Abstract
To examine the outcomes of concurrent versus sequential whole-brain radiotherapy (WBRT) and epidermal growth factor receptor-tyrosine kinase inhibitor (EGFR-TKI) in nonsmall cell lung cancer (NSCLC) patients with EGFR mutation.
Retrospectively 105 patients with NSCLC, brain metastasis, and EGFR mutation (Affiliated Hospital of Guangdong Medical University, 01/2011 to 12/2014) were grouped as: EGFR-TKIs alone (n = 39, group A), EGFR-TKIs + concurrent radiotherapy (n = 34, group B), and radiotherapy followed by EGFR-TKIs (n = 32, group C).
The intracranial objective response rates of groups A, B, and C were 66.7%, 85.3%, and 75%, respectively (P < .05). The median intracranial progression-free survival of groups A, B, and C were 6.8, 12.4, and 9.1 months, respectively (P < .05). The median extracranial progression-free survival of groups A, B, and C were 7.8, 9.4, and 8.3 months, respectively (P > .05).
EGFR-TKIs and WBRT by simultaneous application improved the short- and long-term benefits to patients with NSCLC brain metastasis carrying EGFR mutation compared to concurrent application or EGFR-TKIs alone without additional adverse events.
Keywords: brain metastasis, concurrent, EGFR mutation, nonsmall cell lung cancer, sequential, tyrosine kinase inhibitors, whole brain radiotherapy
1. Introduction
Nonsmall cell lung cancer (NSCLC) is one of the malignancies with the highest morbidity and mortality.[1] NSCLC accounts for 85% to 90% of all lung cancer cases.[2] It mostly affects men ≥65 years of age and cigarette smoking is the main risk factor.[2,3] Its prognosis is usually poor, and the 5-year survival rate is <15% to 18%.[4–6]
In patients with NSCLC, the incidence of first-episode brain metastasis is about 10%, while the incidence of brain metastasis throughout the disease course is around 25% to 38%.[7–10] The median survival of patients with NSCLC is only 1 to 3 months once brain metastasis occurs.[11,12] At present, the primary treatment of brain metastasis is whole brain radiotherapy (WBRT), which only prolongs the median survival of most patients by 4 to 6 months and is considered to have marginal benefit by some authors.[13–18]
An epidermal growth factor receptor (EGFR) mutation is considered a marker of good prognosis of NSCLC because these cancers respond to EGFR tyrosine kinase inhibitors.[19–24] On the other hand, the risk of brain metastasis is considered to be higher for NSCLC patients with EGFR mutation than for those with wild-type EGFR,[25,26] but this is nevertheless controversial.[27] Among patients with NSCLC and EGFR mutations, the presence of brain metastasis leads to worst outcomes compared with extracranial metastases alone.[28]
Even if previous studies demonstrated that EGFR-tyrosine kinase inhibitors (TKIs) can effectively improve the prognosis of NSCLC patients with sensitive EGFR mutations,[19–24] the optimal timing of WBRT and EGFR-TKI remains controversial.[29] Therefore, the aim of this retrospective study was to examine the outcomes of concurrent versus sequential WBRT and EGFR-TKI in NSCLC patients with EGFR mutations. The results could provide some rationale for a prospective multicenter randomized clinical trial.
2. Subjects and methods
2.1. Study design and patients
This was a retrospective study based on the clinical data of 105 patients with NSCLC and brain metastasis who carried an EGFR mutation, and were treated at the Affiliated Hospital of Guangdong Medical University between January 2011 and December 2014. The inclusion criteria were: histopathological diagnosis of NSCLC; patients with metastases confirmed by computed tomography (CT) or magnetic resonance imaging (MRI); Eastern Cooperative Oncology Group (ECOG) score of 0–2; no systemic infections or other malignancies; no history of chemotherapy or radiotherapy to treat brain metastasis; exon 19 deletion or exon 21 L858R mutation in EGFR; and at least one measurable extracranial and intracranial lesion. The exclusion criteria were: incomplete clinical data; or incomplete follow-up data. This study was approved by the Ethics Committee of the Affiliated Hospital of Guangdong Medical University. Informed consent was waived by the committee because of the retrospective nature of the study.
2.2. Treatment methods
The patients received oral gefitinib 250 mg/d, erlotinib 150 mg/d, or icotinib 125 mg/tid until disease progression, death, or adverse events that could not be tolerated. For WBRT, an Elekta linear accelerator was used. Lesion localization was simulated using CT. The clinical target volume (CTV) was plotted, and quantitation limits of the organs at risk were determined. The intersection of brainstem, optic nerve, and optic chiasm was ≤50 Gy, the eyes were ≤45 Gy, and the crystal was ≤8 Gy. Whole brain radiotherapy was performed using 6 to 10 MV X-ray with a total dose of 30 Gy in 10 fractions or 40 Gy/20 fractions.
