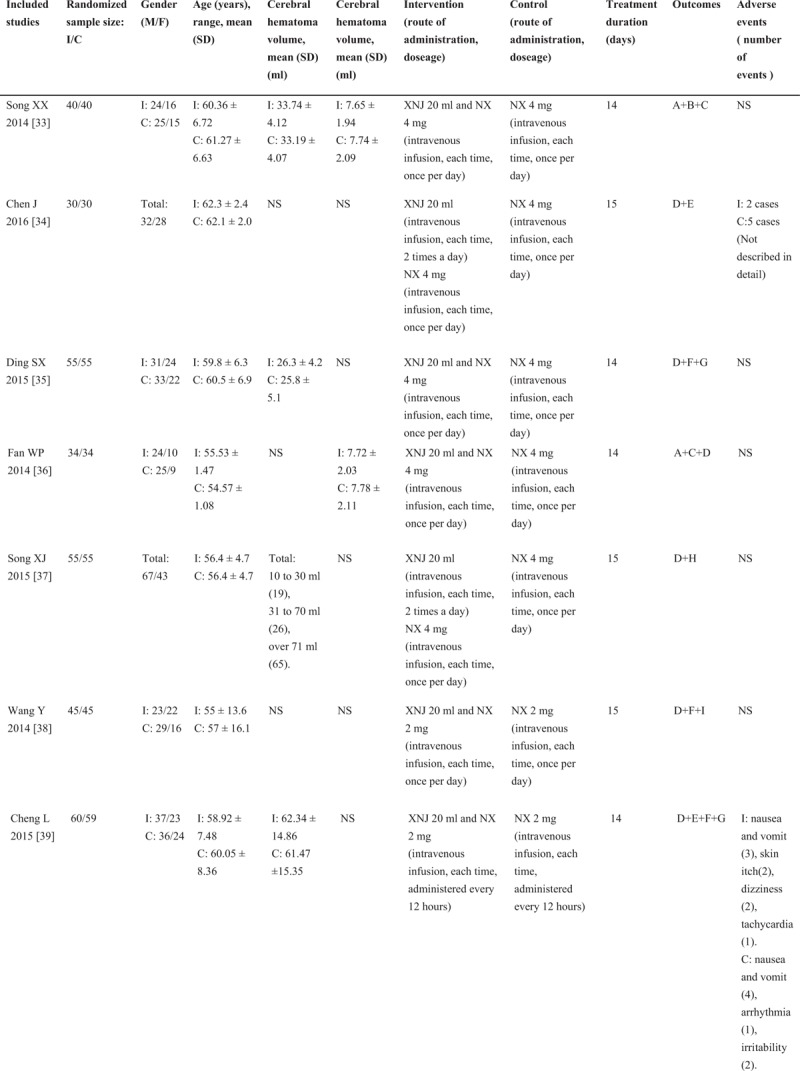
Characteristics of the included studies in the meta-analysis. I: intervention group, C: control group, M/F: male/female, SD: standard deviation, NS: not stated, GCS: Glasgow coma scale, XNJ: xingnaojing, NX: naloxone, A = Glasgow coma scale (GCS), B = cerebral hematoma volume, C = awake time, D = total effective rate (TER), E = adverse events, F = national Institute of Health Stroke Scale (NIHSS), G = the Barthel index, H = the clinical neurological deficit score (NDS), I = activities of daily living (ADL). The average dose of XNJ was 10 to 20 milliliter of intravenously guttae.
Figure 1 (Continued).
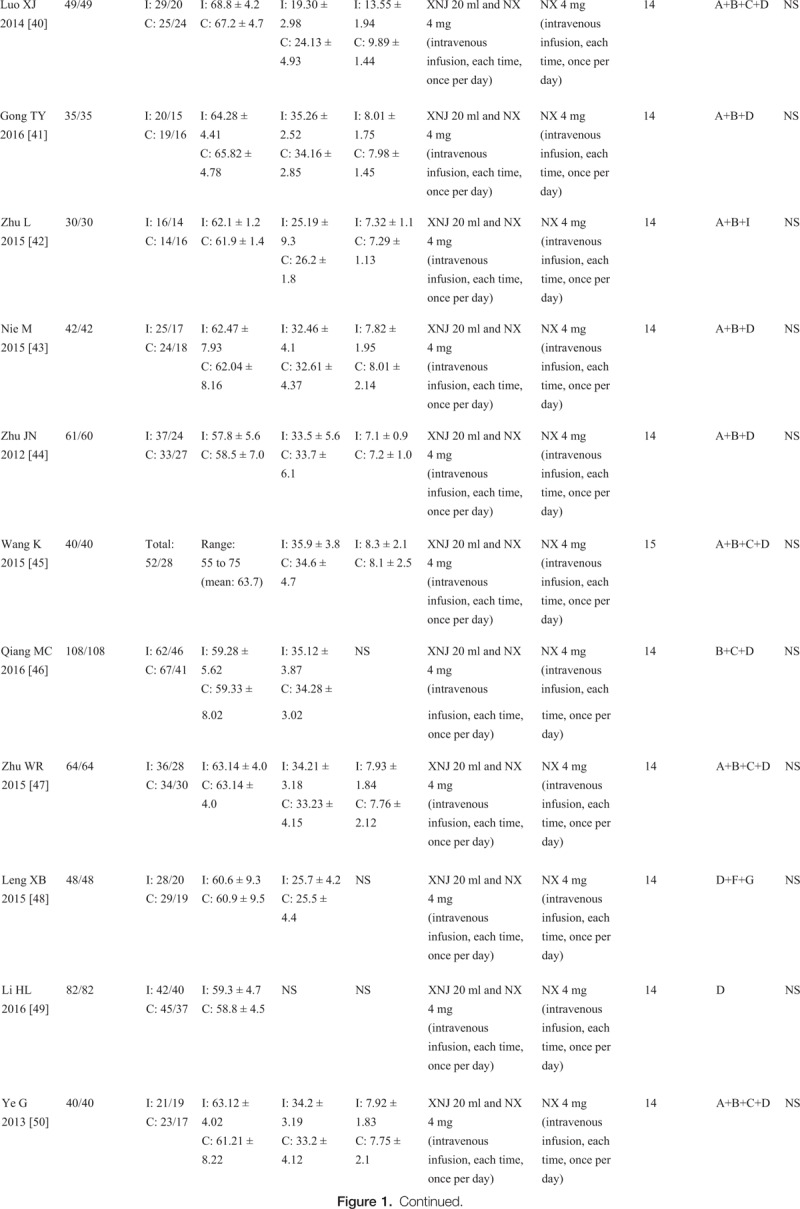
Characteristics of the included studies in the meta-analysis. I: intervention group, C: control group, M/F: male/female, SD: standard deviation, NS: not stated, GCS: Glasgow coma scale, XNJ: xingnaojing, NX: naloxone, A = Glasgow coma scale (GCS), B = cerebral hematoma volume, C = awake time, D = total effective rate (TER), E = adverse events, F = national Institute of Health Stroke Scale (NIHSS), G = the Barthel index, H = the clinical neurological deficit score (NDS), I = activities of daily living (ADL). The average dose of XNJ was 10 to 20 milliliter of intravenously guttae.
