Supplemental Digital Content is available in the text
Keywords: herbal medicine, meta-analysis, moxibustion, polycystic ovary syndrome
Abstract
Background:
Polycystic ovary syndrome (PCOS) is one of the most common disorders of reproductive endocrinology in women of reproductive age. Lifestyle intervention and oral contraceptives are the first-line treatments for PCOS. Recent studies have suggested that complementary and alternative medicine (CAM) therapies including acupuncture, herbal medicine, and mind–body therapy have the potential to alleviate the symptoms and/or pathology of PCOS and to improve the quality of life of women with PCOS. This meta-analysis aimed to quantitatively summarize the efficacy and safety of moxibustion combined with oriental herbal medicine (OHM), common CAM therapies, for treating PCOS.
Methods:
Four databases were searched from their inception to June 22, 2018. Randomized controlled trials (RCTs) and quasi-RCTs using both OHM and moxibustion as experimental intervention, and western medication (WM) as control intervention were included. Studies involving OHM plus moxibustion combined with WM as the experimental intervention were also included. The quality of included studies was assessed using risk of bias tool.
Results:
Owing to the heterogeneity of reporting, meta-analysis was only performed for pregnancy rate, rate of normal biphasic basal body temperature (BBT), and total effective rate (TER). The results showed that compared to the WM group, the OHM combined with moxibustion group was associated with significantly higher pregnancy rate (risk ratio [RR] 1.95, 95% confidence interval [CI] 1.55–2.47; I2 = 0%), normal biphasic BBT rate (RR 1.66, 95% CI 1.34–2.05; I2 = 0%), and TER (RR 1.19, 95% CI 1.08–1.31; I2 = 0%). When OHM combined with moxibustion was used as an adjunctive therapy to WM, pregnancy rate (RR 1.65, 95% CI 1.29–2.11; I2 = 0%), and TER (RR 1.35, 95% CI 1.13–1.61; I2 = 43%) were significantly higher than those of the WM group.
Conclusion:
According to current evidence, OHM combined with moxibustion might be beneficial for treating PCOS. Moreover, the treatment might improve the therapeutic effects of conventional WMs including clomiphene citrate, oral contraceptives, and/or metformin. However, the findings should be interpreted with caution, owing to poor methodological quality of the included studies. Further larger, high-quality, rigorous RCTs should be conducted in this regard.
1. Introduction
Polycystic ovary syndrome (PCOS) is one of the most common disorders of reproductive endocrinology in women of reproductive age. A 5% to 12% prevalence of PCOS is observed among reproductive-aged women, although it varies according to the diagnostic criteria or ethnic groups.[1] PCOS is diagnosed by the presence of 2 of the following 3 criteria: oligo- and/or anovulation, clinical and/or biochemical hyperandrogenism, and ultrasound features of polycystic ovaries, with the exclusion of other etiologies.[2] The major clinical and biochemical features of hyperandrogenism are hirsutism, acne, alopecia, and seborrheic dermatitis; elevated androstenedione, testosterone, and dehydroepiandrosterone sulfate (DHEAS) levels; and decreased sex hormone-binding globulin levels. The syndrome not only presents with reproductive manifestations but also has metabolic implications including insulin resistance, obesity, dyslipidemia, systemic inflammation, and type 2 diabetes.[3–5]
Lifestyle intervention and oral contraceptives (OCPs) are the first-line treatments for hyperandrogenic women with PCOS, as OCPs contain estrogens and progestins to moderate the release of luteinizing hormone (LH).[6] However, the use of OCPs is inappropriate for women wishing to conceive.[6] Moreover, OCPs may increase the risk of cardiovascular complications or aggravate obesity, thereby exacerbating the associated signs and symptoms of PCOS.[7] Clomiphene citrate is used to induce ovulation in women with PCOS; however, it may be associated with a number of side effects including hot flashes, breast discomfort, abdominal distention, nausea, vomiting, nervousness, headache, hair loss, and disturbed vision.[8] The use of metformin, an insulin-sensitizing agent, is also increasing; although this drug is generally considered to be safe, it is associated with a high frequency of gastrointestinal adverse events, sometimes accompanied by the risk of lactic acidosis, which manifests as dizziness, muscle pain, and chills.[9,10]
Recent studies have suggested that complementary and alternative medicine (CAM) therapies including acupuncture, herbal medicine, and mind–body therapy have the potential to alleviate the symptoms and/or pathology of PCOS and to improve the quality of life of women with PCOS.[11–13] Moreover, a survey of Australian women with PCOS has shown that more than 70% of the population uses CAM therapies.[14] Some weak evidence suggests that acupuncture and oriental herbal medicine (OHM), which are famous CAM therapies, might have beneficial effects in treating PCOS.[15–17] Although more clinical evidence is still required, OHM may work on both reproductive and metabolic systems, which may help improve the symptoms of PCOS.[18] However, the use of acupuncture is limited in patients who show acupuncture-associated vasovagal response and in some patients who have severe needle phobia.[19,20] Meanwhile, moxibustion, which is a noninvasive CAM therapy, has long been applied in various obstetrical and gynecological conditions, such as menopausal hot flashes,[21] primary dysmenorrhea,[22] ovulation disorders,[23] breech presentations,[24] and urinary incontinence.[25] In the clinical setting, moxibustion has been used along with OHM in treating gynecological disorders including PCOS, especially in East Asia.[26,27] However, there has been no quantitative synthesis evaluating the efficacy of moxibustion combined with OHM in treating PCOS. This meta-analysis aimed to quantitatively summarize the efficacy and safety of moxibustion combined with OHM for treating PCOS.
2. Materials and methods
We reported this review according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) statement.[28]
2.1. Search strategy
Articles published from their inception to June 22, 2018 were searched in the following core databases: Medline via PubMed, EMBASE via Elsevier, and the Cochrane Central Register of Controlled Trials (CENTRAL). We also searched the Chinese database, China National Knowledge Infrastructure (CNKI). In addition, we searched the reference lists of the relevant articles to identify additional trials. We used “moxibustion” and “PCOS” as the search terms. No search term for OHM was used for comprehensive search. There were no restrictions on language and publication type such as journal article, thesis, and conference proceedings. The search strategy for each database is attached in Supplementary Appendix 1.
2.2. Study selection
Two independent researchers (C-YK and BL) carried out the study search and study selection, and any disagreement was solved by discussion.
2.2.1. Types of studies
We included both randomized controlled trials (RCTs) and quasi-RCTs using a quasirandomization method such as alternate allocation or allocation by birth date. We included parallel as well as crossover studies. Gray literature such as dissertation and conference abstract was also allowed.
2.2.2. Types of participants
Studies involving women with a diagnosis of PCOS were included, regardless of the age or race of the women. Trials that included participants suffering from other serious illnesses such as cancer, liver disease, or kidney disease were excluded.
2.2.3. Types of interventions
Studies using both OHM and moxibustion as experimental intervention, and western medication (WM) as control intervention were included. Studies involving OHM plus moxibustion combined with WM as the experimental intervention were also included if WM was equally used in both experimental and control groups. Studies in which no components of OHM or acupoints of moxibustion were listed were excluded. In this review, OHM was defined as herbal decoctions prescribed according to the theory of East Asian traditional medicines such as traditional Chinese medicine (TCM), traditional Korean medicine, and Kampo medicine.
2.2.4. Types of outcomes
The primary outcome was the ovulation rate of the participants. The secondary outcomes included the level of sex hormones including LH, follicle-stimulating hormone (FSH), estradiol (E2), progesterone, and testosterone; pregnancy rate; rate of normal biphasic basal body temperature (BBT), number of follicles, total effective rate (TER), and incidence of adverse events.
