Abstract
Objective:
To investigate whether patients with emphysema, as evaluated by quantitative CT image measurement, are at risk of developing radiation pneumonitis (RP) after radiotherapy (RT) for non-small cell lung cancer (NSCLC).
Methods:
Between March 2011 and June 2015, 68 consecutive patients with Stage I NSCLC treated with a RT dose of 75 Gy given in 30 fractions were enrolled. The median age was 79 years and there were 45 males and 23 females. The number of patients with T1 and T2 were 49 and 19, respectively. The severity of emphysema was evaluated by the percentages of the low attenuation area (LAA) of ≤−860 or −950 Hounsfield unit (HU) and average HU in the whole lung.
Results:
The mean difference percentages of LAA of ≤−860 (p = 0.0004) or −950 HU (p = 0.005) and average HU (p = 0.001) in patients with RP were significantly lower than those in patients without RP. The area under curve (AUC) of average HU was significantly higher than AUC of LAA of ≤−860 (p < 0.0001) or −950 HU (p < 0.0001). The RP rate after RT was significantly lower when the average HU values were ≤−850 HU (p = 0.0003).
Conclusion:
Patients with emphysema evaluated by average HU (≤–850 HU) in the whole lung were found to be at low risk of RP after RT.
Advances in Knowledge:
Quantitative measurement of average HU from CT images was predicted of RP after RT.
Introduction
Stereotactic body radiotherapy (RT) is the standard care for patients with medically inoperable stage I non-small cell lung cancer (NSCLC), and has been shown to improve survival compared to observation alone.1 The elderly population is increasing rapidly in Japan, and thus the incidence of medically inoperable lung cancer is also expected to increase.2 Chronic obstructive pulmonary disease (COPD), which is one of the reasons for inoperability of NSCLC, is one of the risk factors for radiation pneumonitis (RP).3, 4 RP develops in most patients who receive RT for the lung,5–7 and severe RP develops in a small number of cases.8
However, it is unclear whether NSCLC patients with pulmonary emphysema (PE) can be treated safely by RT. We previously reported that patients with severe PE classified by Goddard’s criteria had a low risk of RP.9 However, in Goddard’s criteria, PE severity is classified based on the appearance of CT images,10 and thus the reproducibility of the classification is limited by interobserver variability.
To overcome the limitations of Godard’s criteria, a method for evaluating PE based on the percentage of low attenuation area (LAA) on CT images of lung has been introduced.11, 12 However, the correlation between the predictions of RP based on this new method of evaluating PE and the actual incidence of RP after RT has not been studied. To address this relation, we examined whether the classification of PE based on the evaluation of LAA on CT images can predict the likelihood of RP following RT.
Methods and Materials
Patients
The ethical committee of Tokyo Medical University, Tokyo, approved this study. Between March 2011 and June 2015, 68 consecutive patients with Stage I NSCLC treated by RT a dose of 75 Gy given in 30 fractions at Tokyo Medical University were enrolled. The patients’ characteristics are shown in Table 1. The median age was 79 (range, 49–90) years. There were 45 male and 23 female patients, and the numbers of patients with T1 and T2 were 49 and 19, respectively. 31 patients (46%) had previously undergone thoracic surgery. There was no significant difference in any of the patients’ characteristics between patients with and without RP. 27 patients were histologically proven NSCLC and remaining 41 patients were diagnosed by fludeoxyglucose-positron emission tomography positive tumor and/or 25% enlarged tumor over a 2 months period on CT image.
Table 1.
Patients’ characteristics
| All (n = 68) | Non-pneumonitis (n = 16) | Pneumonitis (n = 52) | p value | |
| Age (years) | 78.1 ± 8.0 | 77.5 ± 6.2 | 78.2 ± 8.5 | 0.47 |
| T1 : T2 | 49 : 19 | 13 : 3 | 36 : 16 | 0.53 |
| Male : female | 45 : 23 | 10 : 6 | 35 : 17 | 0.77 |
| Surgery + : – | 37 : 31 | 9 : 7 | 28 : 24 | 1.00 |
| Upper and middle/lingual : lower | 30 : 38 | 7 : 9 | 23 : 29 | 1.00 |
PE evaluation and RT method
55 of the patients (80.8%) were participants in a Phase II study at our institute; that study evaluated the local control rate achieved by a dose of 75 Gy given in 30 fractions for patients with stage I NSCLC. The RT procedures were described elsewhere.9, 13 Briefly, the planning target volume was determined by adding 0.5 cm for respiratory uncertainties to the clinical target volume in the craniocaudal direction and 0.5 cm in all other directions. Five non-coplanar ports with or without intensity-modulated filters (decimal Co. Ltd., FL) were typically used. In the three-dimensional conformal RT planning, a 100% dose was prescribed to the iso-center of the tumor. The 95% dose line was set to cover the PTV in intensity-modulated RT planning. None underwent chemotherapy.
