Supplemental Digital Content is available in the text
Keywords: bioelectrical impedance analysis, breast cancer-related lymphedema, rehabilitation
Abstract
Secondary lymphedema is a chronic debilitating lifelong complication and early diagnosis is crucial. The Inbody 720, which is widely used, has no universal index of diagnostic criteria for test results. We aim to determine the normal range, cutoff values, and mean + standard deviation values of extracellular fluid (ECF) and the single frequency bioimpedance (SFBIA) ratios for the diagnosis of lymphedema and suggest the usefulness of these values for detecting lymphedema.
Seventy patients with unilateral breast cancer-related lymphedema and 643 healthy subjects were enrolled. All patients with breast cancer underwent surgeries with dissection of lymph nodes. We analyzed the ECF volume, SFBIA at 1- and 5-kHz frequencies using Inbody 720.
There were significant differences between patients with BCRL and healthy controls. The optimal cutoff values for ECF ratios were 1.010 for both the dominant and non-dominant arms. At 1 kHz, the cutoff values of SFBIA were 1.050 and 1.046, and at 5 kHz, those were 1.070 and 1.030 for the dominant and non-dominant affected arms, respectively. The mean + 2SD values for ECF ratio were 1.018 and 1.020 and at 1 kHz, the mean + 2SD values of SFBIA were 1.144 and 1.0135 and at 5 kHz, the cutoff values of SFBIA were 1.141 and 1.124 for the dominant and non-dominant affected arms, respectively. The mean + 3SD values for ECF ratio were 1.026 and 1.030 and at 1 kHz, the mean + 3SD values of SFBIA were 1.206 and 1.203 and at 5 kHz, those were 1.201 and 1.187 for the arms, respectively. The cutoff, mean + 2SD, and mean + 3SD values were applied to 70 patients with unilateral BCRL. When the cutoff values were applied, a higher proportion of BCRL patients were included.
When these figures were applied to the patient group, the cutoff values included a higher proportion of patients with lymphedema. Further studies are needed to investigate whether bioimpedance analysis can accurately predict the development of lymphedema.
1. Introduction
Lymphedema is defined as the accumulation of protein and fluid in the extravascular interstitium and is commonly known as a chronic disorder of the lymphatic system.[1] Secondary lymphedema is a chronic debilitating lifelong complication that occurs after breast cancer surgery and radiotherapy.[2] This disorder appears as an enlargement and distortion of one limb, and its symptoms manifest as pain, edema with pitting, fibrosis, recurrent infection, limited range of motion, or discomfort.[3] The incidence of lymphedemas following breast cancer treatment has been reported to be about 20% to 30%,[4,5] and they may occur at any time, but they usually occur within the first 1 to 2 years after surgery.[6] Lymphedemas recur frequently and are difficult to treat after fibrosis; thus, early diagnosis is crucial.
Sequential limb circumference measurement, tissue tonometry, tissue indentation force, water displacement volume, and lymphoscintigraphy have been used to detect lymphedema.[7] Currently, there is no definite tool that can be used to accurately diagnose the presence of lymphedema with high specificity and sensitivity.[8] Volume-based evaluation methods do not distinguish bones, muscle, fat, or other soft tissues from lymph or extracellular fluid (ECF).[9] Therefore, such methods are inadequate for precisely measuring lymphedema. Although bioimpedance is commonly used for detecting early lymphedema,[10] it also differentiates lymph or ECF from other tissues. Among several methods, bioelectrical impedance analysis (BIA) is considered an additive tool for measuring lymphedema because current impedance has been found to inversely correlate with fluid accumulation. BIA is easy to use, simple, inexpensive, and noninvasive and can quickly assess changes in body composition.[11–13]
The widely used Imp XCA (Impedimed, Brisbane, Australia) employs a single frequency below 30 kHz to measure impedance and resistance of ECF. The device uses an impedance ratio value, which is relative to normative standards derived from healthy individuals,[14] to calculate a lymphedema index, termed the L-Dex ratio. However, this is not the only Impedimed machine that is used to diagnose and evaluate lymphedema.