For patients with brain metastases, when the disease progressed after first-line therapy, the ECOG PS scores of most patients were 3 to 4. For patients with the ECOG PS score lower than 2, application of second-line drug for mono-chemotherapy could be considered. The other patients continued their initial EGFR-TKIs treatment or received best supportive care. The regimens of the mono-chemotherapy included using Pemetrexed (500 mg/m2, intravenous infusion, d1) or Docetaxel (75 mg/m2, intravenous infusion, d1). Twenty-one days were considered one cycle. Oral administration of dexamethasone was conducted for all the patients before chemotherapy. For the patients who received Pemetrexed therapy, 0.4 mg/d of folic acid was orally administered 1 week before the chemotherapy, and intramuscular injection of 1 mg Vitamin B12 was conducted 1 week before every 3 cycles.
2.3. Grouping
The patients were divided according to the treatment they had received: EGFR-TKI alone (group A); EGFR-TKI combined with concurrent WBRT (group B); or TKI starting one week after the completion of WBRT (sequential treatment; group C).
2.4. Response evaluation
Treatment response evaluation was made using enhanced CT or MRI based on the Response Evaluation Criteria in Solid Tumors (RECIST) (version 1.1).[30] Response was divided into complete remission (CR), partial remission (PR), stable disease (SD), and progressive disease (PD). The intracranial objective response rate (iORR) was calculated according to CR + PR and the intracranial disease control rate (iDCR) was calculated according to CR + PR + SD. Evaluation of all patients was performed by 2 deputy chief physicians from the Oncology Department.
2.5. Data collection
Baseline characteristics of the patients were collected through their medical records. The treatment status was obtained by follow-up records. Brain symptoms were defined as the common symptoms/signs of the central nervous system (headache, dizziness, unilateral limb movement disorders, vomiting, speechlessness, psychiatric disorders, etc.).
2.6. Definitions and follow-up
The overall survival (OS) was defined as the time from the diagnosis of brain metastasis to the time of death due to any causes. Intracranial progression-free survival (iPFS) was the time from the diagnosis of brain metastasis to progression of intracranial lesions or death. Extracranial progression-free survival (ePFS) was defined as the time from starting treatment to disease progression or death due to any causes. Follow-up was censored on June 15, 2017. Intracranial lesions were examined, and response was evaluated by enhanced CT or MRI one month after treatment. Follow-up was performed every 2 months. The OS, iPFS, and ePFS were compared among the 3 groups. The adverse events were evaluated according to the criteria of the NCI CTC 4.0.[31]
2.7. Statistical analysis
SPSS 21.0 (IBM, Armonk, NY) was used for statistical analysis. The categorical variables were expressed as frequency and percentage, and group-wise comparison was performed by Chi-square test. Survival analysis was performed using the Kaplan–Meier method, and the differences among groups were analyzed by the log-rank test. Two-sided P-values <.05 were considered statistically significant.
3. Results
3.1. Baseline characteristics
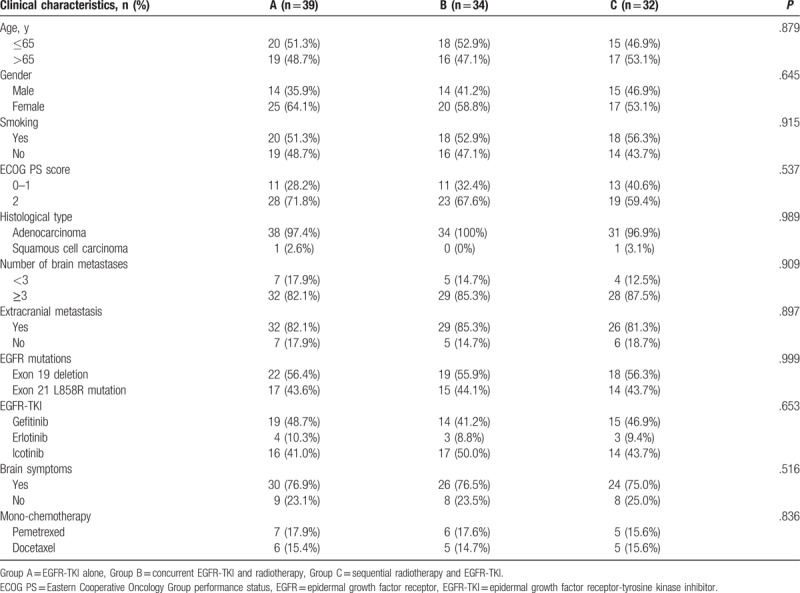
A total of 105 patients were included and divided into group A (n = 39), group B (n = 34), and group C (n = 32). There was no significant difference in clinical data among the 3 groups (Table 1). Two, one, and one patient lost follow-up in group A, B, and C, respectively. Thirty-four (32.4%) of the 105 patients received second-line mono-chemotherapy, among whom 13, 11, and 10 were in group A, B, and C, respectively (P = .836).
