Abstract
Objective:
To prospectively evaluate the incidence of nausea and vomiting after exposure to non-ionic iodinated contrast media (ICM), and to identify potential risk factors, with a focus on fasting duration for solid food and fluids, separately.
Methods:
From January to March 2017, 1175 patients (605 males, 570 females; median age, 60 years; range, 20–91 years) undergoing ICM-enhanced CT were included in this study. Patients received instructions for a 6 h preparatory fast from solid food. Nausea and vomiting after ICM exposure were assessed on a 3-point scale (mild, moderate, severe). Patients’ characteristics and the fasting duration were evaluated to identify risk factors using logistic regression analysis.
Results:
Of the 1175 patients, 34 [2.9%; 95% confidence interval (CI) (2.0–4.0)] experienced mild nausea. No patients experienced vomiting [95% CI (0.0000–0.0005)]. 1173 (99.8%) carried out a 6 h fast, and the median fasting durations were 14 h for solid food (interquartile range, 12.5–15.5 h) and 11 h for fluid (interquartile range, 0–13.5 h), respectively. Fasting durations for solid food and fluids were not associated with nausea on univariate regression analyses (p = 0.282–1.000 and 0.146–1.000, respectively). Multivariate regression analysis revealed that a history of drug hypersensitivity [odds ratio = 4.33; 95% CI (1.85–17.52); p = 0.039] was independent risk factors for nausea, whereas iobitridol was less nauseous [odds ratio = 0.32; 95% CI (0.11–0.90); p = 0.032].
Conclusion:
Mild nausea occurred in 2.9% of patients and none vomited in our study population with a 6 h preparatory fast from solid food. Many patients underwent excessive fasting for fluids as well as solid food and their fasting durations were not associated with nausea.
Advances in knowledge:
We firstly evaluated fasting durations for solid food and fluids, and their impacts on vomiting or nausea after ICM exposure with an instruction of 6 h preparatory fast for solid food: many patients underwent excessive fasting for fluids and the fasting duration was unrelated to nausea.
Introduction
Patients are frequently asked to fast prior to iodinated contrast media (ICM)-enhanced CT.1, 2 Fasting has traditionally been required due to concerns about vomiting, which is one of the most prevalent adverse reactions to ICM and can potentially cause aspiration.3–5 Indeed, approximately up to 24.5% of patients experienced vomiting after intravascular administration of ionic high-osmolality ICM.5–7 However, with the introduction of nonionic low-osmolality ICM, the reported frequency of vomiting has declined with varying range, from 1% or less to 11.7%, lessening the rationale of the fasting.1,5,8–11
Although nearly all radiological clinics in industrial countries only use non-ionic ICM, the current preparatory fasting policy regarding fasting duration and content (solids or fluids) varies considerably across hospital and a 4– 6 h fasting is one of the most common fasting policies.1, 12 Furthermore, fasting duration before CT examination was heterogeneous across countries, with a tendency of longer fasting duration in Korea than in Europe.1 These mixed policy trends presumably originate from a paucity of relevant researches and evidences regarding fasting duration for the prevention of aspiration before the intravascular administration of nonionic low-osmolality ICM, after which vomiting is uncommon.2, 7 In a review of the literature, ingestion of clear inert fluid less than 1 h before ICM-enhanced CT exam rarely induced aspiration.1 Whether preparatory fasting has a beneficial effect against vomiting remains uncertain, but fasting can lead to patient dissatisfaction, dehydration, and exhaustion, especially if patients undergo excessive fasting.13 Some authors have recommended that fluid fasting is not necessary prior to ICM-enhanced CT examination,1 but the impact of duration of fluid fasting on vomiting or nausea has rarely been assessed.
Thus, the aim of this study was to prospectively evaluate the incidence of nausea and vomiting after exposure to non-ionic low-osmolality ICM and to identify potential risk factors, with a focus on fasting duration for solid food and fluids, separately. Based on results of this prospective study, we determine the necessity of fasting prior to the application of ICM and provide the adequate duration of the fasting period.
methods and Materials
This study was approved by our Institutional Review Board (IRB No. 1611-054-807) and was registered with clinicalTrials.gov as NCT03019341. Informed consent was obtained from all patients for participation in this prospective study.
Sample size calculation
We performed a pilot study that included 100 patients undergoing an ICM-enhanced CT scan, and found that 7% experienced sensations of nausea and none vomited. Based on the above expected frequency of nausea, and allowing 5% for Type I error and 3% for a total width of the confidence interval, the sample size estimated using the binominal exact approximation was 1175.