3.2. Risk of bias in the enrolled studies
The risks of bias in the enrolled studies were generalized in Figure 3. Specifically, 10 studies had described the specific methods of random sequence generation, including random number tables [35,37,42,43,46,47,49,53,57] or sealed envelopes[62], while the remaining studies had mentioned “random” without providing detailed methods. All trials failed to mention the allocation concealment and were assessed as unclear risk. Besides, one study [60] applied the double-blind method in participants and personnel, but the details on the blinding of outcome assessments were not provided. Additionally, one study [62] lacked a specific value of the GCS score and was assessed as high risk regarding the incomplete outcome data; meanwhile, the other sources of bias in this study were evaluated as unclear risk. In addition, the remaining trials describing the efficacy indicators and specific values were assessed as low risk. Thirty-2 studies had reported the expected outcomes, and the quality evaluations were rated as low risk. The baseline data of all the enrolled trials were comparable, and the results were evaluated as low risk due to the absence of other sources of bias.
Figure 1 (Continued).
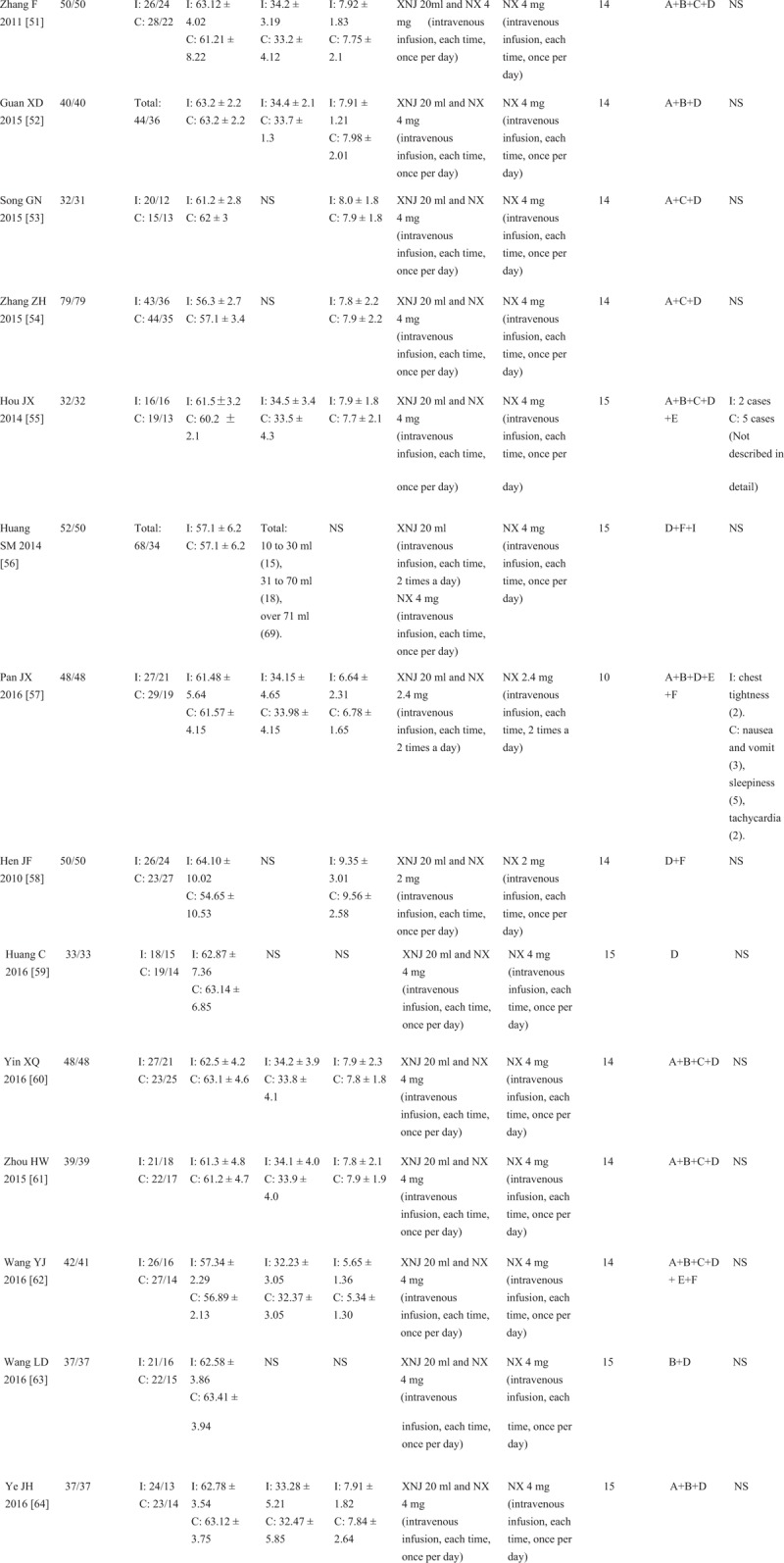
Characteristics of the included studies in the meta-analysis. I: intervention group, C: control group, M/F: male/female, SD: standard deviation, NS: not stated, GCS: Glasgow coma scale, XNJ: xingnaojing, NX: naloxone, A = Glasgow coma scale (GCS), B = cerebral hematoma volume, C = awake time, D = total effective rate (TER), E = adverse events, F = national Institute of Health Stroke Scale (NIHSS), G = the Barthel index, H = the clinical neurological deficit score (NDS), I = activities of daily living (ADL). The average dose of XNJ was 10 to 20 milliliter of intravenously guttae.
3.3. Effect of interventions
3.3.1. Assessment of TER
The TER of XNJ combined with NX on ICH was reported in 30 studies, involving 1467 subjects in experiment groups and 1461 in control groups. [34–41,43–64] As presented in Figure 4, the overall meta-analysis showed that the TER was superior in intervention groups superior to NX application alone (OR 3.78, 95% CI: 3.03–4.73; P < .00001). There was no heterogeneity between trials, and a fixed-effects model was thereby utilized for statistical analysis (χ2 = 7.64, P = 1.00, I2 = 0%). Meanwhile, subgroup analysis between studies was coordinated utilizing different evaluation criteria of GCS, NIHSS and other scores, due to the inconsistent evaluation criteria of TER. Specifically, the subgroup meta-analysis (Fig. 3) suggested that XNJ combined with NX was more effective than NX application alone under the efficacy evaluation criteria of changes in GCS scores (OR 4.09, 95% CI: 3.11–5.38; P < .00001), NIHSS scores (OR 3.24, 95% CI: 2.04–5.13; P < .00001) and the other scales scores (OR 3.21, 95% CI: 1.59–6.46; P = .001).
Figure 2.
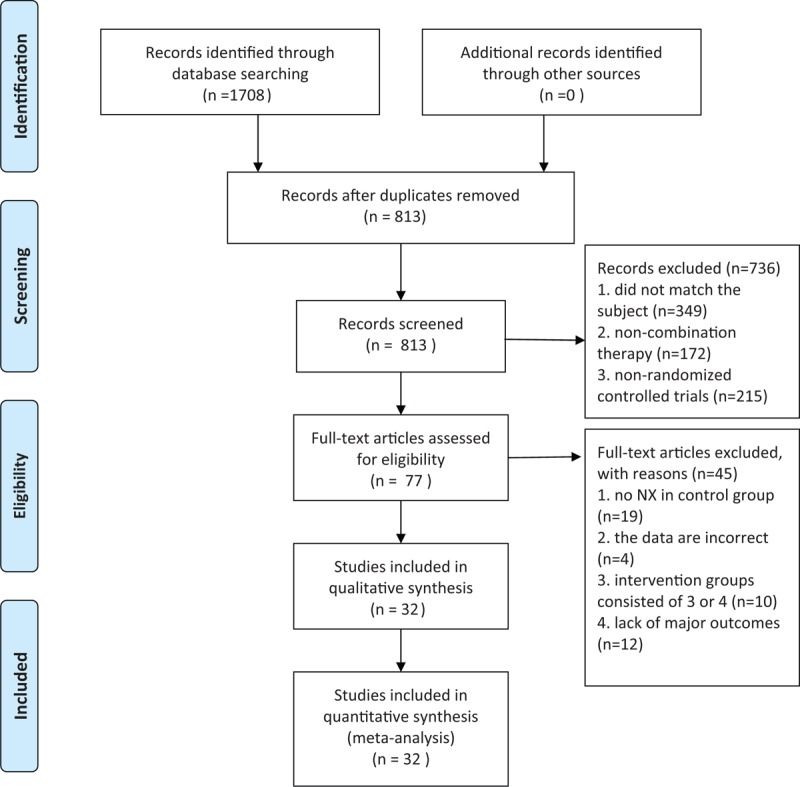
Flow diagram of literature search of systematic review and meta-analysis. NX, Naloxone.
