Abstract
Rationale:
Malignant melanoma (MM) arising in ovarian cystic teratoma (OCT) is a rare disease with poor prognosis. Recently, immune checkpoint inhibitors of cytotoxic T-lymphocyte-associated antigen 4 (CTLA4) and programmed death 1 (PD-1) have shown promising results in MM. Herein we report a case of MM arising in OCT.
Patient concerns:
A 63-year-old Japanese primigravida had lower abdominal pain. Magnetic resonance imaging revealed the presence of an 85-mm mass at the right ovary.
Diagnoses:
The patient underwent right salpingo-oophorectomy for right ovarian tumor, and histopathological examinations revealed MM arising in OCT. On immunohistochemical analysis, the tumor cells were positive for HMB-45, Melan A, and S-100 protein, and negative for programmed death-ligand 1 (PD-L1). BRAF gene mutations were not detected by the Real-Time PCR. Two months after surgery, liver metastasis was detected.
Interventions:
The patient underwent immune checkpoint inhibitors of CTLA4 (ipilimumab) and PD-1 (pembrolizumab and nivolumab). She had interstitial pneumonia associated with ipilimumab, but she safely underwent the immune checkpoint inhibitors therapy along with oral prednisolone. Pembrolizumab, ipilimumab, and nivolumab therapies had poor effect on the tumor.
Outcomes:
Now, the present case has had tumor-bearing survival for 14 months since the initial diagnosis and 12 months since the detection of liver metastasis.
Lessons:
This is the first case of MM arising in OCT treated by immune checkpoint inhibitors, with information of PD-L1 immunohistochemical expression and adverse events. The present case is the longest survivor following the detection of recurrence among all the previous reports. The long survival and slow-growing tumor in the present case may be associated with no PD-L1 expressions.
Keywords: CTLA4, immune checkpoint inhibitor, malignant melanoma, ovary, PD-1, PD-L1, teratoma
1. Introduction
Ovarian cystic teratoma (OCT) is a germ cell tumor that is composed of cells derived from ≥2, but frequently all 3 germ cell layers. They are the most common neoplasm of the ovary and often contain well-differentiated ectodermal or mesodermal structures. Although OCTs are usually benign, 1% to 3% will undergo malignant transformation.[1] Squamous cell carcinoma is the most common malignant transformation to occur in OCTs and represents 80% of the transformations that occur.[2] Other possible malignancies include adenocarcinoma, fibrosarcoma, adenosarcoma, basal cell carcinoma, carcinoid tumor, mixed cell tumors, and malignant melanoma (MM).[3,4] MM arising in OCT was first described by Andrews 1901[5] and is a rare disease with poor prognosis.[6,7] There were 41 cases of MM arising in OCT in English articles with patients’ individual information; the patients often received surgical treatment, radiation therapy, and chemotherapy, including cisplatin, carboplatin, etoposide, vincristine, vinblastine, dacarbazine, bleomycin, peplomycin, paclitaxel, tamoxifen, actinomycin D, nimustine, and imatinib.[6,7]
Cancers harbor numerous genetic and epigenetic alterations, generating neoantigens that are potentially recognizable by the immune system. However, cancers exploit several distinct pathways to actively evade immune destruction, including endogenous “immune checkpoints” that normally terminate immune responses after antigen activation.[8,9] Recently, immune checkpoint inhibitors of cytotoxic T-lymphocyte-associated antigen 4 (CTLA4)[8] and programmed death 1 (PD-1) have shown promising results in MM.[9] To the best of our knowledge, there are no reports about using immune checkpoint inhibitors for MM arising in OCT so far. Herein, we report the first case of MM arising in OCT treated by immune checkpoint inhibitors of CTLA4 (ipilimumab) and PD-1 (pembrolizumab and nivolumab), with information of programmed death-ligand 1 (PD-L1) immunohistochemical expression and adverse events.