Patients
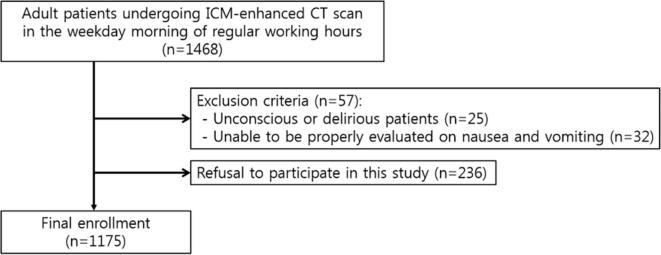
From January to March 2017, we enrolled a total of 1175 patients (605 males, 570 females; median age, 60 years; age range, 20–91 years) in Seoul National University Hospital, Republic of Korea who met the following inclusion criteria: (a) patients who were at least 20 years of age; (b) patients who underwent an ICM-enhanced CT examination on a weekday morning during regular working hours; and (c) patients who were able to sufficiently communicate with the medical staff. Patients who were (a) unconscious or delirious, or (b) unable to be properly evaluated regarding nausea and vomiting due to aspects of a patient’s status such as a recent major operation were excluded (Figure 1). Finally, among 1175 patients, there were 273 inpatients and 902 outpatients. According to our institutional policy, patients received instructions for a 6 h preparatory fast from solid food prior to ICM-enhanced CT scan. In the CT preparation room, a designated nurse surveyed patients willing to participate in this study regarding the following information: age; sex; previous history of ICM usage and adverse reactions to ICM including ICM hypersensitivity; premedication and the methods thereof; type of ICM used; ICM injection rate; the presence of following underlying diseases assessed based on the medical records of the enrolled patients: asthma, drug hypersensitivity excluding ICM hypersensitivity, allergic disease except for drug hypersensitivity, diabetes mellitus, cardiovascular disease including hypertension and heart disease, kidney disease, or hematologic disease; pre-examination sensations of nausea; and fasting duration for solid food and fluids. Fasting duration was evaluated in a 30 min interval and fasting duration less than 30 min was considered to be insufficient fasting.
Figure 1.
Flowchart for selecting the study population. ICM, iodinated contrast media.
CT examination
Various CT protocols on three multidetector CT scanners (Somatom Definition and Somatom Force, Siemens Healthcare, Erlangen, Germany; IQon, Philips Healthcare, Best, Netherlands) were used to examine the enrolled patients. The following types of CT scans were performed: head and neck (3.1%, 36 of 1175), chest (15.3%, 180 of 1175), general abdomen (14.6%, 172 of 1175), gastrointestinal (12.9%, 152 of 1175), liver and pancreatobiliary (28.9%, 339 of 1175), genitourinary (13.1%, 154 of 1175), heart and vascular (12.1%, 142 of 1175). All CT scans were performed with one of five types of non-ionic low-osmolality ICM (details for the usage protocol are shown in Supplementary Table 1): iobitridol (Xenetix®; Guerbet, Aulnay-sous-Bois, France), iohexol (Omnipaque®; GE Healthcare, Milwaukee, WI), iomeprol (Iomeron®; Bracco, Milan, Italy), iversol (Optiray®; Mallinckrodt Medical, St Louis, MO), and iopamidol (Pamiray®; Dongkook Pharm., Seoul, Korea). The type of the ICM was determined by the every CT protocol based on our institutional policy (Supplementary Table 1). The type of CT examination was determined by the clinical physicians who did not know about this study only based on the patients need to be examined. A total dose of ICM was determined by the patient’s body weight and CT protocols. The ICM was administered by an automatic power injector with diverse injection rate in consideration of total dose of ICM and respective CT protocols. A saline chase was performed with the same injection rate for 10 s immediately after ICM administration.
Outcome assessment
A dedicated investigation on nausea and vomiting was conducted using a standardized form which was developed for this study. The investigation was apart from our institutional real-time electronic medical record-based Contrast Safety Monitoring and Management System for various adverse reactions to ICM which might miss minor complaints of nausea.14 Experienced nurses observed patients during the ICM administration and up to 30 min afterwards, and documented the patient’s status and when any complaints of nausea or vomiting events occurred. The severity of nausea and vomiting was categorized on a 3-point scale: mild, minor feeling of nausea and capable of tolerating the CT examination; moderate, unpleasant sensation with an urge to vomit and an inability to tolerate the examination; severe, vomiting.
Statistical analysis
Patients’ characteristics were analyzed using the Fisher exact test and the Χ2 test for categorical variables and the independent t-test or Mann–Whitney test for continuous variables according to the presence of normality, with comparisons made between the participants who experienced nausea or vomiting and those who did not. Univariate and multivariate logistic regression analysis was performed to explore potential risk factors for nausea and vomiting. To further evaluate fasting duration as a risk factor for nausea and vomiting, patients were dichotomized into fasting and non-fasting groups according to fasting duration, and the incidence of nausea and vomiting was compared between groups. For the multivariate logistic regression analysis, variables with a p-value of 0.15 or less were entered into the final model.15 The Fisher exact test and the Χ2 test were used to evaluate the frequency of nausea and vomiting according to the duration of fasting from solid food and fluids. p-values < 0.05 were considered to indicate statistical significance. Statistical analysis was performed using SPSS v. 22.0 (IBM Corp., Armonk, NY) and MedCalc for Windows (v. 8.0.0.1, MedCalc Software, Mariakerke, Belgium).
