Abstract
Rationale:
Programmed cell death-1 (PD-1) or programmed death-ligand 1 (PD-L1) immune checkpoint inhibitors have demonstrated impressive efficacy in patients with nonsmall cell lung cancer (NSCLC). Radiofrequency ablation (RFA) is an alternative locoregional therapy for patients with inoperable NSCLC. We report the role of RFA in a patient with metastasis from advanced stage NSCLC that was managed with checkpoint inhibitors. Therefore, this combination of RFA with subsequent immunotherapy can control NSCLC better than RFA or immunotherapy on their own.
Patient concerns:
We report here a 61-year-old Chinese male who presented with postoperative recurrence squamous cell lung cancer following the left upper lobectomy and 4 cycles of postoperative adjuvant chemotherapy 6 months back.
Diagnosis:
A newly occurring lesion was detected in the left lower lung. Based on computed tomography (CT) and percutaneous lung biopsy enhancement, the patient was diagnosed with stage IV nonsmall cell lung cancer.
Interventions:
The patient refused systemic chemotherapy. And there was no basis for using tyrosine kinase inhibitors. RFA was performed for 3 times at the left lower lung lesion, which was under control. Afterward, an enlargement of the lesion at left lower lung with involvement to chest wall, and new nodules in both lungs were revealed. After that, the patient received intravenous PD-L1 immune checkpoint inhibitors Atezolizumab. Follow-up restaging CT scan showed disease progression in both lungs. However, by treated 4 months later, partial response was observed at the left lower lung lesion, and stable response was observed at the right upper lung lesion.
Outcomes:
The patient displayed a remarkable response to Atezolizumab in one lesion at left lower lung, where he received previous locoregional therapy of RFA. As a comparison, another lesion at right upper lung without RFA history showed little response to Atezolizumab.
Lessons:
Our case suggests a significantly synergistic effect of sequential association of RFA and subsequent immunotherapy. Integrating locoregional therapy such as RFA into anti-PD-1/PD-L1 agent regimens may help to release tumor-associated antigen and mediate T-cell immune enhancement, and on the long run improve the ongoing efficacy of checkpoint inhibitors. The combination of locoregional therapy and immunotherapy represents a potential new treatment option in the management of metastatic NSCLC.
Keywords: immunotherapy, locoregional therapy, non-small cell lung cancer, programmed cell death-1, programmed death-ligand 1, radiofrequency ablation
1. Introduction
Lung cancer remains one of the most malignant tumors, which has poor prognosis, and is one of the leading causes of cancer death in the world.[1] Nonsmall cell lung cancer (NSCLC) accounts for 85% to 90% of lung cancer cases. Advances in gene targeted therapy have obviously improved the prognosis of patients with NSCLC.[2,3] Recently, immune-targeted therapy antiprogrammed cell death-1 (PD-1)/programmed death-ligand 1 (PD-L1) drugs have revolutionized the therapeutic strategies for NSCLC.[4,5] The OAK study showed Atezolizumab, a PD-L1-targeted therapy, resulting in a clinically relevant improvement of overall survival (OS) versus docetaxel in previously treated NSCLC.[6] The IMPOWER150 study showed that the addition of Atezolizumab to bevacizumab plus chemotherapy significantly improved progression-free survival (PFS) and OS among metastatic nonsquamous NSCLC patients, regardless of PD-L1 expression status.[7] The IMPOWER131 study demonstrated that the combination of atezolizumab plus chemotherapy (carboplatin and nanopartical albumin-bound-paclitaxel) improved PFS, higher objective response rate, and longer duration of response compared with chemotherapy alone in the first-line treatment of patients with advanced squamous NSCLC, regardless of PD-L1 expression level.[8] Evidence shows Atezolizumab as first-line therapy in NSCLC is promising safety and efficacy.[9] Immunotherapy targeting programmed cell PD-1 or PD-L1 has become a new option in first-line treatment for NSCLC patients regardless of PD-L1 expression level, and a second-line therapy for all patients owing to its superiority to chemotherapy.[10]
An additional area of clinical interest is the use of the anti-PD-1/PD-L1 therapies in combination with other currently available treatments, such as chemotherapy or radiotherapy. A recent report of a randomized phase III trial, the PACIFIC trial, demonstrated an impressive impovement in median PFS with consolidative durvalumab, another PD-L1 inhibitor, compared with observation after concurrent chemoradiation.[11] The PACIFIC trial indicates association of locoregional therapy and a checkpoint blockade immunotherapy could be a promising treatment regimen for NSCLC.