3.3.2. Assessment of GCS
As shown by the GCS score in 19 studies[33,36,40–45,47,50–55,57,60–62,64], XNJ combined with NX would lead to remarkably improved arousal status (MD 3.86, 95% CI: 3.46–4.25; P < .00001) compared with NX application alone (Fig. 5). In addition, one study [62] did not carry out amalgamation of effect sizes due to the lack of specific GCS values.
Figure 3.
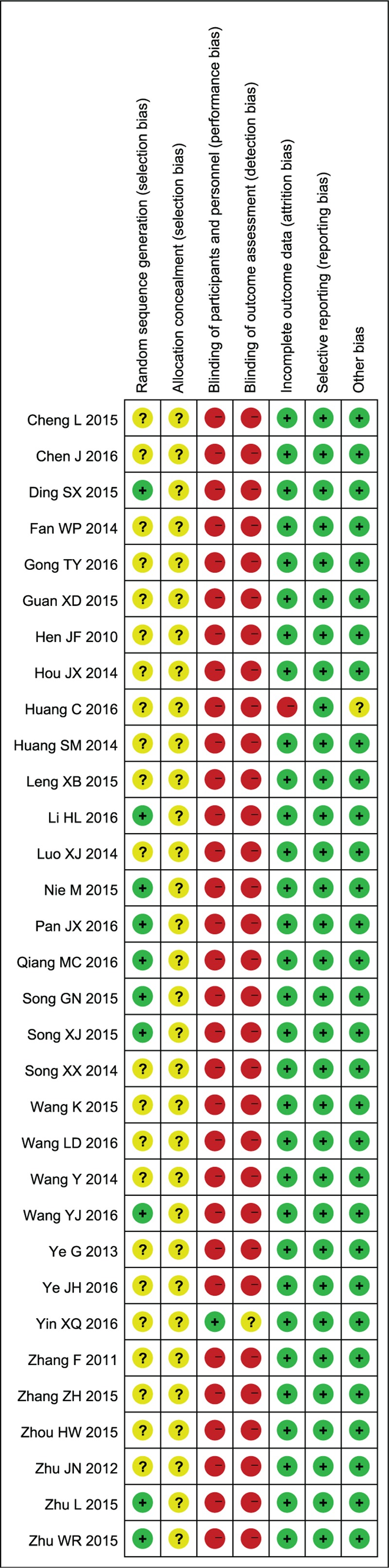
Risk of bias in the enrolled studies. “+”, low risk of bias; “−”, high risk of bias; “?”, unclear risk of bias.
3.3.3. Assessment of coma duration
As presented in Figure 6 ,[33,36,40,45–47,50,51,53–55,60–62] coma duration was markedly decreased after cerebral hemorrhage in 14 studies (MD −5.59, 95% CI: −6.96 to −4.22; P < .00001).
Figure 4.
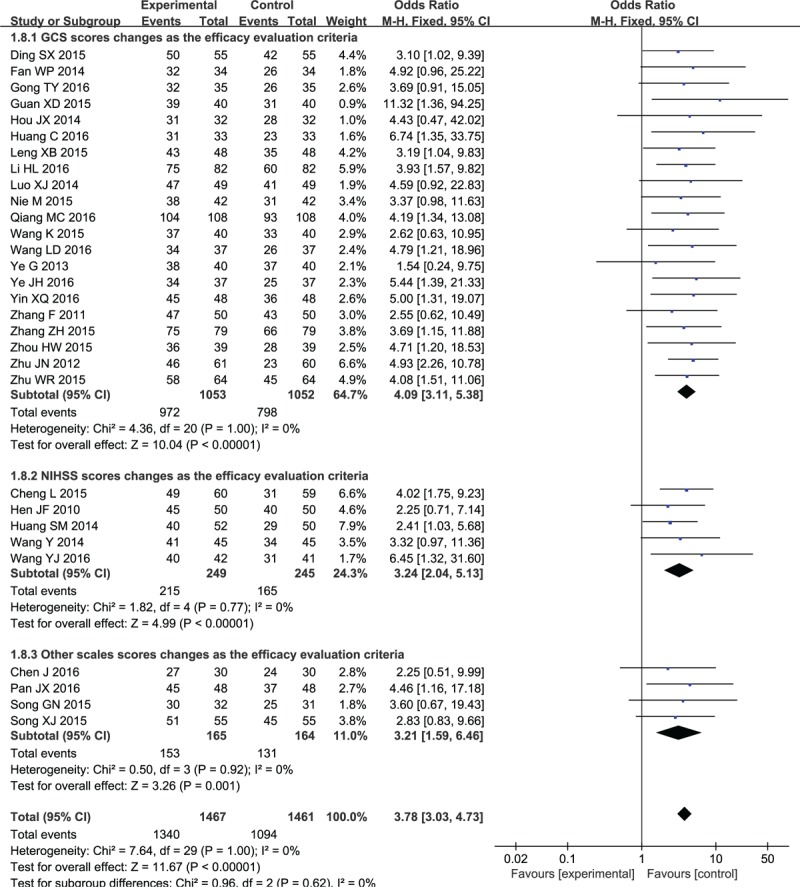
Forest plot and meta-analysis of the total effective rate. CI = confidence interval, GCS = Glasgow coma scale, NIHSS = National Institute of Health Stroke Scale.
3.3.4. Assessment of NIHSS scoring scale
As revealed in Figure 7, there were significant differences among studies,[35,38,39,48,56–58,62] and the NIHSS score was evidently reduced compared with that NX application alone (MD −6.24, 95% CI: −8.05 to −4.42; P < .00001).
Figure 5.
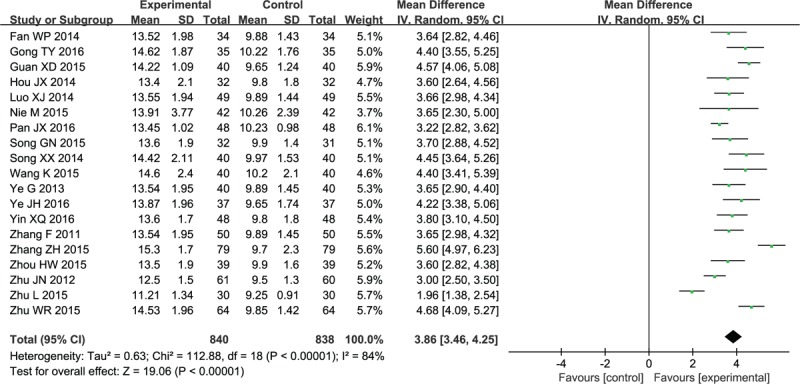
Forest plot and meta-analysis of the Glasgow Coma Scale. CI = confidence interval, SD = standard deviation.