2. Case presentations
A 63-year-old Japanese primigravida, without any history of cancer, had visited another clinic because of lower abdominal pain. Magnetic resonance imaging revealed the presence of an 85 × 84 × 70-mm mass at the right ovary, suspicious of OCT (Fig. 1A and B). No metastasis was detected by systemic computed tomography (CT). The serum levels of tumor markers of squamous cell carcinoma antigen, cancer antigen 125, and cancer antigen 19-9 were 2.0 ng/mL, 62 U/mL, and 10,159 U/mL, respectively. She underwent abdominal right salpingo-oophorectomy. Macroscopically, the right ovarian mass had a cystic appearance without a solid part, but the section was darkly pigmented (Fig. 1C). A histological analysis revealed the tumor had a fibrocystic wall, inside which were detected mature squamous cells, respiratory epithelial cells, hair roots, and bones (Fig. 2A–D). Focally, the tumor had high cellularity, with plump cells containing prominent and pleomorphic nucleoli. The cytoplasm was filled with compact melanosomes (Fig. 2E and F). On immunohistochemical analysis, the tumor cells were strongly positive for HMB-45, Melan A, and S-100 protein (Fig. 3A and B). The tumor was diagnosed as MM arising in OCT. Peritoneal cytology was negative for malignancy. Five weeks after surgery, she was referred to our hospital for reevaluation and treatments of MM. Medical examination of skin over the whole body by a dermatologist showed no evidence of primary cutaneous MM. Finally, we made the diagnosis of MM arising in OCT, International Federation of Gynecology and Obstetrics (FIGO) stage IA (pT1a pNX cM0). On immunohistochemical analysis, the tumor had no expressions of PD-L1 (primary antibodies: 22C3 pharmDx, Dako North America, Carpinteria, CA; and 28-8 pharmDx, Dako North America, Carpinteria, CA) (Fig. 3C and D). BRAF gene mutations (V600E and V600K) were not detected by the Real-Time PCR. Two months after surgery, a 17-mm liver metastasis was detected by FDG PET-CT and serum lactate dehydrogenase (LDH) was elevated (217 U/L). Figure 4 shows the clinical course of the patient from detection of recurrence, including the tumor size, serum LDH levels, and treatments. The patient initially underwent anti-PD-1 therapy with pembrolizumab (2 mg/kg, tri-weekly). After 4 cycles of pembrolizumab, the enhanced CT showed the progressive disease (sum of longest diameter of tumors, 65 mm) and serum LDH increased (1140 U/L). We changed pembrolizumab to anti-CTLA4 therapy with ipilimumab (3 mg/kg, tri-weekly). After 1 cycle of ipilimumab, she had interstitial pneumonia associated with ipilimumab. Ipilimumab was stopped for 3 months, and she took prednisolone orally. In the drug holiday, the sum of the longest diameter of tumors and serum LDH levels were increased (154 mm and 4981 U/L, respectively). After improvement of interstitial pneumonia, ipilimumab (3 mg/kg, tri-weekly) was readministered to her. After 3 cycles of ipilimumab, the enhanced CT showed the progressive disease with new liver lesions (sum of longest diameter of tumors, 248 mm) and serum LDH increased (5586 U/L). We changed ipilimumab to anti-PD-1 therapy with nivolumab (2 mg/kg, tri-weekly). Concurrently, she underwent radiotherapy with 20 Gray/4 fractions for liver metastasis. Although serum LDH level drastically decreased (1202 U/L) after radiotherapy, the enhanced CT showed the progressive disease with new lesions (sum of longest diameter of tumors, 258 mm) and serum LDH rapidly increased (4189 U/L) after 3 cycles of nivolumab. Nivolumab was discontinued and she will undergo dacarbazine monotherapy. Now, she has tumor-bearing survival for 14 months since the initial diagnosis and 12 months since the detection of liver metastasis. We obtained a written informed consent for publications from the patient.
Figure 1.