Radiofrequency ablation (RFA) is an effective percutaneous ablation technique, it was widely applied for treating hepatocellular carcinoma, and significantly improved the prognosis of patients.[12] By using a closed-loop circuit which creates an alternation of electric field agitating ions, reaching a temperature of 50°C to 100 °C during a short time, RFA can lead to tumor cells degeneration and necrosis and kill tumor tissues.[13] In malignant melanoma, RFA has shown a synergistic antitumor activity with immunotherapy.[14] RFA was found to be safe and efficient for the treatment of selected NSCLC.[15,16] However, little is known about the role of RFA played in the combinative administration with immunotherapy against NSCLC. Herein, we report a remarkable response to Atezolizumab after RFA treatment in a 61-year-old patient with metastatic lung squamous cell carcinoma, whose lesion at left lower lung treated by RFA and subsequent Atezolizumab shows a much more significant improvement than the one at right upper lung, which received treatment of Atezolizumab alone.
2. Case report
The patient was a 61-year-old male without smoking history. In August 2014, without any symptom, a chest computed tomography (CT) showed a mass in the left upper lobe of the lung (maximum diameter approximately 6.0 cm). In 21 August 2014, the patient underwent left upper lobectomy and systemic hilar mediastinal lymph nodules section. Pathologic examination confirmed a moderately differentiated squamous cell carcinoma of central left lung (Fig. 1). The margin was negative and no metastasis was found in the lymph nodes. Pathologic stage was pT2bN0M0, stage IIA. The patient then received 4 cycles of postoperative adjuvant chemotherapy with cisplatin 75 mg/m2 on day 1 plus gemcitabine 1250 mg/m2 on days 1 and 8, every 3 weeks.
Figure 1.
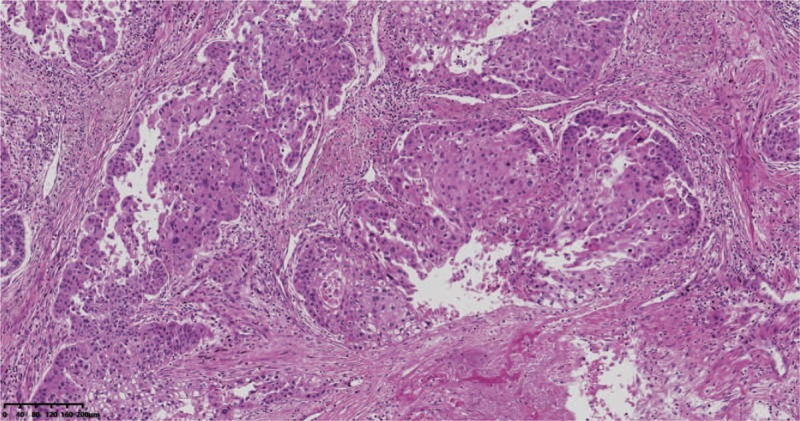
Pathologic examination showed a moderately differentiated squamous cell carcinoma.