Volumetric chest CT images were acquired at the end of expiration and inspiration phases for the treatment planning. No contrast material was administered. CT images acquired during expiration phases were used to quantify PE. Parameters of the 16 multislice CT (Aquilion LB, Toshiba Medical Systems, Tokyo, Japan) were as follows: tube current: 250 mA; tube voltage: 120 KV; gantry rotation time: 0.5 s; CT pitch factor: 15. Row CT data were reconstructed into an axial CT image with 2 mm slice thickness. The analysis of CT images was performed using software (Synapse Vincent, Fujifilm Co., Tokyo, Japan). LAA was calculated as a percentage of lung pixels with an attenuation of ≤−950 Hounsfield unit (HU) or −860 HU in the whole lung of CT images. In addition to LAA, the average lung attenuation value of the whole lung was recorded. When atelectasis or pleural effusion was observed, these areas on CT images were excluded from the measurement.
All patients received physical examinations, blood tests and a chest X-ray every month, and a thoracic CT examination every 3 months for 1 year after completion of RT. Thereafter, they underwent chest X-ray analysis every 3 months and CT examinations every 6 months when chest X-ray analysis did not disclose abnormal shadows. The grade of pulmonary toxicity was determined according to the common terminology criteria for adverse events v. 4.0. by two chest radiologists from different groups in our department. The RP was classified into five grades as follows. Grade 1 RP is asymptomatic, revealed by clinical or diagnostic observation only; intervention is not indicated. Grade 2 is symptomatic, and medical intervention is indicated; the grade shows limited instrumental activities of daily living. Grade 3 is severe symptoms, limiting self-care activities of daily living and oxygen is indicated. Grade 4 is life-threatening respiratory compromise, and urgent intervention is indicated. Grade 5 is death.
Statistical analysis
Categorical variables were evaluated using the chi-squared test and Fisher’s exact test. Continuous variables were evaluated using the Wilcoxon rank-sum test. The time to RP was calculated from the first day of RT. The time to RP was estimated using Kaplan-Meir method and compared by Log-rank test. Cox proportional hazards model was employed to analyze independent variables. The evaluated parameters of CT image were assessed through the non-parametric area under the receiver operating characteristic curve (ROC). The cut-off value was defined as the one point closest to the (0, 1) point. The comparison of the area under the curves (AUC) was performed by methods of DeLong. Statistical analyses were performed using the statistical software of STATA v. 13 (StataCorp, College Station, TX). A probability value of <0.05 with the two-sided test was considered statistically significant.
Results
The median observation period was 18 (range, 3.8–56.2) months. 52 (76.5%) patients had RP on CT image. One patient had been administered a steroid and another patient had undergone oxygen therapy. The remaining of 50 had asymptomatic. So 50 patients, 1 patient and 1 patient had Grade 1, 2 and 3 RP, respectively. None had Grade 4 and more. Because of the low number of high Grade RP, the analysis was performed with 52 patients with RP and 16 patients without RP. The median time of RP was 6 months. The actuarial rate of RP at 6 and 12 months was 52.3% [95% confidence interval (CI) 41.0–64.7%] and 74.7% (95% CI 63.2–84.9%), respectively.
Table 2 showed the mean difference percentages of LAA of ≤−860 or −950 HU, and average HU in the whole lung. The mean different percentages of LAA of ≤−860 HU (p = 0.0004) or −950 HU (p = 0.005), and average HU (p = 0.001) in patients with RP were significantly lower than those in patients without RP. The cut-off of LAA of ≤−860 or −950 HU, and average HU in all patients were determined as 50%, 10% and 850 HU, respectively; these values were taken from respective mean values.
Table 2.