3.3.5. Assessment of Bscoring scale
Three studies had reported the BI scores in the meta-analysis.[35,39,48] As demonstrated in Figure 8, the BI scores in the intervention groups were dramatically improved after 2 weeks (MD 14.12, 95% CI: 6.7–21.54; P < .0002).
Figure 6.
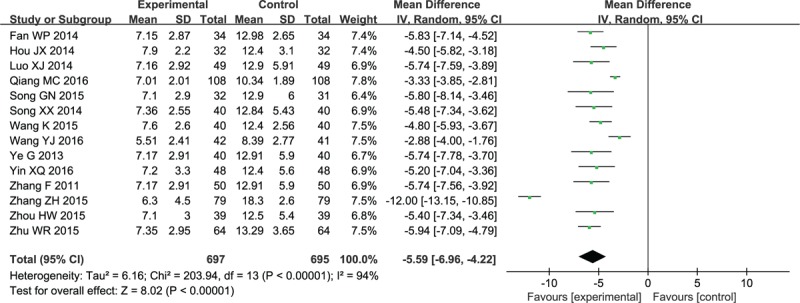
Forest plot and meta-analysis of the coma duration. CI = confidence interval, SD = standard deviation.
3.3.6. Assessment of the cerebral hematoma volume
As shown in Figure 9, 18 studies had reported that XNJ combined with NX could promote the absorption of cerebral hematoma and reduce the inflammation in the surrounding tissues, and the difference between the intervention and control groups was significant (MD −6.05, 95% CI: −6.85 to −5.24; P < .00001).
Figure 7.
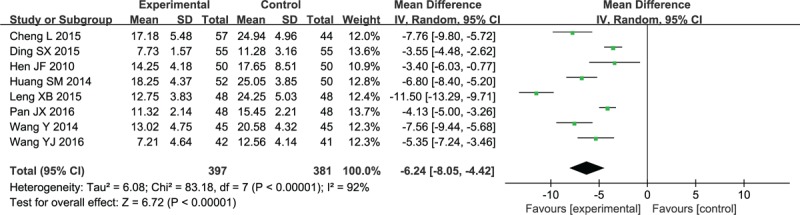
Forest plot and meta-analysis of the NIHSS score. CI = confidence interval, NIHSS = National Institute of Health Stroke Scale, SD = standard deviation.
3.3.7. Adverse events
Adverse events were reported in 4 trials, with the common discomfort symptoms of nausea, vomiting, skin itching, dizziness, palpitations, and irritability. Chen [34] had reported that 5 patients from the control group (16.7%) and 2 from the treatment group had presented adverse reactions (6.7%). Moreover, Cheng[39] had reported 3 cases of nausea and vomiting, 2 of skin itching, 2 of dizziness and 1 of tachycardia in the treatment group (13.11%); whereas 4 of nausea and vomiting, 1 of arrhythmia, and 2 of irritability in the control group (11.86%). There was no significant difference between the 2 groups in the incidence of adverse events. Hou[55] reported that 5 cases in the control group (15.6%) and 2 in the treatment group had developed adverse events (6.3%). Pan[57] had reported 2 cases of chest distress in the treatment group (4.17%), together with 3 of nausea and vomit, 5 of sleepiness and 2 of tachycardia in the control group (20.83%).
3.3.8. Publication bias
The potential publication bias was evaluated using funnel plot. The funnel plot (Fig. 10) indicated that all points had exhibited mild asymmetry, demonstrating potential publication bias in all trials. [34–41,43–64] In addition, the funnel plot of other outcomes (GCS, coma duration, cerebral hematoma volume) illustrated the existence of publication bias in this study. (Figs. 11–13).
Figure 8.
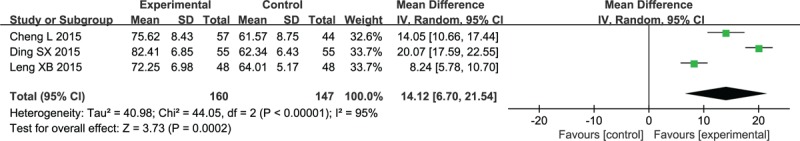
Forest plot and meta-analysis of the Barthel Index score. CI = confidence interval, SD = standard deviation.
Figure 9.
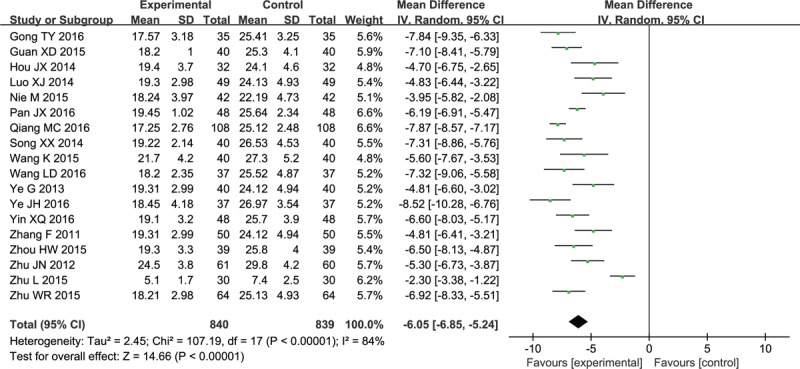
Forest plot and meta-analysis of the cerebral hematoma volume. CI = confidence interval, SD = standard deviation.
Figure 11.
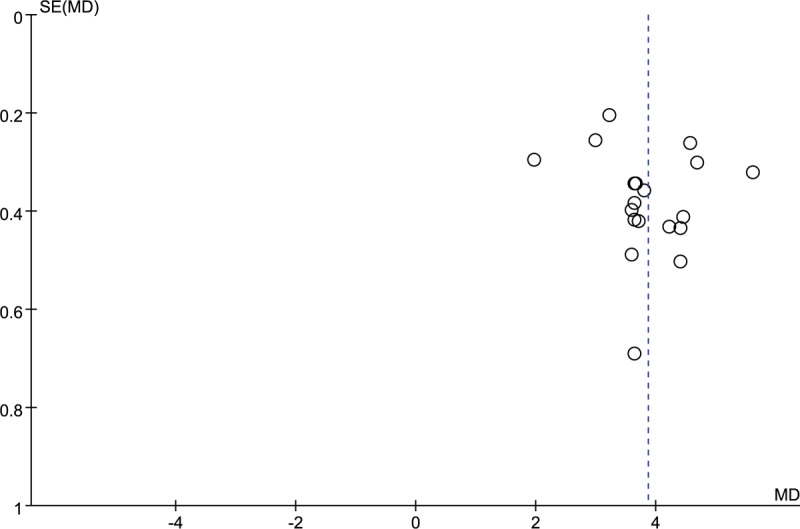
Funnel plot of Glasgow Coma Scale (GCS). MD = mean difference.
Figure 10.
