Supplemental Digital Content is available in the text
Keywords: benign paroxysmal positional vertigo, light cupula, nystagmus, sudden sensorineural hearing loss, vertigo
Abstract
The patients with sudden sensorineural hearing loss (SSNHL) may complain of vertigo. Although there have been many reports on SSNHL with vertigo (SSNHL_V), changes in the pattern of nystagmus have not been studied as yet. This study is a retrospective study and aims to investigate the characteristic changes in type of nystagmus and clinical features in patients with SSNHL_V who experienced a change in their nystagmus pattern during follow-up. Among 50 patients with SSNHL_V between January 2012 and December 2015, we identified 15 patients with SSNHL_V whose pattern of nystagmus changed. Initial nystagmus was classified into 5 subgroups: paretic type, irritative type, persistent geotropic direction-changing positional nystagmus (PG-DCPN), persistent apogeotropic direction-changing positional nystagmus (PA-DCPN), and posterior semicircular canal benign paroxysmal positional vertigo. The most common pattern of initial nystagmus was PG-DCPN (n = 7). The change of initial nystagmus pattern occurred on day 2 to 75 from symptom onset, and 2 (of 15) patients showed further conversion. The most common pattern of final nystagmus was PA-DCPN (n = 9). Hearing improvement after treatment was not significantly different (P = .59) between SSNHL_V patients with nystagmus change (25 ± 17 dB, n = 15) and those without nystagmus change (28 ± 18 dB, n = 35). In conclusion, clinician's attention is required in evaluating the vertigo symptom in patients with SSNHL_V because the initial patterns of nystagmus can be converted to another type of nystagmus. The presence of nystagmus change during follow-up may not be a prognosticator for hearing recovery in patients with SSNHL_V.
1. Introductions
Sudden sensorineural hearing loss (SSNHL) is defined as a subjective sensation of hearing impairment with a rapid onset, usually occurring within a 72-hour period. The most frequently used audiometric criterion is a deterioration of hearing threshold of ≥30 dB in at least 3 consecutive frequencies. Although vascular, viral, or multiple etiologies have been proposed as causes of SSNHL, up to 90% of SSNHL is considered idiopathic.[1] The annual incidence of SSNHL varies from 5 to 20 per 100,000 population, with approximately 4000 new cases per year in the United States.[1]
Vertigo may develop in approximately 30% to 60% of patients with SSNHL,[2–4] and approximately 40% of those suffering from SSNHL with vertigo (SSNHL_V) have ipsilesional canal paresis in a bithermal caloric test.[5] The cochlear and vestibular organs are interconnected by inner ear fluids and surrounding membranes, so disturbances of cochlear and vestibular function may develop concurrently. Because vertigo occurs more frequently in patients with SSNHL with profound hearing loss,[5] it has been suggested that the associated vertigo may indicate a poor prognostic factor for hearing recovery due to widespread and more severe inner ear damage. However, the significance of vertigo as a prognostic factor in terms of hearing recovery remains controversial.[6–8] One of the reasons for this controversy is that vertigo is not a specific disease entity but a symptom, leading to heterogeneous groups of patients being included in the relevant studies. All of SSNHL patients with vertigo exhibited nystagmus with varying types (our unpublished data). Nystagmus is repetitive, to-and-fro movement of the eyes that is initiated by slow phases, and may provide importation information in the diagnosis of vestibular disorders. The nystagmus which has been observed in SSNHL_V can be either spontaneous nystagmus without positional component[9,10] or positional nystagmus similar to benign paroxysmal positional vertigo (BPPV).[11–13]
Although there have been many reports on SSNHL_V, changes in the pattern of nystagmus have not been studied as yet. This study is aimed to obtain an idea whether a nystagmus changes its pattern in SSNHL and attempts to explain as to why that should happen. From the clinical stand point, this study observes that in SSNHL a nystagmus pattern may change yielding important information that in SSNHL, the vestibular system may be affected as well, and shows a dynamic function.
