Supplemental Digital Content is available in the text
Keywords: meta-analysis., metastasis, MMP-9, osteosarcoma, overall survival
Abstract
Background:
Matrix metalloproteinase 9 (MMP-9) is significant in the progression of osteosarcoma (OS) via increasing tumor growth, invasion and metastasis. Although previous reports indicate the prognostic value of MMP-9 in OS, there is still a great degree on inconsistency between studies. Here we report a comprehensive evaluation of the value of MMP-9 in metastasis of OS by conducting a meta-analysis of published studies.
Methods:
The quantity of the studies was evaluated using the Newcastle-Ottawa quality assessment scale (NOS). Sixteen studies with a total of 816 patients with OS were examined and we calculated the pooled odds ratio (OR) with corresponding 95% confidence interval (CI) (95% CI) to evaluate that the positive expression of MMP-9 predicts neoplasm metastasis and poor survival in OS.
Results:
The results of Meta-analysis indicated that patients with positive expression of MMP-9 were significantly associated with neoplasm metastasis (OR = 4.69, 95% CI: 3.05–7.21, P <.001) and poor survival in OS with the pooled OR of 7.19 (95% CI 4.32–11.98, P <.001) when compared to their counterparts with a negative expression of MMP-9. The results of sensitivity analysis showed that the pooled OR was stable. It doesn’t significantly change when a single study was removed.
Conclusions:
The results of meta-analysis indicated that MMP-9 may be a prognostic biomarker guiding the clinical therapy for OS.
1. Introduction
Osteosarcoma (OS), the most common malignant bone tumor, is limited to the metaphysis of long bones and mainly afflicts adolescents.[1,2] Recently, the 5-year survival rate of OS patients has significantly improved to 70% due to the introduction of advanced surgery and combinational chemotherapy.[3] However, with the fact that most of OS patients are involved in fatal metastasis, which dramatically reduces survival rates, OS is still the second leading cause of cancer-related death in adolescents.[4,5] Previous studies showed that approximately 20% to 25% of newly diagnosed patients have detectable lung-related metastasis,[6,7] but at present, the ability to predict the metastasis of OS is limited because the mechanism of oncogenesis is still not fully elucidated and the clinical prognostic factors of OS are still demographics (such as age and sex), tumor size and response to chemotherapy. So to identify prognostic markers in OS may be an informative way for selecting proper management.
The function of zinc-dependent endopeptidases is to degrade the extracellular matrix (ECM). Matrix metalloproteinases (MMPs), a family of zinc-dependent endopeptidases, participate in many pathological and physiological processes, such as tissue repair and remodeling.[8] Moreover, MMPs play a significant role in tumor progression via increasing cell growth, migration, invasion and metastasis.[7] Recently, considerable interest has been focused on an important MMP family member, matrix metalloproteinase 9 (MMP-9) because of its over-expression in various tumors and association with poor disease prognosis in gastric and oral cancers.[9,10] The potential prognostic value of MMP-9 in OS has also been examined. However, no conclusions have been reached due to inconsistent results between studies.[11–13] Like most sarcomas, blood-borne metastases often occur in OS. Metastatic lesions found in the lung, liver, brain, bone, kidney, and local lymph nodes were defined as metastasis.[14–18] In this study, a meta-analysis was conducted to provide a comprehensive evaluation of the relationship between positive expression of MMP-9 and OS metastasis.
2. Materials and methods
2.1. Search strategy and study selection
A systematic search was conducted to search for relevant articles in PubMed, Embase, and China National Knowledge Internet (CNKI) databases. We performed the last search on March 20, 2018. The following terms: “OS” or “osteosarcomas” and “matrix metalloproteinase-9” or “MMP-9” were included in the search strategy without language limitation. Because this analysis was based on previously published studies, the ethics approval was not applicable.
2.2. Inclusion and exclusion criteria
Inclusion criteria:
-
(1)
measurement of MMP-9 in OS using commercial reagents;
-
(2)
pathological diagnosis (gold standard) confirmed for newly diagnosed patients with OS;
-
(3)
the studies had to provide sufficient information to construct the 2 × 2 contingency table;
-
(4)
publications were written in English or Chinese.
