Abstract
Objective:
To assess the accuracy of MRI to predict remaining lymph node metastases in patients with complete pathological luminal response (ypT0) after neoadjuvant therapy.
Methods:
Data from a national registry were used. 19 patients with histopathologically remaining lymph node metastases (ypT0N+) were identified. Another 19 patients without lymph node metastases (ypT0N0) were used as matched controls. Two radiologists blinded to all patient information evaluated staging and restaging MRI that was compared to histopathological findings of the resected specimen.
Results:
The average size of the largest lymph node on restaging MRI was significantly larger (4.5 mm) in the ypT0N+ group than in the ypT0N0 group (2.6 mm) (p = 0.04). Presence of ypN+ was correctly predicted by MRI in 7 of 19 patients. In patients without lymph node metastases (ypT0N0), these were correctly classified by MRI in 16 of 19 patients. All patients who had MR-identified lymph nodes larger than 8 mm at restaging were ypTN+. The sensitivity, specificity, positive predictive value and negative for prediction of remaining lymph node metastasis with MRI were 37, 84, 70 and 57%.
Conclusion:
In patients with ypT0 in rectal cancer after neoadjuvant treatment, remaining regional lymph node metastases cannot safely be predicted by restaging MRI alone using presently known criteria. Presence of a lymph node over 8 mm on restaging MRI strongly indicates yPN+.
Advances in knowledge:
This is one of the first studies on MRI lymph node assessment after chemo-radiotherapy (CRT) in luminal complete response.
Introduction
Colorectal cancer is an age dependent malignancy that is the second most common cause of cancer related death in the western world. The rectum constitutes the last 15 cm of the large bowel above the anal verge. Rectal cancer accounts for approximately 30% of all colorectal malignancies. Surgery has been the mainstay of treatment and is often combined with both neoadjuvant radiotherapy (RT) or chemo-radiotherapy (CRT) and adjuvant chemotherapy. This treatment is associated with a high morbidity rate including a temporary or permanent stoma.1
Post RT/CRT MRI if performed previously has had limited impact on treatment as most patients went on to surgery irrespective of the local restaging, which was mostly used to rule out tumour progression. More advanced neoadjuvant treatment regimens, extended and tailored delays between CRT and surgery results in a higher rate of complete pathological response, pCR (ypT0N0M0) that has widened the treatment options.2–4 Clinical complete response, (cCR, ycT0N0M0) is the absence of remaining tumour in the pelvis after neoadjuvant treatment prior to surgery. While the definition of pCR is undisputed, the clinical staging is based on clinical findings of the mucosa in the lumen together with radiological imaging of the primary tumour and the pelvic lymph nodes.4 In cCR there is no visible or palpable tumour in the rectal lumen and no radiological remnant tumour in the pelvis. Confirmed cCR can be an indication for a non-operative management with a watch and wait policy with regular short interval MRI and endoscopy follow-up instead of standard radical surgery.2–5
Depending on inclusion criteria and treatment protocol, post CRT pCR rates up to 42% have been described in selected studies.2–4,6,7 Both cCR and pCR have also been shown to improve survival and reduce the risk of both local and systemic recurrences.8 However, there is approximately a 20% risk of tumour regrowth in the pelvis after cCR with non-operative management. Even if the outcome after salvage surgery is no worse than for those primarily operated2–5 it would be of interest to predict those with a remaining ycN+ stage to exclude these patients from a non-operative management.
Neoadjuvant treatment has effect both on the primary tumour and the pelvic lymph nodes. Treatment response in the primary tumour and the lymph nodes may show discrepancies. In up to 17% of patients with luminal complete response has been shown to have tumour positive lymph nodes (pN+) in the resected specimen.9, 10 These tumour positive nodes correlate to reduced survival even after surgery10, 11 and MRI detection of tumour positive lymph nodes would therefore be of great importance when deciding upon a non-operative management after CRT treatment.
Several studies, including two meta-analyses, have demonstrated limitations in post CRT MRI-restaging of positive lymph nodes with sensitivities ranging between 37–67% and specificity around 80%, with both over and under staging.12–15 These figures so far indicate that MRI alone cannot be used to safely rule out remnant malignant lymph nodes after CRT.
To our knowledge, no other study has addressed the issue of MRI nodal restaging in luminal complete response after neoadjuvant treatment for rectal cancer. The aim of this study was to retrospectively study MRI nodal staging after RT/CRT in patients with luminal complete response in relation to histopathological findings.
The study was approved by the Regional Ethics Committee and the board of the Swedish Colo-Rectal Cancer Registry.