2. Subjects and methods
2.1. Subjects
Fifty patients who were diagnosed as SSNHL_V between January 2012 and December 2015 were retrospectively reviewed. All patients met the clinical diagnostic criteria for SSNHL, defined as sensorineural hearing loss of ≥30 dB over at least 3 contiguous frequencies in pure tone audiometry (PTA) developing within a period of 3 days.[14] All of 50 patients with SSNHL_V exhibited nystagmus, and the pattern of nystagmus did not change during the disease course in 35 patients. We identified 15 patients with SSNHL_V (8 women and 7 men; age range, 25–65 years) whose pattern of nystagmus changed during follow-up of at least 3 months. The right ear was involved in 8 patients, and the left in 7 patients. The medical history of each patient was reviewed, and an otological examination was performed. Those who experienced multiple attacks of vertigo or recurrent hearing loss in the past were excluded from the study. The study is a retrospective study, and was conducted in a tertiary referral university hospital. The Institutional Review Board approved the study (KUH1110057).
2.2. Audiometric evaluations
The initial PTA average was calculated as the mean threshold at 500, 1000, 2000, and 3000 Hz.[12,15] Follow-up PTAs were checked once daily during the first 4 days and the 14th day, and those at 3 months after treatment were used for the final evaluation of hearing improvement. Audiograms were categorized as high-tone or low-tone, flat-type, or profound hearing loss.[10,12] Hearing loss was classified as high tone if a patient's audiogram showed an average loss of 4 to 8 kHz from the average of 0.25 to 0.5 kHz by ≥30 dB and low tone if the average loss was 0.25 to 0.5 kHz, surpassing the average of 4 to 8 kHz by ≥30 dB. The flat-type group consisted of patients with a ≤20 dB difference between the worst and best hearing levels at 6 frequencies (0.25, 0.5, 1, 2, 4, and 8 kHz). In the group with profound hearing loss, at least 2 frequencies produced results that were off the scale, and the difference between the hearing level and the maximum sound level generated by the audiometer was within 10 dB at all 6 frequencies. Determination of the hearing level of the affected ear was based on the opposite healthy ear. For calculation of the PTA average, the PTA threshold at the frequency where the result was off the scale was regarded as 120 dB.
2.3. Examination of nystagmus and vestibular function tests
The nystagmus findings were serially evaluated in each patient as previously described.[12,16] The patients’ eye movements were examined in various head positions using goggles and recorded with an infrared camera. In all 15 patients, spontaneous nystagmus was checked for with the patient in an upright-seated position, and positional nystagmus was examined during a supine-roll test (SRT) and Dix-Hallpike test (DHT). Nystagmus was examined for 2 minutes if nystagmus persists in each position, and head movement was paused for 1 minute in the supine position without any neck rotation between positioning maneuvers during an SRT. We have classified the patterns of nystagmus based on positional and nonpositional elicitation of nystagmus. The initial nystagmus examination was performed at the first visit; the mean time interval between this examination and onset of vertigo was 1.3 ± 1.5 (range 0–4) days (Table 1). Nystagmus was checked daily or on alternate days during the acute stage (7–14 days after the first examination) and every 3 to 14 days thereafter during the chronic stage in all patients.
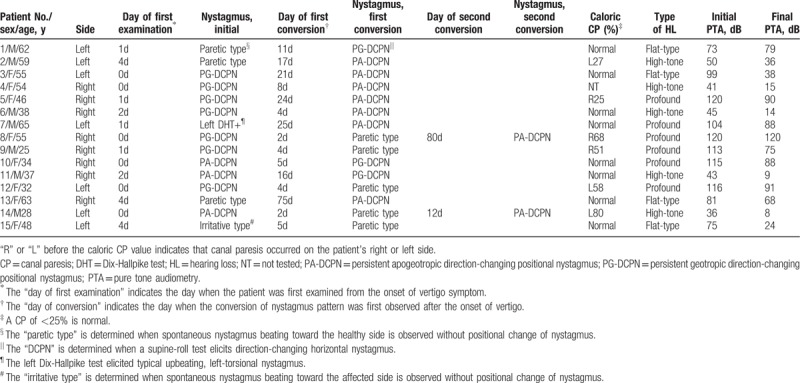
Table 1.