The Inbody 720 is widely used for lymphedema diagnosis, but there is no universal index of diagnostic criteria for test results. The aim of this study was to determine the SFBIA ratio and to obtain cutoff values and mean ± standard deviations (2SDs and 3SDs) of bioimpedance measurement for the diagnosis of lymphedema. In addition, the study aimed to validate the usefulness of these values for detecting lymphedema.
2. Methods
2.1. Subjects
Healthy women who visited Asan Medical Center for regular checkups from June 2010 to August 2011 were enrolled in this study. The charts of these subjects who underwent bioimpedance measurements were retrospectively reviewed. Subjects with kidney disease or diseases that are associated with edema, a history of breast cancer, soft tissue infection, radiotherapy to the upper extremities or the chest wall, pregnancy, or presence of implanted devices (e.g., pacemaker) or orthopedic pins or plates were excluded. Total 643 of women (aged 18–81) were included as a normal data.
Patients with breast cancer who had their breast and lymph nodes removed and received radiotherapy and/or chemotherapy, had differences in arm circumferences of >2 cm at 10 cm either below or above the elbow, and were diagnosed with unilateral upper limb breast cancer-related lymphedema (BCRL) by lymphoscintigraphy at Asan Medical Center from June 2010 to August 2011 were enrolled in this study. Total 70 patients (aged 31–69) with BCRL were included.
Individual date of both healthy woman and patients with BCRL is presented in the Supplement 1. The type of operation, reconstruction surgery, the number of dissected lymph node, the number of metastasized lymph node, chemotherapy, and radiation therapy of the patients with BCRL are presented in the Supplement 2. The study protocol was approved by the Research Ethics Committee of Asan Medical Center (Study number: S2015–1939–0006).
2.2. BIA
We used the Inbody 3.0 system (Biospace Co., Seoul, South Korea), which provides whole-body, trunk, torso, and limb ECF values.[15,16] Body weight and body mass indices (BMIs) were also assessed using the Inbody 3.0 system. Before each measurement, the participants’ palms and soles were wiped with an electrolyte tissue. Then, the participants stood on the InBody 720 scale with their soles in contact with the foot electrodes and body weight was measured. Sex, age, and height were manually entered into the instrument by the investigator. Then, the participant grasped the handles with the palm, fingers, and thumb of each hand making contact with the hand electrodes. The body composition analysis was initiated while the participants remained as motionless as possible. There were a total of 8 electrodes: 2 for each foot and 2 for each hand. Impedance was measured for 5 segments of the body: trunk, right and left arms, and right and left legs. Resistance was measured at 4 surface tactile electrodes placed on the dorsal surface of the hand and foot by the BIA generator. Resistance is the resistance that occurs when alternating current passes through the body water, and reactance indicates the resistance of the cell membrane through which the alternating current passes. Because BIA is sensitive to hydration status, participants were asked to refrain from alcohol consumption or vigorous exercise for 24 hours before the measurement. BIA was measured in the morning after overnight fasting to make the hydration status as uniform as possible. To minimize the contact noise, we cleaned the contacting surface of the electrodes with an alcohol swab before every measurement. In addition, the current electrode and voltage electrode were separated from each other by a total of 8 electrodes because of the structure of the hand. Starting the measurement at the wrist and ankle, where the flow of current and measurement of voltage meet, minimized the influence of the finger and palm, which have high contact resistance.