The difference of low attenuation area on CT images between patients with non-pneumonitis and pneumonitis
| Percentage of area under low attenuation area (mean ± SD) | All | Non-pneumonitis | Pneumonitis | p value |
| −860 HU (%) | 45.3 ± 22.3 | 62.3 ± 15.4 | 40.0 ± 21.5 | 0.0004 |
| −950 HU (%) | 9.1 ± 12.4 | 16.0 ± 16.2 | 6.9 ± 10.3 | 0.005 |
| Average HU (HU) | −845.6 ± 36.3 | −869.8 ± 16.0 | −838.2 ± 37.6 | 0.001 |
HU, Hounsfield unit, SD, standard deviation.
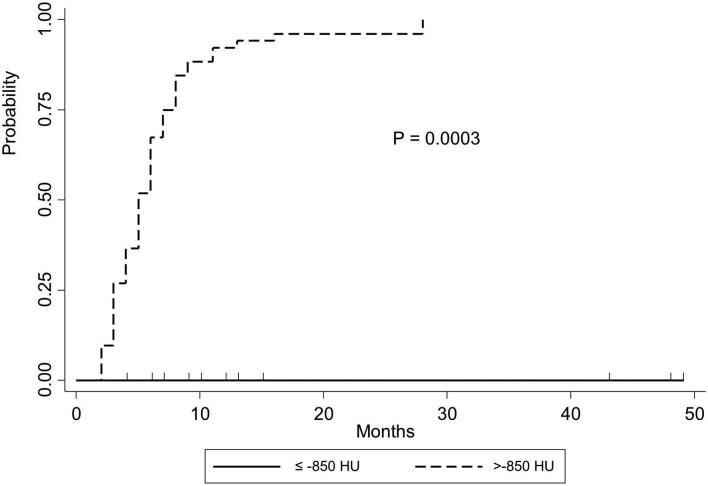
In univariate analysis, when percentages of LAA of less ≤−860 HU were >50%, the incidence (p = 0001) and rate (p = 0.002) of RP were significantly lower than those when the LAA percentage was ≤50% (Table 3). When percentages of LAA of ≤−950 HU were >10%, the incidence (p = 002) and rate (p = 0.03) of RP were statistically significantly lower than those when the LAA percentage was ≤10%. The RP incidence (p = 0.001) and rate (Figure 1, p = 0.0003) were significantly lower when the average HU values were ≤−850 HU.
Table 3.
Risk for radiation pneumonitis by univariate analysis
| Fisher’s exact test p value | Log-rank test p value | |||
| Percentage of LAA −860 HU | ≤50% | >50% | ||
| Pneumonitis – | 3 | 13 | ||
| Pneumonitis + | 35 | 17 | 0.001 | 0.002 |
| Percentage of LAA −950 HU | ≤10% | >10% | ||
| Pneumonitis – | 9 | 7 | ||
| Pneumonitis + | 48 | 4 | 0.002 | 0.03 |
| Average HU | ≤–850 HU | >–850 HU | ||
| Pneumonitis – | 14 | 2 | ||
| Pneumonitis + | 21 | 31 | 0.001 | 0.0003 |
HU, Hounsfield unit; LAA, low attenuation area.
Figure 1.
Kaplan-Meir’s curve of radiation pneumonitis after initiation of RT for patients with non-small cell lung cancer. The actuarial radiation pneumonitis rate is significantly lower when the average HU values were ≤−850 HU compared to >–850 HU (p = 0.0003). HU, Hounsfield unit.
In multivariate analysis, the cut-off values of 50% in the percentage of LAA of ≤−860 HU and average HU value ≤−850 HU were significant risk factors for RP. The location of the tumor, gender and the dose of the T factor were not significant (Table 4).
Table 4.