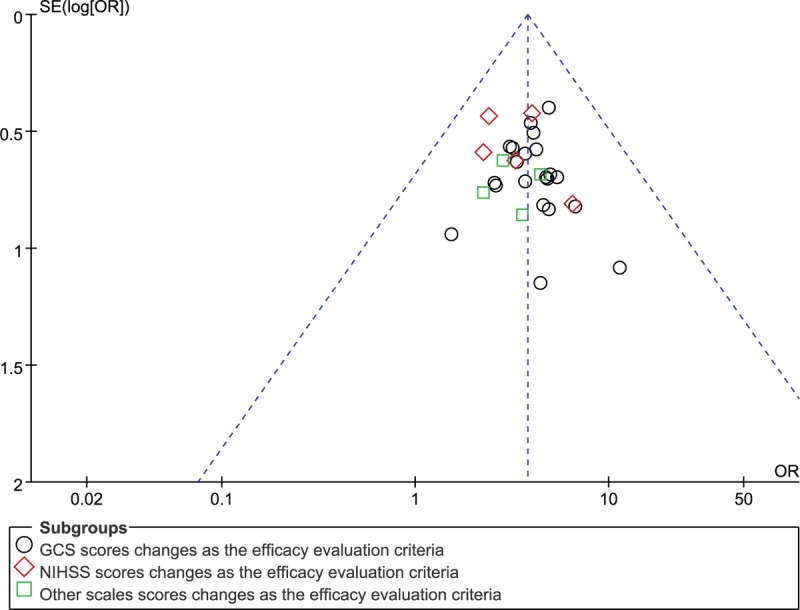
Funnel plot. OR = odds ratio, GCS = Glasgow Coma Scale, NIHSS = National Institute of Health Stroke Scale.
Figure 12.
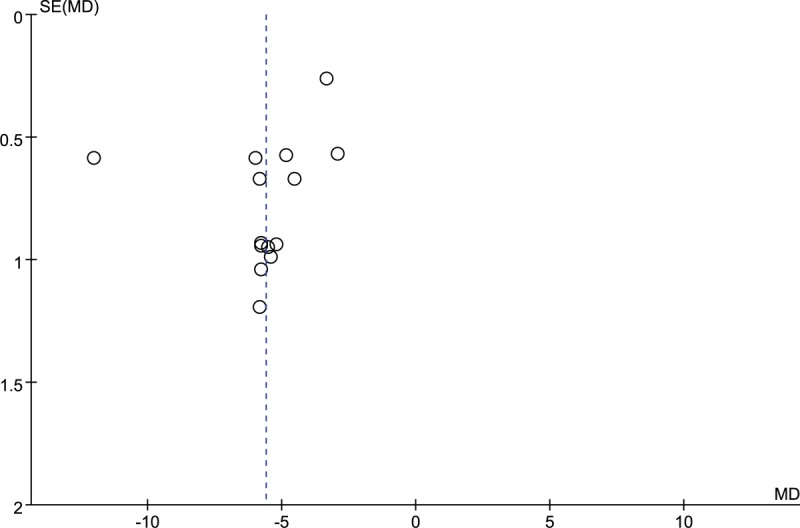
Funnel plot of coma duration. MD = mean difference.
Figure 13.
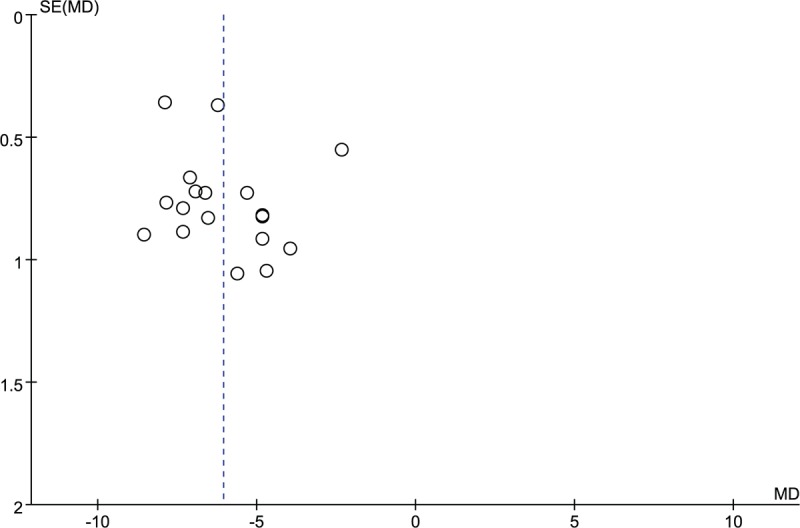
Funnel plot of cerebral hematoma volume. MD = mean difference.
4. Discussion
4.1. Summary of main results
In this study, findings from overall meta-analysis and subgroup meta-analysis indicate that XNJ combined with NX is superior to NX alone for ICH. In this meta-analysis, 32 trials involving 3068 patients have been systemically reviewed, and an intervention therapy of XNJ combined with NX is employed to observe its effectiveness and safety in ICH. The outcomes confirm that XNJ combined with NX shows significant therapeutic effects on clinical effective rates, GCS scores, coma duration, NIHSS score, BI and cerebral haemorrhage volume. Noteworthily, adverse events associated with XNJ combined with NX are not reported in a majority of studies, except for some individual trials. Specifically, the comprehensive outcome indicators make the meta-analysis results more trustworthy in guiding clinical application. Nevertheless, the effects of XNJ combined with NX on ICH can not be recognized yet, due to deficiency in methodological quality, disability to collect the unpublished literature, and the lack of information on other bias evaluation. Therefore, more well-designed research with high quality is demanded in future study.
4.2. Advantages and further elaboration of study
Currently, XNJ combined with NX has been widely used in ICH patients, which has achieved significant effect. Concretely, XNJ is mainly comprised of musk, synthetic borneol, gardenia, and turmeric [5–8], which has the anti-inflammatory pharmacological effects. [65] On the 1 hand, the formed hematoma has multiple effects, including hemiplegia, aphasia, and other neurological deficits.[66] Neurological dysfunction is also caused by the secondary inflammatory injury of cerebral haemorrhage.[67] Moreover, the severity and prognosis of ICH are closely related to the concentrations of these inflammatory factors.[68] Typically, high-sensitivity C-reactive protein (hs-CRP) and tumor necrosis factor-alpha (TNF-α) are the non-specific markers, which serve as the important indicators of inflammatory response.[69,70] XNJ can inhibit the inflammatory response and reduce the serum levels of inflammatory factors, so as to protect the nerve cells. [70] It is also found that XNJ has an adjunctive effect [71,72] mainly reflected by improving the consciousness state, ameliorating the clinical symptoms and reducing brain edema at the acute phase. In experimental research, XNJ plays a role in reducing the release of toll-like receptors-4 (TLR4), TNF-α, and Interleukin-1- beta (interleukin [IL-1β]),[73] increasing the content of superoxide dismutase (SOD), decreasing the concentration of model-driven architecture (MDA),[74] up-regulating the level of matrix metalloprotein-9 (MMP-9) and Complement 3,[75] and inhibiting the expression of aquaporin-4 (AQP-4).[76] On the other hand, NX can reduce the concentration of β-endorphins, decrease the inhibitory effect of endogenous opioid peptides on CNS, improve the state of cerebral ischemia and hypoxia, and unleash the anti-apoptotic effect through down-regulating the expression of Fas-associated with death domain protein (FADD).[77]
4.3. Quality of evidence
Most trials are associated with the drawback of methodological quality deficiencies, which can be attributed to the incapability to collect unpublished literature and the lack of information on other bias evaluation. Simultaneously, the quality of the enrolled trials has restricted us from drawing definite conclusions, due to the existence of the random sequence generation, allocation concealment, as well as blinding of participants, personnel and outcome assessments, incomplete outcome data, and selective reporting bias. Firstly, literature quality is evaluated using the Cochrane risk of bias to avoid the biases of selection, performance, detection and reporting. Secondly, up-to-date literature is searched up to October 2017, and the published time of studies ranges from 2010 to 2016, which has ensured the timeliness and novelty. Thirdly, a comprehensive list of efficacy measurements as outcome indicators can provide more information for clinical practice in this meta-analysis.
