Abstract
Objective:
To investigate the efficacy and safety of ultrasound-guided tissue glue injection for the treatment of iatrogenic femoral artery pseudoaneurysm.
Methods:
The study comprised of nine patients with unsuccessful ultrasound-guided thrombin injection and one patient with rapidly progressing anemia. All patients had undergone recanalization procedures at least twice, including two subjects with a very rapidly enlarging pseudoaneurysm lobe or significant anemia. Tissue glue at a dose of 0.9 ± 0.53 ml was injected under ultrasound guidance in each patient.
Results:
Complete embolization was achieved in all patients. Follow-up ultrasound performed 24 h later as well as at 1 and 2 weeks did not show recurrent reperfusion of the pseudoaneurysm.
Conclusion:
Embolization of iatrogenic pseudoaneurysm using tissue glue seems to be an effective technique for the treatment of this complication. It might be considered as a treatment option in case of unsuccessful primary repair by means of thrombin injection orhemorrhagic shock due to rapid aneurysm progression.
Advances in knowledge:
Patients with multiple recanalizations and those with dynamically enlarging pseudoaneurysm or rapidly progressing anemia are at risk of life-threatening bleeding. An ultrasound-guided tissue glue injection, a novel method for the treatment of femoral artery pseudoaneurysm, might be considered as a treatment option especially in case of primary thrombin injection failure.
Introduction
Iatrogenic pseudoaneurysms (psA) affect about 2.0–6.0% of patients undergoing interventional vessel cannulation in the inguinal area. A pseudoaneurysm forms after disruption of the arterial wall integrity. It is a complication encountered in patients following artery catheterization procedures.1 A puncture site in the arterial wall after arterial sheath removal usually seals under mechanical compression. However, in some cases it remains patent causing blood flow into the perivascular space. Embolization of psA with bovine or recombinant human thrombin under real-time ultrasound guidance is the most commonly used treatment modality (ultrasound-guided thrombin injection, UGTI).2 Percutaneous application of thrombin (human or bovine) into the pseudoaneurysm lobe (or lobes) activates the patient’s coagulation factors and the blood clot is formed, thus avoiding surgical revision which is associated with a risk for intraoperative bleeding (especially in patients receiving antiplatelet therapy), postoperative wound infection and sometimes with the need for rehabilitation.3 Success of UGTI is estimated to be over 97%.4 However, complete or partial reperfusion of a previously thrombosed psA can be observed in some patients.5 Usually, the UGTI procedure is repeated in such cases.1, 4,5 However, we should bear in mind that each time a potent procoagulative agent is injected into the psA lobe communicating with the arterial lumen there is a risk of arterial embolism, which is a potentially life-threatening condition.4 psA fail to thrombose in a small number of patients (about 1–2%),6 usually those with multiple reperfusions after UGTI and those with dynamically enlarging psA and rapidly progressing anemia at risk of hemorrhagic shock. Patients with such clinical presentation were selected for a novel minimally invasive technique: an ultrasound-guided tissue glue injection (UGTGI).
Patients and methods
Patient selection and referral to the psA embolization in Świętokrzyskie Cardiology Centre has been previously described.1 Briefly, all patients who had undergone any percutaneous procedure via femoral access are prospectively screened for the presence of clinical findings suggestive of psA. If present, an ultrasound examination is performed and if psA is confirmed according to the protocol1 the patient is referred for UGTI. In case of UGTI failure, another treatment (either re-UGTI or tissue glue injection) is considered. In the present study, a total of 10 subjects aged 54 to 71 were referred for UGTGI, including nine patients with reperfusion on at least three occasions after ultrasound-guided bovine thrombin injection (Figure 1a–c) and one patient with early signs of hemorrhagic shock due to a dynamically enlarging pseudoaneurysm. In the latter patient, UGTI had been attempted but failed to close the psA, therefore, he was selected for UGTGI. In patients selected for UGTGI in the present study, the percutaneous method was preferred due to the concomitant antithrombotic or dual antiplatelet therapy. The exclusion criteria were more than 2 weeks from the index procedure, the psA border out of the reach of needle, the presence of arteriovenous fistula (AVF), treatment with dabigatran, soft tissue infection or necrosis, lower limb ischemia due to the psA. Patient and procedure characteristics are presented in Table 1.