Exclusion criteria:
-
(1)
OS diagnosed without a biopsy and there was no clear cut-off value in the literature;
-
(2)
similar studies from the same author as well as multiple duplicate data in the different works, excluding earlier and smaller sample data;
-
(3)
cell and animal experiments, reviews, correspondences, case reports, talks, letters, expert opinions, and editorials without original data; and
-
(4)
studies of non-dichotomous MMP-9 expression levels and absence of survival outcome.
2.3. Data extraction
Two investigators (JZ and TL) evaluated the eligibility of all retrieved studies and extracted the relevant data independently. Extracted databases were then crosschecked between the 2 authors to rule out any discrepancy. Data regarding the following for each included studies were extracted independently: first authors’ surname, publication year, MMP-9 assessment methods, and the cut-off definition. Corresponding authors were contacted if further information was needed. The study was excluded if no response was received after sending a reminder.
2.4. Assessment of included studies
The Newcastle-Ottawa quality assessment scale (NOS)[19] was used to assess the quality of included studies. It has 3 categories (selection, comparability, and exposure) and 8 items. The quality assessment values ranged from 0 to 9 stars. Studies that scored more than 6 stars were included for our analysis.
2.5. Statistical analysis
The pooled odds ratio (OR) with corresponding 95% confidence interval (CI) was calculated to evaluate the effect of MMP-9 positive expression on metastasis and poor survival of OS. The heterogeneity between the included studies was assessed by I2 statistics, which quantified the proportion of the total variation in meta-analysis assessment from 0% to 100%.[20] When there was no significant heterogeneity (I2 ≤50%), the fixed effects model was used[21]; otherwise, a random effects model was used for the analysis.[22] Moreover, sensitivity analysis was performed by sequentially omitting individual studies to assess the stability of the results. The possibility of publication bias was assessed via visual assessment of the symmetry of Egger test and Begg funnel plots.[23] All the analyses were performed by using STATA version 12 software (StataCorp LP, College Station, TX). A 2-tailed P <.05 was considered statistically significant.
3. Results
3.1. Selection and characteristics of included studies
Through the primary search in PubMed, Embase, and CNKI databases and further evaluation of full texts, 16 studies[12,13,24–37] with a total of 816 patients with OS were included in the meta-analysis (Fig. 1). Among the 16 studies, 12 were published in Chinese and the other 4 were published in English. There are 3 studies[25,29,32] included in both OS metastasis meta-analysis and overall survival meta-analysis. The sample size of the 11 studies for OS metastasis ranged from 35 to 96 with a mean of 65.5 (Table 1) and the sample size of the 8 studies for OS overall survival ranged from 21 to 96 with a mean of 58.5 (Table 2). The main characteristics of the included studies were summarized in Tables 1 and 2. In summary, immunohistochemistry (IHC) was used for all studies to detect the expression of MMP-9. The results were judged via cut-off in percentage of positivity.
Figure 1.