Results
Patients
Among the 1175 enrolled patients (Table 1), 37 complained of mild nausea, but 3 patients had experienced nausea prior to the administration of non-ionic ICM. Thus, 34 patients (13 males, 21 females; median age, 61 years; age range, 22–80 years) experienced a mild degree of nausea after non-ionic ICM administration [2.9%; 95% CI (2.0–4.0%)] (Table 1). Those 34 patients did not have the known cause for acute nausea such as chemotherapeutic agent, presence of pregnancy, vertigo because of disequilibrium, and psychiatric disease. There was definite causal relationship between acute ICM-induced nausea and ICM administration because of the certain consecutive relationship. Neither vomiting nor nausea causing a patient to be unable to tolerate the examination occurred in the enrolled patients [0.0%; 95% CI (0.0000–0.0005%), respectively]. Among the nausea group, two patients had previous history of allergic-like reactions to ICM which was not gastrointestinal symptom such as nausea and vomiting [5.88% (2/34)]. One of them was administered the alternative contrast agent without any pre-medication and another had antihistamine pre-medication. The latter one had an acute acquired nausea after ICM administration. Among the non-nausea group, the previous history of allergic-like reactions to ICM were existed in 64 patients [5.61% (64/1141)] and 12 of them were pre-medicated (Table 1).
Table 1.
Patients’ characteristics in nausea and non-nausea groups
| Group | Exp(B) (95% CI) | p-value | ||
| Nausea (n = 34) | Non-nausea (n = 1141) | |||
| Sex (M:F) (number) | 34 (13 : 21) | 1141 (592:549) | 1.7420 (0.8640–3.5130) | 0.1210a |
| Age (median, range) | 61 (22–80) | 60 (20–91) | 0.9860 (0.9590–1.0140) | 0.3230 |
| Underlying disease | ||||
| Asthma | 0% (0/34) | 1.75% (20/1141) | 0.0000 (0.0000–0.0000) | 0.9980 |
| Drug hypersensitivity excluding contrast hypersensitivity | 5.88% (2/34) | 1.58% (18/1141) | 3.8990 (0.8680–17.520) | 0.0760a |
| Allergic disease | 0% (0/34) | 4.21% (48/1141) | 0.0000 (0.0000–0.0000) | 0.9980 |
| Diabetes mellitus | 11.76% (4/34) | 14.29% (163/1141) | 0.8000 (0.2780–2.3010) | 0.6790 |
| Cardiovascular disease | 23.53% (8/34) | 27.96% (319/1141) | 0.7930 (0.3550–1.7700) | 0.5710 |
| Chronic renal disease | 2.94% (1/34) | 2.10% (24/1141) | 1.4100 (0.1850–10.7400) | 0.7400 |
| Malignancy | 2.94% (1/34) | 1.67% (19/1141) | 1.7890 (0.2330–13.7680) | 0.5760 |
| Previous history of ICM usage | 85.29% (29/34) | 81.77% (933/1141) | 1.2930 (0.4950–0.3380) | 0.6000 |
| Previous history of allergic-like reactions to ICM | 5.88% (2/34) | 5.61% (64/1141) | 1.0520 (0.2470–4.4870) | 0.9460 |
| Premedication | ||||
| Antihistamine | 2.94% (1/34) | 1.93% (22/1141) | 1.2440 (0.0600–25.5620) | 0.8880 |
| Antihistamine + steroid | 0% (0/34) | 0.88% (10/1141) | 0.9840 (0.9269–1.0421) | 0.5839 |
| Antihistamine + antiemetics | 0% (0/34) | 0.09% (1/1141) | 0.0000 (0.0000–) | 0.9980 |
| Antiemetics | 0% (0/34) | 0.09% (1/1141) | 0.0000 (0.0000–) | 0.9980 |
| Average fasting time (hour) | ||||
| Solid food (median, interquartile range) | 13.75 (12.5–15.0) | 14 (12.5–15.5) | 0.9490 (0.8400–1.0720) | 0.4030 |
| Fluid (median, interquartile range) | 6.5 (2.0–13.0) | 11 (3.0–13.5) | 0.9510 (0.8970–1.0080) | 0.0910 |
| Culprit non-ionic ICM | ||||
| Ioversol | 11.76% (4/34) | 21.30% (243/1141) | 0.4930 (0.1720–1.4120) | 0.1880 |
| Iopamidol | 32.35% (11/34) | 24.89% (284/1141) | 1.4430 (0.6950–2.9980) | 0.3250 |
| Iohexol | 23.53% (8/34) | 12.62% (144/1141) | 2.1300 (0.9460–4.7960) | 0.0680a |
| Iobitridol | 11.76% (4/34) | 29.36% (335/1141) | 0.3210 (0.1120–0.9180) | 0.0340a,b |
| Iomeprol | 20.59% (7/34) | 11.83% (135/1141) | 1.9320 (0.8250–4.5220) | 0.1290a |
| Total dose of ICM administration (ml) (mean ± SD, 95% CI) | 89.71 ± 16.41 (83.98–95.43) |
96.33 ± 17.93 (95.29–97.37) | 0.9790 (0.9600–0.9990) | 0.0360a,b |
| Injection rate (ml/s)(mean ± SD, 95% CI) | 2.93 ± 0.80 (2.65–3.21) |
2.94 ± 0.75 (2.90–2.98) |
0.9830 (0.6230–1.5510) | 0.940 |
CI, confidence interval; ICM, iodinated contrast media; SD, standard deviation.
aIncluded for multivariate regression analysis because of less than 0.15 of the p-value.
bStatistically significant.