4.4. Limitations
There are several limitations in this review, as shown below. First, the differences in bleeding site, hematoma volume and consciousness state between studies may represent sources of heterogeneity. Second, the criteria for efficacy evaluation are varied in the trials. Most studies have referred to the standardized Glasgow outcome scale (GOS),[40,43,46,50,51,59,60,61,63,64] the GCS score[33,35,42,42,44,45,47,48,52,53,54,55,57] or the NIHSS score [38,39,56,58] to assess the clinical efficacy, while the other studies have adopted the hospital questionnaire,[34] NDS[37] and the ADL.[56] Thus, subgroup analysis is performed to avoid potential heterogeneity. Thirdly, methodological heterogeneity or risks of bias exists in the included trials. Most studies do not elaborate the specific methods of random sequence generation, allocation concealment and blinding. Thus, potential risks of selective bias, performance bias and detection bias may exist. Moreover, none of the enrolled studies has reported the follow-up period. In addition, only 4 trials[34,39,55,57] had reported the adverse events, which can not comprehensively reflect the safety of XNJ combined with NX for ICH. Fourthly, all the research sites are in China, and the subjects are domestic patients, which will lead to obvious publication bias. Additionally, all the included studies are derived from the Chinese databases, which is associated with a risk of incomplete retrieval.
4.5. Implications
More prospective and well-designed studies are necessary to assess the efficacy and safety of XNJ combined with NX for ICH. In RCT design, the CONSORT 2010 statement[78,79] serves as a standard used to guide implementation. Typically, the methodology trial design has stressed the importance of randomization, allocation concealment, masking, baseline characteristics, follow-up, intention-to-treat analysis, and other measures. At the same time, strict and unified criteria of inclusion, exclusion, diagnostic and efficacy evaluation should be formulated. Besides, the severity of ICH, disease stage, syndrome, specific treatment, follow-up period, and incidence of adverse events should also be noted. In future studies, rigorous research should be designed to improve the quality of RCTs and evidence, which may result in a higher degree of confidence of systematic reviews.
In conclusions, the effectiveness and safety of XNJ combined with NX for ICH cannot be determined yet, which can be ascribed to the low quality of literature, publication bias and heterogeneity. Consequently, more rigorous RCTs are necessary to verify the role of XNJ combined with NX in treating ICH.
Author contributions
Wei-Xiong Liang, Qi Wang, Yun-Zhi Ma and Ze-Huai Wen select and design scheme in the meta-analysis. The document search, literature screening, formulation of inclusion and exclusion criteria, quality assessment, data extraction were carried out by Yu-Min Xu, Xin-Chen Wang, Shi-Jie Zhang, Ting-Ting Xu, Hong-Ying Li and Shang-Yan Hei were responsible for the data of statistics and analysis. All authors of this article have approved the final version of the manuscript.
Conceptualization: Shi-Jie Zhang, Ze-Huai Wen.
Data curation: Yu-Min XU, Shi-Jie Zhang, Ting-Ting Xu, Hong-Ying Li, Shang-Yan Hei.
Formal analysis: Yu-Min XU, Xin-Chen Wang, Ting-Ting Xu, Shang-Yan Hei.
Funding acquisition: Wei-Xiong Liang.
Investigation: Ting-Ting Xu, Wei-Xiong Liang.
Methodology: Shi-Jie Zhang, Ting-Ting Xu, Ze-Huai Wen, Qi Wang, Yun-Zhi Ma.
Project administration: Wei-Xiong Liang.
Resources: Hong-Ying Li, Shang-Yan Hei, Wei-Xiong Liang.
Software: Shi-Jie Zhang, Hong-Ying Li, Shang-Yan Hei.
Supervision: Yu-Min XU, Ze-Huai Wen, Qi Wang, Wei-Xiong Liang.
Writing – original draft: Yu-Min XU, Xin-Chen Wang.
Writing – review & editing: Yu-Min XU, Xin-Chen Wang, Wei-Xiong Liang.
Footnotes
Abbreviations: ADL = activities of daily living, AKI = acute kidney injury, CI = confidence intervals, CNS = central nervous system, GCS = Glasgow coma scale, GOS = Glasgow outcome scale, ICH= intracerebral hemorrhage, NDS = Neurological Dysfunction Score, NIHSS = National Institute of Health Stroke Scale, OR = odds ratio, RCTs = randomized controlled trials, TCM = traditional Chinese medicine, TER = total effective rates, TNF-α= tumor necrosis factor-alpha, XNJ = xingnaojing injection.
This work was supported by the National Natural Science Foundation of China (No. 81473740, No. 81673627), Guangdong Provincial Major Science and Technology for Special Program of China (No. 2012A080202017, No. 2015A030302072), South China Chinese Medicine Collaborative Innovation Center (No. A1-AFD01514A05), Technology platform of Clinical Trials on New Traditional Medicine (2012ZX09303009-003), Technology platform of Clinical Evaluation on New Traditional Medicine (2008ZX09312-021).
Y-MX and X-CW contributed equally to this work as first authors.
This article does not contain any studies with human or animal subjects performed by any of the authors.
The authors have no conflicts of interest to disclose.
References
- [1].An SJ, Kim TJ, Yoon BW. Epidemiology, risk factors, and clinical features of intracerebral hemorrhage: an update. J Stroke 2017;19:3–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Broderick JP, Brott TG, Duldner JE, et al. Volume of intracerebral haemorrhage. A powerful and easy-to-use predictor of 30-day mortality. Stroke 1993;24:987–93. [DOI] [PubMed] [Google Scholar]
- [3].Thabet AM, Kottapally M, Hemphill JC., 3rd Management of intracerebral hemorrhage. Handb Clin Neurol 2017;140:177–94. [DOI] [PubMed] [Google Scholar]
- [4].Hemphill JC, 3rd, Bonovich DC, Besmertis L, et al. The ICH score: a simple, reliable grading scale for intracerebral haemorrhage. Stroke 2001;32:891–7. [DOI] [PubMed] [Google Scholar]
- [5].Qureshi AI, Mendelow AD, Hanley DF. Intracerebral haemorrhage. Lancet 2009;373:1632–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].van Asch CJ, Luitse MJ, Rinkel GJ, et al. Incidence, case fatality, and functional outcome of intracerebral haemorrhage over time, according to age, sex, and ethnic origin: a systematic review and meta-analysis. Lancet Neurol 2010;9:167–76. [DOI] [PubMed] [Google Scholar]
- [7].Hong KS, Bang OY, Kang DW, et al. Stroke statistics in Korea: part I. Epidemiology and risk factors: a report from the Korean stroke society and clinical research centre for Stroke. J Stroke 2013;15:2–0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Krishnamurthi RV, Moran AE, Forouzanfar MH, et al. The global burden of hemorrhagic stroke: a summary of findings from the GBD 2010 study. Glob Heart 2014;9:101–6. [DOI] [PubMed] [Google Scholar]
- [9].Toyoda K. Epidemiology and registry studies of stroke in Japan. J Stroke 2013;15:21–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Kannel WB, Wolf PA, Verter J, et al. Epidemiologic assessment of the role of blood pressure in stroke. The Framingham study. JAMA 1970;214:301–10. [PubMed] [Google Scholar]