Methods and materials
Patients
Patients registered in the SCRCR between 2007 and 2012 were included in the study and has been presented in detail in a previous study.16 Clinical background, data on surgery and pathology was retrieved from the registry, while data on RT/CRT and MRI imaging was gathered from individual patient records from the 161 patients with ypT0.
The initial study cohort consisted of 1,1226 patients with a median age of 71 years (range 19–99 years) whereof 59 % were male. An abdominal operation was performed on 7885 (70%) of the patients and 56% had pre-operative RT or CRT. The majority of that group had advanced cT3 (48%) and cT4 (36%) cancer. 26 % (2063 patients) were classified as having potential for pCR after receiving LRT or SRT with delay, with or without chemotherapy before abdominal surgery. The average lymph-node yield was 16 over the study period, indicating adequate surgical and pathological quality.
From this group of 2063 patients, 161 patients with luminal complete response (ypT0Nall) were identified. 26 of these patients had ypT0N+ and we were able to retrieve complete medical records and both primary and restaging MRI investigations in 19 of those patients. Another 19 patients with ypT0N0 matched for treating hospitals were selected as a control group, (Table 1).
Table 1.
Description of the study population
| Gender | All | N+ | N0 | p |
| Female | 17 | 11 | 6 | 0.10 |
| Male | 21 | 8 | 13 | |
| Average age at surgery (range), years | 57 (37–74) |
54 (37–69) |
61 (37–74) |
0.05 |
| cT-stage | 0.18 | |||
| T1-T2 | 9 | 5 | 4 | |
| T3 | 14 | 8 | 6 | |
| T4 | 14 | 5 | 9 | |
| Tx | 1 | 1 | ||
| Radiation | 0.73 | |||
| 28 × 1.8 Gy | 27 | 13 | 14 | |
| 5 × 5 Gy with delay | 11 | 6 | 5 | |
| Chemotherapy | 0.18 | |||
| None | 7 | 3 | 4 | |
| 5-FU/capecitabine | 18 | 7 | 11 | |
| 5-FU/capecitabine +oxaliplatiine |
13 | 9 | 4 | |
| Sum | 38 | 19 | 19 |
Pre-operative RT/CRT
Indications for neoadjuvant treatment
Long-term pre-operative radiotherapy (LRT), (e.g. 28 × 1.8 Gy), with 6–8 weeks interval to surgery is given for T4 and T3 tumours with suspected involvement of lateral lymph nodes or an involved circumferential resection margin. An alternative is standard CRT treatment including LRT+ concomitant 5-FU/capacitabine followed by surgery after a delay of 6–8 weeks. Short-term radiotherapy (SRT), 5 × 5 Gy followed by surgery within 5 days is used for less advanced tumours, and has no effect on down staging due to the short time between radiation and surgery. Surgery can, however, be delayed for several reasons, thereby increasing the chance of SRT of having a down staging effect as well. This is termed “SRT with delay”. The standard treatment for T3b tumours in the middle and lower rectum as well as meso-rectal N+ tumours is SRT, provided the circumferential resection margin is clear. Decisions on neoadjuvant therapy, surgery and adjuvant therapy are usually made at a multidisciplinary team conference, and are based on national guidelines and knowledge of the individual patient.
Pre-operative radiotherapy was normally given in a CT-based individual dose regimen to optimize the four-field three-dimensional technique. The target area covered the primary tumour, meso-rectum and secondary glands in the pre-sacral area, along the superior rectal artery and the obturator artery, but normally not the external iliac glands. According to standard protocols, the external beams were delivered with 8–15 MV photons. The daily target dose for SRT was 5 Gy with a total dose of 25 Gy over 5 consecutive days. For LRT the daily dose was 1.8 Gy up to a total dose of 50.4 Gy. The standard treatment for T4 tumours was LRT in combination with 5FU/capacitabin. Individual regimens where chemotherapy was either added to SRT or omitted from LRT were also seen (Table 1).
MR imaging
The MRI examinations were done in different radiological departments in Sweden reflecting the geographical distribution of the patients. Regarding field-strength, 1T, 1.5T as well as 3T systems were used. The examination protocols varied but all included sagittal, transaxial and oblique T2 weighted images in at least two planes including images perpendicular to the rectal wall as well as transaxial T1 weighted images. In 75% of the examinations the perpendicular sequences were thin-section (3 mm slices) T2 weighted sequences.