Of the 34 patients who complained of nausea, 19 patients experienced nausea during ICM administration, and 15 patients reported nausea after the completion of the CT scan. In the latter 15 patients, the average time interval between the ICM injection and the sensation of nausea was 5 min. Among the former 19 patients, nausea persisted after the completion of the CT scan in 8 patients, while nausea spontaneously subsided in the other 11 patients just after the ICM administration. The ICM-induced acquired nausea was an isolated symptom and there was no association to other symptoms such as urticarial, itching, shortness of breathing because of ICM administration. The mean value of ICM total dose was 96.14 ± 17.92 [95% CI (95.11–97.17)] and of injection rate was 2.94 ± 0.75 [95% CI (2.90–2.98)].
Among all patients, the mean value of ICM total dose was 96.14 ± 17.92 [95% CI (95.11–97.17)] and of injection rate was 2.94 ± 0.75 [95% CI (2.90–2.98)]. Total dose and injection rate of ICM is shown in Table 1 based on patients’ group and total dose of ICM showed a statistically significant difference between two groups (Table 1).
Fasting duration and nausea sensation
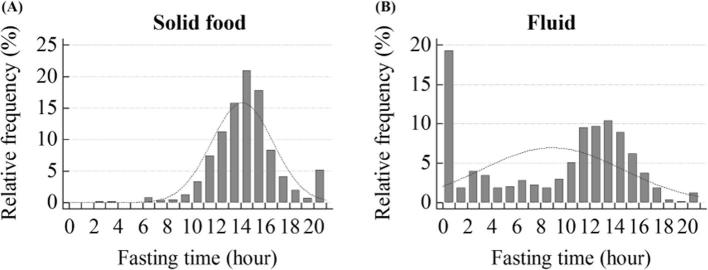
The 6 h solid food fast was kept by 1173 patients. The median fasting duration was 14 h for solid food (interquartile range, 12.5–15.5 h) and 11 h for fluids (interquartile range, 0–13.5 h) (Figure 2). The median fasting duration did not significantly differ between the patients who did or did not experience nausea (solid food, p = 0.4030; fluids, p = 0.9100) (Table 1). When patients were dichotomized at 1 h and every 6 h of fasting duration, the incidence of nausea and vomiting had no significant difference between the two groups based on fasting duration for solid food and fluids (Table 2).
Figure 2.
Histogram of fasting duration for solid food and fluids.
Table 2.
Comparison of nausea frequency for 1 h and every 6 h of fasting duration
| Frequency of nausea | ||||
| Fasting duration | <Duration | ≥Duration | p-valuea | |
| Solid food |
1 h | − (0/0) | 2.89% (34/1175) | NAb |
| 6 h | 0% (0/2) | 2.90% (34/1173) | 1.0000 | |
| 12 h | 1.23% (2/163) | 3.16% (32/1012) | 0.6123 | |
| 18 h | 3.05% (33/1083) | 1.09% (1/92) | 0.4515 | |
| Fluid |
1 h | 3.08% (7/227) | 2.85% (27/948) | 0.9759 |
| 6 h | 3.92% (15/383) | 2.40% (19/792) | 0.2045 | |
| 12 h | 3.42% (23/672) | 2.19% (11/503) | 0.2826 | |
| 18 h | 2.95% (34/1153) | 0% (0/22) | 0.8608 | |
CI, confidence interval.
aFisher exact test and Χ2 test.
bThere was no patient with less than 1 h fasting duration for solid food.
Multivariate regression analysis to identify risk factors for nausea
Among the patients’ clinical and demographic characteristics, the use of iobitridol (p = 0.0340) showed a statistically significant difference between the nausea and non-nausea groups in the univariate analysis (Table 1) and female (p = 0.1210), drug hypersensitivity excluding contrast hypersensitivity (p = 0.0760), the use of iohexol (p = 0.0680), iobitridol (p = 0.0340), and iomeprol (p = 0.1290) were less than 0.15 of the p-value. In addition, total dose of ICM showed a statistically significant difference between two groups (p = 0.0630; Table 1).
Among them, drug hypersensitivity [odds ratio = 4.33; 95% CI (1.85–17.52); p = 0.039] was an independent risk factor for nausea, while the use of iobitridol was less nauseous (odds ratio = 0.32; 95% CI, 0.11–0.90; p = 0.032) (Table 3).
Table 3.