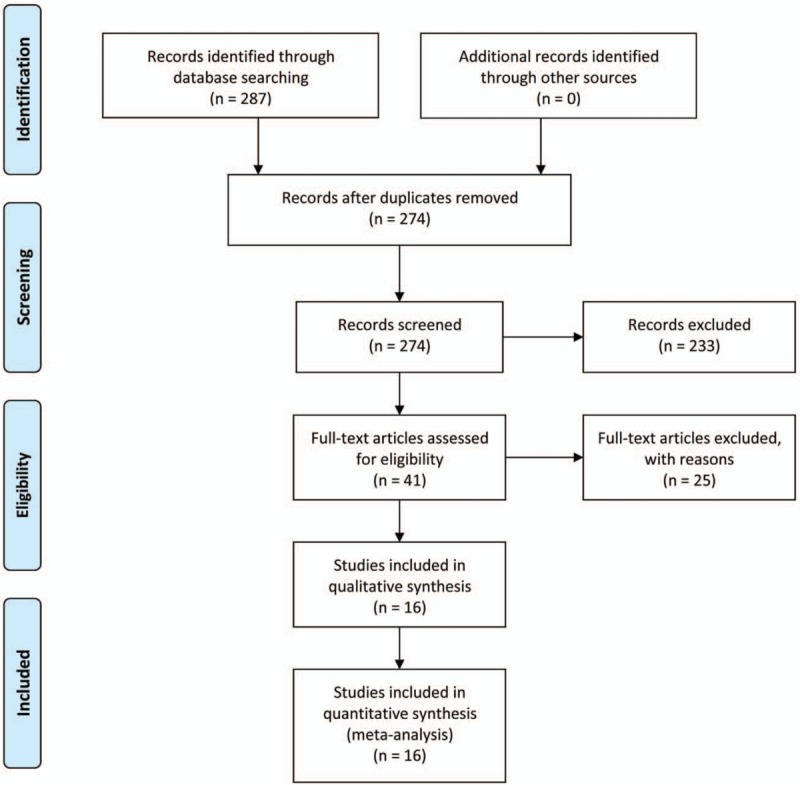
Schematic representation of the study selection.
Table 1.
Characteristics of studies included in the metastasis meta-analysis.

Table 2.
Characteristics of studies included in the 3-year survival meta-analysis.

3.2. Qualitative assessment
The study quality was assessed using the NOS, generating scores ranging from 7 to 8 (with a mean of 7.42). A higher value (0–9) indicates better methodology. The results of the quality assessment are shown in Tables 1 and 2, with detailed information shown in Supplementary Table 1 and Supplementary Table 2.
3.3. Meta-analysis
In the meta-analysis assessment of the effect of MMP-9 positive expression on OS metastasis and overall survival, STATA 12 indicated there was no significant between-study heterogeneity among those studies analyzed for the metastasis or overall survival of MMP-9 (I2 <35%), so the fixed-effect model was used to detect the pooled OR with corresponding 95% CI. The combined OR for all eligible studies evaluating MMP-9 positive expression on metastasis and poor survival in OS was (OR = 4.69, 95% CI: 3.05–7.21, P <.001) and (OR = 7.19, 95% CI 4.32–11.98, P <.001) respectively (Fig. 2).
Figure 2.
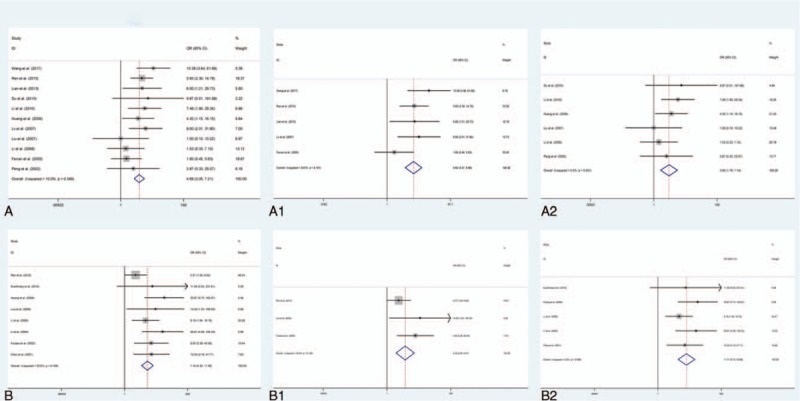
A. MMP-9 expression and metastasis of osteosarcoma patients. A1. MMP-9 expression and metastasis of osteosarcoma patients with cutoff value of MMP-9 >20%. A2. MMP-9 expression and metastasis of osteosarcoma patients with cutoff value of MMP-9 <20%. B. MMP-9 expression and overall survival of osteosarcoma patients. B1. MMP-9 expression and overall survival of osteosarcoma patients with cutoff value of MMP-9 >20%. B2. MMP-9 expression and overall survival of osteosarcoma patients with cutoff value of MMP-9 <20%.