Table 1.
Comparison of the clinical characteristics among the 3 groups.
3.2. Short-term benefits
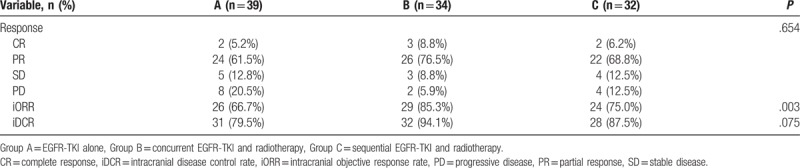
Evaluation at 1 month after treatment showed that iORR was significantly different among the 3 groups (P = .003). The iDCR in group B was significantly higher than that in group A (P < .05) but was not significantly different compared to group C (P > .05). There was no significant difference in iDCR between groups A and C (P > .05) (Table 2).
Table 2.
Comparison of the response among the 3 groups of patients at 1 month after the treatment.
3.3. Survival
The median OS of groups A, B, and C were 16.9, 24.5, and 21.3 months, respectively, with significant differences among the 3 groups (P < .001). The median OS of group B was significantly higher compared to groups A and C (P < .05). The median iPFS of groups A, B, and C were 6.8, 12.4, and 9.1 months, respectively, with significant differences among the 3 groups (P < .001). The median iPFS of group B was significantly higher compared to groups A and C (P < .05). The median ePFS of groups A, B, and C were 7.8, 9.4, and 8.3 months, respectively, without significant difference (P = .852) (Table 3) (Fig. 1).
Table 3.
Comparison of survival among the 3 groups.
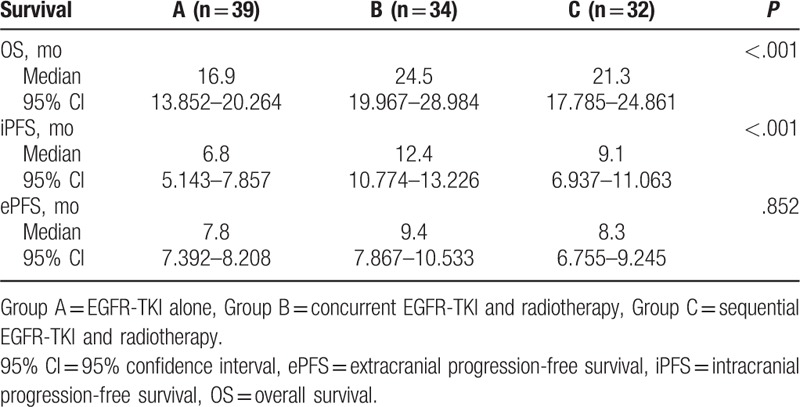
Figure 1.
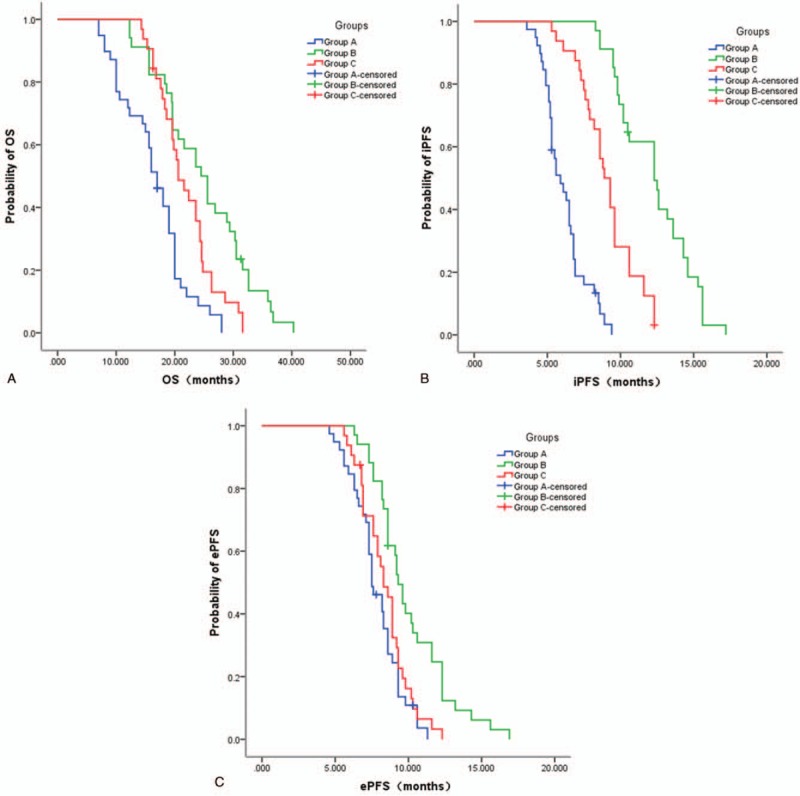
Overall survival (OS) (A), intracranial progression-free survival (iPFS) (B), and extracranial progression-free survival (ePFS) (C) Kaplan–Meier curves of the 3 groups.