2.3. Data extraction
Using predefined extraction forms, 2 independent researchers (C-YK and BL) extracted data from the included studies. The extracted data included the name of the first author; publication year; sample size; participant age; pattern identification; interventions of the experimental group and control group; treatment period; components of OHM; acupoints used for moxibustion; outcomes; results; adverse events; and some information about methodology such as randomization method, allocation concealment, and blinding method.
2.4. Quality assessment
The “risk of bias tool” from the Cochrane Collaboration was used to assess the methodological quality of the included studies.[29] The following items were evaluated as “low risk,” “unclear,” or “high risk”: random sequence generation, allocation concealment, blinding of participants and personnel, blinding of outcome assessment, completeness of outcome data, selective reporting, and other sources of bias. We assessed the item of other sources of bias as “low risk” when the study reported that the characteristics of participants between the 2 groups showed no statistical heterogeneity. The study quality was assessed by 2 independent researchers (C-YK and BL), and any disagreement was solved through their discussion.
2.5. Data analysis and synthesis
The Cochrane Collaboration's software program Review Manager version 5.3 was used to perform the meta-analysis on studies reporting the same comparison and outcome measure, and on studies providing appropriate information to conduct quantitative synthesis. The results were presented as mean difference with 95% confidence interval (CI) for continuous outcomes and as risk ratio (RR) with 95% CI for dichotomous outcomes. Heterogeneity between the studies was assessed by the chi-squared test and the I-squared statistic; I2 value ≥50% indicated substantial heterogeneity and I2 value ≥75% indicated considerable heterogeneity.
The fixed-effects model was used in case of low heterogeneity (I2 < 50%); otherwise, the random-effects model was used. The fixed-effects model was also used when the number of studies included in the meta-analysis was very small, where the estimate of the between-study variance had a poor precision.[30] In case of insufficient or ambiguous data in the included data, the corresponding author was contacted via email to obtain additional information. Subgroup analyses were performed according to the type of primary data that were used to calculate the TER and the type of WM used.
2.6. Publication bias
Publication bias was assessed using funnel plots if 10 or more studies were included in the meta-analysis.
3. Results
3.1. Study description
The database search identified 156 studies, and no additional studies were identified through other sources. After removing 29 duplications, 108 irrelevant documents were excluded by screening the titles and abstracts. Full texts of the remaining 19 studies were reviewed for final inclusion. Barring 1 review article and 9 studies investigating irrelevant comparisons to the inclusion criteria, a total of 9 studies involving 964 participants were finally included in the meta-analysis (Fig. 1).[31–39]
Figure 1.
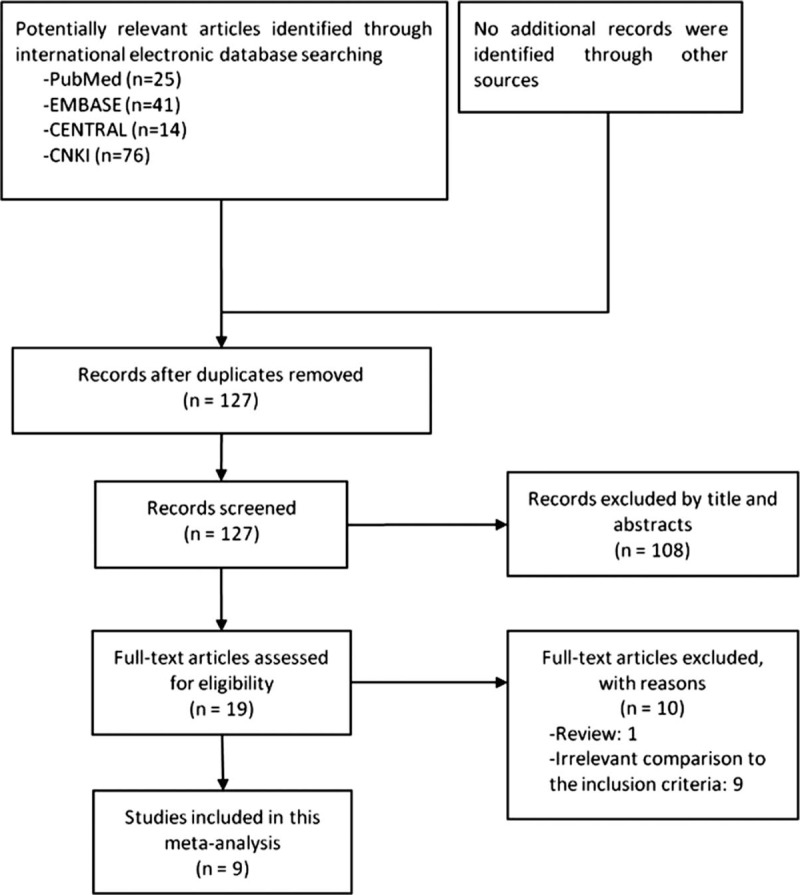
Flow chart of study inclusion.
3.2. Study characteristics
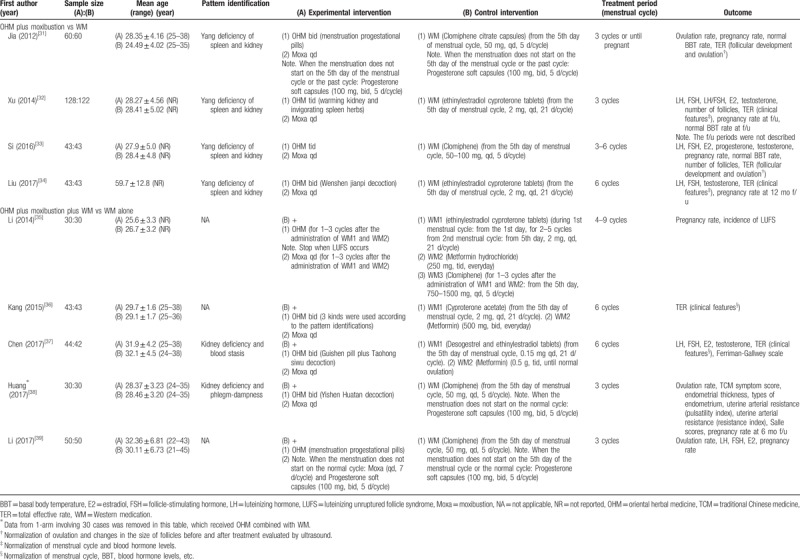
All included studies had been published in China from 2012 to 2017; 8[31–37,39] were journal articles while 1[38] was a dissertation. The mean age of the participants ranged from 21 to 45 years. Six studies[31–34,37,38] recruited participants with a specific TCM pattern, and all patterns were related to renal deficiency. All studies were parallel studies, 8[31–37,39] of which were 2-arm parallel studies. Among them, 4[31–34] compared OHM combined with moxibustion versus WM, and the other 4[35–37,39] compared OHM combined with moxibustion and WM versus WM alone. The remaining study[38] was a 3-arm parallel study comparing OHM combined with moxibustion and WM versus OHM combined with WM versus WM alone.
Generally, 3 types of WM were used: ovulation-inducing agents such as clomiphene citrate; OCPs such as cyproterone acetate, cyproterone acetate combined with ethinylestradiol, and desogestrel combined with ethinylestradiol; and insulin sensitizers such as metformin. Except for 1 study[35] with a treatment period of 4 to 9 menstrual cycles, all of the remaining studies described a treatment period of 3 to 6 cycles. Three studies[31,38,39] reported the ovulation rate of the participants. Pregnancy rate was reported in 7 studies,[31–35,38,39] of which 3[32,34,35] used OCPs as their WMs. Among these 3 studies, 2[32,34] reported pregnancies during the follow-up period after the end of the intervention, and the third one[35] reported pregnancies during clomiphene administration after the OCPs were discontinued. The levels of LH, FSH, E2, progesterone, and testosterone were reported in 5,[32–34,37,39] 5,[32–34,37,39] 4,[32,33,37] 1,[33] and 4 studies,[32–34,37] respectively. Three studies[31–33] reported BBTs of the participants. Safety data were reported in only 1 study (Table 1).[38]
Table 1.