Six months later on September 18, 2015, chest computed tomography detected a newly occurring lesion with diameter of 1.5 cm in the left lower lung. CT-guided percutaneous lung biopsy was performed and metastasis of squamous lung cancer was confirmed. Other distant metastasis in liver, bone or brain was not found. The specimen was submitted for detection of EGFR mutation, ROS1 fusion gene rearrangement and ALK fusion gene rearrangement, yet neither mutation nor gene rearrangement was detected. The patient refused to receive systemic chemotherapy and RFA was intermittently performed for 3 times from October 25, 2015 to September 2, 2016 in his local hospital. The lesion at left lower lung decreased in diameter (0.9 cm) and was temporarily in control (Fig. 2). On March 30, 2017, routine follow-up of chest computed tomography revealed an enlargement of the lesion at left lower lung with involvement to chest wall, and new nodules in both lungs (Fig. 3A). The patient received treatment of intravenous Atezolizumab (1200 mg) every 3 weeks from May 12, 2017. No severe adverse reaction was found during treatment. Radiological evaluation on August 3, 2017 showed disease progression in both lungs (Fig. 3B), however, Atezolizumab was continuously administrated due to his stable physical status. An encouraging partial response was observed at the left lower lung lesion (Fig. 3C), and stable response was observed at the right upper lung lesion on September 14, 2017, when then another radiological evaluation was performed. The patient went on treatment of Atezolizumab, and his improvements on clinical symptoms, health-related quality of life and radiological examination continued until now. A recent evaluation of chest computed tomography on May 24 2018 illustrated a durable clinical benefit (partial or stable response lasting > 6 months), that the lesion in the left lower lung continued to respond to Atezolizumab significantly, while the lesion in the right upper lung remained stable (Fig. 3D).
Figure 2.
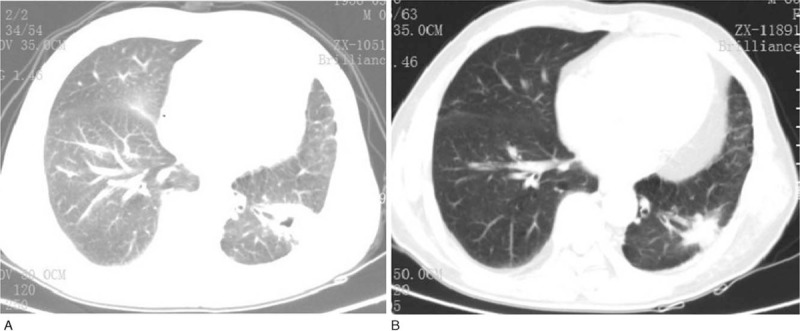
Chest computed tomography scans of the patient during treatment of radiofrequency ablation. After the first time treatment of RFA, a 4.0×3.5 cm thick-walled cavities tumor is seen in the left lower lobe of the lung. Before the third time treatment of RFA, the left lung tumor decreased to 2.0×0.9 cm. RFA = radiofrequency ablation.
Figure 3.
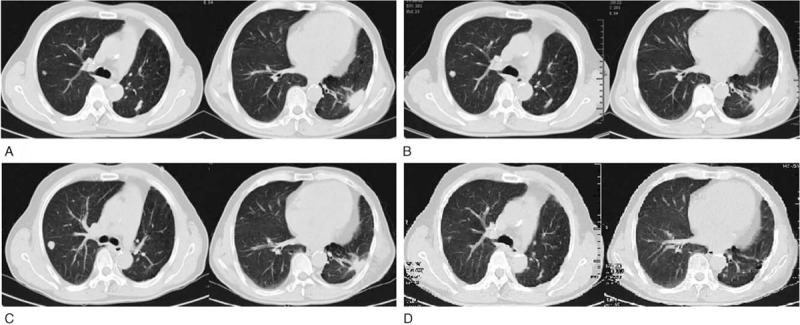
Chest computed tomography scans of the patient during treatment of Atezolizumab. (A) Baseline before treatment of Atezolizumab: a 4.0×1.8 cm tumor is seen in the left lobe of the lung, and multiple nodules in both lungs. (B) After 4 cycles of Atezolizumab therapy, both lung tumors were larger than before, the left lung tumor increased to 4.3×2.0 cm. (C) After 6 cycles of Atezolizumab therapy, the left lung tumor decreased to 3.0×2.0 cm. (D) After 19 cycles of Atezolizumab therapy, the left lung tumor almost disappeared, changing more obvious than the right side.