3.4. Adverse events
The most common adverse events of the 3 groups of patients during the treatment included headache, vomiting, hair loss, rash, diarrhea, and elevated transaminases, which were mostly grade 1 or 2. These symptoms improved after symptomatic treatment, and all the patients could tolerate these events.
4. Discussion
Previous studies demonstrated that EGFR-TKIs can effectively improve the prognosis of NSCLC patients with sensitive EGFR mutation,[19–24] but the optimal timing of WBRT and EGFR-TKI remains controversial.[29] Therefore, this retrospective study aimed to examine the outcomes of concurrent versus sequential WBRT and EGFR-TKI in NSCLC patients with EGFR mutation. The results suggest that the simultaneous application of EGFR-TKIs and WBRT can effectively improve the short- and long-term benefits of patients with brain metastasis of NSCLC carrying EGFR mutation, without additional adverse events.
The prognosis of NSCLS is poor.[4–6] In patients with NSCLC, the incidence of first-episode brain metastasis is about 10%, with the incidence of brain metastasis throughout the disease course around 25% to 38%.[7–10] Because there are often multiple brain metastases and because of the delicate brain structure, surgical resection is often impossible. In addition, most chemotherapy drugs cannot cross the blood–brain barrier, which results in extremely poor therapeutic responses and systemic toxic effects, further affecting the quality of life of the patients.[4,11–13,22–25,30–33]
Radiotherapy is currently the main treatment for brain metastases from NSCLC.[34] With continuous development of molecular biology techniques in the diagnosis and treatment of tumors, the concept of “precision medicine” has been proposed and widely used.[35] EGFR-TKIs are a first-line treatment choice for patients with lung cancer carrying EGFR mutations.[19–24] Multicenter trials like IPASS[36] and WJTOG3405[37] confirmed the role of EGFR-TKIs as first-line treatment of NSCLC patients with EGFR mutation (including patients with brain metastasis). Both European Society for Medical Oncology (ESMO)[2] and National Comprehensive Cancer Network (NCCN)[38] guidelines recommend using EGFR-TKIs as the standard first-line treatment strategy for the advanced stage NSCLC patients with EGFR gene mutations (including those with brain metastases). In addition to conventional chemotherapy and radiotherapy, the presence of an EGFR-mutation also offers the chance of targeted therapy with EGFR-TIKs for the advanced stage NSCLC patients. However, as yet, there is no high-grade evidence available in ESMO[2] or NCCN[38] guidelines regarding selecting and arranging brain radiotherapy to better benefit patients. Currently, the main EGFR-TKI drugs used clinically are gefitinib, erlotinib, and imatinib.[39,40] Because of their small molecular size, EGFR-TKIs can enter the brain through the blood–brain barrier and can effectively control brain metastasis. Nevertheless, the response to EGFR-TKIs alone is not satisfactory in the treatment of patients with brain metastasis from NSCLC.