Multivariate logistic regression analysis of risk factors for nausea
| Exp(B) (95% CI) | p-value | |
| Female | 1.6870 (0.7940–3.5880) | 0.1740 |
| Drug hypersensitivity excluding contrast hypersensitivty | 4.3270 (1.8530–17.5200) | 0.0390a |
| Iohexol | 2.3530 (0.9570–5.7880) | 0.0620 |
| Iobitridol | 0.3150 (0.1100–0.9030) | 0.0320a |
| Iomeprol | 1.4840 (0.3950–5.5720) | 0.5590 |
| Total dose of ICM administration | 0.9820 (0.9550–1.0110) | 0.2180 |
CI, confidence interval; ICM, iodinated contrast media.
astatistically significant
Necessity of concrete fasting duration for solid food and fluid
There was no significant difference between two groups dichotomized based on 1 h of fasting duration criteria for both of solid food and fluid: <1 h group which can be regarded as a control group representing as non-fasting condition vs ≥1 h group (Table 2). Among the 636 patients 60 years or older, most of the patients [99.69% (634/636)] fasted from solid food for longer than instructed 6 h. Among those 636 patients, the fluid fasting duration was diverse because fluid fasting was not mandatory. But most of them [66.04% (420/636)] underwent fasting fluid more than 6 h, the same fasting duration for solid food because of lack of concrete fluid fasting duration.
Discussion
This prospective study confirmed that vomiting was extremely rare [0.0%; 95% CI (0.0000–0.0005%)] and that mild nausea occurred in approximately 3% of patients after exposure to non-ionic ICM; these patients had received instructions to undergo a 6 h preparatory fast from solid food. Our rare incidence of vomiting is within the range reported in the relevant publications (vomiting, 0–0.36%), while the incidence of nausea is higher than has been reported (nausea, 0.05–1.99%) (Table 4).5,7–9,16–24 The latter difference may result from our rigorous investigation of nausea, including a minute transient sensation of nausea, in contrast to other studies where nausea was dealt with as one of various kinds of adverse reactions and sensation of mild nausea might be missed.
Table 4.
Incidence of nausea and vomiting after exposure to non-ionic iodinated contrast media from representative publications where the incidence could be extracted
| Authors | Year | Country | Patient collection | Iodinated contrast media | Incidence | |
| Nausea | Vomiting | |||||
| Katayama et al5 | 1990 | Japan | Retrospective | Iopamidol, Iohexol | 1.04% (1749/168363) | 0.36% (614/168363) |
| Oowaki et al | 1994 | Japan | Unknown | Unknown | 1.4%a (17/1241) | |
| Federle et al16 | 1998 | USA | Prospective | Ioversol | 1.99% (9/452) | 0% (0/452) |
| Nagamoto et al17 | 2006 | Japan | Prospective | Iopamidol | 0.9% (8/940) | 0% (0/940) |
| Wendt-Nordahl et al18 | 2006 | Germany | Prospective (PMS) | Iobitridol | 0.3% | <0.1% |
| Vogl et al19 | 2006 | Germany | Prospective (PMS) | Iobitridol | 0.24% (127/52057) | 0.09% (45/52057) |
| Vijayalakshmi et al20 | 2007 | United Kingdom | Prospective | Iopamidol, Iomeprol | 0.55% (11/1985) | 0.40% (8/1985) |
| Gomi et al21 | 2010 | Japan | Prospective | Iopromide, Iomeprol, Iopamidol, Iohexol, Ioversol |
0.003%a,b (27/8931) | |
| Häussler et al22 | 2010 | Germany | Prospective (PMS) | Iodixanol | 0.12% (11/9515) | 0.08% (8/9515) |
| Maurer et al8 | 2011 | Germany | Prospective (PMS) | Iobitridol | 0.20% (315/160639) | 0.06% (89/160639) |
| Palkowitsch et al9 | 2014 | USA, Europe, Africa, Asia | Prospective (including PMS) | Iopromide | 0.52%a (689/132012) | |
| Zhang et al23 | 2014 | China | Prospective (PMS) | Iodixanol | 0.13% (27/20185) | 0.008% (16/20185) |
| Li et al24 | 2015 | China | Retrospective | Iopromide, Iodixanol, Iopamidol, Iohexol, loversol |
0.05% (51/109255) | 0.006% (7/109255) |
PMS, post-marketing surveillance.
Data in parenthesis indicates the number of patients.
Incidences of nausea and vomiting were not separable.
The incidence of nausea and vomiting which required medical treatment.
Study populations were suspected to be partly overlapped.
Most of the patients fasted from solid food for longer than instructed and underwent fluid fasting, which was not required. Although the reason of patients’ immoderate fasting needs to be identified, it appears that simple instructions for preparatory fasting from solid food can unnecessarily expose patients to potential dehydration and exhaustion. Fluid fasting duration had no significant relationship with the incidence of nausea and vomiting (Table 2), supporting the recent recommendation that fluid fasting is not necessary prior to ICM-enhanced CT examination.1 Thus, when instructions for preparatory fasting from solid foods are provided, patients are recommended to exercise caution against dehydration before undergoing a contrast-enhanced CT examination and fluid hydration may be more important in Summer.25
The fasting duration for solid food more than 6 h did not affect the incidence of nausea in this study. Because there were few patients who fasted for solid food less than 6 h, it could not be determined the relationship between the fasting duration for solid food less than 6 h and the incidence of nausea. Conflicting results exist regarding the relationship between fasting duration and nausea or vomiting. Wagner et al2 reported that there was no significant difference in the incidence of acute adverse reactions between patients who underwent 4 h of fasting and those who did not. Oowaki et al reported that preparatory fasting increased the incidence of nausea and vomiting with high-osmolality ICM but not with low-osmolality ICM.7 Further studies are required to elucidate the relationship between fasting duration for solid food and nausea or vomiting.