Diffusion-weighted images were available in a limited proportion of the patients and were not reviewed
MR Image Interpretation
All images were assessed first independently and then in consensus by two radiologists with 2 years (MS) and more than 20 years of experience (LB) in reading pelvic MRI using a Sectra workstation (Sectra PACS station IDS7TM/DX). The images were reviewed aware of the inclusion criteria but blinded to surgical and pathological findings. The examinations before and after neoadjuvant treatment were assessed together. One of the reviewers (LB) made screenshots of the particular sections containing lymph nodes and marked the lymph nodes with cursors in order to make sure that the other reviewer evaluated the same lymph nodes (Figures 1 and 2).
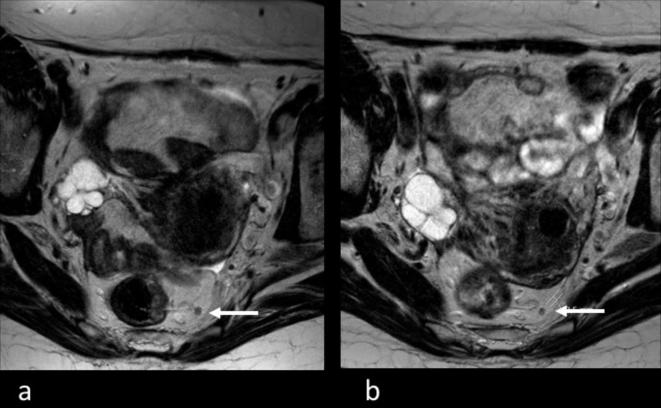
Figure 1.
63-year-old female patient treated with CRT for rectal cancer. Transverse T2 weighted images before (a) and after (b) neoadjuvant treatment. Before treatment, the MRI stage based on morphological criteria was mrN1 and the largest suspicious lymph node 4 mm (a, white arrow). After CRT, the lymph node was reduced to 2.5 mm (b, white arrow) resulting in ymrN0. Pathological staging was ypN1 due to one 2 mm remaining small metastasis. CRT, chemo-radiotherapy.
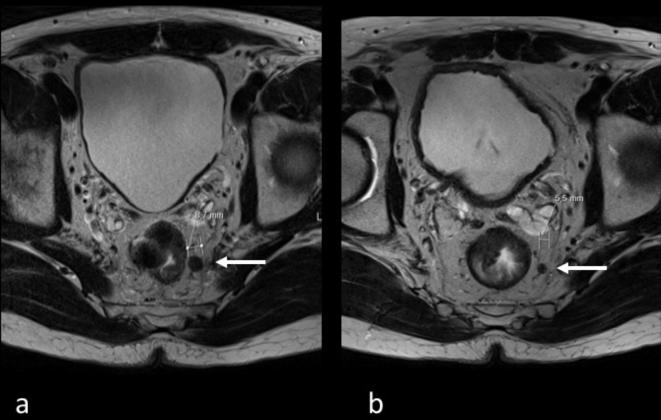
Figure 2.
51-year-old male patient treated with CRT for rectal cancer. Transverse T2 weighted images before (a) and after (b) neoadjuvant treatment. Before treatment, the MRI stage based on morphological criteria was mrN1 and the largest suspicious lymph node 8.7 mm (a, white arrow). After CRT, the lymph node reduced to 5.5 mm (b, white arrow). MRI post CRT stage based on size cut-off criteria was therefore ymrN1 and correlated with pathology (ypN1). CRT, chemo-radiotherapy.
At each imaging review, the following was recorded:
Tumour stage. The TNM T-stage of the tumour as assessed by MRI was recorded. Extramural depth of tumour invasion as well as involvement of the meso-rectal fascia was noted according to previously established criteria.17
Nodal number. The total number of lymph nodes was identified in each patient, including both potentially benign and malignant lymph nodes.
Nodal size. The maximum short-axis diameter of each node was measured in millimeters.
Nodal distribution. The position of the lymph nodes was recorded as located in the meso-rectum or lateral outside the meso-rectum.
Nodal status and stage. The lymph nodes identified at MRI were determined to be malignant or non-malignant using morphological criteria.18, 19 At pre-treatment, lymph node was considered to be malignant if it showed irregular outer border or internal signal heterogeneity at the pre-treatment MRI. On the post CRT MRI, size criteria were used and lymph nodes exceeding 5 mm were regarded as malignant based on discussions in previously reported studies.14, 20
Surgical treatment
An abdominal operation was performed on all patients. Surgery was performed according to the total meso-rectal excision principles of Heald.21 For middle and low rectal cancers, the entire meso-rectum was resected as an intact unit. For high rectal cancers, the meso-rectum was divided 5 cm distal to the lower border of the tumour.