The expression cutoff value of MMP-9 is different among these studies. In order to eliminate the bias caused by different MMP-9 expression cut-off value, we performed a subgroup analysis to eliminate the bias caused by different MMP-9 expression cutoff value. We found significant association in both studies with cut-off value of MMP-9 >20% (OR = 5.62, 95% CI = 3.27–9.66, P <.001) and studies with cutoff value of MMP-9 <20% (OR = 3.55, 95% CI = 1.76–7.14, P <.001) for MMP-9 positive expression on metastasis, as shown in Figure 2. No heterogeneity was found between these 2 groups.
Moreover, for MMP-9 positive expression on overall survival, significant association in both studies with cut-off value of MMP-9 >20% (OR = 4.34, 95% CI = 2.08–9.07, P <.001) and studies with cut-off value of MMP-9 <20% (OR = 11.71, 95% CI = 5.72–23.98, P <.001) (Fig. 2). https://www.baidu.com/javascript: No heterogeneity was found between these 2 groups.
3.4. Sensitivity analysis
We used sensitivity analysis to evaluate the stability of results. As shown in Figure 3, all the heterogeneity did not change significantly no matter which study removed, which suggested that the results of our analysis did not overly rely on a single study and the conclusions are stable. All these results indicated that MMP-9 positive expression was an indicator of metastasis and overall survival for OS patients (Fig. 3).
Figure 3.

Forest plot for the sensitivity analysis in the meta-analysis. A. Metastasis. A1. Metastasis with cutoff value of MMP-9 >20%. A2. Metastasis with cutoff value of MMP-9 <20%. B. Overall survival. B1. Overall survival with cutoff value of MMP-9 >20%. B2. Overall survival with cutoff value of MMP-9 >20%.
3.5. Publication bias
Begg funnel plot and Egger test were performed to assess the publication bias in the meta-analysis. As shown in Figure 4, the funnel plot presented no obvious evidence of asymmetry among the 16 studies. Moreover, Egger test also revealed no significant publication bias in the meta-analysis (P >.05).
Figure 4.
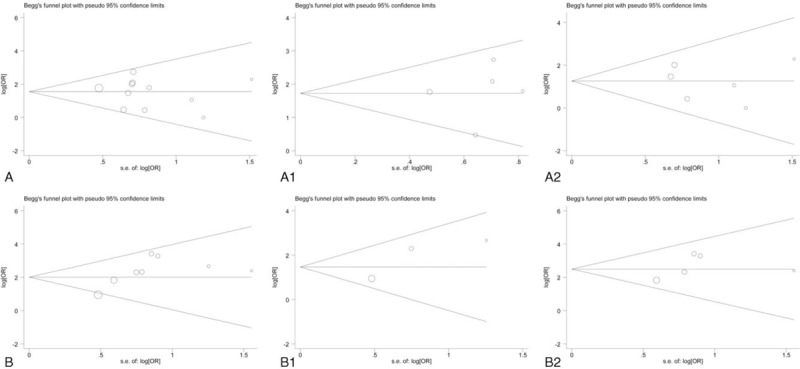
Funnel plot in the meta-analysis of the effect of MMP-9 expression on metastasis and overall survival of OS. A. Metastasis. A1. Metastasis with cutoff value of MMP-9 >20%. A2. Metastasis with cutoff value of MMP-9 <20%. B. Overall survival. B1. Overall survival with cutoff value of MMP-9 >20%. B2. Overall survival with cutoff value of MMP-9 >20%.
4. Discussion
OS is a primary life-threatening malignant bone tumor that often occurs in adolescents and young adults.[38] Disease 5-year survival rate escalated from <20% before the introduction of effective chemotherapy to around 60%.[39,40] With 20% to 25% detected metastases at diagnosis, OS is characterized by a high propensity for metastasis, especially to the lungs.[41] When metastasis is detected at the time of diagnosis, the overall survival rate of OS patients decreases to 30%. Early identification of high-risk patients may improve treatment by allowing clinicians to select the most appropriate therapy. Therefore, it is urgently needed to renew early prognostic biomarkers to adapt the proper therapy for the malignancy.