Based on the results of our study, drug hypersensitivity excluding contrast hypersensitivity was an independent risk factor for nausea, while the use of iobitridol was less nauseous. Nausea and vomiting after ICM exposure mainly result from anaphylactoid reaction which appear clinically identical to anaphylaxis but lack an IgE-dependent mechanism, although vasomotor reaction may be partly involved.26–31 Allergic trait of elevated sensitivity to other drugs is a well-known risk factor for adverse reactions to ICM6, 32 and nausea and vomiting are one of the most common adverse reactions to ICM.33 Thus, the preparative fasting would be cautiously maintained in such patients at risk for nausea sensation. Prophylactic medication with corticosteroid prior to ICM exposure is another option which is effective for reducing anaphylactoid reactions to ICM, including nausea and vomiting.31, 34,35 Such medication may be considered for patients with risk factor for nausea, but, may also cause indirect cost and additional harm. Given that ICM-induced nausea was primarily mild in degree, the necessity of corticosteroid pre-medication should be determined in a risk–benefit perspective.31 The less adverse effect of iobitridol is consistent with the results of other studies that have shown iobitridol to be associated with fewer immediate adverse reactions than other nonionic ICMs.36 Nevertheless, it is not conclusive due to a lack of randomization of ICM for CT examination and less adjustment for potential confounders in this study and further study is needed to confirm the less nauseous effect of iobitridol. Unfortunately, our results do not provide any guidance about whether fasting before ICM-enhanced CT examinations is essential. As vomiting did not occur, we could not sufficiently evaluate any risk factors for vomiting. Patients were enrolled during the regular working hours in the morning due to the practicability of the enrollment process in daily practice. This may have led to an overestimation of fasting duration owing to overnight fasting and having a breakfast or diurnal rhythm may make a difference in an incidence of nausea and vomiting when patients undergo ICM-enhanced CT examination in the afternoon. In this study, the enrolled patients were almost compliant with our instruction, so a less complaint patient population might give different results. We did not enroll patients undergoing emergency CT scans, as they were vulnerable subjects. And there were 273 inpatients and 902 outpatients. Lack of patients in emergency room and fewer inpatients than outpatients may have led to selection bias. As mentioned before, a lack of information on the amount and kinds of solid food and fluids prior to CT examination was another limitation of our study.
Conclusion
In conclusion, mild nausea occurred in 2.9% of patients and none vomited in our study population who was instructed to avoid solid food for 6 h prior to ICM-enhanced CT. There is no significant relationship between fasting duration for not only solid food but fluids and incidence of nausea and vomiting, however, a considerable of patients underwent excessive fasting more than the instructed duration. When patients are instructed to undergo preparatory fasting from solid food before a contrast-enhanced CT examination, they should be recommended to exercise caution against dehydration and not to fast excessively.
Footnotes
Ethical approve: This research was approved by the Institutional Review Board of Seoul National University (IRB No. 1611-054-807).
Contributor Information
Yeon Soo Kim, Email: dustn_727@naver.com.
Soon Ho Yoon, Email: yshoka@gmail.com.
Young Hun Choi, Email: choiyounghun@gmail.com.
Chang Min Park, Email: cmpark.morphius@gmail.com.
Whal Lee, Email: whal.lee@gmail.com.
Jin Mo Goo, Email: jmgoo@plaza.snu.ac.kr.