Pathology
Histopathological assessment was performed on all surgical specimens and staged according to the AJCC Cancer Staging Manual 7th edition.22
Statistics
The Student’s t-test was used to test the significance of the differences between the cases and the control group. Values were given as mean, and range indicated size of individual lymph nodes or number of lymph nodes.
The tests were two-sided, and a value of p < 0.05 was considered statistically significant. Logistic regression was used to analyse the effect of differences between the cases and the control group. Statistical analyses were performed using SPSS©, IBM © (v. 22)(IBM Corp. Armonk, NY).
Results
The mean time between post CRT MRI-staging and surgery was 21 days (range 6–68) with no statistical difference between the N0 and N + group (21 and 25 days, p = 0.27).
Pre CRT MRI staging
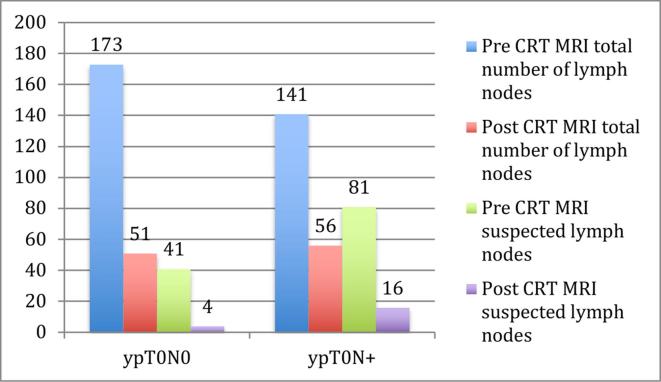
On the pre CRT MRI staging a total number of 314 lymph nodes were detected, and 122 (39%) of them were suspected to be malignant. 29 patients out of the 38 had suspected malignant lymph nodes on the pre CRT MRI staging. Two patients had no detectable lymph nodes and two had 20 or more (20 and 29 lymph nodes).
The mean number of lymph nodes on MRI in the ypN0 group was 9 (range 1–20) and in the ypN+ group 8 (range 0–29) (p = 0.5) (Figure 3).
Figure 3.
Detected lymph nodes in relation to N-stage. CRT, chemo-radiotherapy.
The mean size of the lymph nodes in the ypN0 group was 4.0 mm (range 2–12 mm) and in the ypN+ group it was 3.8 mm (range 2–17 mm) (p = 0.65). The total sum of size was 645 and 612 mm, respectively (p = 0.81).
Post RT/CRT MRI staging vs histopathology (ymrN vs ypN)
At the post RT/CRT restaging MRI (mean 48, median 33 (range 27–184) days after the completion of CRT) 107 lymph nodes were detected and 20 (19%) of them were suspected to be malignant. Eight patients had no detectable lymph nodes, and one had more than 10 (11 lymph nodes). 29 out of 38 patients had no suspected malignant node (ymrN0). The mean number of MR detected lymph nodes was 2.7 (range 0–11) in the ypN0 group compared to 2.9 nodes (range 0–8) in the ypN+group (p = 0.75) (Figure 3).
The mean lymph node size in the ypT0N0 group was 1.9 mm (range 1–7 mm) and 3.0 mm (range 2–12 mm) in the ypT0N+ group (p = 0.12). The total sum size was 144 and 225 mm, respectively (p = 0.21).
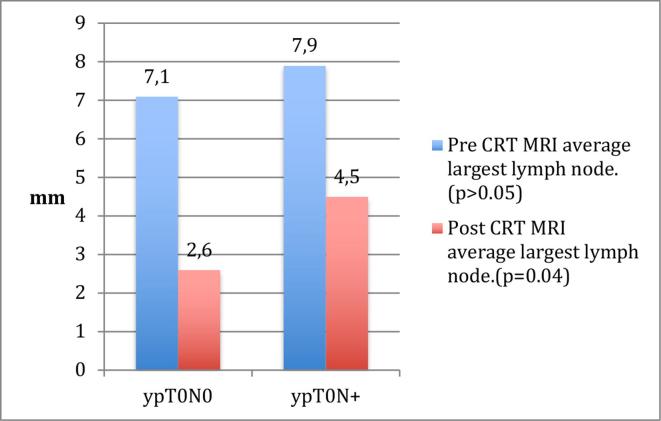
When comparing the mean size for the largest node for every patient there was a significant difference between the ypN+group and the ypN0 group (4.5 mm (range 2–12 mm) vs 2.6 mm (range 2–7 mm) (p = 0.04) (Figure 4).