2.3. Evaluation criteria
Bioimpedance at a frequency of 5 kHz was an index of ECF mainly. This measurement has been validated in the detection and treatment of lymphedema.[17] We obtained values for ECF using bioelectrical impedance spectroscopy (BIS), specific to ECF and more sensitive to localized lymphedema,[18] with multifrequency (1 kHz to 1 MHz) and single frequency bioimpedance analyses (SFBIAs; values at 1- and 5-kHz frequencies) for both upper extremities using the Inbody 720 (Biospace, Seoul, South Korea). At low frequencies, currents flow selectively through the extracellular water compartments, which reflect the lymph volumes. At high frequencies, currents pass through both intracellular fluid and ECF.[19,20] The calculated ECF ratio was defined as a ratio of the affected side to the unaffected side, and the SFBIA ratio was defined as the ratio of the unaffected to the affected side.[21] SFBIA measured impedance at a single, low frequency that is close to zero.[22] SFBIA provides a simple, accurate alternative to BIS for the clinical assessment of unilateral lymphedema, and the results are highly correlated with BIS.[23]
As ECF ratios correlate with the bioimpedance measurements, the ECF ratios of the affected to unaffected arms were calculated. Owing to the wide biological variation between individuals, the absolute measured limb impedance cannot distinguish affected limbs from unaffected, normal limbs. The impedance of a limb with lymphedema is normalized to that of the contralateral unaffected limb, and this ratio is then compared to normative values.[24] Arm dominance was also considered. If the dominant arm was affected, the value of the nondominant arm was divided by that of the dominant arm. If the nondominant arm was affected, the opposite was applied. The affected to unaffected side ratios of SFBIA values at 1 and 5 kHz were also calculated. After obtaining bioimpedance values, mean + 2SD and mean + 3SD of healthy women were applied to the patient group to validate the usefulness of these values compared to receiver-operating characteristic (ROC) curve cutoff values for detecting lymphedemas.
2.4. Statistical analysis
All statistical analyses were performed using IBM SPSS Statistics version 18.0 for Windows (IBM corp., Armonk, NY) and R statistical software v.3.1.2 for Windows (Foundation for Statistical Computing, Vienna, Austria). We used Student t tests to compare the mean values of age, BMI, calculated SFBIA, and ECF ratios between the patient and the control groups. The cutoff values were obtained using ROC curves for calculated ECF, 1 kHz, and 5 kHz SFBIA ratios. Sensitivity and specificity were calculated with cutoff values of the bioimpedance analysis. Statistical significance was determined at P values <.05.
3. Results
3.1. Patient demographic data
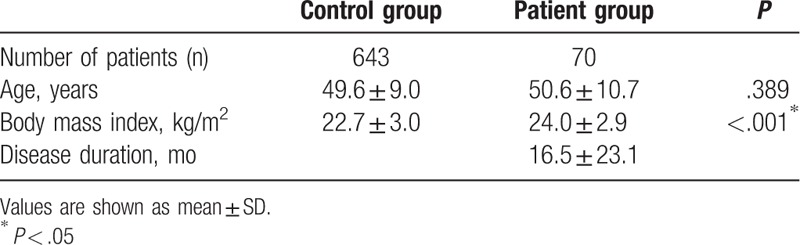
A total of 643 female patients were recruited in the control group, and bioimpedance measurements were reviewed retrospectively. The mean patient age was 49.6 ± 9.0 years, and the mean BMI was 22.7 ± 3.0. The demographic data of the subjects did not show any statistical differences. In the patient group, 70 female patients with unilateral BCRL were enrolled. Their mean age was 50.6 ± 10.7, the mean BMI was 24.0 ± 2.9, and their mean disease duration was 16.5 ± 23.1 months (Table 1). There were statistically significant differences between the 2 groups in terms of BMI and no statistically significant differences in terms of age.
Table 1.
Patients’ demographic characteristics.