Characteristics of included studies.
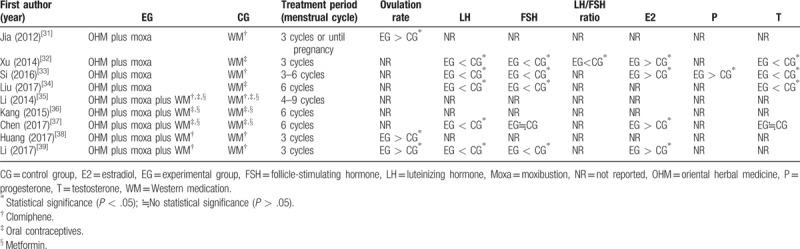
A meta-analysis was performed for pregnancy rate, rate of normal biphasic BBT, and TER. Meta-analysis for the other outcomes was not conducted owing to the heterogeneity of reporting, namely the differences in the units of outcome measures. In particular, with regard to the ovulation rate, which is the primary outcome, one study[39] reported the number of participants who achieved successful ovulation, while another study[38] reported the number of successful menstrual cycles during the entire treatment period; this rendered quantitative synthesis impossible. The results reported in each study are listed in Table 2, along with information on statistical significance.
Table 2.
The effects on the ovulation rate and the levels of sex hormones.
3.3. Risk of bias assessment
Apart from 4 studies[33,35–37] that did not mention the random sequence generation method, 4 studies[31,32,34,39] that used the random number table were assessed to have a low risk of bias in random sequence generation, and 1 study[38] that used inadequate randomization method based on the order of treatment was assessed to have a high risk of bias. No studies reported on allocation concealment. Since none of the included studies used a placebo, all studies were assessed to have a high risk of bias in blinding of the participants and personnel owing to the nature of the intervention. No studies reported on the blinding of outcome assessment, and on the dropouts or withdrawals of the participants. One study[36] that used only TER, a secondary processed datum, as its outcome was assessed to have a high risk of bias in selective reporting. Eight studies[32–39] were assessed to have a low risk of bias in other sources of bias domains, as they reported statistical homogeneity in the characteristics of participants between the groups at baseline, with P value > .05 (Figs. 2 and 3).
Figure 2.
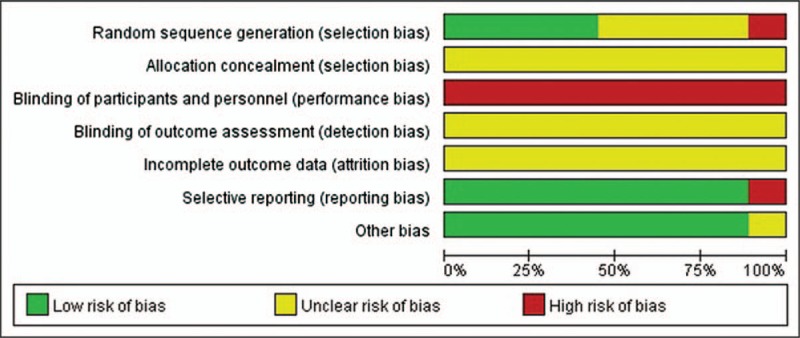
Risk of bias in the included studies.
Figure 3.
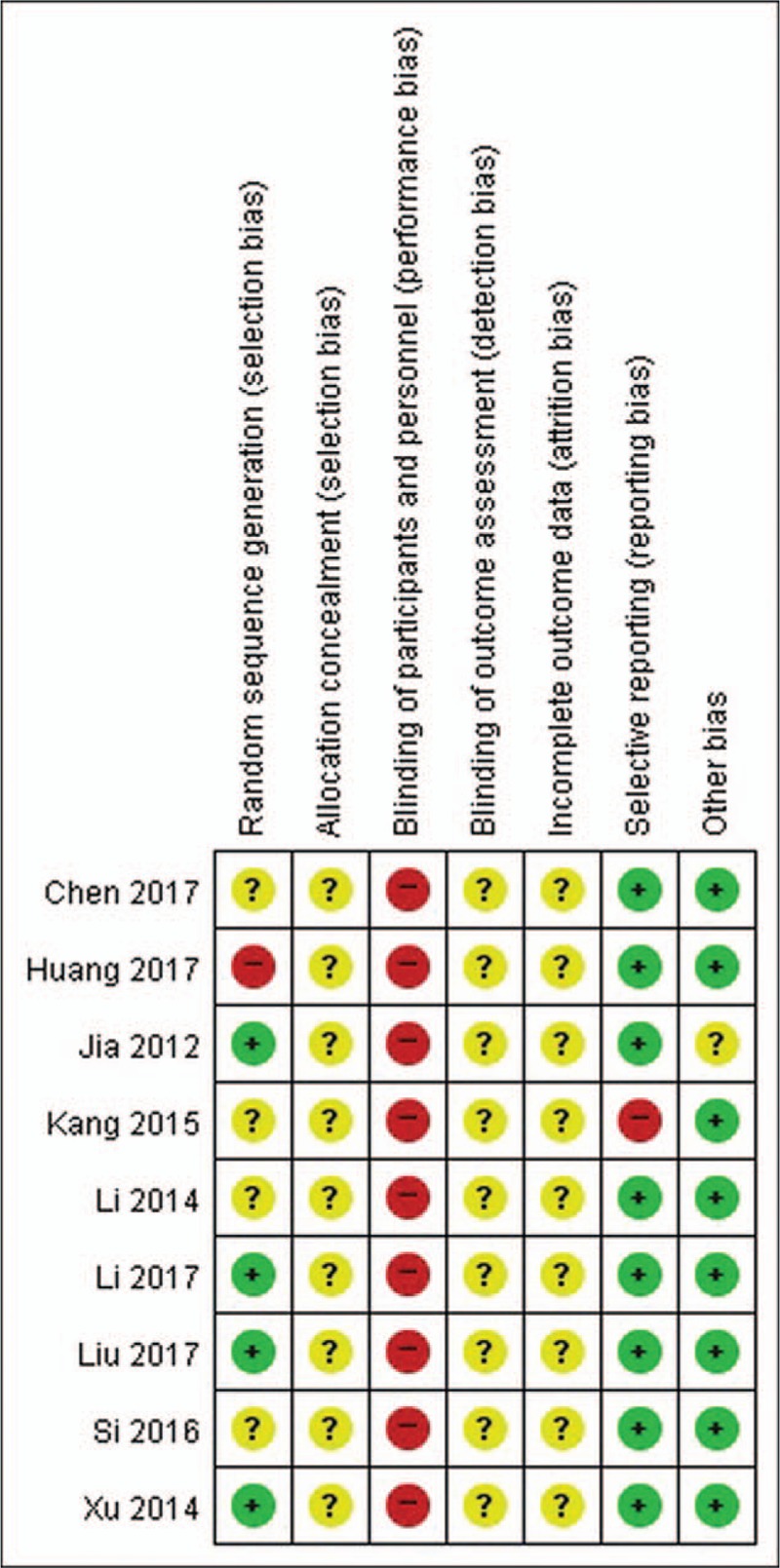
Risk of bias summary.