MMPs play critical roles in tumor cell growth, migration, invasion, metastasis, and angiogenesis.[23,42] MMP-9, a member of MMPs family, mainly functions as a collagenase by degrading type IV collagen which is a major component of basement membrane and ECM.[43,44] According to the results of previous meta-analysis, there is an important correlation between high MMP-9 expression and poor prognosis in breast cancer,[45] gastric cancer,[46] colorectal cancer[47] and non-small cell lung cancer.[48] In this report, a similar approach was used to evaluate the prognostic value of MMP-9 positive expression in OS.
Meta-analysis is a quantitative approach combining information from different studies on the same topic, which has been used to evaluate prognostic markers for several cancers.[49] In order to conduct a precise assessment about the prognostic role of MMP-9 positive expression in OS, a meta-analysis was performed and 16 published studies were included. Our results indicated that MMP-9 positive expression in OS predicted a statistically significant role of MMP-9 on OS metastasis (OR = 4.69, 95% CI: 3.05–7.21, P <.001) and poor survival (OR = 7.19, 95% CI 4.32–11.98, P <.001) in OS (Fig. 2). Considering the expression cutoff value of MMP-9 is different among these studies, we conducted a subgroup analysis to rule out the potential bias caused by different MMP-9 expression cutoff values. We found significant association in both studies with cutoff value of MMP-9 >20% (OR = 5.62, 95% CI = 3.27–9.66, P <.001) and studies with cutoff value of MMP-9 <20% (OR = 3.55, 95% CI = 1.76–7.14, P <.001) for MMP-9 positive expression on metastasis (Fig. 2). Additionally, for MMP-9 positive expression on overall survival, significant association in both studies with cutoff value of MMP-9 >20% (OR = 4.34, 95% CI = 2.08–9.07, P <.001) and studies with cutoff value of MMP-9 <20% (OR = 11.71, 95% CI = 5.72–23.98, P <.001) (Fig. 2). https://www.baidu.com/javascript: No heterogeneity was found between metastasis groups or survival groups. All these results indicated different expression cutoff values of MMP-9 did not affect the results significantly.
Then we conducted a sensitivity analysis to evaluate the stability of results, suggesting the pooled OR was stable and not significantly changed no matter which study removed (Fig. 3). A Begg funnel plot with STATA was performed and no publication bias was found (P >.05) (Fig. 4). This meta-analysis suggests that MMP-9 positive expression is associated with OS metastasis and overall survival. MMP-9 may be used as a prognostic biomarker to guide the clinical therapy for OS.
However, our meta-analysis has its limitations. There are several issues that should be considered.
The sample size of the total patients included in this meta-analysis was relatively small with a mean of 51. Additionally, there were 573 OS patients with MMP-9 positive expression and only 243 patients with MMP-9 negative expression. Random errors and sample bias are unavoidably produced due to the relatively small size.
In this meta-analysis, only articles published in English or Chinese were included, which may cause additional bias.
There was not any unified cut-off value for defining MMP-9 positive expression. Although we conducted a subgroup analysis to eliminate the potential bias, a standard threshold would be beneficial to make precise evaluation about the prognostic role of MMP-9 positive expression.
There is no publication bias. However, potential publication bias may still exist. These studies with desirable results may be published more easily, which may cause an over-estimation of overall accuracy.
We cannot stratify patient data by age, tumor stage, tumor size, and histological types due to lack of sufficient data. In order to strengthen our findings, well-designed clinical studies with larger sample size are needed to be performed in the future before the application of MMP-9 on the metastasis of OS patients. Although the inherent limitations of this meta-analysis are still exist, this meta-analysis presents a quantified synthesis of published studies, which may draw more attention on new prognostic biomarkers of OS.
In conclusion, a meta-analysis was conducted to evaluate the association between MMP-9 positive expression and prognosis, included metastasis and overall survival, of patients with OS. According to the results from the meta-analysis, MMP-9 is an effective biomarker that correlates with OS metastasis and poor survival. In order to obtain a more comprehensive evaluation about the prognostic role of MMP-9 positive expression in OS patients, more well-designed studies with larger sample sizes are still needed.
Acknowledgments
The authors thank Dr. Ayub Abdulle nur and Dr. Chenxi Li for English language support in preparing manuscript.
