Abstract
Objective:
To test, through tridimensional analysis, whether (1) cartilage thickness at the posterior aspect of femoral condyles differs in knees with medial femorotibial osteoarthritis (OA) compared to non-OA knees; (2) the location of the thickest cartilage at the posterior aspect of femoral condyles differs between OA and non-OA knees.
Methods:
CT arthrograms of knees without radiographic OA (n = 30) and with severe medial femorotibial OA (n = 30) were selected retrospectively from patients over 50 years of age. The groups did not differ in gender, age and femoral size. CT arthrograms were segmented to measure the mean cartilage thickness, the maximal cartilage thickness and its location in a region of interest at the posterior aspect of condyles.
Results:
For the medial condyle, mean and maximum cartilage thicknesses were statistically significantly higher in OA knees compared to non-OA knees [1.66 vs 1.46 mm (p = 0.03) and 2.56 vs 2.14 mm (p = 0.003), respectively]. The thickest cartilage was located in the half most medial aspect of the posterior medial condyle for both groups, without significant difference between groups. For the lateral condyle, no statistically significant difference between non-OA and OA knees was found (p ≥ 0.17).
Conclusion:
Cartilage at the posterior aspect of the medial condyle, but not the lateral condyle, is statistically significantly thicker in advanced medial femorotibial OA knees compared to non-OA knees. The thickest cartilage was located in the half most medial aspect of the posterior medial condyle. These results will serve as the basis for future research to determine the histobiological processes involved in this thicker cartilage.
Advances in knowledge:
This study, through a quantitative tridimensional approach, shows that cartilage at the posterior aspect of the medial condyles is thicker in severe femorotibial osteoarthritic knees compared to non-OA knees. In the posterior aspect of the medial condyle, the thickest cartilage is located in the vicinity of the center of the half most medial aspect of the posterior medial condyle. These results will serve as the basis for future research to determine the histobiological processes involved in this thicker cartilage.
BACKGROUND AND OBJECTIVES
Osteoarthritis (OA) has long been considered as a “wear and tear” process, where articular cartilage is assumed to have limited healing capacity.1 In this model, the disease is believed to develop due to an imbalance that occurs between increased cartilage degradation and insufficient cartilage synthesis, leading to gradual destruction and thinning of cartilage as the disease progresses.2
However, recent data showed that this traditional model of a one-way road toward progressive destruction of cartilage might not be true for certain areas of the knee joint to which little attention had been paid so far. Indeed, cartilage at the most posterior aspect of the medial condyle was found to be thicker in OA knees, including in advanced stages, compared to non-OA knees.3 This finding could have important implications for the understanding and treatment of OA since it could point at potential regenerative capacities of cartilage. However, in this previous report, cartilage thickness was manually measured at a single focal point, on the midsagittal plane of each femoral condyle. Since cartilage thickness is known to vary throughout the articular surface,4–6 it is necessary to validate these potentially important results on another dataset, using a three-dimensional analysis that would take into account the entire articular surface at the posterior aspect of the condyles. Furthermore, it is important to determine the location of the thickest cartilage in these posterior regions to help enhance our understanding of morphological differences related to OA and provide a basis for future analyses, including histobiological assays.
Therefore, in the current study, we aimed
to test, through tridimensional analysis, whether cartilage thickness at the posterior aspect of the medial and lateral condyles differs in medial femorotibial OA knees compared to non-OA knees;
to compare the location of the thickest cartilage between non-OA and OA knees within the posterior aspect of the condyles showing differences in cartilage thickness.
METHODS AND MATERIALS
Patient population
Two groups of patients with non-radiographic OA (n = 30; mean age = 61.6 ± 5.14 years; 13 males) and severe medial femorotibial OA (n = 30; mean age = 62.3 ± 9.35 years; 13 males) (one knee per individual) were selected retrospectively and randomly from our institution's clinical database.
The indications to perform the CT examinations included the diagnostic workup of suspected menisco-cartilaginous lesions, as well as the preoperative workup of knee arthroplastic procedures to determine the number of compartments to be replaced.