REFERENCES
- 1.Lee BY, Ok JJ, Abdelaziz Elsayed AA, Kim Y, Han DH. Preparative fasting for contrast-enhanced CT: reconsideration. Radiology 2012; 263: 444–50. doi: 10.1148/radiol.12111605 [DOI] [PubMed] [Google Scholar]
- 2.Wagner HJ, Evers JP, Hoppe M, Klose KJ. Must the patient fast before intravascular injection of a non-ionic contrast medium? Results of a controlled study. Rofo 1997; 166: 370–5. doi: 10.1055/s-2007-1015444 [DOI] [PubMed] [Google Scholar]
- 3.Shehadi WH. Contrast media adverse reactions: occurrence, recurrence, and distribution patterns. Radiology 1982; 143: 11–17. doi: 10.1148/radiology.143.1.7063711 [DOI] [PubMed] [Google Scholar]
- 4.Bush WH, Swanson DP. Acute reactions to intravascular contrast media: types, risk factors, recognition, and specific treatment. AJR Am J Roentgenol 1991; 157: 1153–61. doi: 10.2214/ajr.157.6.1950858 [DOI] [PubMed] [Google Scholar]
- 5.Katayama H, Yamaguchi K, Kozuka T, Takashima T, Seez P, Matsuura K, Hitoshi Katayama M. Adverse reactions to ionic and nonionic contrast media. A report from the Japanese committee on safety of contrast media. Radiology 1990; 175: 621–8. doi: 10.1148/radiology.175.3.2343107 [DOI] [PubMed] [Google Scholar]
- 6.Manhire AR, Dawson P, Dennet R. Contrast agent-induced emesis. Clin Radiol 1984; 35: 369–70. doi: 10.1016/S0009-9260(84)80186-5 [DOI] [PubMed] [Google Scholar]
- 7.Oowaki K, Saigusa H, Ojiri H, Ariizumi M, Yamagisi J, Fukuda K, et al. Relationship between oral food intake and nausea caused by intravenous injection of iodinated contrast material. Nihon Igaku Hoshasen Gakkai Zasshi 1994; 54: 476–9. [PubMed] [Google Scholar]
- 8.Maurer M, Heine O, Wolf M, Freyhardt P, Schnapauff D, Hamm B, et al. Safety and tolerability of iobitridol in general and in patients with risk factors: results in more than 160,000 patients. Eur J Radiol 2011; 80: 357–62. doi: 10.1016/j.ejrad.2010.03.018 [DOI] [PubMed] [Google Scholar]
- 9.Palkowitsch PK, Bostelmann S, Lengsfeld P. Safety and tolerability of iopromide intravascular use: a pooled analysis of three non-interventional studies in 132,012 patients. Acta Radiol 2014; 55: 707–14. doi: 10.1177/0284185113504753 [DOI] [PubMed] [Google Scholar]
- 10.de Geeter P, Melchior H. Iomeprol versus iopromide for intravenous urography. Br J Radiol 1994; 67: 958–63. doi: 10.1259/0007-1285-67-802-958 [DOI] [PubMed] [Google Scholar]
- 11.García M, Aguirre U, Martinez A, Ruiz B, Lertxundi U, Aguirre C. Acute adverse reactions to iopromide vs iomeprol: a retrospective analysis of spontaneous reporting from a radiology department. Br J Radiol 2014; 87: 20130511. doi: 10.1259/bjr.20130511 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Barbosa P, Bitencourt AGV, Tyng CJ, Cunha R, Travesso DJ, Almeida MFA, et al. JOURNAL CLUB: preparative fasting for contrast-enhanced CT in a cancer center: a new approach. AJR Am J Roentgenol 2018; 210: 941–7. doi: 10.2214/AJR.17.19061 [DOI] [PubMed] [Google Scholar]
- 13.Nygren J, Thorell A, Ljungqvist O. Are there any benefits from minimizing fasting and optimization of nutrition and fluid management for patients undergoing day surgery? Curr Opin Anaesthesiol 2007; 20: 540–4. doi: 10.1097/ACO.0b013e3282f15493 [DOI] [PubMed] [Google Scholar]
- 14.Lee SY, Yang MS, Choi YH, Park CM, Park HW, Cho SH, et al. Stratified premedication strategy for the prevention of contrast media hypersensitivity in high-risk patients. Ann Allergy Asthma Immunol 2017; 118: 339–44. doi: 10.1016/j.anai.2016.11.027 [DOI] [PubMed] [Google Scholar]
- 15.Bursac Z, Gauss CH, Williams DK, Hosmer DW. Purposeful selection of variables in logistic regression. Source Code Biol Med 2008; 3: 17. doi: 10.1186/1751-0473-3-17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Federle MP, Willis LL, Swanson DP. Ionic versus nonionic contrast media: a prospective study of the effect of rapid bolus injection on nausea and anaphylactoid reactions. J Comput Assist Tomogr 1998; 22: 341–5. doi: 10.1097/00004728-199805000-00001 [DOI] [PubMed] [Google Scholar]
- 17.Nagamoto M, Gomi T, Terada H, Terada S, Kohda E. Evaluation of the acute adverse reaction of contrast medium with high and moderate iodine concentration in patients undergoing computed tomography. Radiat Med 2006; 24: 669–74. doi: 10.1007/s11604-006-0087-1 [DOI] [PubMed] [Google Scholar]
- 18.Wendt-Nordahl G, Rotert H, Trojan L, Michel MS, Peters CR, Alken P, et al. Intravenous contrast media in uroradiology: evaluation of safety and tolerability in almost 50,000 patients. Med Princ Pract 2006; 15: 358–61. doi: 10.1159/000094269 [DOI] [PubMed] [Google Scholar]