Author contributions
Data curation: Jian Zhou, Tang Liu, wanchun wang.
Formal analysis: Tang Liu, wanchun wang.
Funding acquisition: Jian Zhou, wanchun wang.
Investigation: Jian Zhou, wanchun wang.
Methodology: Jian Zhou.
Writing – original draft: Jian Zhou, wanchun wang.
Writing – review & editing: Jian Zhou, wanchun wang.
Supplementary Material
Footnotes
Abbreviations: CI = confidence interval, CNKI = China National Knowledge Internet, ECM = extracellular matrix, MMP-9 = matrix metalloproteinase 9, MMPs = matrix metalloproteinases, NOS = Newcastle-Ottawa quality assessment scale, OR = odds ratio, OS = osteosarcoma.
This work was supported by the Fundamental Research Funds for the Central Universities of Central South University (Grant No. 2018zzts930), the Central South University Sports Medicine Scholarship, the National College Students’ Innovation and Entrepreneurship Training Program (Grant No. 201710422116) and the National Natural Science Foundation of China (Grant Nos. 81000821 and 81672176), the Natural Science Foundation of Hunan Province, China (Grant No. 2018JJ2565) and the National Science Foundation for Post-doctoral Scientists of China (Grant No. 2017M622601).
The authors have no conflicts of interest to disclose.
Supplemental Digital Content is available for this article.
References
- [1].Siclari VA, Qin L. Targeting the osteosarcoma cancer stem cell. J Orthop Surg Res 2010;5:78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Picci P, Mercuri M, Ferrari S, et al. Survival in high-grade osteosarcoma: improvement over 21 years at a single institution. Ann Oncol 2010;21:1366–73. [DOI] [PubMed] [Google Scholar]
- [3].Hameed M, Dorfman H. Primary malignant bone tumors–recent developments. Semin Diagn Pathol 2011;28:86–101. [DOI] [PubMed] [Google Scholar]
- [4].Weeden S, Grimer RJ, Cannon SR, et al. The effect of local recurrence on survival in resected osteosarcoma. Eur J Cancer 2001;37:39–46. [DOI] [PubMed] [Google Scholar]
- [5].Harting MT, Blakely ML, Jaffe N, et al. Long-term survival after aggressive resection of pulmonary metastases among children and adolescents with osteosarcoma. J Pediatr Surg 2006;41:194–9. [DOI] [PubMed] [Google Scholar]
- [6].Ta HT, Dass CR, Choong PF, et al. Osteosarcoma treatment: state of the art. Cancer Metastasis Rev 2009;28:247–63. [DOI] [PubMed] [Google Scholar]
- [7].Roy R, Yang J, Moses MA. Matrix metalloproteinases as novel biomarkers and potential therapeutic targets in human cancer. J Clin Oncol 2009;27:5287–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Nagase H, Visse R, Murphy G. Structure and function of matrix metalloproteinases and TIMPs. Cardiovasc Res 2006;69:562–73. [DOI] [PubMed] [Google Scholar]
- [9].Viros D, Camacho M, Zarraonandia I, et al. Prognostic role of MMP-9 expression in head and neck carcinoma patients treated with radiotherapy or chemoradiotherapy. Oral Oncol 2013;49:322–5. [DOI] [PubMed] [Google Scholar]
- [10].Vilen ST, Salo T, Sorsa T, et al. Fluctuating roles of matrix metalloproteinase-9 in oral squamous cell carcinoma. ScientificWorldJournal 2013;2013:920595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Foukas AF, Deshmukh NS, Grimer RJ, et al. Stage-IIB osteosarcomas around the knee. A study of MMP-9 in surviving tumour cells. J Bone Joint Surg Br 2002;84:706–11. [DOI] [PubMed] [Google Scholar]
- [12].Kushlinsky NE, Solovyov YN, Babkina IV, et al. Matrix metalloproteinases 2, 7, 9 and tissue inhibitor of matrix metalloproteinase-1 in the sera of patients with bone tumors. Bull Exp Biol Med 2010;149:233–5. [DOI] [PubMed] [Google Scholar]