3.2. Bioimpedance values
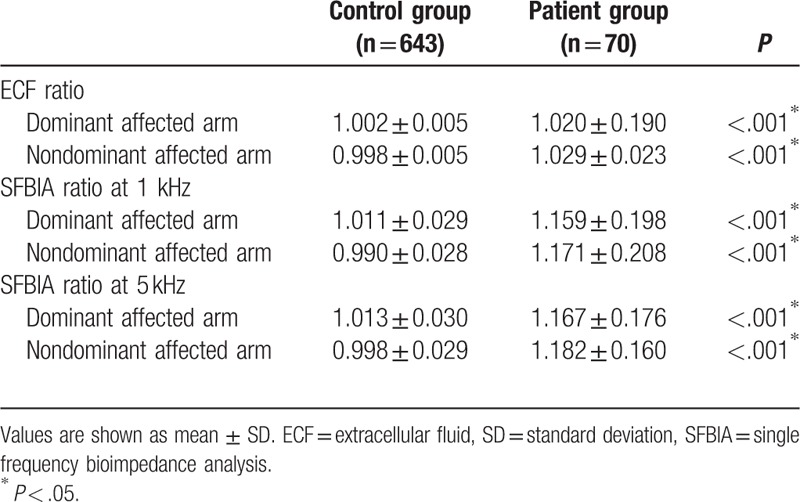
The ECF and SFBIA ratios at 1 and 5 kHz were calculated, and the arm dominance was investigated (Table 2). There were significant differences in ECF and SFBIA ratios between patients with breast cancer and healthy controls. The ECF ratios of the dominant affected arms were 1.020 ± 0.190 for the patient group and 1.002 ± 0.005 for the control group. The ECF ratios of the nondominant affected arms of these 2 groups were 1.029 ± 0.023 and 0.998 ± 0.005, respectively. The SFBIA ratios at 1 kHz of the dominant affected arms were 1.159 ± 0.198 for the patient group and 1.011 ± 0.029 for the control group, and the values were 1.171 ± 0.208 and 0.990 ± 0.028 for nondominant affected arms, respectively. The SFBIA ratios at 5 kHz of the dominant affected arms were 1.167 ± 0.176 and 1.013 ± 0.030 for the patient and control groups, respectively, and for the nondominant arms, the values were 1.182 ± 0.160 and 0.998 ± 0.029, respectively.
Table 2.
Impedance values of patients with lymphedema and healthy controls.
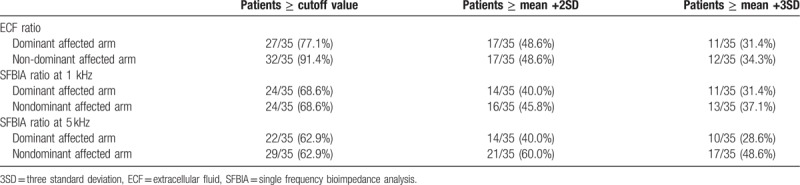
The mean + 2SD value for ECF ratio was 1.018 for the dominant affected arms and 1.020 for the nondominant affected arms. The cutoff value for SFBIA ratio at 1 kHz was 1.144 for dominant affected arms and 1.135 for nondominant affected arms. The cutoff value at 5 kHz was 1.141 for dominant affected arms and 1.124 for nondominant affected arms. The sensitivity and specificity are presented. The mean + 3SD value for ECF ratio was 1.026 for the dominant affected arms and 1.030 for the nondominant affected arms. The cutoff value for SFBIA ratio at 1 kHz was 1.206 for dominant affected arms and 1.203 for nondominant affected arms. The cutoff value at 5 kHz was 1.201 for dominant affected arms and 1.187 for nondominant affected arms. The sensitivity and specificity are presented (Table 3). The optimal cutoff value for the ECF ratio was 1.010 for both dominant and nondominant arms. The cutoff value for the SFBIA ratio at 1 kHz was 1.050 for dominant affected arms and 1.046 for nondominant affected arms. The cutoff value at 5 kHz was 1.070 for dominant affected arms and 1.030 for nondominant affected arms. The sensitivity and specificity (Table 4), the value of the area under the curb (Table 4), and the ROC curbs (Fig. 1) are also demonstrated. When the cutoff values, mean + 2SD, and mean + 3SD were applied to patients, the cutoff values rather than the mean + 2SD and mean + 3SD values included a higher number of patients with BCRL (Table 5).
Table 3.
Mean +2 and mean +3 standard deviation values for lymphedema diagnosis.
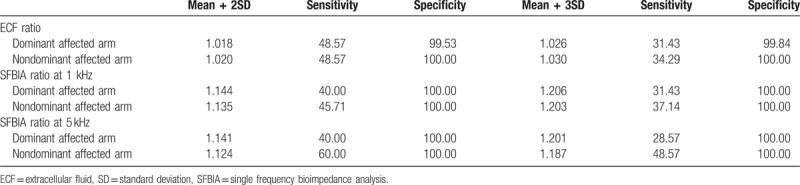
Table 4.
Cutoff values for lymphedema diagnosis.