Inclusion criteria were age of 50 years or above, and CT arthrogram and lateral and posteroanterior weight-bearing radiographs of the knees available from the same day.
Any knees presenting imaging signs of the following conditions were excluded from the initial set of examinations: previous bone fractures, previous knee surgery (including knee replacement procedures, ligamentoplasty and cartilage repair procedures), inflammatory joint disease, crystal arthropathy or poor image quality.
We randomly included the patients until the sample size, which was determined based on data from a previous report, was reached.3
The non-OA group was defined by a Kellgren–Lawrence (K/L) grade <2 for all three compartments at knee radiography, while the severe OA group was defined by a K/L grade ≥3 for the medial femorotibial compartment and a K/L grade <2 for the patellofemoral and the lateral femorotibial compartments.7
Independent t-tests indicated no statistically significant difference between groups for age (p = 0.49) and biepicondylar femoral diameter, as measured following previously published methodology (8.1 ± 0.6 vs 8.1 ± 0.4 cm for the non-OA and OA groups respectively, p = 0.80).3
This study was approved by the institutional ethical committee without requirement for informed consent from the participants due to the retrospective study design.
Imaging protocol
Radiography
Knee radiographs, obtained immediately before the arthrographic examinations, included lateral and frontal weight-bearing views. Radiographs were graded by a musculoskeletal radiologist (PO) with 8 years of experience, using a modified K/L grade allowing the separate analysis of each compartment of the knee (medial, lateral and patellofemoral) to identify medial femorotibial OA knees.7 The reader was blinded to the CT arthrographic findings while analyzing the radiographs.
CT arthrography
CT arthrograms were performed following the standard procedure used in clinical practice at the institution: CT examinations were performed after intra-articular injection of 10 ml of ionic contrast material (320 mg of iodine per milliliter of meglumine ioxaglate and sodium ioxaglate) under fluoroscopy using a lateral patellofemoral approach, by practicing radiologists from the musculoskeletal imaging unit of the institution. Examinations were performed on a 40-detector row CT scanner (Somatom Definition AS; Siemens Healthcare, Forchheim, Germany). Patients were positioned supine, with extension of the knee. Acquisition parameters were tube voltage, 120 kVp; reference tube current–time product, 350 mAs with the application of a dose modulation protocol (Care Dose 4D; Siemens Healthcare); detector configuration: 16 × 0.6 mm; pitch: 0.85; gantry rotation time: 1 s. The following image reconstruction parameters were used: field of view = 15 cm; matrix = 5122; section thickness/increment = 0.6/0.3 mm; bone convolution kernel. The CT examination was performed within 15 min of the arthrographic procedure, to avoid any significant penetration of contrast media into the cartilage.
Image post-processing
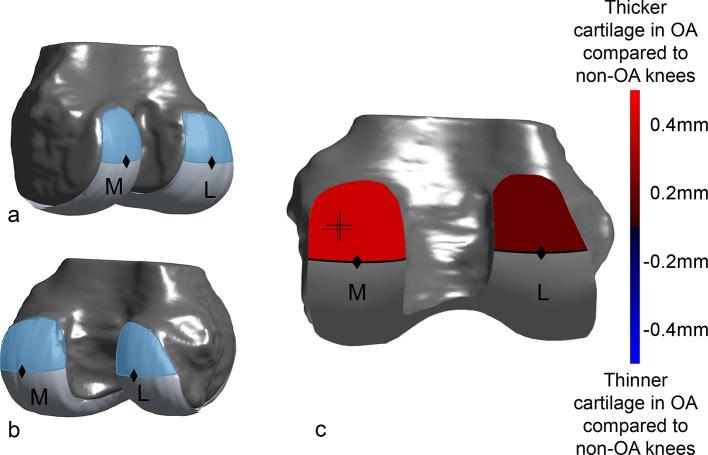
The femoral bone and cartilage were segmented in the CT images by two trained researchers under the supervision of a musculoskeletal radiologist with 8 years of experience (PO), using a previously validated semi-manual method allowing subpixel resolution based on B-spline.8 This method reconstructs tridimensional mesh models of the femoral bones and cartilages, and calculates tridimensional cartilage thickness maps corresponding to the thickness of cartilage covering the subchondral bone.6 The individual cartilage thickness maps were then standardized across knees using a previously described method based on a matching of the shape of the subchondral bone.5, 9 This process provided an anatomically standardized cartilage thickness map for each knee, allowing spatial comparison among samples. Two regions of interest (ROI) corresponding to the posterior aspect of each condyle were identified in the standardized thickness map, and the mean cartilage thickness, the maximal cartilage thickness and the location of the point of maximal cartilage thickness were determined for each ROI.6 The ROIs were defined independently for each condyle as the areas of the thickness maps passed the most posterior point of each condyle (Figure 1).