Figure 4.
Average size of the largest lymph node. CRT, chemo-radiotherapy.
In multiple regression analysis different forms of chemotherapy or radiotherapy did not alter the results for the size of the largest lymph node.
Nodes larger than 8 mm were only found in N+ patients (n = 3).
Two patients with no detected lymph nodes on MRI staging were still staged ypN+ at the pathology report.
Of the 19 patients who were ypN+12 were MRI staged as N0, and 3 of the 19 ypN0 were on MRI staged as N+. Sensitivity, specificity, positive predictive value and negative predictive value for the radiological prediction of N+ in ypT0 patients were 37, 84, 70 and 57%.
Discussion
The aim of this study was to assess the accuracy of MRI to predict the presence of remaining meso-rectal lymph node metastases in patients with complete pathological luminal response after neoadjuvant radiotherapy (RT) or CRT. Using 5 mm as a cut-off post CRT, we found a sensitivity of 37% and a specificity of 84% indicating low sensitivity of post CRT imaging for nodal staging in rectal cancer. This is known from earlier studies. A focus on specificity would render a larger cut-off size and opposite with a better sensitivity, a smaller cut-off size. Although the sensitivity was low as compared to the specificity in the current study, a high specificity may be the preferred alternative in to select patients for a watch and wait approach.18, 23 The strong correlation of nodes larger than 8 mm and a node positive specimen indicates a cut-off size with high positive predictive value.
The detection of tumour positive lymph nodes or tumour remnants in radiated tissue can change the path from no surgery at all to major curative surgery. MRI is currently the gold standard in restaging neoadjuvant treated rectal cancer both for the primary tumour and the pelvic lymph nodes. Endo-luminal examination and rectal digital palpation is mandatory to confirm luminal complete response but for lymph node staging there is no other available modality proven better than MRI.24, 25 Following RT/CRT there is usually a shrinking of the primary tumour, reduction of both number and size of lymph nodes and variable replacement of tumour by fibrosis.9, 26 The rate of involved loco-regional lymph nodes is related to the tumour stage and the level of tumour differentiation.27, 28 After CRT 84% of all lymph nodes tend to decrease in size or disappear, while 16% has no change in size or even increase in size.20 After RT/CRT it is well-known that lymph nodes with histologically confirmed tumour growth are significantly larger on MRI than uninvolved lymph nodes at histology.29 CRT has been shown to reduce the rate of tumour positive lymph nodes at histopathology from around 40 to 25% in a group of almost 800 patients when comparing pre-operative or post-operative CRT.30
Several studies have pinpointed challenges of MRI in detecting tumour positive lymph nodes after CRT with a variation from 72–96% accuracy for lymph node restaging and different conclusion on the value of MRI in clinical practice.12, 13,31 There is still to find a threshold value for size that can rule out malignant lymph nodes by sheer size on post CRT MRI. Defining the optimal cut-off size is challenged by differences in initial tumour stage, CRT regimens and time between CRT treatment, reevaluation MRI and the following surgery in the reported studies. The role of remnant tumour positive lymph nodes is not clear-cut as up to 20% of node negative patients develop metastases, while only 50% of the node positive patients develop metastases.32 Few, if any other study has focused exclusively on ypT0 and nodal staging where the issue of surgery or non-operative management is depending on lymph node status when the primary tumour is eradicated. The current study on ypT0 and lymph node staging shows that CRT has a substantial effect on the size and number of detected lymph nodes, which is in accordance with other studies.20
Regarding suspected lymph nodes we could see a trend towards more suspected lymph nodes both before and after CRT in the ypT0N+ group (Figure 3). Furthermore, there was no significant difference in size of MRI identified average size of meso-rectal lymph nodes in the N+ and N0 groups as assessed by post CRT MRI that is in contrast to other studies that has reported differences of 2.5 and 4.5 mm.20, 33 To note is also that two patients (5%) with no identified lymph nodes on post CRT MRI were still pN+. In contrast to previous studies, we excluded all but complete responders in our study population. This makes comparison with other studies difficult, as they comprise a greater variety in the post CRT T-stage since T-stage is related to lymph node status.27, 28 When we analysed the average size of the largest lymph node we did find that the largest lymph node was significantly larger in the ypT0N+ group than in the ypT0N0 group. Thus, presence of the largest lymph node >4.5 mm in size may indicate N+ stage.