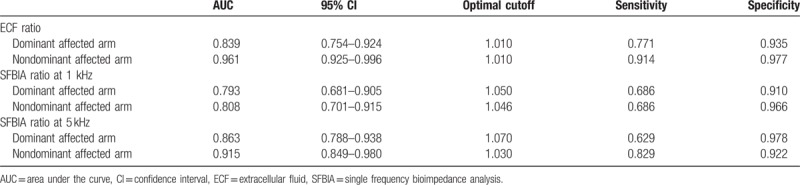
Figure 1.
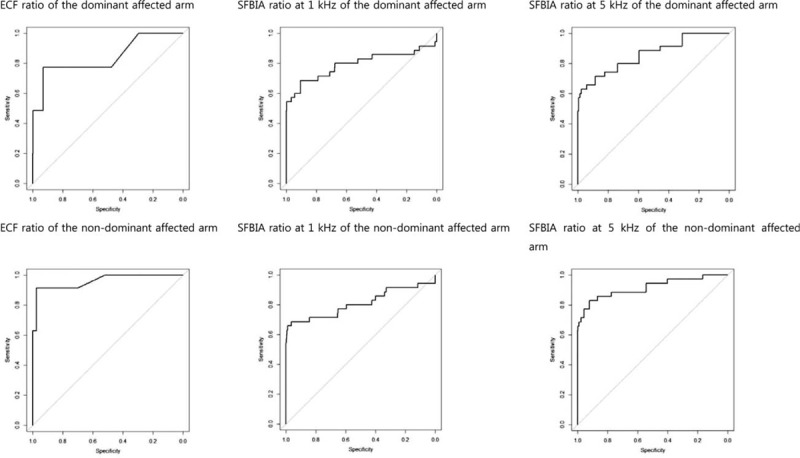
The receiver-operating characteristic curve with sensitivity and specificity for each cutoff value.
Table 5.
Prediction of patients with lymphedema, cutoff versus mean +2 and mean +3 standard deviation values.
4. Discussion
Many institutions and hospitals use the Inbody 720 to obtain values such as the ECF volume using BIS, SFBIA at 1- and 5-kHz frequencies, and BMI in patients with breast cancer with unilateral lymphedema. However, there are no standard normal or predictive values for detecting lymphedema. Our study is the first to suggest cutoff and mean + 2SD and mean + 3SD values. From our comparative analysis, ROC curve cutoff values were lower than mean + 2SD and mean + 3SD values. Our study shows that bioimpedance measurements significantly differed between patients with lymphedema and healthy controls. This supports the results of other studies that revealed that bioimpedance measurements can be used to assess and rule out the presence of lymphedema. Moreover, to the best of our knowledge, no previous study has suggested cutoff and mean + 2SD and mean + 3SD values using such a large number of normal, healthy people (n = 643).
Ward et al[25] determined the inter-arm impedance ratio thresholds according to the mean and SDs of 172 healthy patients and explained that these values are suitable for the early clinical detection of lymphedema. Furthermore, Ward[21] suggested that bioimpedance measurement is an accurate and sensitive early detection method for identifying people at a risk for developing lymphedema. In Ward's study, before clinical diagnoses of lymphedema, patients with abnormal impedance ratios were confirmed to have lymphedema. Cornish et al[3] showed that patients with BIS values >3 standard deviations from those of healthy controls were diagnosed with lymphedema after 10 months. In our study, the cutoff and mean + 2SD and mean + 3SD values of bioimpedance measurements were obtained and applied for patients with lymphedema.
The Imp XCA uses an impedance ratio value, relative to normative standards that are derived from healthy individuals,[14] to calculate a lymphedema index, termed the L-Dex ratio. This value ranges from −10 to +10, which is equivalent to impedance ratios from 0.935 to 1.139 for at-risk dominant arms and 0.862 to 1.066 for at-risk nondominant arms.[24] However, these figures are incompatible with those of the Inbody 3.0 system, and their availability is limited; hence, there has been a demand for reference values that can be used in other systems, which we present in the present study.