Figure 1.
Illustration of the regions of interest (ROIs) at the posterior aspect of the medial (M) and lateral (L) femoral condyles. The ROIs were determined on a 3D anatomically standardized model of subchondral bone,9 and corresponded to the region of cartilage located cranially and posteriorly to the most posterior point (black diamonds) of each condyle. (a, b): 3D volume rendering oblique views of a standard knee showing these ROIs for both the medial (M) and lateral (L) condyles. (c) 3D volume rendering posterior view of a standard knee showing ROIs color-coded according to the differences in mean cartilage thickness between the non-osteoarthritis (OA) and OA groups of knees (red and blue indicating thicker and thinner cartilage in OA compared to non-OA knees, respectively). The cartilage on the medial condyle was statistically significantly thicker in OA knees compared to non-OA knees (p < 0.001). The location of the points of thickest cartilage for the medial condyle is also represented using crosses indicating the mean and standard deviation of the locations of thickest cartilage (the location of the cross indicates the location of thickest cartilage averaged for non-OA knees (full cross) and OA knees (dotted cross), the width of the cross in the medial–lateral and anteroposterior axes represents the standard deviations of the coordinates).
Statistical analyses
The normal distribution of the data samples was confirmed using the one-sample Kolmogorov–Smirnov test. Student’s t-tests for independent samples were then performed to compare the mean cartilage thickness, maximal cartilage thickness and location of thickest cartilage between the non-OA and OA groups of knees. Since the locations of thickest cartilage were measured on anatomically standardized thickness maps, the coordinates of the thickest points could be directly compared between groups along the medial–lateral and anteroposterior directions.5, 6 All statistical analyses were performed using Matlab (MATLAB Release 2014b, The MathWorks, Inc., Natick, Massachusetts, United States) and an alpha-level of 5% was considered for all tests.
RESULTS
Comparison of cartilage thickness between non-OA and OA-groups
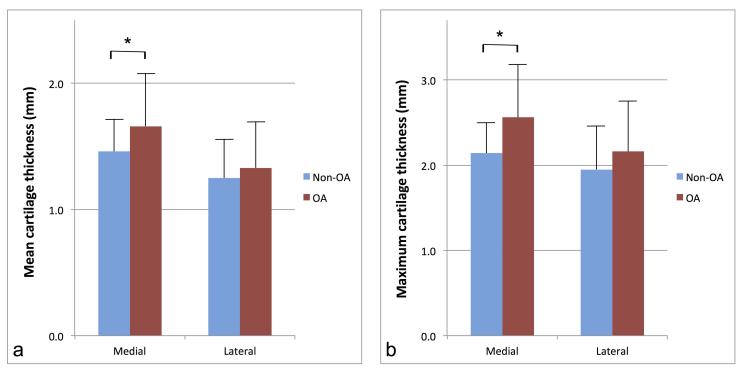
For the medial condyle, mean cartilage thickness and maximum cartilage thickness were statistically significantly higher in OA knees compared to non-OA knees [1.66 ± 0.42 vs 1.46 ± 0.26 mm (p = 0.03) and 2.56 ± 0.62 vs 2.14 ± 0.36 mm (p = 0.003), respectively] (Figures 2 and 3)(Table 1). For the lateral condyle, no statistically significant difference between OA and non-OA knees was found for either the mean or the maximum cartilage thickness [1.33 ± 0.37 vs 1.25 ± 0.31 mm (p = 0.40) and 2.16 ± 0.60 vs 1.95 ± 0.50 mm (p = 0.17), respectively] (Figure 3)(Table 1).