There are some limitations with this study. A node-per-node pathological-radiological matching could not be performed since pathology data was retrieved from the existing registry and pathology reports were not reviewed. Another limitation is the small number of patients, making the study prone to a Type 2-error with difficulty to state significant changes between the two groups. The strengths of the study are: (1) The quality of the patient registry. (2) The exclusive group of patients with luminal complete response. (3) The quality of the surgical and pathological procedures. (4) A standardised examination protocol for the MRI re-evaluation.
The implications of this study need to be confirmed in future prospective studies. This study confirms results from earlier studies with relatively low accuracy in lymph node staging. However, restaging is better than the initial staging and can be iterated during the waiting time for surgery for better accuracy. Clinical staging is also combined with endoscopic and digital examination as a combined method. As there is no better option today, we should interpret MR findings of lymph nodes with caution after CRT. Large lymph nodes over 8 mm could indicate node positivity. Despite the limitations of MRI staging, non-operative management is so far safe with very low numbers of local or distant metastases.
In summary, this is one of the first studies on assessment of remaining lymph node metastases by MRI in patients with luminal complete response after RT/CRT of rectal cancer. We showed a large reduction of both of size and number of suspected malignant and benign lymph nodes after CRT compared to pre-treatment. The size of the largest lymph node seems to predict lymph node positivity at histopathology in this study; but size as the only criterion is not a safe predictor of nodal involvement.18 The study also highlights the need to standardise all image acquisition, interpretation, treatment, time between RT/CRT and imaging and surgery in prospective cohorts of patients, both in wait and watch approaches or and in patients surgically treated to be able to better predict lymph nodal status in patients with luminal complete response in rectal cancer.
Contributor Information
Per Loftås, Email: Per.loftas@liu.se.
Margrét Sturludóttir, Email: margret.sturludottir@gmail.com.
Olof Hallböök, Email: Olof.hallbook@liu.se.
Karin Almlöv, Email: Karin.almlov@regionostergotland.se.
Gunnar Arbman, Email: Gunnar.arbman@regionostergotland.se.
Lennart Blomqvist, Email: Lennart.k.blomqvist@ki.se.
References
- 1.Glimelius B, Grönberg H, Järhult J, Wallgren A, Cavallin-Ståhl E. A systematic overview of radiation therapy effects in rectal cancer. Acta Oncol 2003; 42: 476–92. doi: 10.1080/02841860310012301 [DOI] [PubMed] [Google Scholar]
- 2.Glynne-Jones R, Hughes R. Critical appraisal of the 'wait and see' approach in rectal cancer for clinical complete responders after chemoradiation. Br J Surg 2012; 99: 897–909. doi: 10.1002/bjs.8732 [DOI] [PubMed] [Google Scholar]
- 3.Habr-Gama A, Perez RO. Non-operative management of rectal cancer after neoadjuvant chemoradiation. Br J Surg 2009; 96: 125–7. doi: 10.1002/bjs.6470 [DOI] [PubMed] [Google Scholar]
- 4.Habr-Gama A, Perez RO, São Julião GP, Proscurshim I, Gama-Rodrigues J. Nonoperative approaches to rectal cancer: a critical evaluation. Semin Radiat Oncol 2011; 21: 234–9. doi: 10.1016/j.semradonc.2011.02.010 [DOI] [PubMed] [Google Scholar]
- 5.Maas M, Beets-Tan RG, Lambregts DM, Lammering G, Nelemans PJ, Engelen SM, et al. Wait-and-see policy for clinical complete responders after chemoradiation for rectal cancer. J Clin Oncol 2011; 29: 4633–40. doi: 10.1200/JCO.2011.37.7176 [DOI] [PubMed] [Google Scholar]
- 6.Glynne-Jones R, Hughes R. Complete response after chemoradiotherapy in rectal cancer (Watch-and-Wait): have we cracked the code? Clin Oncol 2016; 28: 152–60. doi: 10.1016/j.clon.2015.10.011 [DOI] [PubMed] [Google Scholar]