3. Discussion
We describe a case of a 61-year-old male who presented with metastatic squamous cell lung cancer, without targeted gene mutation, whose left lower lung lesion where he received previous locoregional therapy of RFA displayed a remarkable response to Atezolizumab. As a comparison, the lesion at right upper lung without RFA history showed little response to Atezolizumab.
Among reported cases and researches, no definitive direct evidence is available supporting the efficacy of the association of RFA and PD-1/PD-L1 blockade in patients with NSCLC. There are some relevant reports in other types of cancer. In hepatocellular carcinoma, combined therapy of RFA and anti-PD-1 antibodies has been proved to significantly promote stronger antitumor immunity and prolonged survival.[17] In intestinal liver metastases, RFA treatment of liver metastases increased not only T-cell infiltration, but also PD-L1 expression in primary human colorectal tumors.[18] In advanced stage melanoma, RFA and PD-1 have a synergistic antitumor effect on melanoma spleen oligopotency.[14] Regarding the remarkable response in other solid tumors to the combination of checkpoint inhibitors with RFA, we presume that synergetic correlation between RFA and immune checkpoint inhibitors could also be observed in NSCLC. And this conjecture is confirmed in the present case due to the different response to Atezolizumab between 2 lung lesions that one has undergone RFA and another has not. Furthermore, these clues validate the combination of RFA and PD-1/PD-L1 blockade superior to monotherapy.
To date, the mechanism of synergy between RFA and PD-L1 blockade is complicated and remains unclear. Typically, RFA waves are converted into heat to achieve local temperatures sufficient to induce tissue destruction, which can induce necrotic tumor debris serving as an in situ antigen source to stimulate an systemic autologous antitumor immune response,[19–21] and can control distant metastasis,[22] just as radiation-derived antitumor response, not only for the local treatment area, but also for tumors from distance, the distant antitumor activity induced by local treatments is called abscopal effect.[23,24] RFA may function as an in situ vaccine and enhance immune control of distant disease. Besides, many experts believe T lymphocyte-mediated antitumor immunity is an important theory. RFA affects the proliferation of T cells. After RFA in lung cancer patients, the level of tumor-specific T cells, helper T (Th) 1 cells, and Th1/Th2 increased, whereas Th2, Th17, and regulatory T cells decreased.[25,26] RFA also induce the migration of cytotoxic T lymphocytes to metastatic nodules.[17] These findings indicate that RFA mediate an improvement of antitumor immunity. In addition, RFA may promote long-term immunity against NSCLC by leading to an activated and highly T-cell-stimulatory phenotype of dendritic cells[20] and enhancing dendritic cells maturation.[26] Actually, PD-L1 expression increase after RFA[18] and anti-PD-1 therapy boosts neoantigen-specific T cell reactivity.[27] RFA induces changes in the tumor environment and surviving cancer cells that can result in immune mediated clearance of residual local disease. It proves that RFA and immunotherapy could be an appealing combination approach for cancer therapy. The remarkable response to association of RFA with subsequent Atezolizumab in the present case is in favor of this hypothesis. The PACIFIC Clinical Trial[11] that PD-L1 inhibitor combined with local treatment significantly prolongs PFS in NSCLC patients. And the latest research shows that pembrolizumab after stereotactic body radiotherapy improved the overall response rate compared to pembrolizumab alone in patients with advanced NSCLC,[28] which also verifies this conjecture. Generally, systemic therapy is emphasized for patients with advanced NSCLC.[10] However, if there are some oligo-metastases or metastases with slow progression or few numbers, local treatments such as surgery or radiotherapy may be more suitable. Individualized precision RFA and other local treatment should be considered to combine with immunotherapy, which may obtain better clinical benefits.
To the best of our knowledge, this is the first case report where RFA-based Atezolizumab immunotherapy has been used to control the stage IV NSCLC. There is a need for more clinical observations and further exploration to better define the efficacy and mechanism of synergistic effects of AFR and PD-1/PD-L1 inhibitors as well as the therapeutic model, the timing of treatment and the biomarker for predicting efficacy. It is hoped our case report will help to provide different ideas for the clinician in determining the optimal approach to the medical management of advanced/metastatic squamous NSCLC.