- [11].Broderick J, Connolly S, Feldmann E, et al. Guidelines for the management of spontaneous intracerebral hemorrhage in adults: 2007 update: a guideline from the American Heart Association/American Stroke Association Stroke Council, High Blood Pressure Research Council, and the Quality of Care and Outcomes in Research Interdisciplinary Working Group. Stroke 2007;38:2001–23. [DOI] [PubMed] [Google Scholar]
- [12].Davis SM, Broderick J, Hennerici M, et al. Hematoma growth is a determinant of mortality and poor outcome after intracerebral haemorrhage. Neurology 2006;66:1175–81. [DOI] [PubMed] [Google Scholar]
- [13].Cen ML, Jiang LY, Chen G, et al. Clinical progress of hypertensive intracerebral haemorrhage. Clin Res Pract 2016;186–7. [Google Scholar]
- [14].Caglar Nil Sayiner, Akin Turkan, Erdem Ibrahim Halil, et al. Where are we in terms of poststroke functional outcomes and risk factors. Neuro Rehabil 2014;34:391–9. [DOI] [PubMed] [Google Scholar]
- [15].Ye Hong, Su, Yingying Hemodynamic effects of mannitol infusion in patients with acute intracerebral haemorrhage. Acta Cir Bras 2013;28:106–11. [DOI] [PubMed] [Google Scholar]
- [16].Kim Min Young, Park Ji Hyeon, Kang Na Ree, et al. Increased risk of acute kidney injury associated with higher infusion rate of mannitol in patients with intracranial haemorrhage. J Neurosurg 2014;120:1340–8. [DOI] [PubMed] [Google Scholar]
- [17].Lin Shin-Yi, Tang Sung-Chun, Tsai Li-Kai, et al. Incidence and risk factors for acute kidney injury following mannitol infusion in patients with acute stroke: a retrospective cohort study. Medicine (Baltimore) 2015;94:e2032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Wu Bo, Liu Ming, Liu Hua, et al. Meta-analysis of traditional Chinese patent medicine for ischemic stroke. Stroke 2007;38:1973–9. [DOI] [PubMed] [Google Scholar]
- [19].Yu Jiegen, Li Rong, Liang Wei, et al. Effects of Xingnaojing on hypertensive cerebral haemorrhage: a Meta-analysis. Chin J Clin Pharmacol Ther 2016;21:417–24. [Google Scholar]
- [20].Wu Lijun, Zhang Hua, Xing Yanwei, et al. Meta-analysis of the effects of Xingnaojing injection on consciousness disturbance. Medicine (Baltimore) 2016;95:e2875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Ma Xiao, Yang Yu X, Chen Nian, et al. Meta-analysis for clinical evaluation of Xingnaojing injection for the treatment of cerebral infarction. Front Pharmacol 2017;8:485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Peng Weijun, Yang Jingjing, Wang Yang, et al. Systematic review and meta-analysis of randomized controlled trials of xingnaojing treatment for stroke. Evid Based Complement Alternat Med 2014;2014:210851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Nishikawa T, Ueba T, Kajiwara M, et al. A priority treatment of the intraventricular haemorrhage (IVH) should be performed in the patients suffering intracerebral haemorrhage with large IVH. Clin Neurol Neurosurg 2009;111:450–3. [DOI] [PubMed] [Google Scholar]
- [24].Zhang DK. Effect of Xingnaojing injection on IL-1β, IL-6 and TNF-α in serum and intracranial hematoma of patients with acute intracerebral hemorrhage. Chin J Gerontol 2015;35:6421–2. [Google Scholar]
- [25].Gu HJ, Li G, Wan D. Clinical effect of Xingnaojing injection on acute intracerebral haemorrhage and its effect on serum hs-CRP and NSE. Chin J Exp Tradit Med Formulae 2014;178–82. [Google Scholar]
- [26].Huang YJ. Effect of Xingnaojing injection combined with minimally invasive puncture and drainage on cerebral edema and serum AQP4 in patients with moderate hypertensive basal intracerebral hemorrhage. Chin J Chin Materia Medica 2014;2564–8. [PubMed] [Google Scholar]
- [27].Guo Feng, Lu Xiao-Wei, Xu Qiu-Ping. Protective effect of Xingnaojing and Xuesaitong injections on cerebral ischemic reperfusion injury in rats. Zhonghua Yi Xue Za Zhi 2010;90:1645–7. [PubMed] [Google Scholar]
- [28].Wei Gang, Huang YueChun, Li Fei, et al. Xingnaojing, prescription of traditional Chinese medicine, prevents autophagy in experimental stroke by repressing p53-DRAM pathway. BMC Complement Altern Med 2015;15:377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Feng Yuan, He Xiaozhou, Yang Yilin, et al. Current research on opioid receptor function. Curr Drug Targets 2012;13:230–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Moher D, Liberati A, Tetzlaff J, et al. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Ann Intern Med 2009;151:264–9. [DOI] [PubMed] [Google Scholar]
- [31].Hemphill JC, 3rd, Greenberg SM, Anderson CS, et al. Guidelines for the management of spontaneous intracerebral hemorrhage. Stroke 2015;46:2032–60. [DOI] [PubMed] [Google Scholar]
- [32].Rao ML. Summary of guidelines for the prevention and treatment of cerebrovascular diseases in China (4). J Apoplexy Nervous Dis 2006;2:132–6. [Google Scholar]
- [33].Song XX, Cui Y, Zhang L, et al. Clinical application of Xingnaojing combined with naloxone hydrochloride for coma after intracerebral haemorrhage. Chin J Trauma Disabil Med 2014;140–140. [Google Scholar]
- [34].Chen J. Clinical observation of naloxone combined with Xingnaojing in treating hypertensive intracerebral haemorrhage. World Latest Med Info 2016;16:151–4. [Google Scholar]
- [35].Ding SX. Clinical observation of the combination of Xingnaojing and naloxone hydrochloride for coma after intracerebral haemorrhage. Guide Chin Med 2015;21:80. [Google Scholar]
- [36].Fan WP. Clinical analysis of combined use of Xingnaojing and naloxone hydrochloride for coma after intracerebral haemorrhage. Con MedSym 2014;7:14–5. [Google Scholar]
- [37].Song XJ, Jia YH. Clinical observation of naloxone combined with Xingnaojing for hypertensive intracerebral haemorrhage. Mod Health 2015;12:141. [Google Scholar]
- [38].Wang Y, Wei B, Zhang GF, et al. Clinical observation of naloxone combined with Xingnaojing for acute intracerebral haemorrhage. World Latest Med Info 2014;30:16–7. [Google Scholar]
- [39].Cheng L. Effect of naloxone combined with Xingnaojing on hypertensive intracerebral haemorrhage after minimally invasive hematoma evacuation and its effect on neurological function. Chin J Pract Nerv Dis 2015;11:102–3. [Google Scholar]
- [40].Luo XJ. Analysis of clinical effect of dementia combined with naloxone hydrochloride in coma patients after the cerebral haemorrhage. Love and Health 2014;4:8–9. [Google Scholar]
- [41].Gong TY, Geng T. Evaluation of the effect of Xingnaojing combined with naloxone hydrochloride for coma patients after intracerebral haemorrhage. Guide Chin Med 2016;14:190–1. [Google Scholar]
- [42].Zhu L, Yue C, Yu Q. Effect of Xingnaojing combined with naloxone on GCS and ADL in patients with hypertensive intracerebral haemorrhage. J Nanchang Univ (Med Sci) 2015;48–50. [Google Scholar]
- [43].Nie M. Clinical analysis of Xingnaojing combined with naloxone in patients with coma after intracerebral haemorrhage. World Clin Med 2016;10:126. [Google Scholar]