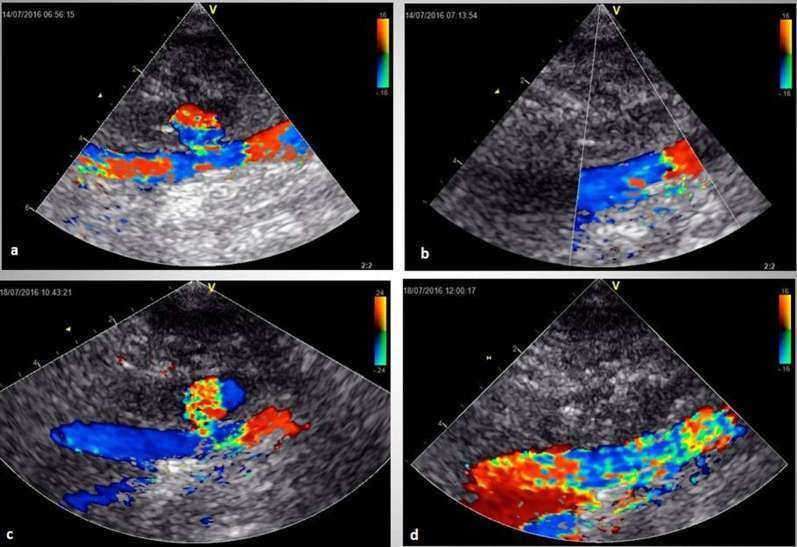
Figure 1.
Ultrasound imaging of the pseudoaneurysm treatment using thrombin and tissue glue. Recanalization of pseudoaneurysm after two attempts of thrombin injection (a). The third attempt of thrombin embolization (b). Recurrent recanalization after the third thrombin injection (c). Final successful treatment with tissue glue injection (d). (1) psA cavity, (2) RSFA. RFSA, right superficial femoral artery.
Table 1.
Patients’ and procedure characteristics
| Parameter | Number (%) |
| Sex | |
| Male | 4 (40) |
| Female | 6 (60) |
| Index procedure type | |
| Angiography | 1 (10) |
| Coronary angioplasty | 8 (80) |
| Electrophysiology | 1 (10) |
| Punctured vessel | |
| Femoral artery | 9 (90) |
| Femoral vein | 1 (1) |
| Sheath size | |
| French 6 | 2 (20) |
| French > 6 | 8 (80) |
| Concomitant therapy | |
| Acetyl salicylic acid | 9 (90) |
| Clopidogrel | 8 (80) |
| Unfractionated heparin | 4 (40) |
| Vitamin K antagonist | 2 (20) |
| Time from puncture to treatment | |
| <7 days | 4 (40) |
| ≥7 days | 6 (60) |
| Number of psA compartments | |
| 1 | 9 (90) |
| >1 | 1 (10) |
| Procedure characteristics | |
| psA volume, ml | 2.86 (1.13–7.24)a |
| Channel length, mm | 5 (2–8)a |
| Number of initial UGTI | 3.6 (1–5)a |
| UGTI, total thrombin, IU | 670 (380–960)a |
| Tissue glue injection | |
| Total dose of tissue glue, ml | 0.9 ± 0.53b |
| Thrombin, IU | 450 ± 256b |
| Fibrinogen, mg | 81.9 ± 48.23b |
IU, international unit; psA, pseudoaneurysm; UGTI, ultrasound-guided thrombin injection.
Data are presented as numbers and percentages or mean and minimum–maximum range.
Mean ± standard deviation.