- [13].Uchibori M, Nishida Y, Nagasaka T, et al. Increased expression of membrane-type matrix metalloproteinase-1 is correlated with poor prognosis in patients with osteosarcoma. Int J Oncol 2006;28:33–42. [PubMed] [Google Scholar]
- [14].Tian H, Guan D, Li J. Identifying osteosarcoma metastasis associated genes by weighted gene co-expression network analysis (WGCNA). Medicine (Baltimore) 2018;97:e10781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Wang X, Wang D, Yuan N, et al. The prognostic value of PCNA expression in patients with osteosarcoma: A meta-analysis of 16 studies. Medicine (Baltimore) 2017;96:e8254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Tang Y, Yang C, Guo Z, et al. P16 protein expression as a useful predictive biomarker for neoadjuvant chemotherapy response in patients with high-grade osteosarcoma: a systematic meta-analysis under guideline of PRISMA. Medicine (Baltimore) 2017;96:e6714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Zhang Q, Liu F, Wang B, et al. HER-2 expression in biopsy and surgical specimen on prognosis of osteosarcoma: A systematic review and meta-analysis of 16 studies. Medicine (Baltimore) 2016;95:e3661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Wang S, Zheng S, Hu K, et al. A predictive model to estimate the pretest probability of metastasis in patients with osteosarcoma. Medicine (Baltimore) 2017;96:e5909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Wells GA, Shea B, O’Connell D, et al. The Newcastle–Ottawa Scale (NOS) for assessing the quality of non-randomised studies in meta-analyses; 2011. Available at: http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp. [Google Scholar]
- [20].Higgins JP, Thompson SG, Deeks JJ, et al. Measuring inconsistency in meta-analyses. BMJ 2003;327:557–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Mantel N, Haenszel W. Statistical aspects of the analysis of data from retrospective studies of disease. J Natl Cancer Inst 1959;22:719–48. [PubMed] [Google Scholar]
- [22].DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials 1986;7:177–88. [DOI] [PubMed] [Google Scholar]
- [23].Egger M, Davey SG, Schneider M, et al. Bias in meta-analysis detected by a simple, graphical test. BMJ 1997;315:629–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Wang J, Yi Z, Pu Y, et al. Expression of ERK5 and MMP-9 in osteosarcoma and its clinical significance (article in Chinese). Chinese tumor clinic 2017;689–94. [Google Scholar]
- [25].Ren Z, Liang S, Yang J, et al. Coexpression of CXCR4 and MMP9 predicts lung metastasis and poor prognosis in resected osteosarcoma. Tumour Biol 2016;37:5089–96. [DOI] [PubMed] [Google Scholar]
- [26].Lian H, Li Y, Zhao M, et al. Expression of cyclooxygenase-2 and matrix metalloproteinase-9 in osteosarcoma and its clinical significance (Article in Chinese). Hebei Tradit Chin Med 2013;1105–7. [Google Scholar]
- [27].Du H, Liu X, Zhang Y, et al. Expression of RhoC and MMP-9 in osteosarcoma and its clinicopathological significance (Article in Chinese). Modern Prevent Med 2010;1794–07. [Google Scholar]
- [28].Li R. Expression and significance of MMP-9 and VEGF in osteosarcoma (article in Chinese). Shandong Med 2010;50:67–8. [Google Scholar]
- [29].Huang Y, Wang J. Expression and clinical significance of MDM2 and MMP-9 in osteosarcoma (article in Chinese). J Pract Oncol 2009;1:35–7. [Google Scholar]
- [30].Lv H, Chen M, Li S, et al. Expression of MMP-9 in osteosarcoma and its clinical significance (article in Chinese). Med Exam Clin 2007;18:57–8. [Google Scholar]