Summary of clinical characteristics and nystagmus findings in patients who had sudden sensorineural hearing loss with vertigo and showed a change in pattern of nystagmus during 3 months of follow-up (n = 15).
A bithermal caloric test was performed while recording eye movements using an infrared video-based system. Each ear was irrigated with a constant flow of water at alternating temperatures of 30°C and 44°C for a constant period of 30 seconds. The maximum slow-phase velocity of nystagmus was calculated after each irrigation, and the Jongkees formula was used to determine canal paresis. Canal paresis ≥25% was considered to be abnormal.
3. Results
The patterns of nystagmus were classified into 2 groups according to the presence of positional nystagmus. Nystagmus without positional component was defined when nystagmus direction was not changed by positioning maneuvers, and classified into 2 subgroups: paretic type in which the direction of spontaneous nystagmus is toward the healthy side (Supplemental video 1) and irritative type in which spontaneous nystagmus beats toward the affected side (Supplemental video 2). Nystagmus with positional component was defined when the direction of nystagmus was changed by positioning maneuvers, and classified into 3 subgroups: persistent geotropic direction-changing positional nystagmus (PG-DCPN) in which positional nystagmus beats toward the lowermost ear (geotropic) for a prolonged duration in an SRT (Supplemental video 3), persistent apogeotropic direction-changing positional nystagmus (PA-DCPN) in which positional nystagmus beats toward the uppermost ear (apogeotropic) for a prolonged duration in an SRT (Supplemental video 4), and torsional, upbeating nystagmus is elicited by a DHT on the same side as the hearing loss (Supplemental video 5).
Paretic type, in which spontaneous nystagmus beats toward the unaffected side without positional change of the nystagmus, was observed initially in 3 patients (Fig. 1, Table 1). This converted to PG-DCPN in 1 patient on day 11 after onset of vertigo and to PA-DCPN in the other 2 patients on days 17 and 75 after onset of vertigo.
Figure 1.
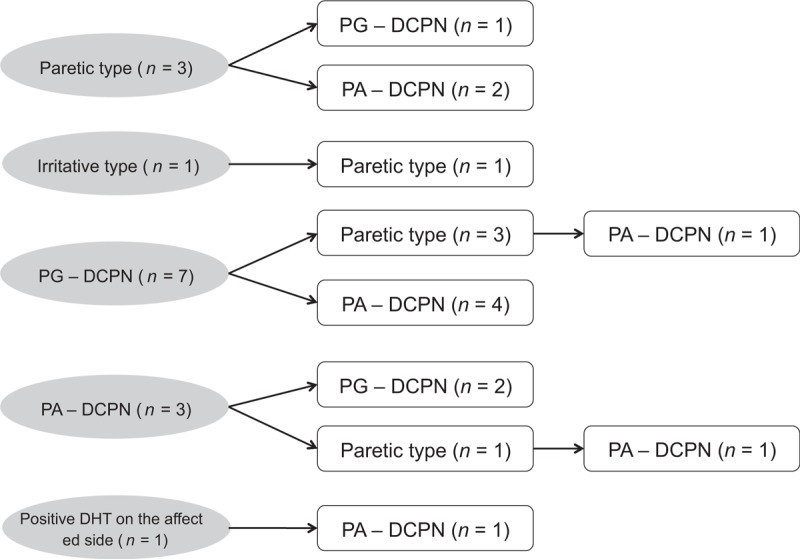
A diagram demonstrating the change of nystagmus pattern in patients with sudden sensorineural hearing loss with vertigo who showed the conversion of nystagmus findings during follow-up period (n = 15). DHT = Dix-Hallpike test; PA-DCPN = persistent apogeotropic direction–changing positional nystagmus; PG-DCPN = persistent geotropic direction–changing positional nystagmus.