- 7.Habr-Gama A, Sabbaga J, Gama-Rodrigues J, São Julião GP, Proscurshim I, Bailão Aguilar P, et al. Watch and wait approach following extended neoadjuvant chemoradiation for distal rectal cancer: are we getting closer to anal cancer management? Dis Colon Rectum 2013; 56: 1109–17. doi: 10.1097/DCR.0b013e3182a25c4e [DOI] [PubMed] [Google Scholar]
- 8.Maas M, Nelemans PJ, Valentini V, Das P, Rödel C, Kuo LJ, et al. Long-term outcome in patients with a pathological complete response after chemoradiation for rectal cancer: a pooled analysis of individual patient data. Lancet Oncol 2010; 11: 835–44. doi: 10.1016/S1470-2045(10)70172-8 [DOI] [PubMed] [Google Scholar]
- 9.Bedrosian I, Rodriguez-Bigas MA, Feig B, Hunt KK, Ellis L, Curley SA, et al. Predicting the node-negative mesorectum after preoperative chemoradiation for locally advanced rectal carcinoma. J Gastrointest Surg 2004; 8: 56–63. doi: 10.1016/j.gassur.2003.09.019 [DOI] [PubMed] [Google Scholar]
- 10.Yeo SG, Kim DY, Kim TH, Chang HJ, Oh JH, Park W, et al. Pathologic complete response of primary tumor following preoperative chemoradiotherapy for locally advanced rectal cancer: long-term outcomes and prognostic significance of pathologic nodal status (KROG 09-01). Ann Surg 2010; 252: 998–1004. doi: 10.1097/SLA.0b013e3181f3f1b1 [DOI] [PubMed] [Google Scholar]
- 11.Loftås P, Arbman G, Fomichov V, Hallböök O. Nodal involvement in luminal complete response after neoadjuvant treatment for rectal cancer. Eur J Surg Oncol 2016; 42: 801–7. doi: 10.1016/j.ejso.2016.03.013 [DOI] [PubMed] [Google Scholar]
- 12.Memon S, Lynch AC, Bressel M, Wise AG, Heriot AG. Systematic review and meta-analysis of the accuracy of MRI and endorectal ultrasound in the restaging and response assessment of rectal cancer following neoadjuvant therapy. Colorectal Dis 2015; 17: 748–61. doi: 10.1111/codi.12976 [DOI] [PubMed] [Google Scholar]
- 13.Huh JW, Kim HC, Lee SJ, Yun SH, Lee WY, Park YA, et al. Diagnostic accuracy and prognostic impact of restaging by magnetic resonance imaging after preoperative chemoradiotherapy in patients with rectal cancer. Radiother Oncol 2014; 113: 24–8. doi: 10.1016/j.radonc.2014.08.023 [DOI] [PubMed] [Google Scholar]
- 14.Koh DM, Chau I, Tait D, Wotherspoon A, Cunningham D, Brown G. Evaluating mesorectal lymph nodes in rectal cancer before and after neoadjuvant chemoradiation using thin-section T2-weighted magnetic resonance imaging. Int J Radiat Oncol Biol Phys 2008; 71: 456–61. doi: 10.1016/j.ijrobp.2007.10.016 [DOI] [PubMed] [Google Scholar]
- 15.Zhao RS, Wang H, Zhou ZY, Zhou Q, Mulholland MW. Restaging of locally advanced rectal cancer with magnetic resonance imaging and endoluminal ultrasound after preoperative chemoradiotherapy: a systemic review and meta-analysis. Dis Colon Rectum 2014; 57: 388–95. doi: 10.1097/DCR.0000000000000022 [DOI] [PubMed] [Google Scholar]
- 16.Loftås P, Arbman G, Fomichov V, Hallböök O. Nodal involvement in luminal complete response after neoadjuvant treatment for rectal cancer. Eur J Surg Oncol 2016; 42: 801–7. doi: 10.1016/j.ejso.2016.03.013 [DOI] [PubMed] [Google Scholar]
- 17.MERCURY Study Group Extramural depth of tumor invasion at thin-section MR in patients with rectal cancer: results of the MERCURY study. Radiology 2007; 243: 132–9. doi: 10.1148/radiol.2431051825 [DOI] [PubMed] [Google Scholar]
- 18.Brown G, Richards CJ, Bourne MW, Newcombe RG, Radcliffe AG, Dallimore NS, et al. Morphologic predictors of lymph node status in rectal cancer with use of high-spatial-resolution MR imaging with histopathologic comparison. Radiology 2003; 227: 371–7. doi: 10.1148/radiol.2272011747 [DOI] [PubMed] [Google Scholar]
- 19.Kim JH, Beets GL, Kim MJ, Kessels AG, Beets-Tan RG. High-resolution MR imaging for nodal staging in rectal cancer: are there any criteria in addition to the size? Eur J Radiol 2004; 52: 78–83. doi: 10.1016/j.ejrad.2003.12.005 [DOI] [PubMed] [Google Scholar]