- [44].Zhu JN. 61 cases of intracerebral haemorrhage coma with Xingnaojing combined with naloxone. J Commun Med 2012;10:12–3. [Google Scholar]
- [45].Wang K, Xing MR. Clinical observation of Xingnaojing combined with naloxone for coma patients with intracerebral haemorrhage. Pharmacol Clin Chin Materia Medica 2015;2:203–4. [Google Scholar]
- [46].Qiang MC, Dang YD. Clinical effect of Xingnaojing combined with naloxone hydrochloride on intracerebral haemorrhage with coma. Clin Res Pract 2016;1:21–2. [Google Scholar]
- [47].Zhu WR. Clinical analysis of Xingnaojing combined with naloxone hydrochloride for coma after intracerebral haemorrhage. Chin Foreign Med Treat 2015;5:109–10. [Google Scholar]
- [48].Leng XB, Yin YX. Clinical observation of Xingnaojing combined with naloxone hydrochloride for coma after intracerebral haemorrhage. Chin Pract Med 2015;10:112–3. [Google Scholar]
- [49].Li HL. Efficacy of Xingnaojing combined with naloxone hydrochloride for coma after intracerebral haemorrhage. Guide Chin Med 2016;5:203. [Google Scholar]
- [50].Ye G. Efficacy of Xingnaojing combined with naloxone hydrochloride for coma after intracerebral haemorrhage. Strait Pharma J 2013;25:114–5. [Google Scholar]
- [51].Zhang F, Cao JM. Efficacy of Xingnaojing combined with naloxone hydrochloride for coma after intracerebral haemorrhage. Pract Prevent Med 2011;18:1090–2. [Google Scholar]
- [52].Guan XD, Wang X. Clinical efficacy of Xingnaojing combined with naloxone hydrochloride for coma after intracerebral haemorrhage. World Latest Med Info 2015;15:51–2. [Google Scholar]
- [53].Song GN, Zhu FQ. Clinical efficacy of Xingnaojing combined with naloxone hydrochloride for coma after intracerebral haemorrhage. Chin J Pharma Economics 2015;10:63–4. [Google Scholar]
- [54].Zhang ZH. Clinical observation of Xingnaojing combined with naloxone hydrochloride for coma after intracerebral haemorrhage. E-J Trans Med 2015;3:11–2. [Google Scholar]
- [55].Hou JX. Efficacy of Xingnaojing combined with naloxone hydrochloride for patients with intracerebral haemorrhage. Chin J Trauma Disabil Med 2014;8:125–6. [Google Scholar]
- [56].Huang SM, Guo SQ. Efficacy and safety of Xingnaojing and naloxone for hypertensive intracerebral haemorrhage. Chin Modn Doc 2014;52:115–7. [Google Scholar]
- [57].Pan JX, Wu Y, Chen X, et al. Effect of Xingnaojing Injection combined with Naloxone on neurological function in patients with acute intracerebral haemorrhage and consciousness disorder. Mod J Integrated Tradit Chin Western Med 2016;25:2829–31. [Google Scholar]
- [58].Heng JF. Efficacy of Xingnaojing injection combined with naloxone for acute intracerebral haemorrhage. J Emergency Tradit Chin Med 2010;19:1521–2. [Google Scholar]
- [59].Huang C, Wang YP, Ren XY. Clinical efficacy of Xingnaojing injection combined with naloxone hydrochloride for intracerebral haemorrhage with coma. China Rural Health 2016;15:53–5. [Google Scholar]
- [60].Yin XQ. Clinical effect of Xingnaojing injection combined with naloxone hydrochloride injection in treating intracerebral hemorrhage with coma. Med Hygiene 2016;35:253. [Google Scholar]
- [61].Zhou HW. Clinical efficacy of Xingnaojing injection combined with naloxone hydrochloride for intracerebral haemorrhage with coma. Chin J Clin Rational Drug Use 2015;27:1–2. [Google Scholar]
- [62].Wang YJ. Clinical efficacy of Xingnaojing injection combined with naloxone hydrochloride for intracerebral haemorrhage with coma. Chin Foreign Med Treatment 2016;35:138–40. [Google Scholar]
- [63].Wang LD. Clinical efficacy of Xingnaojing injection combined with naloxone hydrochloride for cerebral haemorrhage with coma. Chin Rural Health 2016;23:20. [Google Scholar]
- [64].Ye JH, Ou LZ, Li GY, et al. Clinical efficacy of Xingnaojing injection combined with naloxone hydrochloride for intracerebral haemorrhage with coma. Clin Med Eng 2016;23:1085–6. [Google Scholar]
- [65].Ziai WC. Hematology and inflammatory signaling of intracerebral haemorrhage. Stroke 2013;44:S74–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [66].Zhang Xuxin, Li Yanzhao, Liang Yan, et al. Distinguishing intracerebral haemorrhage from acute cerebral infarction through metabolomics. Rev Invest Clin 2017;69:319–28. [DOI] [PubMed] [Google Scholar]
- [67].Brouwers HB, Greenberg SM. Hematoma expansion following acute intracerebral haemorrhage. Cerebrovasc Dis 2013;35:195–201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [68].Zhang T. Predictive value of serum inflammatory cytokine levels in acute intracerebral haemorrhage. Chin J Lab Diagnosis 2014;18:1077–9. [Google Scholar]
- [69].Liu QJ. Changes of inflammatory factors and C-reactive protein in patients with acute intracerebral haemorrhage and its correlation with disease prognosis and recovery of neurological function. J Clin Emergency 2017;1:55–7. [Google Scholar]
- [70].Zhu BX, Zhang BG. Neuroprotective effects and mechanism of Xingnaojing injection on patients with acute intracerebral haemorrhage. Shandong Med J 2015;33:38–9. [Google Scholar]
- [71].Ma YJ, Li JH. Clinical analysis of modified decoctomy combined with Xingnaojing injection for moderate to severe craniocerebral injury. Pharmacol Clin Chin Materia Medica 2015;1:315–6. [Google Scholar]
- [72].Bo ZS. Clinical observation of modified decoctomy combined with Xingnaojing injection for moderate to severe craniocerebral injury. J Emergency Tradit Chin Med 2013;22:1792–3. [Google Scholar]
- [73].Zheng MH, Fan XZ. Effects of ventricle injection of Xingnaojing on expression of TLR4 and inflammatory factors in rats with intracerebral haemorrhage. Chin Mod Med 2015;20:4–7. [Google Scholar]
- [74].Xu YH. Pharmacological pharmacodynamics study and Clinical Application of Xingnaojing Injection. Mod J Integr Tradit Chin Western Med 2010;19:507–10. [Google Scholar]
- [75].Fan ML, Zhou GX. Effects of Xingnaojing on cerebral edema and matrix metalloproteinase-9 and complement C3 in brain edema and hematoma in rats with intracerebral haemorrhage. Chin J Contemporary Neurol Neurosurg 2009;9:284–9. [Google Scholar]
- [76].Li GL, Ma CT. Effect of Xingnaojing and Shengmai Injection on PAR1 and AQP4 expression in brain tissue after intracerebral haemorrhage in rats. Clin Med Eng 2014;21:1401–3. [Google Scholar]
- [77].Sun XP, Huang HS, Liu C, et al. Effects of naloxone on expression of FADD and cerebral oedema around cerebral hematoma in rats with intracerebral haemorrhage. Hebei Med J 2011;33:810–2. [Google Scholar]
- [78].Moher D, Hopewell S, Schulz KF, et al. CONSORT 2010 explanation and elaboration: Updated guidelines for reporting parallel group randomized trials. Int J Surg 2012;10:28–55. [DOI] [PubMed] [Google Scholar]
- [79].Schulz KF, Altman DG, Moher D, et al. CONSORT 2010 statement: updated guidelines for reporting parallel group randomized trials. Obstet Gynecol 2010;115:1063–70. [DOI] [PubMed] [Google Scholar]