3.4. Efficacy and safety of OHM combined with moxibustion
3.4.1. OHM combined with moxibustion versus WM
There were 4 studies[31–34] comparing OHM combined with moxibustion versus WM. In 1 study,[31] the experimental group was associated with a significantly higher ovulation rate than that of the control group (P < .05).
With regard to the levels of sex hormones, the experimental group was associated with significantly lower levels of LH (3 studies[32–34]), FSH (3 studies[32–34]), LH/FSH ratio (1 study[32]), and testosterone (3 studies[32–34]), and significantly higher levels of E2 (2 studies[32,33]) and progesterone (1 study[33]) (all P < .05). We did not conduct a meta-analysis for the levels of sex hormones owing the heterogeneity of the units.
Meta-analysis was conducted for pregnancy rate, normal biphasic BBT rate, and TER (Figs. 4–7). According to the pooled results, the experimental group was associated with a significantly higher pregnancy rate (4 RCTs; RR 1.95, 95% CI 1.55–2.47; I2 = 0%), rate of normal biphasic BBT (2 RCTs; RR 1.66, 95% CI 1.34–2.05; I2 = 0%), and TER (3 RCTs; RR 1.19, 95% CI 1.08–1.31; I2 = 0%) than that of the controls, without heterogeneity. Although Jia[31] reported a normal rate of biphasic BBT in favor of the experimental group, this study was not included in the meta-analysis, as it reported the ratio of normal menstrual cycle in terms of BBT to the total menstrual cycle. Two studies[32,33] reported the number of follicles after intervention, and both of these reported significantly lower number of follicles in the experimental groups (both P < .05). However, one study[32] reported the number of follicles in the left and right ovary, while the other study[33] did not mention these; therefore, we could not quantitatively synthesize the results of these 2 studies.
Figure 4.
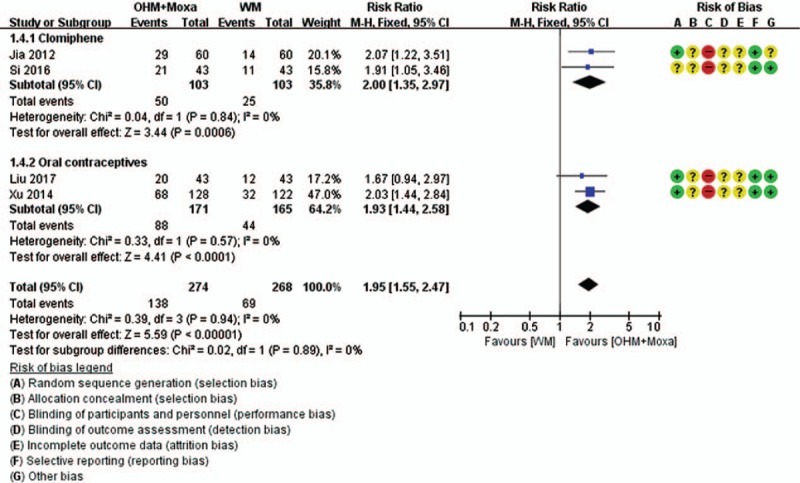
Forest plots of pregnancy rate. Comparison: OHM combined with moxibustion versus WM. Subgroup analysis according to the types of WM used. OHM = oriental herbal medicine, WM = Western medication.
Figure 7.
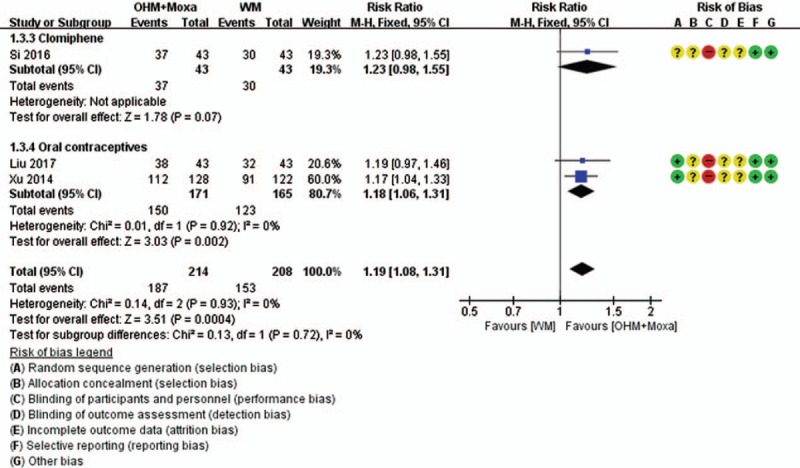
Forest plots of TER. Comparison: OHM combined with moxibustion versus WM. Subgroup analysis according to the types of WM used. OHM = oriental herbal medicine, TER = total effective rate, WM = Western medication.
Figure 5.
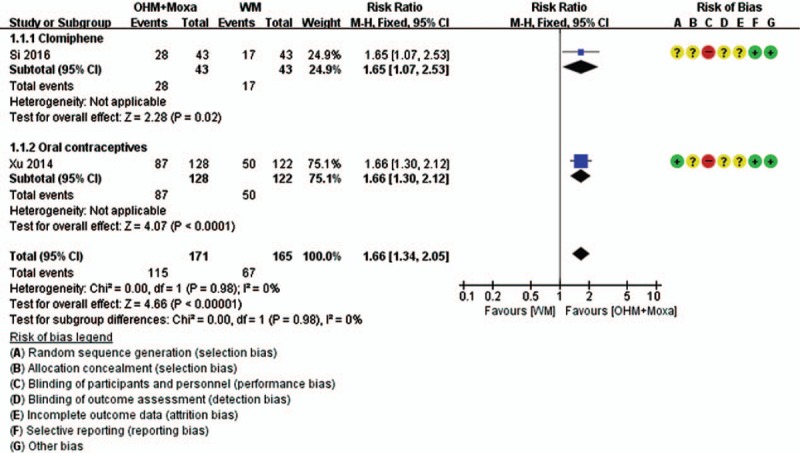
Forest plots of normal biphasic BBT rate. Comparison: OHM combined with moxibustion versus WM. Subgroup analysis according to the types of WM used. BBT = basal body temperature, OHM = oriental herbal medicine, WM = Western medication.
Figure 6.
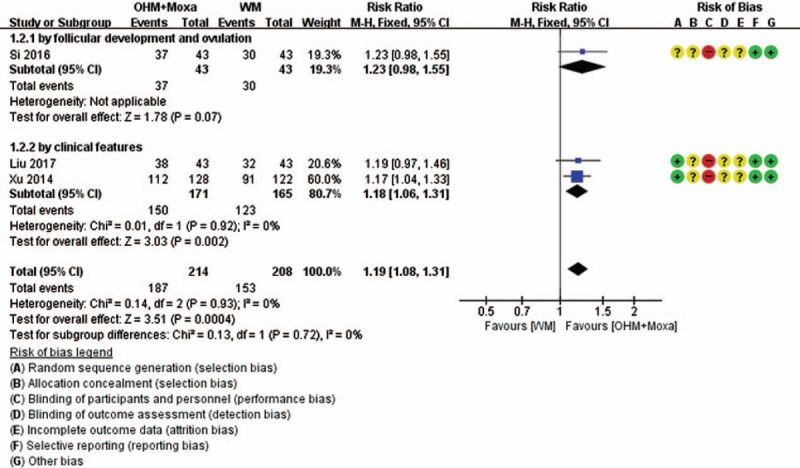
Forest plots of TER. Comparison: OHM combined with moxibustion versus WM. Subgroup analysis according to the type of primary data that were used to calculate the TER. OHM = oriental herbal medicine, TER = total effective rate, WM = Western medication.