Irritative type, in which spontaneous nystagmus beats toward the affected side without positional change of the nystagmus, was initially observed in 1 patient (Fig. 1, Table 1). This changed to the paretic type on day 5 after onset of vertigo.
PG-DCPN in an SRT was the most commonly observed initial nystagmus pattern (n = 7); in these cases, the pattern changed to paretic type and PA-DCPN in 3 and 4 patients, respectively (Fig. 1, Table 1). The change in nystagmus pattern from PG-DCPN to paretic type occurred on days 2 to 4 after onset of vertigo, and 1 of these 3 patients showed a further conversion of nystagmus to PA-DCPN on day 80 after onset of vertigo. Conversion from PG-DCPN (n = 4) to PA-DCPN occurred on days 4 to 24 after onset of vertigo.
Three patients showed PA-DCPN in an SRT as the initial nystagmus (Fig. 1, Table 1), which changed to PG-DCPN on days 15 to 16 after onset of vertigo in 2 patients and to paretic type on day 2 after onset of vertigo in 1 patient who showed a further change to PA-DCPN (patient 14).
One patient showed a positive DHT on the affected side at initial examination, and the nystagmus pattern changed to PA-DCPN on day 25 after onset of vertigo (Fig. 1, Table 1).
Profound hearing loss was the most commonly observed type of hearing loss (n = 6) followed by high-tone (n = 5) and flat-type (n = 4) hearing loss. Low-tone hearing loss was not observed in any patient. In all patients, treatment for SSNHL consisted of high-dose systemic steroids (prednisolone 1 mg/kg/day for 4 days, tapered during the subsequent 10 days). Some patients subsequently received an intratympanic steroid injection as a salvage treatment. A modified Epley maneuver was performed in 1 patient (patient 6 in Table 1) with a positive DHT for treatment of positional vertigo. The initial average PTA threshold was 86 ± 32 dB, which was significantly higher than the final average PTA threshold (62 ± 36 dB) after treatment (P < .001, paired t-test). The speech discrimination score was significantly improved after treatment (32% ± 44% at the initial test vs 50% ± 45% after treatment; P = .048, paired t-test). Hearing improvement in PTA threshold after treatment was not significantly different (P = .59) between SSNHL_V patients with nystagmus change (25 ± 17 dB, n = 15) and those without nystagmus change (28 ± 18 dB, n = 35).
Neurologic examinations revealed no focal neurologic sign in any patient except for dysfunction of VIIIth cranial nerve. Fourteen of 15 patients underwent brain or internal auditory canal magnetic resonance imaging, which revealed no acute brain lesions or inner ear abnormality. Bithermal caloric test was performed in 14 of 15 patients. Of the 14 patients who underwent testing, 6 showed canal paresis on the affected side and 8 showed no canal paresis (Table 1).
4. Discussion
The etiology of SSNHL is considered to be idiopathic, although viral neurolabyrinthitis, labyrinthine ischemia, labyrinthine hemorrhage, and disruption of the cochlear membrane have been proposed as the most likely pathophysiology for SSNHL_V.[10,17] Khetarpal[18] suggested that vertigo occurs via biochemical changes of the inner ear fluid in patients with SSNHL_V, which was supported by the histologic study demonstrating that patients with SSNHL_V showed only atrophic changes in the extracellular suprastructure of the labyrinth without loss of sensory cells.[19] If vertigo and nystagmus are caused by the biochemical changes of the inner ear fluids in SSNHL_V, it is reasonable to assume that the patterns of nystagmus may be various and change during the course of the disease.