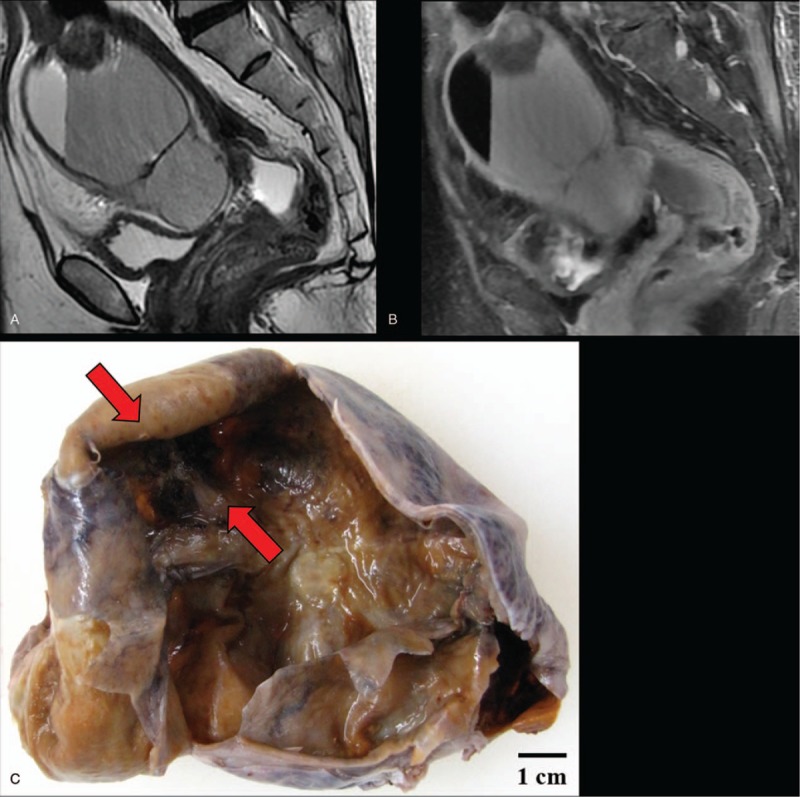
Magnetic resonance imaging and macroscopy of malignant melanoma arising in ovarian cystic teratoma: (A) T2-weighted image and (B) FAT SAT image revealed an 85 × 84 × 70-mm ovarian cystic tumor with fat. (C) The right ovarian mass had cystic appearance without solid part, but the section is darkly pigmented (red arrows) on macroscopy.
Figure 2.
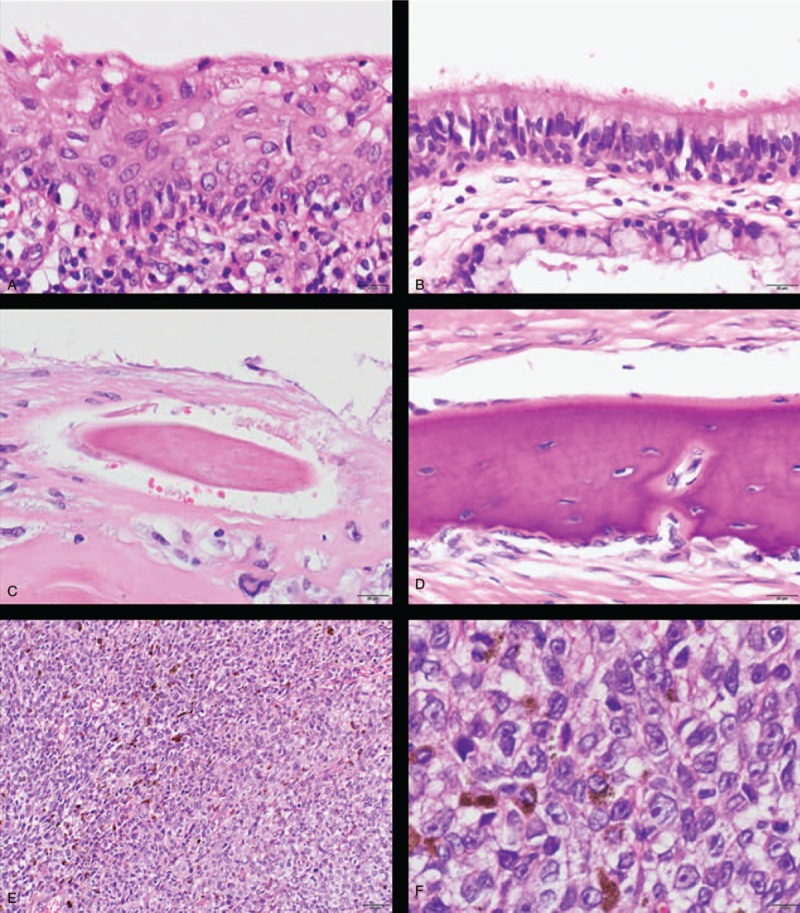
Histology of malignant melanoma arising in ovarian cystic teratoma (H&E): The tumor had (A) squamous epithelium, (B) respiratory epithelium, (C) hair root, and (D) bones. Focally, the tumor had high cellularity, with plump cells containing prominent and pleomorphic nucleoli. The cytoplasm was filled with compact melanosomes (E, low power view; F, high power view).
Figure 3.