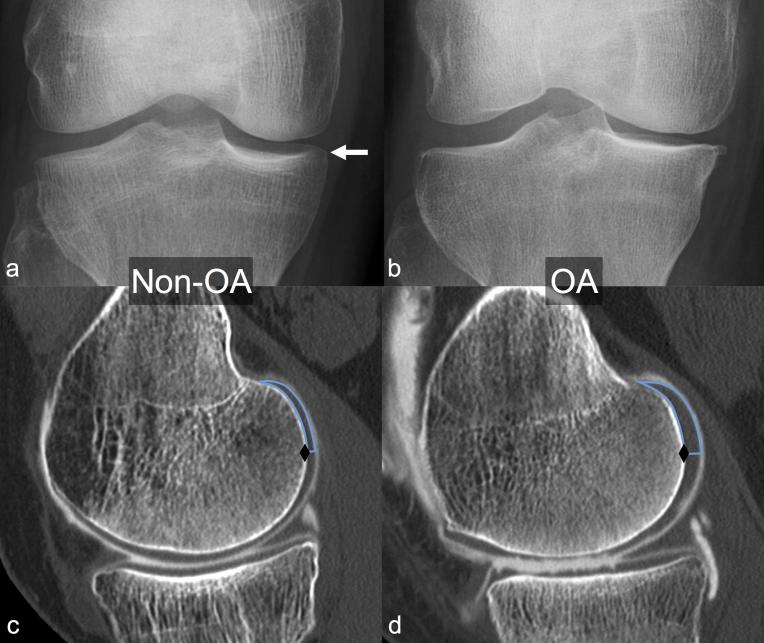
Figure 2.
Frontal weight-bearing radiographs (a and b) and sagittal reformats (c, d) from two 57-year-old females [(a, c): patient from the non-osteoarthritis (OA) group; (b, d): patient from the OA group]. (a) Doubtful osteophyte at the medial aspect of the medial tibial plateau (arrow): Kellgren–Lawrence Grade 1 medial femorotibial OA. (b) Moderate osteophyte at the medial aspect of the medial tibial plateau, definite narrowing of medial femorotibial joint space, and slight sclerosis of the medial tibial plateau: Kellgren–Lawrence Grade 3 medial femorotibial OA. Sagittal reformats (c, d) through the point of maximum cartilage thickness in the half most medial portion of the posterior aspect of the medial condyle show the regions of interest (ROIs) used to determine cartilage thickness at the most posterior aspect of the condyles (blue line), cranially and posteriorly to the most posterior point of the condyle (black diamond). (c) Patient taken from the non-OA group: mean cartilage thickness for the entire medial condyle ROI: 1.03 mm, maximum cartilage thickness: 1.63 mm. (d) Patient taken from the OA group: mean cartilage thickness for the entire medial condyle ROI 2.16 mm, maximum cartilage thickness 3.48 mm.
Figure 3.
Charts illustrate the comparison of the mean cartilage thickness (a) and maximum cartilage thickness (b), between non-osteoarthritis (blue) and osteoarthritis (red) groups, for both the medial and lateral condyles. Results are expressed in millimeters, and error bars correspond to standard deviations of the means. Asterisks indicate statistically significant differences.
Table 1.
Comparison of mean cartilage thickness and maximum cartilage thickness at the posterior aspect of the medial and lateral condyles between non-OA and OA knees
| Mean cartilage thickness | Maximum cartilage thickness | |||||
| Non-OA (n = 30) | OA (n = 30) | p-value | Non-OA (n = 30) | OA (n = 30) | p-value | |
| Medial | 1.46 (0.26) | 1.66 (0.42) | 0.03 | 2.14 (0.36) | 2.56 (0.62) | 0.003 |
| Lateral | 1.25 (0.31) | 1.33 (0.37) | 0.40 | 1.95 (0.50) | 2.16 (0.60) | 0.17 |
OA, osteoarthritis.