Technique of ultrasound-guided tissue glue injection
The injection set (Tisseel Lyo, Baxter AG, Vienna, Austria) consists of four ampoules: one (2 ml) containing human lyophilized fibrinogen 91 mg ml−1, one (2 ml) with human thrombin (500 IU ml−1), one with synthetic aprotinin (3000 KIU ml−1) and one with aqueous calcium chloride 40 µmol ml−1. Immediately before the procedure, the components should be warmed to 37 °C (storage temperature is below 25 °C). Then, thrombin is added to aqueous calcium chloride and separately fibrinogen to aprotinin and stirred. Each solution is drawn into 2 ml syringes which are then placed in a special delivery device enabling simultaneous injection from the two syringes. Both syringes are connected with a collector which mixes the dilutions immediately before placement into the application needle. The needle is advanced under ultrasound guidance, similar to thrombin injection procedure, and positioned in the patent psA chamber, as far from the psA inflow orifice as possible. The content of the two syringes is delivered simultaneously. One application may suffice but a second (or third) injection may need to be performed in case of only partial thrombosis of the pseudoaneurysm lobe or lobes. Coagulation begins when the contents of one syringe is mixed with the contents of the second syringe, although it may start already in the application needle (Figure 1d). For this reason, the solution should be applied quite rapidly, but in small doses. In case a second injection is performed, the application needle may be changed if required. Follow-up ultrasound was performed 24 h later, and then at 1 and 2 weeks after the procedure.
Results
Ultrasound-guided tissue glue injection resulted in complete psA obliteration in all patients. The dose of the agent was 0.9 ± 0.53 ml (thrombin 450 ± 256 IU and fibrinogen 81.9 ± 48.23 mg). Follow-up ultrasound scanning at 24 h as well as at 1 and 2 weeks did not show reperfusion of the previously thrombosed pseudoaneurysm lobes in any of the patients undergoing UGTGI. Even in the patient with dynamic enlargement of the psA tissue glue injection resulted in stable thrombus formation. Although the morphological characteristics of the clot formed after bovine thrombin injection were similar to that formed after tissue glue injection the clot in the latter case had more contrast-enhanced and granular appearance (Figure 1).
DiscussioN
A number of therapeutic strategies have been proposed for the management of psA. Initially, the only treatment available was surgery associated with a degree of risk.3 In 1986, Copie and Zeit were the first to use a new minimally invasive technique for successful clotting by percutaneous thrombin injection directly into the psA lobe (or lobes).6 Ultrasound-guided compression repair was first performed by Fellmeth et al7. Unfortunately, this new procedure was less effective than UGTI and additionally caused patient discomfort, especially in case of the concomitant antiplatelet therapy. A simple procedure of para-aneurysmal injection of physiological saline followed by manual pressure did not gain popularity.8 Some investigators proposed endovascular treatment with stent implantation in patients with the presence of a pseudoaneurysm and a concomitant AVF at the site of flow into the psA lobe.9 Percutaneous injection of bovine or recombinant human thrombin is still the most commonly used technique for aneurysm clotting under the guidance of ultrasound. As a result a psA, which is a potentially serious complication is converted into a benign hematoma, which undergoes retraction and phagocytosis. An alternative to minimally invasive techniques is surgical treatment but this approach is significantly limited due to the need for using antiplatelet agents in such patients. A multicomponent tissue glue has not been proposed as a technique of psA embolization as yet. Matson et al10 were first to attempt a pretty complicated procedure of fibrin adhesive injection as an alternative to ultrasound-guided compression repair.This approach necessitated balloon inflation across the neck of the pseudoaneurysm, some patients (11%) required a second procedure and surgical treatment was a necessity in 7% of patients. Furthermore, fibrin