- 19.Vogl TJ, Honold E, Wolf M, Mohajeri H, Hammerstingl R. Safety of iobitridol in the general population and at-risk patients. Eur Radiol 2006; 16: 1288–97. doi: 10.1007/s00330-005-0061-9 [DOI] [PubMed] [Google Scholar]
- 20.Vijayalakshmi K, Kunadian B, Wright RA, Hall JA, Stewart MJ, Davies A, et al. A prospective randomised controlled trial to determine the early and late reactions after the use of iopamidol 340 (Niopam) and iomeprol 350 (Iomeron) in cardiac catheterisation. Eur J Radiol 2007; 61: 342–50. doi: 10.1016/j.ejrad.2006.09.013 [DOI] [PubMed] [Google Scholar]
- 21.Gomi T, Nagamoto M, Hasegawa M, Katoh A, Sugiyama M, Murata N, et al. Are there any differences in acute adverse reactions among five low-osmolar non-ionic iodinated contrast media? Eur Radiol 2010; 20: 1631–5. doi: 10.1007/s00330-009-1698-6 [DOI] [PubMed] [Google Scholar]
- 22.Häussler MD. Safety and patient comfort with iodixanol: a postmarketing surveillance study in 9515 patients undergoing diagnostic CT examinations. Acta Radiol 2010; 51: 924–33. doi: 10.3109/02841851.2010.504739 [DOI] [PubMed] [Google Scholar]
- 23.Zhang BC, Hou L, Lv B, Xu YW. Post-marketing surveillance study with iodixanol in 20 185 Chinese patients from routine clinical practices. Br J Radiol 2014; 87: 20130325. doi: 10.1259/bjr.20130325 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Li X, Chen J, Zhang L, Liu H, Wang S, Chen X, et al. Clinical observation of the adverse drug reactions caused by non-ionic iodinated contrast media: results from 109,255 cases who underwent enhanced CT examination in Chongqing, China. Br J Radiol 2015; 88: 20140491. doi: 10.1259/bjr.20140491 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Motoi R, Yano I, Ozaki J, Hokoyama K, Yamamoto T, Fukatsu S, et al. Effect of water intake on allergy-like events associated with non-ionic Iodine contrast agents. Yakugaku Zasshi 2015; 135: 1177–84. doi: 10.1248/yakushi.15-00111 [DOI] [PubMed] [Google Scholar]
- 26.Cochran ST. Anaphylactoid reactions to radiocontrast media. Curr Allergy Asthma Rep 2005; 5: 28–31. doi: 10.1007/s11882-005-0051-7 [DOI] [PubMed] [Google Scholar]
- 27.Brockow K, Romano A, Aberer W, Bircher AJ, Barbaud A, Bonadonna P, et al. Skin testing in patients with hypersensitivity reactions to iodinated contrast media - a European multicenter study. Allergy 2009; 64: 234–41. doi: 10.1111/j.1398-9995.2008.01832.x [DOI] [PubMed] [Google Scholar]
- 28.Romano A, Artesani MC, Andriolo M, Viola M, Pettinato R, Vecchioli-Scaldazza A, et al. Effective prophylactic protocol in delayed hypersensitivity to contrast media: report of a case involving lymphocyte transformation studies with different compounds. Radiology 2002; 225: 466–70. doi: 10.1148/radiol.2251011654 [DOI] [PubMed] [Google Scholar]
- 29.Vernassiere C, Trechot P, Commun N, Schmutz JL, Barbaud A. Low negative predictive value of skin tests in investigating delayed reactions to radio-contrast media. Contact Dermatitis 2004; 50: 359–66. doi: 10.1111/j.0105-1873.2004.00367.x [DOI] [PubMed] [Google Scholar]
- 30.Kanny G, Pichler W, Morisset M, Franck P, Marie B, Kohler C, et al. T cell-mediated reactions to iodinated contrast media: evaluation by skin and lymphocyte activation tests. J Allergy Clin Immunol 2005; 115: 179–85. doi: 10.1016/j.jaci.2004.09.012 [DOI] [PubMed] [Google Scholar]
- 31.Bottinor W, Polkampally P, Jovin I. Adverse reactions to iodinated contrast media. Int J Angiol 2013; 22: 149–54. doi: 10.1055/s-0033-1348885 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Davenport MS, Cohan RH, Caoili EM, Ellis JH. Repeat contrast medium reactions in premedicated patients: frequency and severity. Radiology 2009; 253: 372–9. doi: 10.1148/radiol.2532090465 [DOI] [PubMed] [Google Scholar]
- 33.Mikkonen R, Kontkanen T, Kivisaari L. Acute and late adverse reactions to low-osmolal contrast media. Acta Radiol 1995; 36: 72–6. doi: 10.1177/028418519503600113 [DOI] [PubMed] [Google Scholar]
- 34.Greenberger PA, Patterson R, Radin RC. Two pretreatment regimens for high-risk patients receiving radiographic contrast media. J Allergy Clin Immunol 1984; 74: 540–3. doi: 10.1016/0091-6749(84)90391-9 [DOI] [PubMed] [Google Scholar]
- 35.Mervak BM, Cohan RH, Ellis JH, Khalatbari S, Davenport MS. Intravenous corticosteroid premedication administered 5 hours before CT compared with a traditional 13-hour oral regimen. Radiology 2017; 285: 425–33. doi: 10.1148/radiol.2017170107 [DOI] [PubMed] [Google Scholar]
- 36.Kim SR, Lee JH, Park KH, Park HJ, Park JW. Varied incidence of immediate adverse reactions to low-osmolar non-ionic iodide radiocontrast media used in computed tomography. Clin Exp Allergy 2017; 47: 106–12. doi: 10.1111/cea.12803 [DOI] [PubMed] [Google Scholar]