Prognostic factor associated with radiation pneumonitis
| HR | 95% CI | p value | HR | 95% CI | p value | HR | 95% CI | p value | |||
| Average HU | Percentage of area under −950 HU |
Percentage of area under −860 HU |
|||||||||
| ≤ −850 HU : >−850 HU | 0.40 | 0.22–0.73 | 0.003 | ≤10% :>10% | 0.38 | 0.13–1.10 | 0.08 | ≤50% :>50% | 0.41 | 0.21–0.79 | 0.008 |
| Location | Location | Location | |||||||||
| Upper : middle or linguinal and lower lobe | 0.80 | 0.43–1.47 | 0.47 | Upper : middle or linguinal and lower lobe | 0.82 | 0.44–1.51 | 0.52 | Upper : middle or linguinal and lower lobe | 0.93 | 0.50–1.72 | 0.81 |
| Gender | Gender | Gender | |||||||||
| Male: female | 0.79 | 0.44–1.44 | 0.45 | Male: female | 0.81 | 0.44–1.47 | 0.48 | Male: female | 0.66 | 0.36–1.21 | 0.18 |
| Dose | Dose | Dose | |||||||||
| ≤78 Gy :>78 Gy | 0.80 | 0.43–1.49 | 0.49 | ≤78 Gy :>78 Gy | 0.72 | 0.39–1.33 | 0.3 | ≤78 Gy :>78 Gy | 0.94 | 0.48–1.83 | 0.85 |
| T factor | T factor | T factor | |||||||||
| 1 : 2 | 0.89 | 0.46–1.73 | 0.73 | 1 : 2 | 0.95 | 0.50–1.81 | 0.87 | 1 : 2 | 0.89 | 0.46–1.71 | 0.72 |
CI, confidence interval; HU, Hounsfield unit; HR, hazard ratio.
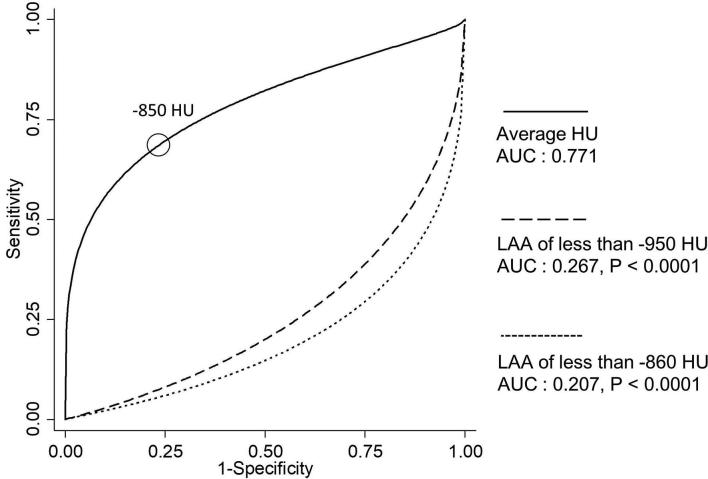
Figure 2 showed the ROC curves of LAA of ≤−860 or −950 HU, and average HU. The cut-off value of average HU was determined as −850 HU by ROC. AUC of average HU was significantly higher than that of LAA of ≤−860 (p < 0.0001) or −950 HU (p < 0.0001).
Figure 2.
ROC curve of LAA of ≤−860 or ≤−950 HU, and average HU estimated by binomial distribution. AUC of LAA of ≤−860 or ≤−950 HU, and average HU are 0.207, 0.267 and 0.771, respectively. The cut-off value is determined as –850 HU, which is closest distance from the maximum vertical (0,1) point. AUC of average HU is significantly higher than those of ≤−860 (p < 0.0001) or ≤−950 HU (p < 0.0001). AUC, Area under curve; HU, Hounsfield unit; LAA, low attenuation area; ROC, Receiver operating characteristic.
Discussion
Although stereotactic body RT is the standard care for stage I NSCLC, there has been no randomized trial to compare directly large dose hypofraction to conventional fraction. The trans-tasman radiation oncology group conducts randomized trial comparing hypofraction with conventional fraction for stage I NSCLC.14 This study showed that based on CT images, 75% of patients had Grade 1 RP and only 3% of patients had Grade 2 or 3 RP. In our current results, conventional fraction achieved less RP compared to other hypofractionated stereotactic studies.5–7
Pulmonary emphysema is characterized by anatomical alterations enlargement of the air spaces distal from the terminal bronchiole. The LAA value, which is an index of the enlargement of air spaces, can be concretely calculated from the CT value and is now available for clinical use.11, 12 The presence or absence of emphysema can then be determined based on the thus-calculated LAA values. Most quantitative CT studies have defined emphysema according to a threshold value ranging between −856 HU and −950 HU. Wang et al defined emphysema as an LAA value above −950 HU,15 while Busacker et al used a threshold of −850 HU.16 Some researchers stated that LAA values above −856 HU strongly correlated with physiological measurements of airway obstruction.11, 17 Thus, we set thresholds of −860 HU and –950 HU to find the optimal cut-off values. To the best of our knowledge, there was no report concerning the association between average HU in the whole lung and RP after RT. In the present study, our ROC analysis showed that average HU was better predicted of RP than was an LAA of ≤−860 HU or an LAA of ≤−950 HU.