4. Conclusion
NSCLC is a malignant disease with high incidence. The locoregional therapy-based (radiofrequency ablation or radiotherapy) anti-PD-1/PD-L1immunotherapy for NSCLC is a feasible approach as it not only releases tumor-associated antigen and mediates T-cell immune enhancement, but improves the ongoing efficacy of checkpoint inhibitors by increasing PD-L1 expression. Combined therapy of RFA and anti-PD-1/PD-L1 immunotherapy can produces potentially additional therapeutic benefit.
4.1. Consent
Written informed consent to publication was obtained from the patient.
Acknowledgment
The authors would like to thank the patient for allowing them to publish the case report and to use the images taken during his hospital admission.
Author contributions
Conceptualization: Yina Wang.
Funding acquisition: Yina Wang, Jingyin Dong.
Investigation: Jingyin Dong.
Resources: Jie Yin.
Supervision: Wei Gao.
Writing – original draft: Jie Yin.
Writing – review & editing: Yina Wang.
Footnotes
Abbreviations: CT = computed tomography, NSCLC = non-small-cell lung cancer, OS = overall survival, PD-1 = programmed cell death-1, PD-L1 = programmed death-ligand 1, PFS = progression-free survival, RFA = radiofrequency ablation, Th = helper T cell.
Consent: Written informed consent to publication was obtained from the patient.
This study was supported by grants from Zhejiang Provincial Natural Science Foundation of China (LY16H160006) and Hangzhou Science and Technology Bureau Grant (20160533 B72).
The authors have no conflicts of interest to disclose.
References
- [1].Torre LA, Bray F, Siegel RL, et al. Global cancer statistics, 2012. CA Cancer J Clin 2015;65:87–108. [DOI] [PubMed] [Google Scholar]
- [2].Maemondo M, Inoue A, Kobayashi K, et al. Gefitinib or chemotherapy for non-small-cell lung cancer with mutated EGFR. N Engl J Med 2010;362:2380–8. [DOI] [PubMed] [Google Scholar]
- [3].Popat S. Osimertinib as first-line treatment in EGFR-mutated non-small-cell lung cancer. N Engl J Med 2018;378:192–3. [DOI] [PubMed] [Google Scholar]
- [4].Bianco A, Malapelle U, Rocco D, et al. Targeting immune checkpoints in non small cell lung cancer. Curr Opin Pharmacol 2018;40:46–50. [DOI] [PubMed] [Google Scholar]
- [5].Jain P, Jain C, Velcheti V. Role of immune-checkpoint inhibitors in lung cancer. Ther Adv Respir Dis 2018;12:1753465817750075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Rittmeyer A, Barlesi F, Waterkamp D, et al. Atezolizumab versus docetaxel in patients with previously treated non-small-cell lung cancer (OAK): a phase 3, open-label, multicentre randomised controlled trial. Lancet 2017;389:255–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Socinski MA, Jotte RM, Cappuzzo F, et al. Atezolizumab for first-line treatment of metastatic nonsquamous NSCLC. N Engl J Med 2018;378:2288–301. [DOI] [PubMed] [Google Scholar]
- [8].Squamous NSCLC improves with Atezolizumab plus chemo. Cancer Discov 2018;8:OF10. [DOI] [PubMed] [Google Scholar]
- [9].Ryu R, Ward KE. Atezolizumab for the first-line treatment of non-small cell lung cancer (NSCLC): current status and future prospects. Front Oncol 2018;8:277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Ettinger DS, Wood DE, Aisner DL, et al. Non-small cell lung cancer, version 5.2017, NCCN Clinical Practice Guidelines in Oncology. J Natl Compr Canc Netw 2017;15:504–35. [DOI] [PubMed] [Google Scholar]
- [11].Antonia SJ, Villegas A, Daniel D, et al. Durvalumab after chemoradiotherapy in stage III non-small-cell lung cancer. N Engl J Med 2017;377:1919–29. [DOI] [PubMed] [Google Scholar]