In the present study, we demonstrate 15 patients with SSNHL_V who showed a change in their nystagmus pattern during 3 months of follow-up. Initial examination revealed various types of initial nystagmus: 2 types without positional component (paretic-type and irritative-type) and 3 types with positional component (PG-DCPN, PA-DCPN, and positive DHT on the affected side) (Table 1 and Fig. 1). Initial nystagmus with positional component (PG-DCPN, PA-DCPN, and positive DHT on the affected side) was more common than those without positional component (n = 11 vs n = 4). Conversion of the nystagmus pattern occurred on days 2 to 75 from the vertigo onset in these patients, of which 2 patients showed further conversion. The most common pattern of nystagmus at the final examination was PA-DCPN (n = 9, Fig. 1).
The observation of paretic-type and irritative-type nystagmus indicates ipsilateral vestibular hypofunction and hyperfunction at the time of examination, respectively. The observation of nystagmus with positional component (PG-DCPN, PA-DCPN, and positive DHT on the affected side) can be explained as coexistence of BPPV and SSNHL on the same side.[11–13,17,20,21] For the etiology of BPPV in SSNHL, it has been suggested that selective damage to the cochlea and the utricle causes sudden hearing loss and detachment of otoconial particles, respectively,[11,20,22] and utricular ischemia may be a cause of BPPV in patients with SSNHL_V. When otoconial particles enter the PSCC, a DHT provokes vertigo and upbeating, torsional nystagmus. When otoconial particles are attached on the cupula of the lateral semicircular canal (LSCC), PA-DCPN is observed in an SRT (cupulolithiasis). When otoconial particles enter the LSCC, geotropic DCPN is observed in an SRT (canalolithiasis). On the contrary, other hypotheses have been proposed recently. Kim et al[15] suggested that blood debris within the PSCC, if inner ear hemorrhage is a cause of SSNHL, may act as an otolith particles eliciting typical positional nystagmus. In contrast to LSCC canalolithiasis which shows transient geotropic DCPN, geotropic DCPN in SSNHL has prolonged duration,[12,21,22] and minor hemorrhage or an increased concentration of proteins in the inner ear fluids was suggested as a cause of this PG-DCPN.
The mechanism underlying the conversion of nystagmus pattern in SSNHL_V is not clear. Change in nystagmus pattern between PG-DCPN and PA-DCPN in SSNHL patients has been reported previously,[16] with the following mechanisms suggested for nystagmus conversion: otoconial particles attached on the LSCC cupula are changed in density because of degeneration over time, biochemical change in the inner ear fluids alters over time, and central reorganization due to vestibular compensation. Irritative type was observed only at the initial examination (n = 1), which was converted to paretic type. It can be assumed that biochemical alteration in the inner ear fluid which initially exerted excitotoxic effect to the vestibular function has been changed to that exerting inhibito-toxic effect to the vestibular function. Conversion between paretic type and PG-DCPN or PA-DCPN was observed in 7 patients, of which the underlying mechanism is unknown. It can be assumed that biochemical alteration of the inner ear fluids has been changed during the course of the disease, or BPPV takes over after an assault of SSNHL in patients with preexisting vestibular pathology such as autoimmune inner ear disease.
Although the peripheral end organ is thought to be the most common site of pathology in SSNHL_V, the exact location of pathologic change in SSNHL_V has long been controversial while the pathologic change in vestibular neuritis is believed to lie in the vestibular nerve. Vestibular neuritis exhibits horizontal nystagmus beating toward the healthy side (paretic type), and the pattern or direction of nystagmus does not change during the disease course only decreasing the nystagmus intensity over time. On the contrary, the present study shows that some patients with SSNHL_V exhibit the change in nystagmus pattern that is presumably caused by biochemical alteration in the inner ear fluids, which may support that vestibular deficit in SSNHL arises from the inner ear end organs.