Location of thickest cartilage
The location of the point of thickest cartilage was determined for the posterior aspect of the medial condyle (which showed statistically significantly thicker cartilage in the OA knees). The points of thickest cartilage in non-OA and OA knees (black and blue crosses in Figure 1, respectively) were primarily located in the vicinity of the center of the half most medial portion of the posterior aspect of the medial condyle (crosses in Figure 1c). The medial–lateral and cranial–distal coordinates of the points of thickest cartilage did not differ between the two groups (p ≥ 0.56) [as shown in Figure 1, the locations of the black (non-OA) and blue (OA) crosses were similar].
DISCUSSION
The tridimensional quantitative analysis performed in this study showed thicker cartilage at the posterior aspect of the medial condyle in severe medial tibiofemoral OA compared to non-OA knees.
This finding of thicker cartilage in advanced OA knees questions the common understanding that cartilage undergoes progressive destruction and thinning as OA progresses.
There is very little data in the literature pointing to thicker cartilage in advanced OA knees. In a first report dating back to the early 80s, Vignon and Arlot noted on 32 femoral head samples from OA hips that cartilage at the periphery of the cartilage surface showed increased thickness, which was associated to increased cell density and proteoglycan synthesis.10 Shortly later, the same authors and colleagues confirmed these results using an experimental model of OA, consisting of transection of the anterior cruciate ligament, which they performed in 11 mature dogs.11 Morphometric, histological and biochemical analysis of the patellofemoral joints was performed 0.5 to 8 months after surgery, with the contralateral side taken as control. They confirmed increased thickness and hypertrophic regenerative changes with cell proliferation and increased matrix synthesis.
About a decade later, another team of researchers used the same experimental model of OA on three mature dogs, and performed an in vivo assessment of cartilage thickness 36 months after ACL transection. Using MRI, they confirmed an increase in cartilage thickness at the periphery of advanced OA knees, but not at the weight-bearing regions, 36 months after transection.12 Of note, as described in a following paper, the authors followed up the same dogs up to 45 months and showed that eventually, areas of cartilage hypertrophy presented with cartilage breakdown on the medial condyle, but not on the lateral condyle, where it remained thicker.13
Apart from these four reports, including one on hip specimens and three on animal models of OA, there has been, to the best of our knowledge, no report confirming this concept of thicker cartilage in OA joints. Indeed, most research on non-invasive assessment of the human cartilage, either indirectly with radiographs or directly with MRI, has so far been focusing on areas of cartilage breakdown and thinning on the weight-bearing regions of the femorotibial joint. In particular, a large number of cohort studies have used coronal imaging protocols, only covering 60% of the articular surface (roughly corresponding to the central weight-bearing region).14 As a consequence, areas of thicker cartilage in advanced OA have gone undetected for a number of years.
Recently, one paper, focusing on the analysis of cartilage from the entire articular surface, did point to subregional variations of cartilage thickness, showing some areas of cartilage thickening.15 The authors studied 267 knee MRIs from 135 participants, corresponding to 308 non-OA and 227 OA knees, followed-up at 21 months, and showed some areas of cartilage thickening at follow-up in the medial femorotibial compartment.15 Finally, a recent report specifically aimed at confirming the presence of thicker cartilage in preserved areas of OA knees. In a report on 535 consecutive CT arthrograms (308 non-OA and 227 OA knees), the authors measured cartilage thickness manually on a single standardized point at the posterior aspect of the medial condyle, and found significantly thicker cartilage in OA compared to non-OA knees [2.43 mm, 95% CI (2.36, 2.51 vs 2.13 mm), 95% CI (2.02, 2.17) respectively, p < 0.001].3 This area of the posterior aspect of the medial condyle seems interesting because it represents a non-weight-bearing area in most day-to-day activities (these areas only bear weight in activities such as squatting).16
It is not clear why cartilage in the posterior aspect of the medial condyle is thicker in advanced OA knees. It could correspond to swollen cartilage as classically described in the early stages of OA.17, 18 In our sample of knees with advanced OA, the cartilage we focused on at the posterior aspect of the condyles was located at the periphery of the weight-bearing regions, which could be in an early phase of the disease. Another explanation, which is supported by the previously mentioned studies using animal models, could be that cartilage in these areas is truly hypertrophic, as a consequence of anabolic processes initiated in reaction to OA, potentially representing a reparative answer to the disease.10, 11 In weight-bearing areas, these processes could be overcome by the mechanical strains to which cartilage is exposed, leading to tissue destruction and thinning. However, the anabolic reactions could manifest in non-weight-bearing areas, such as the posterior aspect of the medial condyle. If this hypothesis stands true, determining the mediators responsible for these anabolic reactions could open new doors to develop novel therapeutic pathways for the management of OA. Therefore, future research should investigate the histobiological characteristics of this thicker cartilage to determine whether it is simply swollen or truly hypertrophic.