- 20.Heijnen LA, Maas M, Beets-Tan RG, Berkhof M, Lambregts DM, Nelemans PJ, et al. Nodal staging in rectal cancer: why is restaging after chemoradiation more accurate than primary nodal staging? Int J Colorectal Dis 2016; 31: 1157–62. doi: 10.1007/s00384-016-2576-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Heald RJ, Ryall RD. Recurrence and survival after total mesorectal excision for rectal cancer. Lancet 1986; 1: 1479–82. doi: 10.1016/S0140-6736(86)91510-2 [DOI] [PubMed] [Google Scholar]
- 22.Hari DM, Leung AM, Lee JH, Sim MS, Vuong B, Chiu CG, et al. AJCC cancer staging manual 7th edition criteria for colon cancer: do the complex modifications improve prognostic assessment? J Am Coll Surg 2013; 217: 181–90. doi: 10.1016/j.jamcollsurg.2013.04.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Vogl TJ, Pegios W, Mack MG, Hünerbein M, Hintze R, Adler A, et al. Accuracy of staging rectal tumors with contrast-enhanced transrectal MR imaging. AJR Am J Roentgenol 1997; 168: 1427–34. doi: 10.2214/ajr.168.6.9168702 [DOI] [PubMed] [Google Scholar]
- 24.Maretto I, Pomerri F, Pucciarelli S, Mescoli C, Belluco E, Burzi S, et al. The potential of restaging in the prediction of pathologic response after preoperative chemoradiotherapy for rectal cancer. Ann Surg Oncol 2007; 14: 455–61. doi: 10.1245/s10434-006-9269-4 [DOI] [PubMed] [Google Scholar]
- 25.Perez RO, Habr-Gama A, Gama-Rodrigues J, Proscurshim I, Julião GP, Lynn P, et al. Accuracy of positron emission tomography/computed tomography and clinical assessment in the detection of complete rectal tumor regression after neoadjuvant chemoradiation: long-term results of a prospective trial (National Clinical Trial 00254683). Cancer 2012; 118: 3501–11. doi: 10.1002/cncr.26644 [DOI] [PubMed] [Google Scholar]
- 26.Caricato M, Ausania F, De Dominicis E, Vincenzi B, Rabitti C, Tonini G, et al. Tumor regression in mesorectal lymphnodes after neoadjuvant chemoradiation for rectal cancer. Eur J Surg Oncol 2007; 33: 724–8. doi: 10.1016/j.ejso.2007.01.023 [DOI] [PubMed] [Google Scholar]
- 27.Brodsky JT, Richard GK, Cohen AM, Minsky BD. Variables correlated with the risk of lymph node metastasis in early rectal cancer. Cancer 1992; 69: 322–6. doi: [DOI] [PubMed] [Google Scholar]
- 28.Minsky BD, Rich T, Recht A, Harvey W, Mies C. Selection criteria for local excision with or without adjuvant radiation therapy for rectal cancer. Cancer 1989; 63: 1421–9. doi: [DOI] [PubMed] [Google Scholar]
- 29.Heijnen LA, Lambregts DM, Lahaye MJ, Martens MH, van Nijnatten TJ, Rao SX, et al. Good and complete responding locally advanced rectal tumors after chemoradiotherapy: where are the residual positive nodes located on restaging MRI? Abdom Radiol 2016; 41: 1245–52. doi: 10.1007/s00261-016-0640-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sauer R, Becker H, Hohenberger W, Rödel C, Wittekind C, Fietkau R, et al. Preoperative versus postoperative chemoradiotherapy for rectal cancer. N Engl J Med 2004; 351: 1731–40. doi: 10.1056/NEJMoa040694 [DOI] [PubMed] [Google Scholar]
- 31.van Heeswijk MM, Lambregts DM, Palm WM, Hendriks BM, Maas M, Beets GL, et al. DWI for assessment of rectal cancer nodes after chemoradiotherapy: is the absence of nodes at DWI proof of a negative nodal status? AJR Am J Roentgenol 2017; 208: W79–W84. doi: 10.2214/AJR.16.17117 [DOI] [PubMed] [Google Scholar]
- 32.Nicastri DG, Doucette JT, Godfrey TE, Hughes SJ. Is occult lymph node disease in colorectal cancer patients clinically significant? A review of the relevant literature. J Mol Diagn 2007; 9: 563–71. doi: 10.2353/jmoldx.2007.070032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kim IK, Kang J, Lim BJ, Sohn SK, Lee KY. The impact of lymph node size to predict nodal metastasis in patients with rectal cancer after preoperative chemoradiotherapy. Int J Colorectal Dis 2015; 30: 459–64. doi: 10.1007/s00384-014-2099-0 [DOI] [PubMed] [Google Scholar]