The limitations of this study are as follows. First, the number of patients who were enrolled in our study was small (n = 70), especially patients whose initial SFBIA and BIA values were applied to predict lymphedema occurrence. Second, we could only calculate the ratio of ECF to the total arm volume and could not measure the exact ECF volume directly. Additionally, it should be noted that bioimpedance measurements are not definite tools to assess lymphedema and cannot detect lymphedema accurately in all patients with lymphedema. In the early stage of lymphedema, BIA measurements may reflect some degree of edema, but it is difficult to predict this degree when edema becomes chronic. That explained sensitivity is not high enough. The BIA measurements are related to chronicity of the lymphedema. Third, there are also male patients with breast cancer, but the result of this study generalized for only female patients with breast cancer not for male patients.
Further studies with longer observation periods are recommended to accurately define the relationships between SFBIA and BIA values with lymphedema and investigate whether bioimpedance measurements can accurately predict the presence of lymphedema in the future.
5. Conclusions
Our study provides trustable references of 643 healthy subjects, cutoff values, mean + 2SD and mean + 3SD values of the ECF volume from SFBIA at 1- and 5-kHz frequencies for both dominant and nondominant arms. The results of our comparative analysis showed that ROC curve cutoff values were lower than mean + 2SD and mean + 3SD values. When these figures (cutoff, mean + 2SD and mean + 3SD values) were assigned to the patient group, the cutoff value included a higher proportion of patients with lymphedema. The cutoff values can be more useful for distinguishing between patients and healthy subjects than mean + 2SD and mean + 3SD values.
Author contributions
Conceptualization: Jae Yong Jeon, Gi Jeong Yun.
Data curation: Minji Jung, Seoyon Yang.
Formal analysis: Minji Jung, Seoyon Yang.
Methodology: Minji Jung.
Supervision: Jae Yong Jeon.
Writing – original draft: Minji Jung, Seoyon Yang.
Writing – review & editing: Minji Jung, Jae Yong Jeon, Sara Kwon, Yu Jin Seo.
Jae Yong Jeon orcid: 0000-0003-1534-7931.
Supplementary Material
Footnotes
Abbreviations: 3SD = three standard deviations, BCRL = breast cancer-related lymphedema, BIS = bioelectrical impedance spectroscopy, BMI = body mass index, ECF = extracellular fluid, SFBIA = single frequency bioimpedance analysis.
JYJ and GJY conceived the design of the study. SY collected the data and contributed to the design of the study. MJ wrote the manuscript. SK and YJS reviewed the manuscript.
The authors report no conflicts of interest.
Supplemental Digital Content is available for this article.
References
- [1].Warren AG, Brorson H, Borud LJ, et al. Lymphedema: a comprehensive review. Ann Plast Surg 2007;59:464–72. [DOI] [PubMed] [Google Scholar]
- [2].Horan D, McMullen M. Assessment and management of the woman with lymphedema after breast cancer. J Am Acad Nurse Pract 1998;10:155–9. [PubMed] [Google Scholar]
- [3].Cornish BH, Chapman M, Hirst C, et al. Early diagnosis of lymphedema using multiple frequency bioimpedance Lymphology 2001;34:2–11. [PubMed] [Google Scholar]
- [4].Leigh C, Ward SC, MHI, Sharon Sci, et al. Operational equivalence of bioimpedance indices and perometry for the assessment of unilateral arm lymphedema. Lymphat Res Biol 2009;7:82–5. [DOI] [PubMed] [Google Scholar]
- [5].DiSipio T, Rye S, Newman B, et al. Incidence of unilateral arm lymphoedema after breast cancer: a systematic review and meta-analysis. Lancet Oncol 2013;14:500–15. [DOI] [PubMed] [Google Scholar]
- [6].Petrek JA, Senie RT, Peters M, et al. Lymphedema in a cohort of breast carcinoma survivors 20 years after diagnosis. Cancer 2001;92:1368–77. [DOI] [PubMed] [Google Scholar]