The second main finding of our study was that the thickest cartilage was primarily located in the vicinity of the center of the half most medial portion of the posterior aspect of the medial condyle. This has potential implications for future research aiming to characterize the histobiological properties of this thicker cartilage, by providing a target area for such analyses, possibly on samples from total knee replacement surgeries.
We found cartilage to be significantly thicker at the posterior aspect of the medial condyle, but not of the lateral condyle, which is in agreement with previously published data.3 The absence of any significant difference in cartilage thickness for the lateral condyle could have several explanations. One hypothesis is that the increased thickness for the medial condyle could be the consequence of local factors that only act on the affected compartment (in the current study, we only analyzed medial femorotibial OA knees). However, it has been previously shown that this hypothesis does not hold true for the lateral compartment: when considering lateral femorotibial OA knees, cartilage thickness at the posterior aspect of the lateral condyle decreased with increasing K/L grade.3 A more likely explanation would be the presence of cartilage lesions on the lateral compartment, decreasing the mean cartilage thickness in that compartment, and to a lesser degree, the maximum cartilage thickness, as illustrated by our results. These cartilage lesions have previously been shown to be more frequent laterally than medially, probably due to the different mechanical constraints sustained by the two condyles.19, 20 In fact, studies have found a high frequency of cartilage lesions at the posterior aspect of the lateral condyle (up to 24% of 654 consecutive knee MRIs), while in the great majority of cases, cartilage is preserved at the most posterior aspect of the medial condyle until the latest stages of OA (up to 87% of 41 consecutive pre-total knee arthroplasty knees).19, 20
This study has several limitations. First, although most potential confounders that have been previously described to correlate with cartilage thickness, including age, gender and bone size, were not different between the non-OA and OA groups, other parameters related to the physical size of the participants (such as height, weight and body mass index) were not analyzed as these data were unavailable. An association between cartilage thickness and physical size, however, remains controversial.21, 22 Second, the results are limited by the retrospective nature of this study and longitudinal and prospective research is necessary to confirm that cartilage does actually thicken with the development of OA. Third, participants were selected based on a well-accepted radiographic definition of OA, but the results could be different if a clinical definition of OA was used. Nevertheless, the fact that the radiographic definition of OA does not consider cartilage in the posterior aspect of the condyle strengthens the results, avoiding a selection bias. Fourth, while the cartilage thickness measurement method was previously validated and successfully used in several studies using MRI data, the method has not been specifically assessed with CT arthrography images.6, 8,23 Nevertheless, it is expected that the reliability of the method should be at least as good as with MRI data as the contrast between bone, cartilage and synovial fluid is higher with CT arthrography.24 Although one cannot exclude any measurement error, the error should have affected both groups equally. Therefore, the differences identified in this study are likely to be true. It is, however, possible that other differences existed but were not detected. Finally, it is worth mentioning that the present results concur with a prior report using a different measurement method on a different population.
CONCLUSION
In conclusion, this study confirmed that cartilage at the posterior aspect of the medial condyle, but not at the posterior aspect of the lateral condyle, is statistically significantly thicker in OA knees compared to non-OA knees, using a tridimensional approach. Second, the results showed that the thickest cartilage was primarily located in the half most medial aspect of the posterior medial condyle for both non-OA and OA knees.