Using EGFR-TKIs alone for the treatment of patients with brain metastases of NSCLC involves relatively high intracranial recurrence rate, and the PFS is also short. Park et al[41] prospectively analyzed the data of using EGFR-TKIs alone for the treatment of brain metastases of lung cancer in 28 patients with EGFR mutation, and found tumor progression in 21 patients (13 patients with only intracranial progression, 4 with intra- and extracranial progression, and 4 with only extracranial progression). In addition, 14 of the 17 patients with intracranial progression received radiotherapy. The PFS of all the patients was 6.6 months, and OS was 15.9 months. The relatively long survival time in these patients was probably due to the radiotherapy after tumor progression. These findings highlight several drawbacks in using EGFR-TKIs alone for the treatment, which could be associated with the function of blood–brain barrier that restrict the transfer of EGFR-TKIs into brain, and therefore lead to low dose of EGFR-TKIs in the central nervous system. Yang et al[42] reported that the median iPFS was 4.8 and 10.0 months for patients with brain metastasis of NSCLC carrying EGFR mutation in the WBRT and icotinib groups, respectively (P < .05), while the median OS was 20.5 and 18.0 months, respectively (P > .05). Therefore, treating brain metastases from NSCLC using TKI therapy alone seems insufficient. On the other hand, WBRT is not restricted by the blood–brain barrier and may facilitate the intracranial transfer of EGFR-TKIs. Previous studies have already demonstrated that using EGFR-TKIs could increase the sensitivity of tumor cells to radiotherapy[43–45] thus concurrent application might exert a synergistic effect between EGFR-TKIs and radiotherapy. Supporting our finding that concurrently using WBRT and EGFR-TKIs was the most effective treatment.
A phase II clinical trial conducted by Welsh et al[43] revealed that ORR of patients with brain metastasis from NSCLC can be improved to 86% using WNRT combined with erlotinib, and the median survival of patients carrying EGFR mutation was up to 19.1 months. Zeng et al[44] reported ORR and DCR of patients with brain metastasis of NSCLC carrying EGFR mutation of 71.4% and 85.7%, respectively, when using gefitinib combined with WBRT, with median OS of 23.4 months. In the present study, the iORR of patients with brain metastasis from NSCLC treated with EGFR-TKIs alone was 66.7%, while the median OS and iPFS were 16.9 months and 6.8 months, respectively. iORR, median OS, and iPFS were significantly better when EGFR-TKIs were used with WBRT, suggesting that WBRT combined with EGFR-TKIs can improve the prognosis and prolong survival of patients with brain metastasis of NSCLC.
In the present study, the timing (concurrent vs sequential) of WBRT and EGFR-TKI was also studied. The results showed that iORR, median iPFS, and OS were better for patients in the concurrent group compared with the sequential group. This is supported by a previous study that showed that EGFR-TKI combined with WBRT not only prolong the iPFS of the patients, but also improve the OS, compared to EGFR-TKI sequential therapy after WBRT.[46] Previous studies demonstrated that EGFR-TKIs can significantly improve the sensitivity of tumor cells to radiotherapy.[47,48] The possible mechanisms include: promotion of tumor cell apoptosis; reduction of the radiation resistance of tumor cells; inhibition of tumor angiogenesis; inhibition of radiation injury repair; and inhibition of the re-proliferation of tumor cells after radiation injury.[47,48] At present, few studies examined the timing of EGFR-TKIs and WBRT. The results of the present study showed that WBRT combined with EGFR-TKI had better iPFS (12.4 months vs 9.1 months) and higher survival benefit (24.5 months vs 21.1 months) compared to WBRT and EGFR-TKI sequential therapy. As shown in the above mechanisms, this may be due to the synergistic effects from the 2 therapeutic approaches.[47,48] The present study also revealed that adverse events were fewer during the simultaneous application of WBRT and EGFR-TKI than during their sequential use. These results are supported by Magnuson et al[49,50] who showed that the upfront use of EGFR-TKI and deferring WBRT resulted in poorer outcomes compared with radiotherapy followed by EGFR-TKI.
The present study is not without limitations. First, this was a retrospective study with a number of confounding factors. Secondly, the sample size was small. Thirdly, this study only included 2 clinically common EGFR mutations, and there was no comprehensive analysis of the EGFR mutations. In the future, more rigorous and prospective clinical studies with large sample size should be designed to further confirm the optimal timing, modes, and doses of WBRT and EGFR-TKI in the treatment of patients with brain metastasis of NSCLC with EGFR mutation, to provide a higher level of evidence-based treatment for those patients.
In conclusion, the concurrent application of EGFR-TKIs and WBRT effectively improved the iORR, median iPFS, and OS of patients with brain metastasis of NSCLC with EGFR mutation compared to concurrent application or EGFR-TKIs alone without additional adverse events.
Author contributions
Conceptualization: Hualin Chen, Ming Chen.
Data curation: Hualin Chen, Aibing Wu, Hua Tao, Donghong Yang, Yiping Luo, Shujun Li, Zhixiong Yang.
Formal analysis: Hualin Chen, Aibing Wu, Hua Tao, Donghong Yang, Yiping Luo, Shujun Li, Zhixiong Yang, Ming Chen.
Project administration: Ming Chen.
Writing – original draft: Hualin Chen.