- [7].Mayrovitz MN. Assessing lymphedema by tissue indentation force and local tissue water. Lymphology 2009;42:88–98. [PubMed] [Google Scholar]
- [8].Ward LC, Czerniec S, Kilbreath SL. Quantitative bioimpedance spectroscopy for the assessment of lymphoedema. Breast Cancer Res Treat 2009;117:541–7. [DOI] [PubMed] [Google Scholar]
- [9].Smoot BJ, Wong JF, Dodd MJ. Comparison of diagnostic accuracy of clinical measures of breast cancer related lymphedema: area under the curve. Arch Phys Med Rehabil 2011;92:603–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Wang H, Li D, Liuya J, et al. Reference ranges using bioimpedance for detection of lymphedema in chinese women. Lymphat Res Biol 2017;15:268–73. [DOI] [PubMed] [Google Scholar]
- [11].Gupta D, Lammersfeld CA, Vashi PG, et al. Bioelectrical impedance phase angle as a prognostic indicator in breast cancer. BMC Cancer 2008;8:249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Tuorkey MJ. Bioelectrical impedance as a diagnostic factor in the clinical practice and prognostic factor for survival in cancer patients: prediction, accuracy and reliability. J Biosens Bioelectron 2012;3:4. [Google Scholar]
- [13].Qiao G, Wang W, Duan W, et al. Bioimpedance analysis for the characterization of breast cancer cells in suspension. IEEE Trans Biomed Eng 2012;59:2321–9. [DOI] [PubMed] [Google Scholar]
- [14].Czerniec SA, Ward LC, Refshauge KM, et al. Assessment of breast cancer related arm lymphedema - comparison of physical measurement methods and self report. Cancer Invest 2010;28:54–62. [PubMed: 19916749]. [DOI] [PubMed] [Google Scholar]
- [15].Cha K, Chertow G, Gonzalez J, et al. Multifrequency bioelectrical impedance estimates the distribution of body water. J Appl Physiol 1995;79:1316–9. [DOI] [PubMed] [Google Scholar]
- [16].Moseley A, Piller N, Carati C. Combined opto-electronic perometry and bioimpedance to measure objectively the effectiveness of a new treatment intervention for chronic secondary leg lymphedema. Lymphology 2002;35:136–43. [PubMed] [Google Scholar]
- [17].Kim L, Jeon JY, Sung IY, et al. Prediction of treatment outcome with bioimpedance measurements in breast cancer related lymphedema patients. Ann Rehabil Med 2011;35:687–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Dylke ES, Schembri GP, Bailey DL, et al. Diagnosis of upper limb lymphoedema: development of an evidence-based approach. Acta Oncol 2016;55:1477–83. [DOI] [PubMed] [Google Scholar]
- [19].Ward LC, Bunce IH, Cornish BH, et al. Multi-frequency bioelectrical impedance augments the diagnosis and management of lymphoedema in post-mastectomy patients. Eur J Clin Ivest 1992;751–4. [DOI] [PubMed] [Google Scholar]
- [20].Warren AG, Janz BA, Slavin SA, et al. The use of bioimpedance analysis to evaluate lymphedema. Ann Plast Surg 2007;58:541–3. [DOI] [PubMed] [Google Scholar]
- [21].Ward LC. Bioelectrical impedance analysis: proven utility in lymphedema risk assessment and therapeutic monitoring. Lymphat Res Biol 2006;4:51–6. [DOI] [PubMed] [Google Scholar]
- [22].Cornish B. Bioimpedance analysis: scientific background. Lymphat Res Biol 2006;4:47–50. [DOI] [PubMed] [Google Scholar]
- [23].York SL, Ward LC, Czerniec S, et al. Single frequency versus bioimpedance spectroscopy for the assessment of lymphedema. Breast Cancer Res Treat 2009;117:177–82. [DOI] [PubMed] [Google Scholar]
- [24].Fu MR, Guth AA, Cleland CM, et al. The Effects of symptomatic seroma on lymphedema symptoms following breast cancer treatment. Lymphology 2011;44:134–43. [PubMed] [Google Scholar]
- [25].Ward LC, Dylke E, Czerniec S, et al. Confirmation of the reference impedance ratios used for assessment of breast cancer-related lymphedema by bioelectrical impedance spectroscopy. Lymphat Res Biol 2011;9:47–51. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