The limitations of this study are as follows: the number of patients included was not large enough to provide information for clinical decision-making or to present conclusive data; the initial nystagmus presented in Table 1 can be not really initial because the initial examination was performed on the day of each patient's first visit to the hospital, which was 0 to 4 days after onset of vertigo; and there is the possibility that some of the changes in the pattern of nystagmus went undetected because serial examination of nystagmus was performed every 1 to 2 days (acute stage) and every 3 to 14 days (chronic stage) during follow-up. However, the present study is the first, as far as we know, to demonstrate that the patterns of nystagmus can change over time in patients with SSNHL_V, suggesting that the biochemical changes in the inner ear fluids causing vertigo and nystagmus in those patients may become various during the course of the disease.
5. Conclusions
Among 50 patients with SSNHL_V, 15 patients showed conversion of their pattern of nystagmus. Nystagmus pattern was classified into 2 groups: nystagmus without positional component (paretic type, irritative type) and nystagmus with positional component (PG-DCPN, PA-DCPN, and positive DHT on the affected side). Nystagmus with positional component was more commonly observed both at the initial and follow-up examination. The mechanism underlying the nystagmus conversion is still unclear, biochemical changes in the inner ear fluids or central reorganization due to vestibular compensation are assumed to be a possible cause.
Author contributions
Conceptualization: Chang-Hee Kim.
Data curation: Hye Rang Choi, Seongjun Choi, Jung Eun Shin.
Formal analysis: Chang-Hee Kim, Hye Rang Choi, Seongjun Choi, Yong Sik Lee, Jung Eun Shin.
Investigation: Chang-Hee Kim, Yong Sik Lee, Jung Eun Shin.
Methodology: Hye Rang Choi, Yong Sik Lee.
Supervision: Chang-Hee Kim.
Validation: Seongjun Choi.
Writing – original draft: Chang-Hee Kim.
Writing – review and editing: Chang-Hee Kim.
Supplementary Material
Supplementary Material
Supplementary Material
Supplementary Material
Supplementary Material
Footnotes
Abbreviations: BPPV = benign paroxysmal positional vertigo, CP = canal paresis, DHT = Dix-Hallpike test, LSCC = lateral semicircular canal, PA-DCPN = persistent apogeotropic direction-changing positional nystagmus, PG-DCPN = persistent geotropic direction-changing positional nystagmus, PSCC = posterior semicircular canal, PTA = pure tone audiometry, SRT = supine-roll test, SSNHL = sudden sensorineural hearing loss, SSNHL_V = sudden sensorineural hearing loss with vertigo.
This paper was supported by Konkuk University in 2017.
The authors have no conflicts of interest to disclose.
Supplemental Digital Content is available for this article.
References
- [1].Stachler RJ, Chandrasekhar SS, Archer SM, et al. Clinical practice guideline: sudden hearing loss. Otolaryngol Head Neck Surg 2012;146:S1–35. [DOI] [PubMed] [Google Scholar]
- [2].Park HM, Jung SW, Rhee CK. Vestibular diagnosis as prognostic indicator in sudden hearing loss with vertigo. Acta Otolaryngol Suppl 2001;545:80–3. [PubMed] [Google Scholar]
- [3].Rauch SD. Clinical practice. Idiopathic sudden sensorineural hearing loss. N Engl J Med 2008;359:833–40. [DOI] [PubMed] [Google Scholar]
- [4].Moskowitz D, Lee KJ, Smith HW. Steroid use in idiopathic sudden sensorineural hearing loss. Laryngoscope 1984;94:664–6. [PubMed] [Google Scholar]
- [5].Shaia FT, Sheehy JL. Sudden sensori-neural hearing impairment: a report of 1,220 cases. Laryngoscope 1976;86:389–98. [DOI] [PubMed] [Google Scholar]