Writing – review & editing: Aibing Wu, Hua Tao, Donghong Yang, Yiping Luo, Shujun Li, Zhixiong Yang, Ming Chen.
Footnotes
Abbreviations: CR = complete remission, CT = computed tomography, CTV = clinical target volume, ECOG = Eastern Cooperative Oncology Group, EGFR = epidermal growth factor receptor, ePFS = extracranial progression-free survival, ESMO = European Society for Medical Oncology, iDCR = intracranial disease control rate, iORR = intracranial objective response rate, iPFS = intracranial progression-free survival, MRI = magnetic resonance imaging, NCCN = National Comprehensive Cancer Network, NSCLC = nonsmall cell lung cancer, OS = overall survival, PD = progressive disease, PR = partial remission, RECIST = Response Evaluation Criteria in Solid Tumors, SD = stable disease, TKI = tyrosine kinase inhibitor, WBRT = whole-brain radiotherapy.
The authors have no conflicts of interest to disclose.
References
- [1].Siegel RL, Miller KD, Jemal A. Cancer statistics, 2017. CA Cancer J Clin 2017;67:7–30. [DOI] [PubMed] [Google Scholar]
- [2].Novello S, Barlesi F, Califano R, et al. Metastatic non-small-cell lung cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol 2016;27:v1–27. [DOI] [PubMed] [Google Scholar]
- [3].Ferlay J, Soerjomataram I, Dikshit R, et al. Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer 2015;136:E359–86. [DOI] [PubMed] [Google Scholar]
- [4].Govindan R. Overcoming resistance to targeted therapy for lung cancer. N Engl J Med 2015;372:1760–1. [DOI] [PubMed] [Google Scholar]
- [5].Goldstraw P, Chansky K, Crowley J, et al. The IASLC Lung Cancer Staging Project: proposals for Revision of the TNM Stage Groupings in the Forthcoming (Eighth) Edition of the TNM Classification for Lung Cancer. J Thorac Oncol 2016;11:39–51. [DOI] [PubMed] [Google Scholar]
- [6].Sause W, Kolesar P, Taylor SI, et al. Final results of phase III trial in regionally advanced unresectable non-small cell lung cancer: Radiation Therapy Oncology Group, Eastern Cooperative Oncology Group, and Southwest Oncology Group. Chest 2000;117:358–64. [DOI] [PubMed] [Google Scholar]
- [7].Bhatt VR, Kedia S, Kessinger A, et al. Brain metastasis in patients with non-small-cell lung cancer and epidermal growth factor receptor mutations. J Clin Oncol 2013;31:3162–4. [DOI] [PubMed] [Google Scholar]
- [8].Owen S, Souhami L. The management of brain metastases in non-small cell lung cancer. Front Oncol 2014;4:248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].D’Antonio C, Passaro A, Gori B, et al. Bone and brain metastasis in lung cancer: recent advances in therapeutic strategies. Ther Adv Med Oncol 2014;6:101–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Chi A, Komaki R. Treatment of brain metastasis from lung cancer. Cancers (Basel) 2010;2:2100–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Ebert BL, Niemierko E, Shaffer K, et al. Use of temozolomide with other cytotoxic chemotherapy in the treatment of patients with recurrent brain metastases from lung cancer. Oncologist 2003;8:69–75. [DOI] [PubMed] [Google Scholar]
- [12].Rangachari D, Yamaguchi N, VanderLaan PA, et al. Brain metastases in patients with EGFR-mutated or ALK-rearranged non-small-cell lung cancers. Lung Cancer 2015;88:108–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Soffietti R, Ruda R, Mutani R. Management of brain metastases. J Neurol 2002;249:1357–69. [DOI] [PubMed] [Google Scholar]
- [14].Loganadane G, Hendriks L, Le Pechoux C, et al. The current role of whole brain radiation therapy in non-small cell lung cancer patients. J Thorac Oncol 2017;12:1467–77. [DOI] [PubMed] [Google Scholar]
- [15].Zhu J, Kang M, Fan X. Whole brain radiotherapy for non-small cell lung cancer. Lancet 2017;389:1395. [DOI] [PubMed] [Google Scholar]
- [16].Langley RE, Nankivell M, Barton R, et al. Whole brain radiotherapy for non-small cell lung cancer—authors’ reply. Lancet 2017;389:1395–6. [DOI] [PubMed] [Google Scholar]
- [17].Cagney DN, Alexander BM, Aizer AA. Whole brain radiotherapy for non-small cell lung cancer. Lancet 2017;389:1394–5. [DOI] [PubMed] [Google Scholar]