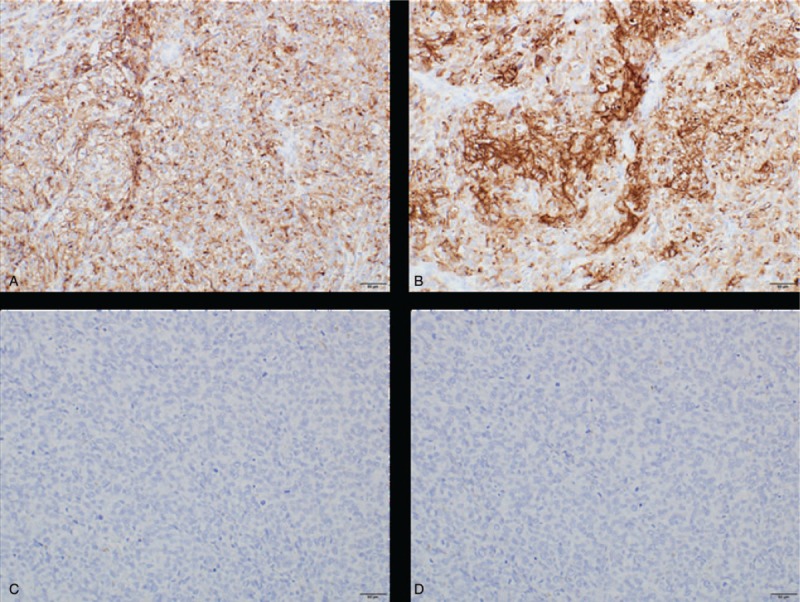
Immunohistochemical analysis of malignant melanoma arising in ovarian cystic teratoma: positive for (A) HMB-45 and (B) Melan A. Negative for PD-L1 clone (C) 22C3 and (D) 28-8.
Figure 4.
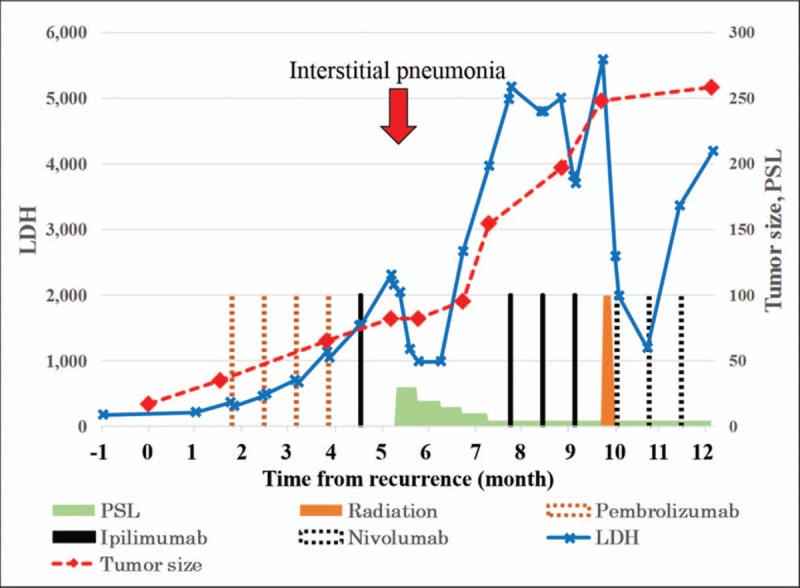
Clinical course of the present case from recurrence: red dotted line, sum of longest diameter of tumors (mm); blue solid line, serum LDH level (U/mL); brown dotted bar, pembrolizumab (2 mg/kg); black solid bar, ipilimumab (3 mg/kg); black dotted bar, nivolumab (2 mg/kg); brown solid bar, radiation therapy; green stair graph, oral prednisolone (PSL) (mg/d); red arrow, interstitial pneumonia.