was the only component of the injection, and the procedure was successful only in 82% of patients. Tissue glues have not been used as a minimally invasive treatment of psA. They are well-known in surgical operations, where they are applied directly onto the surgical field or sprayed with a device. They are used to improve regional hemostasis and as a sealant or a substance facilitating adhesion of tissue planes.11 They act very rapidly, thus shortening the duration of surgical operation and improving prognosis. Tissue glues in a short time generate the formation of a clot, which is additionally stabilized by the presence of aprotinin, a protease inhibitor with antifibrinolytic activity. Aprotinin acts as an inhibitor of plasmin, plasma kallikrein and trypsin, in this way inhibiting fibrinolysis and stabilizing the clot. Clots that are formed in response to thrombin (human or bovine) more easily undergo endogenous fibrinolysis. At present, there are two types of tissue glues used in surgery: synthetic and biological. Cyanoacrylates are synthetic adhesives with the ability to polymerize in aqueous environment. Polymer chains are not easily biodegradable, and their degradation products may have toxic effects on living organisms.12 The longer chain polymers such as n-butyl cyanoacrylate glue demonstrate slow degradation and thus weaker toxic effects. Fibrin glue is a biological tissue adhesive. Fibrin may be the only component or it may be combined with thrombin resulting in the coagulation cascade and formation of a stable clot. The resultant fibrin clot degrades physiologically (slowly) similar to native clots, and its biodegradation products do not have any significant toxic effects. Tissue glues are manufactured in a more complex way and for this reason, they are more expensive than the synthetic ones. Synthetic glues have been used in medicine since the 60s.12 Biological adhesives attracted the medical community in the beginning of the 70s when Matras et al demonstrated experimentally that the stability of the clot improved after addition of fibrin and Factor XIII.13 Synthetic glues are most frequently used in ophthalmology and microsurgery where the least possible doses are applied, whereas biological adhesives in larger doses are applied in surgery and neurosurgery.14 The use of n-butyl cyanoacrylate glue was described mainly in the treatment of iatrogenic AVF as a complication of endovascular interventions.15 The treatment of psA using biological adhesives has not been reported as yet.
In summary, although a vast majority of psA is successfully managed with thrombin only, the use of tissue glues appears warranted in the treatment of clinically challenging psA. Tissue adhesives might be considered as a treatment option if thrombin injection fails as it occurs in patients with recurrent recanalization of a previously thrombosed pseudoaneurysm and a possible hemorrhagic shock. Small differences in substance application and its cost may favor this approach. Tissue glues for embolization of psA appear superior to human or bovine thrombin because of more rapid effects and weaker susceptibility of the clot to endogenous fibrinolysis. They also lack the cyanoacrylate toxicity. Potential complications and their rates are similar to those accompanying to the UGTI procedure. However, the two main disadvantages are the higher costs of treatment (ca USD 200 per 4 ml) and the need for placement of the needle in the psA chamber each time the substance is applied to avoid the active mixture uncontrolled injection if the canula has not been changed.
Study limitations
The main limitations of the present study are the small number of patients and the lack of a long term follow-up. Although final results are encouraging, they should be interpreted with caution.
Conclusion
Embolization of iatrogenic psA using tissue glues seems to be an effective technique for the treatment of this complication. It might be considered as a treatment option in case of unsuccessful primary psA repair by means of thrombin injection or hemorrhagic shock due to rapid psA progression.
Contributor Information
Jacek Kurzawski, Email: jkurzawski@op.pl.
Agnieszka Janion-Sadowska, Email: ajanion@o2.pl.
Marcin Sadowski, Email: emsad@o2.pl.