In a subgroup analysis according to the type of primary data, TER measured by clinical features, indicating normalization of menstrual cycle and blood hormone levels, remained statistically significant (2 RCTs; RR 1.18, 95% CI 1.06–1.31; I2 = 0%), while there was no significant difference in the TER measured by follicular development and ovulation, indicating normalization of ovulation and changes in the size of follicles after treatment, as evaluated by ultrasound, between the groups (1 RCT; RR 1.23, 95% CI 0.98–1.55). In subgroup analysis according to the type of WM, the significances of pregnancy rate (2 RCTs for clomiphene; RR 2.00, 95% CI 1.35–2.97; I2 = 0%) (2 RCTs for OCPs; RR 1.93, 95% CI 1.44–2.58; I2 = 0%) and BBT (1 RCT for clomiphene; RR 1.65, 95% CI 1.07–2.53) (1 RCT for OCPs; RR 1.66, 95% CI 1.30–2.12) remained in both cases. However, the significance of TER remained only in the case of women taking OCPs (2 RCTs; RR 1.18, 95% CI 1.06–1.31; I2 = 0%) but not in those taking clomiphene (1 RCT; RR 1.23, 95% CI 0.98–1.55).
3.4.2. OHM combined with moxibustion and WM versus WM alone
There were 5 studies[35–39] comparing OHM combined with moxibustion and WM versus WM alone. In 2 studies,[38,39] the experimental groups were associated with a significantly higher ovulation rate than that of the controls (both P < .05). With regard to the levels of sex hormones, the experimental group was associated with significantly lower levels of LH (2 studies[37,39]) and significantly higher levels of E2 (2 studies[37,39]) (all P < .05). Of the 2 studies[37,39] reporting the level of FSH, one[39] reported that the level of FSH in the experimental group was significantly lower (P < .05), while the other study[37] reported no significant difference in the levels of FSH between the groups. In 1 study[37] reporting the level of testosterone, there was no significant difference in the levels between the 2 groups (Table 2).
Meta-analysis was conducted for pregnancy rate and TER (Figs. 8 and 9). According to the pooled results, compared with the control group, the experimental group was associated with a significantly higher pregnancy rate (3 RCTs; RR 1.65, 95% CI 1.29–2.11; I2 = 0%) and TER, as calculated by the improvement of clinical features (2 RCTs; RR 1.35, 95% CI 1.13–1.61; I2 = 43%), indicating normalization of menstrual cycle, BBT, blood hormone levels, etc. In subgroup analysis according to the type of WM, the significance of pregnancy rate remained only in the case of women taking clomiphene alone (2 RCTs; RR 1.62, 95% CI 1.27–2.07; I2 = 25%) but not in those taking clomiphene combined with OCPs and metformin (1 RCT; RR 1.83, 95% CI 0.78–4.32).
Figure 8.
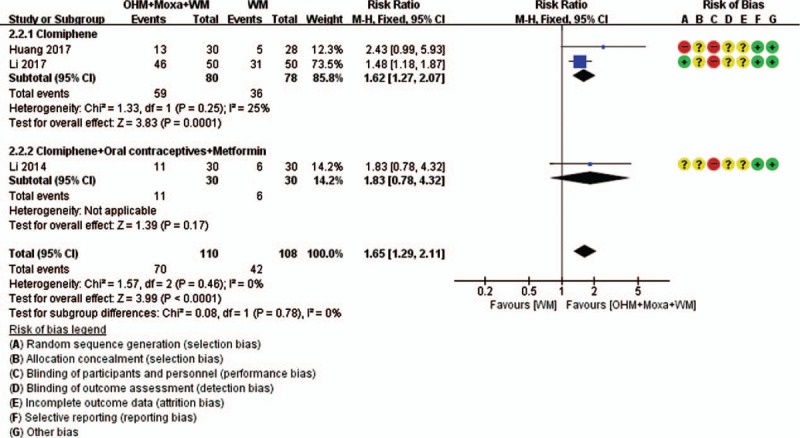
Forest plots of pregnancy rate. Comparison: OHM combined with moxibustion and WM versus WM. Subgroup analysis according to the types of WM used. OHM = oriental herbal medicine, WM = Western medication.
Figure 9.
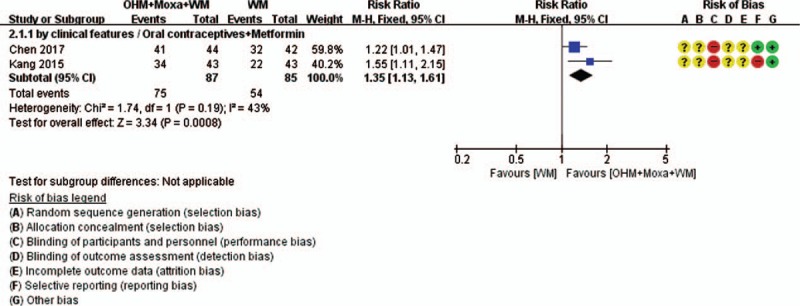
Forest plots of TER. Comparison: OHM combined with moxibustion and WM versus WM. OHM = oriental herbal medicine, TER = total effective rate, WM = Western medication.
3.4.3. Safety
Only 1 study[38] that compared OHM combined with moxibustion versus WM reported adverse events related to the interventions. Huang[38] reported that 6 cases of gastric pain and mild nausea, and 1 case of diarrhea were reported in the OHM combined with moxibustion group. However, no abnormalities were observed in blood, urine, and liver and kidney function tests, and no serious adverse events were reported.
3.5. OHM administered for PCOS
In 4 studies,[31,32,36,39] OHM administration was initiated on the 5th day of the menstrual cycle. In 3 studies,[33,35,37] OHM was administered everyday within the treatment period. One study[34] reported the initiation of OHM at the end of menstrual bleeding. The remaining study[28] did not report the time of initiation of OHM administration. Except for the 3 studies[33,35,37] that prescribed OHM everyday, 5 studies[31,32,36,38,39] reported a 20- or 21-day period of OHM administration in a menstrual cycle. One study[34] reported a period of OHM administration from the end of menstrual bleeding to the start of the next menstrual bleeding (Table 3).
Table 3.
OHM administered for PCOS.
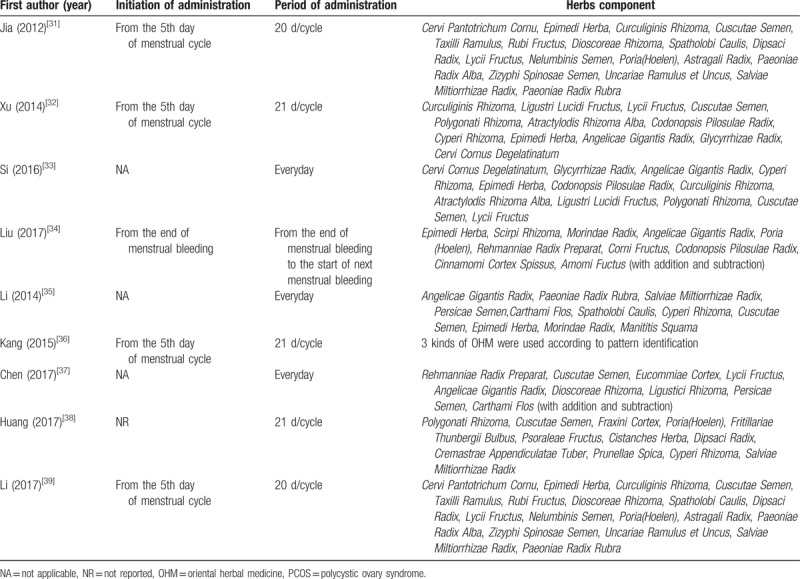
The components of OHM used in the included RCTs, except for 1 study[36] that used 3 kinds of OHM according to pattern identification, were analyzed. The median number of herbs constituting the OHM was 12; 43 different kinds of herbs were used in the 8 studies.[31–35,37–39] Among them, Semen Cuscutae (7 studies,[31–33,35,37–39] 87.5%) was the most frequently used, followed by Epimedii Herba (6 studies,[31–35,39] 75.0%), Lycii Fructus (5 studies,[31–33,37,39] 62.5%), and Angelicae Gigantis Radix (5 studies,[32–35,37] 62.5%) (Table 5).