Contributor Information
Patrick Omoumi, Email: pomoumi@gmail.com.
Hugo Babel, Email: hugo.babel@chuv.ch.
Brigitte M Jolles, Email: Brigitte.Jolles-Haeberli@chuv.ch.
Julien Favre, Email: Julien.Favre@chuv.ch.
REFERENCES
- 1.Hunziker EB, Lippuner K, Keel MJB, Shintani N. An educational review of cartilage repair: precepts & practice – myths & misconceptions – progress & prospects. Osteoarthritis Cartilage 2015; 23: 334–50. doi: 10.1016/j.joca.2014.12.011 [DOI] [PubMed] [Google Scholar]
- 2.Abramson SB, Attur M. Developments in the scientific understanding of osteoarthritis. Arthritis Res Ther 2009; 11: 227. doi: 10.1186/ar2655 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Omoumi P, Michoux N, Roemer FW, Thienpont E, Vande Berg BC. Cartilage thickness at the posterior medial femoral condyle is increased in femorotibial knee osteoarthritis: a cross-sectional CT arthrography study (Part 2). Osteoarthritis Cartilage 2015; 23: 224–31. doi: 10.1016/j.joca.2014.08.017 [DOI] [PubMed] [Google Scholar]
- 4.Cohen ZA, Mow VC, Henry JH, Levine WN, Ateshian GA. Templates of the cartilage layers of the patellofemoral joint and their use in the assessment of osteoarthritic cartilage damage. Osteoarthritis Cartilage 2003; 11: 569–79. doi: 10.1016/S1063-4584(03)00091-8 [DOI] [PubMed] [Google Scholar]
- 5.Favre J, Fasel B, Andriacchi TP. Pattern in femoral cartilage thickness map allows subtle scoring of medial compartment knee osteoarthritis severity. Osteoarthritis and Cartilage 2013; 21: S231–S232. doi: 10.1016/j.joca.2013.02.477 [DOI] [Google Scholar]
- 6.Favre J, Scanlan SF, Erhart-Hledik JC, Blazek K, Andriacchi TP. Patterns of femoral cartilage thickness are different in asymptomatic and osteoarthritic knees and can be used to detect disease-related differences between samples. J Biomech Eng 2013; 135: 101002–10. doi: 10.1115/1.4024629 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Felson DT, McAlindon TE, Anderson JJ, Weissman BW, Aliabadi P, Evans S, et al. Defining radiographic osteoarthritis for the whole knee. Osteoarthritis Cartilage 1997; 5: 241–50. doi: 10.1016/S1063-4584(97)80020-9 [DOI] [PubMed] [Google Scholar]
- 8.Koo S, Gold GE, Andriacchi TP. Considerations in measuring cartilage thickness using MRI: factors influencing reproducibility and accuracy. Osteoarthritis Cartilage 2005; 13: 782–9. doi: 10.1016/j.joca.2005.04.013 [DOI] [PubMed] [Google Scholar]
- 9.Favre J, Erhart-Hledik JC, Blazek K, Fasel B, Gold GE, Andriacchi TP. Anatomically standardized maps reveal distinct patterns of cartilage thickness with increasing severity of medial compartment knee osteoarthritis. J Orthop Res 2017; 35: 2442–51. doi: 10.1002/jor.23548 [DOI] [PubMed] [Google Scholar]
- 10.Vignon E, Arlot M. Macroscopically normal cartilage from the human osteoarthritic femoral head. II. Measurement of cartilage thickness and cell density. J Rheumatol 1981; 8: 447–50. [PubMed] [Google Scholar]
- 11.Vignon E, Arlot M, Hartmann D, Moyen B, Ville G. Hypertrophic repair of articular cartilage in experimental osteoarthrosis. Ann Rheum Dis 1983; 42: 82–8. doi: 10.1136/ard.42.1.82 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Braunstein EM, Brandt KD, Albrecht M. MRI demonstration of hypertrophic articular cartilage repair in osteoarthritis. Skeletal Radiol 1990; 19: 335–9. doi: 10.1007/BF00193086 [DOI] [PubMed] [Google Scholar]