REFERENCES
- 1.Kurzawski J, Sadowski M, Janion-Sadowska A. Complications of percutaneous thrombin injection in patients with postcatheterization femoral pseudoaneurysm. J Clin Ultrasound 2016; 44: 188–95. doi: 10.1002/jcu.22274 [DOI] [PubMed] [Google Scholar]
- 2.Vázquez V, Reus M, Piñero A, Abellán D, Canteras M, Espinosa de Rueda M, et al. Human thrombin for treatment of pseudoaneurysms: comparison of bovine and human thrombin sonogram-guided injection. AJR Am J Roentgenol 2005; 184: 1665–71. doi: 10.2214/ajr.184.5.01841665 [DOI] [PubMed] [Google Scholar]
- 3.San Norberto García EM, González-Fajardo JA, Gutiérrez V, Carrera S, Vaquero C. Femoral pseudoaneurysms post-cardiac catheterization surgically treated: evolution and prognosis. Interact Cardiovasc Thorac Surg 2009; 8: 353–7. doi: 10.1510/icvts.2008.188623 [DOI] [PubMed] [Google Scholar]
- 4.Webber GW, Jang J, Gustavson S, Olin JW. Contemporary management of postcatheterization pseudoaneurysms. Circulation 2007; 115: 2666–74. doi: 10.1161/CIRCULATIONAHA.106.681973 [DOI] [PubMed] [Google Scholar]
- 5.Krüger K, Zähringer M, Söhngen FD, Gossmann A, Schulte O, Feldmann C, et al. Femoral pseudoaneurysms: management with percutaneous thrombin injections--success rates and effects on systemic coagulation. Radiology 2003; 226: 452–8. doi: 10.1148/radiol.2262012107 [DOI] [PubMed] [Google Scholar]
- 6.Cope C, Zeit R. Coagulation of aneurysms by direct percutaneous thrombin injection. AJR Am J Roentgenol 1986; 147: 383–7. doi: 10.2214/ajr.147.2.383 [DOI] [PubMed] [Google Scholar]
- 7.Fellmeth BD, Roberts AC, Bookstein JJ, Freischlag JA, Forsythe JR, Buckner NK, et al. Postangiographic femoral artery injuries: nonsurgical repair with US-guided compression. Radiology 1991; 178: 671–5. doi: 10.1148/radiology.178.3.1994400 [DOI] [PubMed] [Google Scholar]
- 8.Gehling G, Ludwig J, Schmidt A, Daniel WG, Werner D. Percutaneous occlusion of femoral artery pseudoaneurysm by para-aneurysmal saline injection. Catheter Cardiovasc Interv 2003; 58: 500–4. doi: 10.1002/ccd.10485 [DOI] [PubMed] [Google Scholar]
- 9.Onal B, Kosar S, Gumus T, Ilgit ET, Akpek S. Postcatheterization femoral arteriovenous fistulas: endovascular treatment with stent-grafts. Cardiovasc Intervent Radiol 2004; 27: 453–8. doi: 10.1007/s00270-004-0176-4 [DOI] [PubMed] [Google Scholar]
- 10.Matson MB, Morgan RA, Belli AM. Percutaneous treatment of pseudoaneurysms using fibrin adhesive. Br J Radiol 2001; 74: 690–4. doi: 10.1259/bjr.74.884.740690 [DOI] [PubMed] [Google Scholar]
- 11.Orci LA, Oldani G, Berney T, Andres A, Mentha G, Morel P, et al. Systematic review and meta-analysis of fibrin sealants for patients undergoing pancreatic resection. HPB 2014; 16: 3–11. doi: 10.1111/hpb.12064 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Coover HN, Joyner FB, Sheerer NH. Chemistry and performance of a cyanoacrylate adhesive. J Spec Tech Pap 1959; 5: 413–7. [Google Scholar]
- 13.Diao E, Peimer C. Sutureless methods of nerve repair. Operative nerve repair and reconstruction. JB Lippincott Philadelphia 1991; 20: 305–14. [Google Scholar]
- 14.Narakas A. The use of fibrin glue in repair of peripheral nerves. Orthop Clin North Am 1988; 19: 187–99. [PubMed] [Google Scholar]
- 15.Rathod JR, Dhomne S, Taori K, Prasad KP, Guha A. Endovascular stent graft for post-traumatic superficial femoral artery pseudoaneurysms with arteriovenous fistula: 6 months follow-up of 2 cases. J Radiol Case Rep 2011; 5: 26–34. doi: 10.3941/jrcr.v5i11.776 [DOI] [PMC free article] [PubMed] [Google Scholar]