Table 5.
The most frequently used herbs and acupoint.

3.6. Moxibustion performed for PCOS
The initiation of moxibustion was reported on the 5th day of the menstrual cycle in 2 studies,[32,38] the 10th day of the menstrual cycle in 1 study,[36] and the 12th day of the menstrual cycle in 1 study.[31] In 3 studies,[34,35,37] moxibustion was performed everyday within the treatment period. In 1 study,[33] moxibustion on CV4 and ST36 was performed everyday within the treatment period, while moxibustion on CV8 was initiated on the 7th day of the menstrual cycle. In the remaining study,[39] moxibustion was initiated “when the menstruation does not start on the normal cycle.” Besides the 3 studies[34,35,37] in which moxibustion was performed every day, in 3 studies,[31,36,39] moxibustion was performed for 7 days in a menstrual cycle. In 1 study,[33] moxibustion on CV4 and ST36 was performed every day, while moxibustion on CV8 was performed for 21 days in a menstrual cycle. In the remaining 2 studies, moxibustion was performed for 21 days[32] or 10 to 15 days[38] in a menstrual cycle. Except 1 study that did not report the duration of moxibustion in a treatment session,[38] in most of the studies,[31–34,37,39] moxibustion was performed for 20 minutes in a treatment session. Except for 3 studies,[31,36,39] 6 studies[32–35,37,38] reported the moxibustion method, which was using the indirect moxa stick (Table 4).
Table 4.
Moxibustion performed for PCOS.
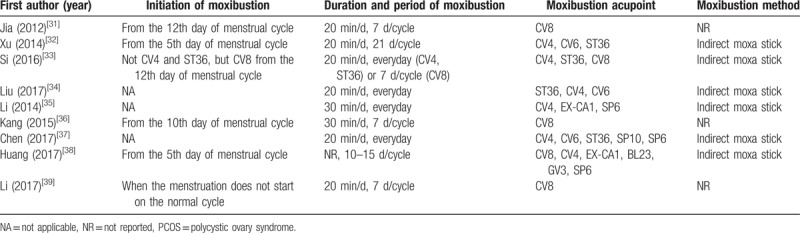
The moxibustion acupoints used in the included RCTs were analyzed. The average number of acupoints was 2.89. Nine kinds of acupoints were used in the 9 studies. Among them, CV4 (6 studies,[32–34,35,37,38] 66.7%) was the most frequently used, followed by CV8 (5 studies,[31,33,36,38,39] 55.6%), ST36 (4 studies,[32–34,37] 44.4%), CV6 (3 studies,[32,34,37] 33.3%), and SP6 (3 studies,[35,37,38] 33.3%) (Table 5).
3.7. Publication bias
The publication bias was not evaluated because the analysis contained fewer than 10 studies.
4. Discussion
In the present study, through comprehensive searches in English and Chinese databases, we conducted a meta-analysis of 9 RCTs to evaluate the efficacy and safety of OHM plus moxibustion for PCOS. In addition, we also included gray literature to comprehensively collect and evaluate evidence, and to minimize publication bias.
The results suggested that compared with the WM group, the OHM combined with moxibustion group was associated with significantly higher ovulation rate, levels of E2 and progesterone, pregnancy rate, normal biphasic BBT rate, and TER. The latter group also showed lower levels of LH, FSH, and testosterone, and fewer follicles. When OHM combined with moxibustion was used as an adjunctive therapy to WM, ovulation rate, E2 levels, pregnancy rate, and TER were significantly higher and LH levels were lower than those of the WM group. However, the FSH levels showed inconsistent results between the groups, and no difference in testosterone levels was observed between the groups. In subgroup analysis, when OHM combined with moxibustion was used as an alternative therapy to WM, it showed inconsistent results according to both the primary data of TER and the type of WM used. We also found that OHM plus moxibustion showed inconsistent results in the subgroup analysis of pregnancy rate according to the type of WM used, when added to WM. However, the results of these subgroup analyses should be interpreted with caution owing to the small number of studies included in each subgroup analysis.
Within the study documenting the safety data,[38] no serious adverse events or abnormalities in blood, urine, and liver and kidney function tests were reported. However, conclusions regarding the safety of OHM plus moxibustion could not be drawn, as only 1 study reported on this method.
OHM has been used with the aim of improving ovarian function, hyperandrogenism, and subfertility in women with PCOS.[40,41] In laboratory studies, OHM seemed to normalize the levels of female hormones,[42–45] decrease the levels of the male hormone[42,43] and recover the estrous cycle in a rat model of PCOS.[43–46] Moxibustion is a noninvasive CAM therapy that involves the burning of dried mugwort (i.e., Artemisia vulgaris) directly or indirectly at acupoints. Various methods of moxibustion have been applied in the clinical field; however, it can be divided into direct and indirect methods, in general. Although the underlying mechanisms of moxibustion for PCOS are not yet established, animal studies have shown that this treatment may have therapeutic effects by improving insulin resistance through upregulation of adiponectin levels.[47] Moreover, it has been reported that moxibustion can activate inflammatory responses and induce vascular changes around the sites of moxibustion, with releases of mediators including histamine and substance P, and induction of vasodilatation in mice.[48] Currently, abnormal vascular responses and inflammation, especially low-grade inflammation, are considered to play an important role in relation to cardiovascular complications and hyperandrogenism or insulin resistance, respectively.[49,50]
The studies included in this review reported the improvement of ovulation rate and reduction of LH, LH/FSH ratio, and testosterone levels after OHM plus moxibustion. This improvement in ovarian function and the normalization of sex hormone levels are consistent with the results of previously published studies on OHMs.[40,42–46] In addition, moxibustion may have reinforced the therapeutic effect of OHMs by improving insulin resistance, activating inflammatory responses, and inducing vascular changes.[47,48] OCPs are currently being used as the first-line treatment for PCOS.[6] However, considering the inability to use OCPs in women planning a pregnancy, and the possibility of cardiovascular complications, CAM therapies such as OHM and moxibustion, which could normalize the sex hormones of women with PCOS, may be useful.
In this review, we observed that OHM combined with moxibustion as an alternative or adjunctive therapy to WM had a relatively consistent efficacy, especially for ovulation rate, levels of E2 and LH, pregnancy rate, and TER. However, we were unable to draw a firm conclusion about the efficacy of OHM combined with moxibustion owing to the following limitations. The methodological quality of the included studies was generally low. There were no studies reporting allocation concealment method, blinding of participants, personnel, and outcome assessors. This leads to high risks of selection bias, performance bias, and detection bias, which can lead to overestimation of the efficacy of OHM combined with moxibustion. Meta-analyses showed that the statistical heterogeneity of the effect estimates was low, because I2 value was lower than 50%; however, the clinical heterogeneity remained owing to the wide age range of the participants, diversity of the OHM used, diversity of acupoints used in moxibustion, etc. Despite having searched 3 English databases and 1 Chinese database, all included studies were published in China. Therefore, this review may have potential reporting biases. However, owing to the insufficient number of studies included in the meta-analysis, we could not evaluate the publication bias, one of the types of reporting biases, using funnel plots.