Rancati et al performed multivariate analysis in patients with NSCLC who underwent RT and reported that the existence of COPD was a risk factor for RP but not dosimetric parameters.4 They denoted that patient with COPD was contra-indicated for RT because of the risk of RP. Kimura et al investigated the risk of RP in patients with PE, but the results of their two reports were contradictory.6, 18 When patients with NSCLC underwent stereotactic body RT, most patients with PE did not suffer from RP.6 In contrast, when patients with NSCLC and small cell lung cancer received involved field RT, PE was a risk factor for RP.18 In the letter study, 80% of patients underwent chemotherapy, and thus they considered that chemotherapy might increase the risk of RP. Our present findings indicated that patient with PE was not contra-indicated for RT.
RT induces cytokine cascades in parenchymal lung.19 The total amount of cytokine production in PE appears lower than that in chronic bronchitis, because the lungs of patient with emphysema have less parenchymal tissue than those of individuals without PE. Our study showed patient with PE had a low rate of RP.
This research had limitation. First, the study is limited by its retrospective design. In addition, few of the enrolled patients experienced clinically relevant RP. However, approximately three-quarters of the patients had Grade 1 RP, which was predicted by the quantitative measurement of the average HU.
Conclusion
The quantitative measurement of average HU in the whole lung from CT images is a useful tool to predict RP after RT. Further studies using quantitative CT images are needed to confirm whether PE patients can be treated safety by a standard hypofractionated dose of stereotactic.
Contributor Information
Tatsuhiko Saito, Email: saito19880704@gmail.com.
Hidetsugu Nakayama, Email: hnakayama@hosp.ncgm.go.jp.
Takafumi Yamada, Email: yamada.takafumi.1214@gmail.com.
Sachica Shiraishi, Email: s-nogi@tokyo-med.ac.jp.
Koichi Tokuuye, Email: ktokuue@tokyo-med.ac.jp.
REFERENCES
- 1.Nanda RH, Liu Y, Gillespie TW, Mikell JL, Ramalingam SS, Fernandez FG, et al. . Stereotactic body radiation therapy versus no treatment for early stage non-small cell lung cancer in medically inoperable elderly patients: A National Cancer Data Base analysis. Cancer 2015; 121: 4222–30. doi: 10.1002/cncr.29640 [DOI] [PubMed] [Google Scholar]
- 2.Hori M, Matsuda T, Shibata A, Katanoda K, Sobue T, Nishimoto H, et al. . Japan Cancer Surveillance Research Group Cancer incidence and incidence rates in Japan in 2009: a study of 32 population-based cancer registries for the Monitoring of Cancer Incidence in Japan (MCIJ) project. Jpn J Clin Oncol 2015; 45: 884–91. doi: 10.1093/jjco/hyv088 [DOI] [PubMed] [Google Scholar]
- 3.Palma D, Lagerwaard F, Rodrigues G, Haasbeek C, Senan S. Curative treatment of Stage I non-small-cell lung cancer in patients with severe COPD: stereotactic radiotherapy outcomes and systematic review. Int J Radiat Oncol Biol Phys 2012; 82: 1149–56. doi: 10.1016/j.ijrobp.2011.03.005 [DOI] [PubMed] [Google Scholar]
- 4.Rancati T, Ceresoli GL, Gagliardi G, Schipani S, Cattaneo GM. Factors predicting radiation pneumonitis in lung cancer patients: a retrospective study. Radiother Oncol 2003; 67: 275–83. doi: 10.1016/S0167-8140(03)00119-1 [DOI] [PubMed] [Google Scholar]
- 5.Aoki T, Nagata Y, Negoro Y, Takayama K, Mizowaki T, Kokubo M, et al. . Evaluation of lung injury after three-dimensional conformal stereotactic radiation therapy for solitary lung tumors: CT appearance. Radiology 2004; 230: 101–8. doi: 10.1148/radiol.2301021226 [DOI] [PubMed] [Google Scholar]