- [31].Liu Y, Wu H, Cheng G, et al. Expression and clinical significance of MMP-9, TIMP-1 and CD44 in osteosarcoma (article in Chinese). Northwest Natl Defense Med J 2007;28:427–8. [Google Scholar]
- [32].Li W, Ye Z, Yang D, et al. Correlation between osteosarcoma and expression of vascular endothelial growth factor and matrix metalloproteinase 2, 9 (article in Chinese). Zhejiang Med 2006;28:691–4. [Google Scholar]
- [33].Ferrari C, Benassi S, Ponticelli F, et al. Role of MMP-9 and its tissue inhibitor TIMP-1 in human osteosarcoma: findings in 42 patients followed for 1-16 years. Acta Orthop Scand 2004;75:487–91. [DOI] [PubMed] [Google Scholar]
- [34].Peng T, Qiu J, Wu H, et al. Relationship between expression of CD44 s, MMP-9 and Ki-67 and invasion, metastasis and recurrence of osteosarcoma (article in Chinese). Cancer 2002;21:745–50. [PubMed] [Google Scholar]
- [35].Luo X, Ni J. Expression of MMP-9 and PCNA in osteosarcoma and its clinical significance (article in Chinese). Med Clin Res 2006;23:682–4. [Google Scholar]
- [36].Li X, Li J, Yang Z, et al. Relationship between MMP-7, MMP-9 and MMP-10 and invasion and metastasis of osteosarcoma and its clinical significance (article in Chinese). J Shandong Univ Med Edition 2004;42:593–5. [Google Scholar]
- [37].Chen L, Lin J, Zhang S. Expression of MMP-9 in osteosarcoma and its clinical significance (article in Chinese). Chinese tumor clinic 2001;32–4. [Google Scholar]
- [38].Geller DS, Gorlick R. Osteosarcoma: a review of diagnosis, management, and treatment strategies. Clin Adv Hematol Oncol 2010;8:705–18. [PubMed] [Google Scholar]
- [39].Osborne TS, Khanna C. A review of the association between osteosarcoma metastasis and protein translation. J Comp Pathol 2012;146:132–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Zhang Y, Ding C, Wang J, et al. Prognostic significance of CD44V6 expression in osteosarcoma: a meta-analysis. J Orthop Surg Res 2015;10:187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Jaffe N. Osteosarcoma: review of the past, impact on the future. The American experience. Cancer Treat Res 2009;152:239–62. [DOI] [PubMed] [Google Scholar]
- [42].Li H, Zhang K, Liu LH, et al. A systematic review of matrix metalloproteinase 9 as a biomarker of survival in patients with osteosarcoma. Tumour Biol 2014;35:5487–91. [DOI] [PubMed] [Google Scholar]
- [43].Liotta LA, Tryggvason K, Garbisa S, et al. Metastatic potential correlates with enzymatic degradation of basement membrane collagen. Nature 1980;284:67–8. [DOI] [PubMed] [Google Scholar]
- [44].Stetler-Stevenson WG. Type IV collagenases in tumor invasion and metastasis. Cancer Metastasis Rev 1990;9:289–303. [DOI] [PubMed] [Google Scholar]
- [45].Song J, Su H, Zhou YY, et al. Prognostic value of matrix metalloproteinase 9 expression in breast cancer patients: a meta-analysis. Asian Pac J Cancer Prev 2013;14:1615–21. [DOI] [PubMed] [Google Scholar]
- [46].Zhang QW, Liu L, Chen R, et al. Matrix metalloproteinase-9 as a prognostic factor in gastric cancer: a meta-analysis. Asian Pac J Cancer Prev 2012;13:2903–8. [DOI] [PubMed] [Google Scholar]
- [47].Li CY, Yuan P, Lin SS, et al. Matrix metalloproteinase 9 expression and prognosis in colorectal cancer: a meta-analysis. Tumour Biol 2013;34:735–41. [DOI] [PubMed] [Google Scholar]
- [48].Peng WJ, Zhang JQ, Wang BX, et al. Prognostic value of matrix metalloproteinase 9 expression in patients with non-small cell lung cancer. Clin Chim Acta 2012;413:1121–6. [DOI] [PubMed] [Google Scholar]
- [49].Fu HL, Shao L, Wang Q, et al. A systematic review of p53 as a biomarker of survival in patients with osteosarcoma. Tumour Biol 2013;34:3817–21. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