- [18].Mulvenna P, Nankivell M, Barton R, et al. Dexamethasone and supportive care with or without whole brain radiotherapy in treating patients with non-small cell lung cancer with brain metastases unsuitable for resection or stereotactic radiotherapy (QUARTZ): results from a phase 3, non-inferiority, randomised trial. Lancet 2016;388:2004–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Inoue A, Yoshida K, Morita S, et al. Characteristics and overall survival of EGFR mutation-positive non-small cell lung cancer treated with EGFR tyrosine kinase inhibitors: a retrospective analysis for 1660 Japanese patients. Jpn J Clin Oncol 2016;46:462–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Inoue A, Kobayashi K, Maemondo M, et al. Updated overall survival results from a randomized phase III trial comparing gefitinib with carboplatin-paclitaxel for chemo-naive non-small cell lung cancer with sensitive EGFR gene mutations (NEJ002). Ann Oncol 2013;24:54–9. [DOI] [PubMed] [Google Scholar]
- [21].Takano T, Fukui T, Ohe Y, et al. EGFR mutations predict survival benefit from gefitinib in patients with advanced lung adenocarcinoma: a historical comparison of patients treated before and after gefitinib approval in Japan. J Clin Oncol 2008;26:5589–95. [DOI] [PubMed] [Google Scholar]
- [22].Ma S, Xu Y, Deng Q, et al. Treatment of brain metastasis from non-small cell lung cancer with whole brain radiotherapy and Gefitinib in a Chinese population. Lung Cancer 2009;65:198–203. [DOI] [PubMed] [Google Scholar]
- [23].Iuchi T, Shingyoji M, Sakaida T, et al. Phase II trial of gefitinib alone without radiation therapy for Japanese patients with brain metastases from EGFR-mutant lung adenocarcinoma. Lung Cancer 2013;82:282–7. [DOI] [PubMed] [Google Scholar]
- [24].Cai Y, Wang JY, Liu H. Clinical observation of whole brain radiotherapy concomitant with targeted therapy for brain metastasis in non-small cell lung cancer patients with chemotherapy failure. Asian Pac J Cancer Prev 2013;14:5699–703. [DOI] [PubMed] [Google Scholar]
- [25].Iuchi T, Shingyoji M, Itakura M, et al. Frequency of brain metastases in non-small-cell lung cancer, and their association with epidermal growth factor receptor mutations. Int J Clin Oncol 2015;20:674–9. [DOI] [PubMed] [Google Scholar]
- [26].Shin DY, Na II, Kim CH, et al. EGFR mutation and brain metastasis in pulmonary adenocarcinomas. J Thorac Oncol 2014;9:195–9. [DOI] [PubMed] [Google Scholar]
- [27].Stanic K, Zwitter M, Hitij NT, et al. Brain metastases in lung adenocarcinoma: impact of EGFR mutation status on incidence and survival. Radiol Oncol 2014;48:173–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Noronha V, Joshi A, Gokarn A, et al. The importance of brain metastasis in EGFR mutation positive NSCLC patients. Chemother Res Pract 2014;2014:856156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Dziadziuszko R. Whole brain radiotherapy for patients with non-small cell lung cancer with EGFR mutations—why and when? J Thorac Oncol 2016;11:1604–5. [DOI] [PubMed] [Google Scholar]
- [30].Eisenhauer EA, Vermorken JB. The taxoids. Comparative clinical pharmacology and therapeutic potential. Drugs 1998;55:5–30. [DOI] [PubMed] [Google Scholar]
- [31].Chen AP, Setser A, Anadkat MJ, et al. Grading dermatologic adverse events of cancer treatments: the Common Terminology Criteria for Adverse Events Version 4.0. J Am Acad Dermatol 2012;67:1025–39. [DOI] [PubMed] [Google Scholar]
- [32].Lu Y, Fan Y. Combined action of EGFR tyrosine kinase inhibitors and whole-brain radiotherapy on EGFR-mutated non-small-cell lung cancer patients with brain metastasis. Onco Targets Ther 2016;9:1135–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Xiang Z, Chen J, Zhang H, et al. Whole brain radiotherapy-based combined modality treatment of brain metastases from non-small cell lung cancer: a retrospective analysis of prognostic factors. Oncol Res Treat 2015;38:35–40. [DOI] [PubMed] [Google Scholar]
- [34].Putora PM, Ess S, Panje C, et al. Prognostic significance of histology after resection of brain metastases and whole brain radiotherapy in non-small cell lung cancer (NSCLC). Clin Exp Metastasis 2015;32:143–9. [DOI] [PubMed] [Google Scholar]