- 6.Kimura T, Matsuura K, Murakami Y, Hashimoto Y, Kenjo M, Kaneyasu Y, et al. . CT appearance of radiation injury of the lung and clinical symptoms after stereotactic body radiation therapy (SBRT) for lung cancers: are patients with pulmonary emphysema also candidates for SBRT for lung cancers? Int J Radiat Oncol Biol Phys 2006; 66: 483–91. doi: 10.1016/j.ijrobp.2006.05.008 [DOI] [PubMed] [Google Scholar]
- 7.Takeda T, Takeda A, Kunieda E, Ishizaka A, Takemasa K, Shimada K, et al. . Radiation injury after hypofractionated stereotactic radiotherapy for peripheral small lung tumors: serial changes on CT. AJR Am J Roentgenol 2004; 182: 1123–8. doi: 10.2214/ajr.182.5.1821123 [DOI] [PubMed] [Google Scholar]
- 8.Roach M, Gandara DR, Yuo HS, Swift PS, Kroll S, Shrieve DC, et al. . Radiation pneumonitis following combined modality therapy for lung cancer: analysis of prognostic factors. J Clin Oncol 1995; 13: 2606–12. doi: 10.1200/JCO.1995.13.10.2606 [DOI] [PubMed] [Google Scholar]
- 9.Ishijima M, Nakayama H, Itonaga T, Tajima Y, Shiraishi S, Okubo M, et al. . Patients with severe emphysema have a low risk of radiation pneumonitis following stereotactic body radiotherapy. Br J Radiol 2015; 88: 20140596. doi: 10.1259/bjr.20140596 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Goddard PR, Nicholson EM, Laszlo G, Watt I. Computed tomography in pulmonary emphysema. Clin Radiol 1982; 33: 379–87. doi: 10.1016/S0009-9260(82)80301-2 [DOI] [PubMed] [Google Scholar]
- 11.Schroeder JD, McKenzie AS, Zach JA, Wilson CG, Curran-Everett D, Stinson DS, et al. . Relationships between airflow obstruction and quantitative CT measurements of emphysema, air trapping, and airways in subjects with and without chronic obstructive pulmonary disease. AJR Am J Roentgenol 2013; 201: W460–W470. doi: 10.2214/AJR.12.10102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Washko GR, Hunninghake GM, Fernandez IE, Nishino M, Okajima Y, Yamashiro T, et al. . Lung volumes and emphysema in smokers with interstitial lung abnormalities. N Engl J Med 2011; 364: 897–906. doi: 10.1056/NEJMoa1007285 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tajima Y, Nakayama H, Itonaga T, Shiraishi S, Okubo M, Mikami R, et al. . Dosimetric evaluation of compensator intensity modulation-based stereotactic body radiotherapy for Stage I non-small-cell lung cancer. Br J Radiol 2015; 88: 20150122. doi: 10.1259/bjr.20150122 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Trans Tasman Radiation Oncology Group. Hypofractionated image guidedradiotherapy versus conventionalradiotherapy for inoperable early stage Inon small cell lung cancer. 2017. Available from: https://www.trog.com.au/TROG-0902-CHISEL [17 Apr 2018].
- 15.Wang Z, Gu S, Leader JK, Kundu S, Tedrow JR, Sciurba FC, et al. . Optimal threshold in CT quantification of emphysema. Eur Radiol 2013; 23: 975–84. doi: 10.1007/s00330-012-2683-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Busacker A, Newell JD, Keefe T, Hoffman EA, Granroth JC, Castro M, et al. . A multivariate analysis of risk factors for the air-trapping asthmatic phenotype as measured by quantitative CT analysis. Chest 2009; 135: 48–56. doi: 10.1378/chest.08-0049 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Morgan MD. Detection and quantification of pulmonary emphysema by computed tomography: a window of opportunity. Thorax 1992; 47: 1001–4. doi: 10.1136/thx.47.12.1001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kimura T, Togami T, Takashima H, Nishiyama Y, Ohkawa M, Nagata Y. Radiation pneumonitis in patients with lung and mediastinal tumours: a retrospective study of risk factors focused on pulmonary emphysema. Br J Radiol 2012; 85: 135–41. doi: 10.1259/bjr/32629867 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mehta V. Radiation pneumonitis and pulmonary fibrosis in non-small-cell lung cancer: pulmonary function, prediction, and prevention. Int J Radiat Oncol Biol Phys 2005; 63: 5–24. doi: 10.1016/j.ijrobp.2005.03.047 [DOI] [PubMed] [Google Scholar]