- [6].Byl FM., Jr Sudden hearing loss: eight years’ experience and suggested prognostic table. Laryngoscope 1984;94:647–61. [PubMed] [Google Scholar]
- [7].Wang CT, Huang TW, Kuo SW, et al. Correlation between audiovestibular function tests and hearing outcomes in severe to profound sudden sensorineural hearing loss. Ear Hear 2009;30:110–4. [DOI] [PubMed] [Google Scholar]
- [8].Watanabe Y, Aso S, Ohi H, et al. Vestibular nerve disorder in patients suffering from sudden deafness with vertigo and/or vestibular dysfunction. Acta Otolaryngol Suppl 1993;504:109–11. [DOI] [PubMed] [Google Scholar]
- [9].Kim CH, Jeong KH, Ahn SH, et al. Vibration- and hyperventilation-induced nystagmus in patients with Ramsay hunt syndrome with vertigo. Otolaryngol Head Neck Surg 2015;152:912–8. [DOI] [PubMed] [Google Scholar]
- [10].Kim CH, Na BR, Park HJ, et al. Impairment of static vestibular function is limited in patients with sudden sensorineural hearing loss with vertigo. Audiol Neurootol 2013;18:208–13. [DOI] [PubMed] [Google Scholar]
- [11].El-Saied S, Joshua BZ, Segal N, et al. Sudden hearing loss with simultaneous posterior semicircular canal BPPV: possible etiology and clinical implications. Am J Otolaryngol 2014;35:180–5. [DOI] [PubMed] [Google Scholar]
- [12].Kim CH, Shin JE, Yang YS, et al. Sudden sensorineural hearing loss with positional vertigo: initial findings of positional nystagmus and hearing outcomes. Int J Audiol 2016;55:541–6. [DOI] [PubMed] [Google Scholar]
- [13].Kim MB, Ban JH. Benign paroxysmal positional vertigo accompanied by sudden sensorineural hearing loss: a comparative study with idiopathic benign paroxysmal positional vertigo. Laryngoscope 2012;122:2832–6. [DOI] [PubMed] [Google Scholar]
- [14].Whitaker S. Idiopathic sudden hearing loss. Am J Otol 1980;1:180–3. [PubMed] [Google Scholar]
- [15].Kim CH, Shin JE, Park HJ, et al. Concurrent posterior semicircular canal benign paroxysmal positional vertigo in patients with ipsilateral sudden sensorineural hearing loss: is it caused by otolith particles? Med Hypotheses 2014;82:424–7. [DOI] [PubMed] [Google Scholar]
- [16].Shin JE, Jeong KH, Ahn SH, et al. Conversion between geotropic and apogeotropic persistent direction-changing positional nystagmus. Acta Otolaryngol 2015;135:1238–44. [DOI] [PubMed] [Google Scholar]
- [17].Iwasaki S, Takai Y, Ozeki H, et al. Extent of lesions in idiopathic sudden hearing loss with vertigo: study using click and galvanic vestibular evoked myogenic potentials. Arch Otolaryngol Head Neck Surg 2005;131:857–62. [DOI] [PubMed] [Google Scholar]
- [18].Khetarpal U. Investigations into the cause of vertigo in sudden sensorineural hearing loss. Otolaryngol Head Neck Surg 1991;105:360–71. [DOI] [PubMed] [Google Scholar]
- [19].Inagaki T, Cureoglu S, Morita N, et al. Vestibular system changes in sudden deafness with and without vertigo: a human temporal bone study. Otol Neurotol 2012;33:1151–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Karlberg M, Halmagyi GM, Buttner U, et al. Sudden unilateral hearing loss with simultaneous ipsilateral posterior semicircular canal benign paroxysmal positional vertigo: a variant of vestibulo-cochlear neurolabyrinthitis? Arch Otolaryngol Head Neck Surg 2000;126:1024–9. [DOI] [PubMed] [Google Scholar]
- [21].Kim CH, Choi JM, Jung HV, et al. Sudden sensorineural hearing loss with simultaneous positional vertigo showing persistent geotropic direction-changing positional nystagmus. Otol Neurotol 2014;35:1626–32. [DOI] [PubMed] [Google Scholar]
- [22].Rambold H, Heide W, Helmchen C. Horizontal canal benign paroxysmal positioning vertigo with ipsilateral hearing loss. Eur J Neurol 2004;11:31–5. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