- [35].Garraway LA, Verweij J, Ballman KV. Precision oncology: an overview. J Clin Oncol 2013;31:1803–5. [DOI] [PubMed] [Google Scholar]
- [36].Wu YL, Chu DT, Han B, et al. Phase III, randomized, open-label, first-line study in Asia of gefitinib versus carboplatin/paclitaxel in clinically selected patients with advanced non-small-cell lung cancer: evaluation of patients recruited from mainland China. Asia Pac J Clin Oncol 2012;8:232–43. [DOI] [PubMed] [Google Scholar]
- [37].Mitsudomi T, Morita S, Yatabe Y, et al. Gefitinib versus cisplatin plus docetaxel in patients with non-small-cell lung cancer harbouring mutations of the epidermal growth factor receptor (WJTOG3405): an open label, randomised phase 3 trial. Lancet Oncol 2010;11:121–8. [DOI] [PubMed] [Google Scholar]
- [38].Ettinger DS, Aisner DL, Wood DE, et al. NCCN Guidelines Insights: Non-Small Cell Lung Cancer, Version 5.2018. J Natl Compr Canc Netw 2018;16:807–21. [DOI] [PubMed] [Google Scholar]
- [39].Yusuf SW, Kim P, Durand JB. Erlotinib or gefitinib for non-small-cell lung cancer. N Engl J Med 2011;364:2367author reply 2368. [DOI] [PubMed] [Google Scholar]
- [40].Shi Y, Zhang L, Liu X, et al. Icotinib versus gefitinib in previously treated advanced non-small-cell lung cancer (ICOGEN): a randomised, double-blind phase 3 non-inferiority trial. Lancet Oncol 2013;14:953–61. [DOI] [PubMed] [Google Scholar]
- [41].Park SJ, Kim HT, Lee DH, et al. Efficacy of epidermal growth factor receptor tyrosine kinase inhibitors for brain metastasis in non-small cell lung cancer patients harboring either exon 19 or 21 mutation. Lung Cancer 2012;77:556–60. [DOI] [PubMed] [Google Scholar]
- [42].Yang JJ, Zhou C, Huang Y, et al. Icotinib versus whole-brain irradiation in patients with EGFR-mutant non-small-cell lung cancer and multiple brain metastases (BRAIN): a multicentre, phase 3, open-label, parallel, randomised controlled trial. Lancet Respir Med 2017;5:707–16. [DOI] [PubMed] [Google Scholar]
- [43].Welsh JW, Komaki R, Amini A, et al. Phase II trial of erlotinib plus concurrent whole-brain radiation therapy for patients with brain metastases from non-small-cell lung cancer. J Clin Oncol 2013;31:895–902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Zeng YD, Liao H, Qin T, et al. Blood-brain barrier permeability of gefitinib in patients with brain metastases from non-small-cell lung cancer before and during whole brain radiation therapy. Oncotarget 2015;6:8366–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Park SY, Kim YM, Pyo H. Gefitinib radiosensitizes non-small cell lung cancer cells through inhibition of ataxia telangiectasia mutated. Mol Cancer 2010;9:222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Sperduto PW, Wang M, Robins HI, et al. A phase 3 trial of whole brain radiation therapy and stereotactic radiosurgery alone versus WBRT and SRS with temozolomide or erlotinib for non-small cell lung cancer and 1 to 3 brain metastases: Radiation Therapy Oncology Group 0320. Int J Radiat Oncol Biol Phys 2013;85:1312–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Zhang J, Shi X, Cai D, et al. MINI01.11: radiotherapy plus EGFR TKIs for brain metastasis in EGFR-mutant non-small cell lung cancer: a retrospective analysis of a single institution: topic: medical oncology. J Thorac Oncol 2016;11:S263. [Google Scholar]
- [48].Ulahannan D, Lee SM. Erlotinib plus concurrent whole-brain radiation therapy for non-small cell lung cancers patients with multiple brain metastases. Transl Lung Cancer Res 2016;5:208–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Magnuson WJ, Lester-Coll NH, Wu AJ, et al. Management of brain metastases in tyrosine kinase inhibitor-naive epidermal growth factor receptor-mutant non-small-cell lung cancer: a retrospective multi-institutional analysis. J Clin Oncol 2017;35:1070–7. [DOI] [PubMed] [Google Scholar]
- [50].Magnuson WJ, Yeung JT, Guillod PD, et al. Impact of deferring radiation therapy in patients with epidermal growth factor receptor-mutant non-small cell lung cancer who develop brain metastases. Int J Radiat Oncol Biol Phys 2016;95:673–9. [DOI] [PubMed] [Google Scholar]