Future clinical studies should be performed with the following considerations in mind. RCTs with a high-quality and larger sample sizes should be conducted. A strict protocol should be planned prior to future trials, and the reporting should be done in accordance with a systematic reporting guideline such as Consolidated Standards of Reporting Trials (CONSORT) Extension for Chinese Herbal Medicine Formulas 2017.[51] In addition, just 1 study[34] included in this review conducted a follow-up after 12 months to investigate the pregnancy rate. We suggest that long-term clinical trials with longer follow-up periods should be conducted to determine the efficacy of OHM plus moxibustion over time. Moreover, systematic monitoring and reporting of adverse reactions related to OHM or moxibustion using CONSORT for Harms Data Recommendations are required.[52] Lastly, further studies on the underlying mechanisms of OHM plus moxibustion on PCOS-related pathologies such as inflammation and insulin resistance are warranted.
5. Conclusion
According to current evidence, OHM combined with moxibustion might be beneficial for treating PCOS. Moreover, the treatment might improve the therapeutic effects of conventional WMs including clomiphene citrate, OCPs, and/or metformin. However, the findings should be interpreted with great caution, owing to poor methodological quality of the included studies, especially the high risks of selection bias, performance bias, and detection bias. Further larger, high-quality, rigorous RCTs should be conducted in this regard.
Author contributions
Chan-Young Kwon and Boram Lee performed the literature search, study selection, data extraction, and methodological quality assessment using the risk of bias tool. Chan-Young Kwon and Boram Lee participated in the analysis. All authors interpreted the data and drafted the manuscript. Chan-Young Kwon and Kyoung Sun Park critically reviewed the manuscript. All authors approved the final manuscript.
Conceptualization: Chan-Young Kwon, Boram Lee, Kyoung Sun Park.
Data curation: Chan-Young Kwon, Boram Lee.
Formal analysis: Chan-Young Kwon, Boram Lee.
Funding acquisition: Kyoung Sun Park.
Investigation: Chan-Young Kwon.
Supervision: Kyoung Sun Park.
Writing – original draft: Chan-Young Kwon, Boram Lee, Kyoung Sun Park.
Writing – review & editing: Chan-Young Kwon, Kyoung Sun Park.
Supplementary Material
Footnotes
Abbreviations: BBT = basal body temperature, CAM = complementary and alternative medicine, CENTRAL = Cochrane Central Register of Controlled Trials, CI = confidence interval, CNKI = China National Knowledge Infrastructure, CONSORT = Consolidated Standards of Reporting Trials, DHEAS = dehydroepiandrosterone sulfate, E2 = estradiol, FSH = follicle-stimulating hormone, LH = luteinizing hormone, OCP = oral contraceptive, OHM = oriental herbal medicine, PCOS = polycystic ovary syndrome, PRISMA = Preferred Reporting Items for Systematic Reviews and Meta-Analyses, RCT = randomized controlled trial, RR = risk ratio, TCM = traditional Chinese medicine, TER = total effective rate, WM = Western medication.
This work was supported by National Research Foundation of Korea (NRF) grant funded by the Korea government (MSIP) (No. NRF-2017R1C1B1006387).
The authors have no conflicts of interest to disclose.
Supplemental Digital Content is available for this article.
References
- [1].Ding T, Hardiman PJ, Petersen I, et al. The prevalence of polycystic ovary syndrome in reproductive-aged women of different ethnicity: a systematic review and meta-analysis. Oncotarget 2017;8:96351–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Rotterdam ESHRE/ASRM-Sponsored, PCOS, Consensus Workshop Group. Revised 2003 consensus on diagnostic criteria and long-term health risks related to polycystic ovary syndrome. Fertil Steril 2004;81:19–25. [DOI] [PubMed] [Google Scholar]
- [3].DeUgarte CM, Bartolucci AA, Azziz R. Prevalence of insulin resistance in the polycystic ovary syndrome using the homeostasis model assessment. Fertil Steril 2005;83:1454–60. [DOI] [PubMed] [Google Scholar]
- [4].Sartor BM, Dickey RP. Polycystic ovarian syndrome and the metabolic syndrome. Am J Med Sci 2005;330:336–42. [DOI] [PubMed] [Google Scholar]
- [5].Escobar-Morreale HF, Luque-Ramírez M, González F. Circulating inflammatory markers in polycystic ovary syndrome: a systematic review and metaanalysis. Fertil Steril 2011;95:1048–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Rocca ML, Venturella R, Mocciaro R, et al. Polycystic ovary syndrome: chemical pharmacotherapy. Expert Opin Pharmacother 2015;16:1369–93. [DOI] [PubMed] [Google Scholar]
- [7].Diamanti-Kandarakis E, Baillargeon JP, Iuorno MJ, et al. A modern medical quandary: polycystic ovary syndrome, insulin resistance, and oral contraceptive pills. J Clin Endocrinol Metab 2003;88:1927–32. [DOI] [PubMed] [Google Scholar]
- [8].Legro RS, Barnhart HX, Schlaff WD, et al. Clomiphene, metformin, or both for infertility in the polycystic ovary syndrome. N Engl J Med 2007;356:551–66. [DOI] [PubMed] [Google Scholar]
- [9].Costello M, Shrestha B, Eden J, et al. Insulin-sensitising drugs versus the combined oral contraceptive pill for hirsutism, acne and risk of diabetes, cardiovascular disease, and endometrial cancer in polycystic ovary syndrome. Cochrane Database Syst Rev 2007;1:CD005552. [DOI] [PubMed] [Google Scholar]
- [10].Nasri H, Rafieian-Kopaei M. Metformin: current knowledge. J Res Med Sci 2014;19:658–64. [PMC free article] [PubMed] [Google Scholar]
- [11].Raja-Khan N, Stener-Victorin E, Wu X, et al. The physiological basis of complementary and alternative medicines for polycystic ovary syndrome. Am J Physiol Endocrinol Metab 2011;301:E1–0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Arentz S, Abbott JA, Smith CA, et al. Herbal medicine for the management of polycystic ovary syndrome (PCOS) and associated oligo/amenorrhoea and hyperandrogenism; a review of the laboratory evidence for effects with corroborative clinical findings. BMC Complement Altern Med 2014;14:511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Stefanaki C, Bacopoulou F, Livadas S, et al. Impact of a mindfulness stress management program on stress, anxiety, depression and quality of life in women with polycystic ovary syndrome: a randomized controlled trial. Stress 2015;18:57–66. [DOI] [PubMed] [Google Scholar]
- [14].Arentz S, Smith CA, Abbott JA, et al. A survey of the use of complementary medicine by a self-selected community group of Australian women with polycystic ovary syndrome. BMC Complement Altern Med 2014;14:472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Lim CE, Ng RW, Xu K, et al. Acupuncture for polycystic ovarian syndrome. Cochrane Database Syst Rev 2016;5:CD007689. [DOI] [PubMed] [Google Scholar]
- [16].Jo J, Lee YJ, Lee H. Acupuncture for polycystic ovarian syndrome: a systematic review and meta-analysis. Medicine (Baltimore) 2017;96:e7066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Ma QW, Tan Y. Effectiveness of co-treatment with traditional Chinese medicine and letrozole for polycystic ovary syndrome: a meta-analysis. J Integr Med 2017;15:95–101. [DOI] [PubMed] [Google Scholar]
- [18].Ong M, Peng J, Jin X, et al. Chinese herbal medicine for the optimal management of polycystic ovary syndrome. Am J Chin Med 2017;45:405–22. [DOI] [PubMed] [Google Scholar]