3. Discussion
MM arising in OCT is a rare disease with poor prognosis, even with patients receiving surgical treatment, radiation therapy, and chemotherapy.[6,7] Immune checkpoint inhibitor is an additional, relatively new treatment modality for MM. Ipilimumab, anti-CTLA4 therapy,[8] and nivolumab and pembrolizumab, 2 anti-PD-1 therapies, have shown promising results in MM.[9] To the best of our knowledge, the present case is the first case of MM arising in OCT treated by immune checkpoint inhibitors so far. In cutaneous MM, anti-PD-1 therapy provides superior progression-free as well as overall survival, compared with anti-CTLA4 therapy, with fewer grade 3 to 4 treatment-related adverse events.[10] Anti-PD-1 therapy followed by anti-CTLA4 therapy appears to be a more clinically beneficial option compared with the reverse sequence, albeit with a higher frequency of adverse events.[11] Therefore, the present case was treated by pembrolizumab followed by ipilimumab. Unfortunately, this sequential treatment was ineffective in the present case, and the patient had interstitial pneumonia associated with ipilimumab, but she safely underwent immune checkpoint inhibitors under oral prednisolone. In MM, immune checkpoint inhibitors cause immune-related adverse events, including colitis (41%), hepatitis (36%), and pneumonitis (4%). Of these, 96% received corticosteroids and 21% received additional immunosuppression. Resumption of immune checkpoint inhibitors had 18% recurrent immune-related adverse events.[12]
More than half of patients with MM arising in OCT died of their disease, and the average time from diagnosis to death was 9.3 months. In addition, patients with recurrence and distant metastasis had worse prognosis. The average interval from recurrence presentation to death was 3 months (range 0–8).[6] The present case has survived 14 months after the initial diagnosis and 12 months since the detection of liver metastasis. To the best of our knowledge, the present case is the longest survivor from recurrence among all the previous reports. Low expression of PD-L1 is associated with good prognosis in several malignant tumors, including cutaneous melanoma,[13] breast cancer,[14] and non-small cell lung cancer.[15] The correlations between immunohistochemical overexpression of PD-L1 and immune checkpoint inhibitors outcome are positive in non-small cell lung cancer,[16,17] but controversial in MM[10,18,19] and ovarian cancer.[20] The present case had no immunohistochemical expression of PD-L1, and poor effects of immune checkpoint inhibitors. The long survival and slow-growing tumor in the present case may be associated with no PD-L1 expressions. Immune checkpoint inhibitors are one of the expected treatments for MM arising in OCT, but further studies are needed to clarify the effectiveness.
In conclusion, we reported the first case of MM arising in an OCT treated by immune checkpoint inhibitors, with information of PD-L1 immunohistochemical expression and adverse events. Further studies are needed to clarify the association among effectiveness of immune checkpoint inhibitors, PD-L1 expression, and patient survival.
Acknowledgments
We thank the Departments of Clinical Laboratory, and Obstetrics and Gynecology, Kumagaya General Hospital, for the provision of clinical specimens.
Author contributions
Conceptualization: Mitsutake Yano, Tomomi Katoh, Masanori Yasuda.
Data curation: Mitsutake Yano, Yuri Asami, Yukiko Teramoto, Masanori Yasuda.
Formal analysis: Mitsutake Yano, Masanori Yasuda.
Funding acquisition: Masanori Yasuda.
Investigation: Mitsutake Yano, Saori Yoshida, Kouichi Kamada, Tomomi Katoh, Yukiko Teramoto, Yasuhiro Nakamura, Masanori Yasuda.
Methodology: Mitsutake Yano, Saori Yoshida, Yasuhiro Nakamura.
Project administration: Mitsutake Yano, Saori Yoshida.
Resources: Mitsutake Yano, Saori Yoshida.
Supervision: Tadaaki Nishikawa, Yasuhiro Nakamura, Masanori Yasuda.
Validation: Yuri Asami, Saori Yoshida.
Visualization: Mitsutake Yano, Saori Yoshida, Tomomi Katoh.
Writing – original draft: Mitsutake Yano.
Writing – review & editing: Tadaaki Nishikawa, Masanori Yasuda.
Mitsutake Yano orcid: 0000-0002-4436-838X.
Footnotes
Abbreviations: CT = computed tomography, CTLA4 = cytotoxic T-lymphocyte-associated antigen 4, FIGO = International Federation of Gynecology and Obstetrics, LDH = lactate dehydrogenase, MM = malignant melanoma, OCT = ovarian cystic teratoma, PD-1 = programmed death 1, PD-L1 = programmed death-ligand 1.
This study was supported by Grants-in-Aid from the Ministry of Education, Science, Sports and Culture of Japan (Research Project Numbers: 15K08355 and 18K06997).
The authors have no conflicts of interest to disclose.