- 13.Brandt KD, Braunstein EM, Visco DM, O'Connor B, Heck D, Albrecht M. Anterior (cranial) cruciate ligament transection in the dog: a bona fide model of osteoarthritis, not merely of cartilage injury and repair. J Rheumatol 1991; 18: 436–46. [PubMed] [Google Scholar]
- 14.Wirth W, Benichou O, Kwoh CK, Guermazi A, Hunter D, Putz R, et al. Spatial patterns of cartilage loss in the medial femoral condyle in osteoarthritic knees: data from the osteoarthritis initiative. Magn Reson Med 2010; 63: 574–81. doi: 10.1002/mrm.22194 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jørgensen DR, Lillholm M, Genant HK, Dam EB. On subregional analysis of cartilage loss from knee MRI. Cartilage 2013; 4: 121–30. doi: 10.1177/1947603512474265 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Andriacchi TP, Koo S, Scanlan SF. Gait mechanics influence healthy cartilage morphology and osteoarthritis of the knee. J Bone Joint Surg Am 2009; 91(Suppl 1): 95–101. doi: 10.2106/JBJS.H.01408 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Calvo E, Palacios I, Delgado E, Ruiz-Cabello J, Hernández P, Sánchez-Pernaute O, et al. High- resolution MRI detects cartilage swelling at the early stages of experimental osteoarthritis. Osteoarthritis Cartilage 2001; 9: 463–72. doi: 10.1053/joca.2001.0413 [DOI] [PubMed] [Google Scholar]
- 18.Hunter DJ, Niu JB, Zhang Y, LaValley M, McLennan CE, Hudelmaier M, et al. Premorbid knee osteoarthritis is not characterised by diffuse thinness: the Framingham osteoarthritis study. Ann Rheum Dis 2008; 67: 1545–9. doi: 10.1136/ard.2007.076810 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Omoumi P, Michoux N, Thienpont E, Roemer FW, Vande Berg BC. Anatomical distribution of areas of preserved cartilage in advanced femorotibial osteoarthritis using CT arthrography (Part 1). Osteoarthritis Cartilage 2015; 23: 83–7. doi: 10.1016/j.joca.2014.10.006 [DOI] [PubMed] [Google Scholar]
- 20.Ogino S, Huang T, Watanabe A, Iranpour-Boroujeni T, Yoshioka H. Magnetic resonance imaging of articular cartilage abnormalities of the far posterior femoral condyle of the knee. Acta Radiol 2010; 51: 52–7. doi: 10.3109/02841850903307566 [DOI] [PubMed] [Google Scholar]
- 21.Eckstein F, Winzheimer M, Westhoff J, Schnier M, Haubner M, Englmeier KH, et al. Quantitative relationships of normal cartilage volumes of the human knee joint-assessment by magnetic resonance imaging. Anat Embryol 1998; 197: 383–90. doi: 10.1007/s004290050149 [DOI] [PubMed] [Google Scholar]
- 22.Karvonen RL, Negendank WG, Teitge RA, Reed AH, Miller PR, Fernandez-Madrid F. Factors affecting articular cartilage thickness in osteoarthritis and aging. J Rheumatol 1994; 21: 1310–8. [PubMed] [Google Scholar]
- 23.Chehab EF, Favre J, Erhart-Hledik JC, Andriacchi TP. Baseline knee adduction and flexion moments during walking are both associated with 5 year cartilage changes in patients with medial knee osteoarthritis. Osteoarthritis Cartilage 2014; 22: 1833–9. doi: 10.1016/j.joca.2014.08.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Omoumi P, Mercier GA, Lecouvet F, Simoni P, Vande Berg BC. CT arthrography, MR arthrography, PET, and scintigraphy in osteoarthritis. Radiol Clin North Am 2009; 47: 595–615. doi: 10.1016/j.rcl.2009.04.005 [DOI] [PubMed] [Google Scholar]