- [19].Christensen KA, Gosse BJ, Hildebrand C, et al. Acupuncture-associated vasovagal response: revised terminology and hospital experience. Med Acupunct 2017;29:366–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Lu DP, Lu GP. Clinical management of needle-phobia patients requiring acupuncture therapy. Acupunct Electrother Res 1999;24:189–201. [DOI] [PubMed] [Google Scholar]
- [21].Park JE, Lee MS, Jung S, et al. Moxibustion for treating menopausal hot flashes: a randomized clinical trial. Menopause 2009;16:660–5. [DOI] [PubMed] [Google Scholar]
- [22].Kim SO, Cho SH. The effect of hand acupuncture therapy and moxibustion heat therapy on dysmenorrhea. Korean Acad Womens Health Nurs 2001;7:610–21. [Google Scholar]
- [23].Peng Y, Hou LH, Wu XK. Advances of modern studies of acupuncture and moxibustion for treatment of ovulation disorders. Zhongguo Zhen Jiu 2006;26:756–9. [PubMed] [Google Scholar]
- [24].van den Berg I, Bosch JL, Jacobs B, et al. Effectiveness of acupuncture-type interventions versus expectant management to correct breech presentation: a systematic review. Complement Ther Med 2008;16:92–100. [DOI] [PubMed] [Google Scholar]
- [25].Kim PW, Lee CW, Kim WI, et al. The effects of moxibustion on the stress urinary incontinence in middle-aged women. J Korean Acupunct Moxibustion Soc 2004;21:93–106. [Google Scholar]
- [26].Zhang CR, Shen T. Thirty cases of PCOS treated by the according to stage moxibustion on medicinal cake and acupuncture. Zhongguo Zhen Jiu 2012;32:42. [PubMed] [Google Scholar]
- [27].Zhou J, Qu F. Treating gynaecological disorders with traditional Chinese medicine: a review. Afr J Tradit Complement Altern Med 2009;6:494–517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Moher D, Liberati A, Tetzlaff J, et al. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med 2009;6:e1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Higgins JPT, Altman DG. The Cochrane Collaboration. Chapter 8: Assessing risk of bias in included studies. In: Higgins J, Green S, Eds., Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0, 2011. Available from: http://www.handbook-5-1.cochrane.org/ Accessed August 29, 2018. [Google Scholar]
- [30].Borenstein M, Hedges LV, Higgins J, et al. A basic introduction to fixed-effect and random-effects models for meta-analysis. Res Synth Methods 2010;1:97–111. [DOI] [PubMed] [Google Scholar]
- [31].Jia CM. Clinical observation on treating 60 cases of spleen deficiency type PCOS infertility in TCM and moxibustion. CJCM 2012;4:47–8. [Google Scholar]
- [32].Xu Y. Observation on therapeutic effect of traditional Chinese medicine and moxibustion in treating 128 cases of spleen kidney yang deficiency type polycystic ovary syndrome induced infertility. World Chin Med 2014;9:1079–82. [Google Scholar]
- [33].Si Q. Observation on the clinical effect of Chinese herbal medicine plus moxibustion on infertility of polycystic ovary syndrome with spleen and kidney yang deficiency. World Latest Med Inf 2016;16:93–4. [Google Scholar]
- [34].Liu ZF, Chen HQ. Effect of Wenshen Jianpi Recipe combined with acupoint moxibustion on infertility patients with polycystic ovary syndrome with spleen and kidney yang deficiency. Women's Heal Res 2017;1:153–4. [Google Scholar]
- [35].Li BL, Xie H, Ma WX. Traditional Chinese medicine plus moxibustion combined with western medicine to prevent luteinized unrupture follicle syndrome in 30 cases. TCM Res 2014;27:59–61. [Google Scholar]
- [36].Kang L, Li Y, Zhou H. Syndrome differentiation combining moxibustion in the treatment of polycystic ovary syndrome infertility for 43 cases. Chin Med Mod Distance Educ Chin 2015;13:61–2. [Google Scholar]
- [37].Chen Q, Tao Y, Liu J, et al. Influence of Bushen Huoxue Chinese herbs combined with moxibustion on sex hormone levels for polycystic ovarian syndrome patients. J Sichuan TCM 2017;35:154–7. [Google Scholar]
- [38].Huang YF. Effect of Yishen Huatan decoction and moxibustion of temperature sensitive acupoint on endometrial receptivity in polycystic ovary syndrome. Master's thesis in Guangxi University of Chinese Medicine 2017. [Google Scholar]
- [39].Li J, Tang Y, Yao L. Clinical effect of Chinese medicine, moxibustion and western medicine in treatment of obese patients with infertility caused by polycystic ovary syndrome. World Chin Med 2017;12:331–4. [Google Scholar]
- [40].Kuek S, Wang WJ, Gui SQ. Efficacy of Chinese patent medicine Tian Gui Capsule in patients with polycystic ovary syndrome: a randomized controlled trial. Zhong Xi Yi Jie He Xue Bao 2011;9:965–72. [DOI] [PubMed] [Google Scholar]
- [41].Zhou K, Zhang J, Xu L, et al. Chinese herbal medicine for subfertile women with polycystic ovarian syndrome. Cochrane Database Syst Rev 2016;10:CD007535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Zhao H, Zhou D, Chen Y, et al. Beneficial effects of Heqi San on rat model of polycystic ovary syndrome through the PI3K/AKT pathway. DARU J Pharmaceut Sci 2017;25:21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Rezvanfar M, Ahmadi A, Shojaei-Saadi H, et al. Molecular mechanisms of a novel selenium-based complementary medicine which confers protection against hyperandrogenism-induced polycystic ovary. Theriogenology 2012;78:620–31. [DOI] [PubMed] [Google Scholar]
- [44].Jang M, Lee MJ, Lee JM, et al. Oriental medicine Kyung-Ok-Ko prevents and alleviates dehydroepiandrosterone-induced polycystic ovarian syndrome in rats. PLoS ONE 2014;9:e87623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Sun HY, Li Q, Liu YY, et al. Xiao-Yao-San, a Chinese medicine formula, ameliorates chronic unpredictable mild stress induced polycystic ovary in rat. Front Physiol 2017;8:729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Lee MJ, Jang M, Bae CS, et al. Effects of oriental medicine Kyung-Ok-Ko on uterine abnormality in hyperandrogenized rats. Rejuvenation Res 2016;19:456–66. [DOI] [PubMed] [Google Scholar]
- [47].Legro RS, Zaino RJ, Demers LM, et al. The effects of metformin and rosiglitazone, alone and in combination, on the ovary and endometrium in polycystic ovary syndrome. Am J Obstet Gynecol 2007;196:402.e1–0. [DOI] [PubMed] [Google Scholar]
- [48].Okazaki M, Aizawa S, Yamauchi M, et al. Effects of single moxibustion on cutaneous blood vessel and microvascular permeability in mice. Am J Chin Med 1990;18:121–30. [DOI] [PubMed] [Google Scholar]
- [49].Repaci A, Gambineri A, Pasquali R. The role of low-grade inflammation in the polycystic ovary syndrome. Mol Cell Endocrinol 2011;335:30–41. [DOI] [PubMed] [Google Scholar]
- [50].Labruijere S, van Houten EL, de Vries R, et al. Analysis of the vascular responses in a murine model of polycystic ovary syndrome. J Endocrinol 2013;218:205–13. [DOI] [PubMed] [Google Scholar]
- [51].Cheng CW, Wu TX, Shang HC, et al. CONSORT extension for Chinese herbal medicine formulas 2017: recommendations, explanation, and elaboration. Ann Intern Med 2017;167:112–21. [DOI] [PubMed] [Google Scholar]
- [52].Ioannidis JP, Evans SJ, Gøtzsche PC, et al. Better reporting of harms in randomized trials: an extension of the CONSORT statement. Ann Intern Med 2004;141:781–8. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