References
- [1].Griffiths D, Wass J, Look K, et al. Malignant degeneration of a mature cystic teratoma five decades after discovery. Gynecol Oncol 1995;59:427–9. [DOI] [PubMed] [Google Scholar]
- [2].Hackethal A, Brueggmann D, Bohlmann MK, et al. Squamous-cell carcinoma in mature cystic teratoma of the ovary: systematic review and analysis of published data. Lancet Oncol 2008;9:1173–80. [DOI] [PubMed] [Google Scholar]
- [3].Hyun HS, Mun ST. Primary malignant melanoma arising in a cystic teratoma. Obstet Gynecol Sci 2013;56:201–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Stamp GW, McConnell EM. Malignancy arising in cystic ovarian teratomas. A report of 24 cases. Br J Obstet Gynaecol 1983;90:671–5. [DOI] [PubMed] [Google Scholar]
- [5].Andrews H. Primary melanotic sarcoma of the ovary. Trans Obstet Soc 1901;43:228–31. [Google Scholar]
- [6].Zikry J, Korta DZ, Chapman LW, et al. Melanoma arising in an ovarian cystic teratoma: a systematic review of presentation, treatment, and outcomes. Arch Gynecol Obstet 2017. [DOI] [PubMed] [Google Scholar]
- [7].Shen X, Fan Y, Cao S. Primary malignant melanoma arising in an ovarian cystic teratoma. Melanoma Res 2017;27:601–6. [DOI] [PubMed] [Google Scholar]
- [8].Hodi FS, O’Day SJ, McDermott DF, et al. Improved survival with ipilimumab in patients with metastatic melanoma. N Engl J Med 2010;363:711–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Topalian SL, Hodi FS, Brahmer JR, et al. Safety, activity, and immune correlates of anti-PD-1 antibody in cancer. N Engl J Med 2012;366:2443–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Robert C, Schachter J, Long GV, et al. Pembrolizumab versus ipilimumab in advanced melanoma. N Engl J Med 2015;372:2521–32. [DOI] [PubMed] [Google Scholar]
- [11].Weber JS, Gibney G, Sullivan RJ, et al. Sequential administration of nivolumab and ipilimumab with a planned switch in patients with advanced melanoma (CheckMate 064): an open-label, randomised, phase 2 trial. Lancet Oncol 2016;17:943–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Pollack MH, Betof A, Dearden H, et al. Safety of resuming anti-PD-1 in patients with immune-related adverse events (irAEs) during combined anti-CTLA-4 and anti-PD1 in metastatic melanoma. Ann Oncol 2018;29:250–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Massi D, Brusa D, Merelli B, et al. PD-L1 marks a subset of melanomas with a shorter overall survival and distinct genetic and morphological characteristics. Ann Oncol 2014;25:2433–42. [DOI] [PubMed] [Google Scholar]
- [14].Muenst S, Schaerli AR, Gao F, et al. Expression of programmed death ligand 1 (PD-L1) is associated with poor prognosis in human breast cancer. Breast Cancer Res Treat 2014;146:15–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Cooper WA, Tran T, Vilain RE, et al. PD-L1 expression is a favorable prognostic factor in early stage non-small cell carcinoma. Lung Cancer 2015;89:181–8. [DOI] [PubMed] [Google Scholar]
- [16].Borghaei H, Paz-Ares L, Horn L, et al. Nivolumab versus docetaxel in advanced nonsquamous non-small-cell lung cancer. N Engl J Med 2015;373:1627–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Garon EB, Rizvi NA, Hui R, et al. Pembrolizumab for the treatment of non-small-cell lung cancer. N Engl J Med 2015;372:2018–28. [DOI] [PubMed] [Google Scholar]
- [18].Robert C, Long GV, Brady B, et al. Nivolumab in previously untreated melanoma without BRAF mutation. N Engl J Med 2015;372:320–30. [DOI] [PubMed] [Google Scholar]
- [19].Abdel-Rahman O. PD-L1 expression and outcome of advanced melanoma patients treated with anti-PD-1/PD-L1 agents: a meta-analysis. Immunotherapy 2016;8:1081–9. [DOI] [PubMed] [Google Scholar]
- [20].Webb JR, Milne K, Kroeger DR, et al. PD-L1 expression is associated with tumor-infiltrating T cells and favorable prognosis in high-grade serous ovarian cancer. Gynecol Oncol 2016;141:293–302. [DOI] [PubMed] [Google Scholar]