- [12].Crocetti L, Bargellini I, Cioni R. Loco-regional treatment of HCC: current status. Clin Radiol 2017;72:626–35. [DOI] [PubMed] [Google Scholar]
- [13].Facciorusso A, Serviddio G, Muscatiello N. Local ablative treatments for hepatocellular carcinoma: an updated review. World J Gastrointest Pharmacol Ther 2016;7:477–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Mudan S, Kumar J, Mafalda NC, et al. Case report on the role of radiofrequency-assisted spleen-preserving surgery for splenic metastasis in the era of check-point inhibitors. Medicine (Baltimore) 2017;96:e9106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Hiraki T, Gobara H, Mimura H, et al. Radiofrequency ablation of lung cancer at Okayama University Hospital: a review of 10 years of experience. Acta Med Okayama 2011;65:287–97. [DOI] [PubMed] [Google Scholar]
- [16].Li G, Xue M, Chen W, et al. Efficacy and safety of radiofrequency ablation for lung cancers: a systematic review and meta-analysis. Eur J Radiol 2018;100:92–8. [DOI] [PubMed] [Google Scholar]
- [17].Kudo M. Immuno-Oncology in Hepatocellular Carcinoma: 2017 Update. Oncology 2017;93 suppl 1:147–59. [DOI] [PubMed] [Google Scholar]
- [18].Shi L, Chen L, Wu C, et al. PD-1 blockade boosts radiofrequency ablation-elicited adaptive immune responses against tumor. Clin Cancer Res 2016;22:1173–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Fagnoni FF, Zerbini A, Pelosi G, et al. Combination of radiofrequency ablation and immunotherapy. Front Biosci 2008;13:369–81. [DOI] [PubMed] [Google Scholar]
- [20].Schneider T, Hoffmann H, Dienemann H, et al. Immune response after radiofrequency ablation and surgical resection in nonsmall cell lung cancer. Semin Thorac Cardiovasc Surg 2016;28:585–92. [DOI] [PubMed] [Google Scholar]
- [21].Mizukoshi E, Yamashita T, Arai K, et al. Enhancement of tumor-associated antigen-specific T cell responses by radiofrequency ablation of hepatocellular carcinoma. Hepatology 2013;57:1448–57. [DOI] [PubMed] [Google Scholar]
- [22].Deng Z, Zhang W, Han Y, et al. Radiofrequency ablation inhibits lung metastasis of breast cancer in mice. Zhonghua Zhong Liu Za Zhi 2015;37:497–500. [PubMed] [Google Scholar]
- [23].Siva S, Callahan J, MacManus MP, et al. Abscopal [corrected] effects after conventional and stereotactic lung irradiation of non-small-cell lung cancer. J Thorac Oncol 2013;8:e71–2. [DOI] [PubMed] [Google Scholar]
- [24].Cong Y, Shen G, Wu S, et al. Abscopal regression following SABR for non-small-cell-lung cancer: a case report. Cancer Biol Ther 2017;18:1–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Shaobin W, Yu X, Jiatian L, et al. Changes of CD4(+) T-cell subsets after radiofrequency ablation in lung cancer and its significance. J Cancer Res Ther 2016;12 supplement:C166–70. [DOI] [PubMed] [Google Scholar]
- [26].Higgins JP, Bernstein MB, Hodge JW. Enhancing immune responses to tumor-associated antigens. Cancer Biol Ther 2009;8:1440–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Rizvi NA, Hellmann MD, Snyder A, et al. Cancer immunology. Mutational landscape determines sensitivity to PD-1 blockade in non-small cell lung cancer. Science 2015;348:124–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Willemijn T, Ferry L, Joop DL, et al. Randomized phase II study of pembrolizumab after stereotactic body radiotherapy (SBRT) versus pembrolizumab alone in patients with advanced non-small cell lung cancer: The PEMBRO-RT study. J Clin Oncol 2018;36(15_suppl):9023. [Google Scholar]


