Abstract
Objective:
The main importance of imaging breast cancer is to guide conservative surgeries. In this study, we evaluated the role of contrast-enhanced spectral mammogram (CESM) in correlation with three-dimensional (3D) breast ultrasound in characterizing the extension of the intramammary cancer in view of the: (i) the size of the main tumor, (ii) the multiplicity of the breast cancer, and (iii) the peri-tumoral stromal involvement (i.e. free or intraductal extension of the cancer).
Methods:
The study is a prospective analysis that included 300 breast masses proved to be malignant. The masses were evaluated for their size, multiplicity and surrounding stromal involvement. Contrast-based mammography performed with low (22–33 kVp) and high (44–49 kVp) energy exposures that were taken after i.v. injection of contrast agent and followed by bilateral 3D breast ultrasound. Operative data were the gold standard reference.
Results:
There was no significant difference between the sizes of the included cancers as measured by CESM and 3D ultrasound and that measured at the pathological analysis. CESM showed higher accuracy (32.7%, n = 98) than 3D ultrasound (24.7%, n = 74) in the size agreement within 5% range. CESM was the most accurate modality (94%, n = 282) in detecting tumor multiplicity, followed by traditional sonomammogram (88%, n = 264), then 3D breast ultrasound (84%, n = 252). Intraductal extension of the breast cancer was best evaluated by the 3D ultrasound with an accuracy value of 98% (n = 294) compared to only 60% (n = 180) by CESM.
Conclusion:
CESM is a recommended investigation in breast cancer to increase the accuracy of size measurement and the detection of multiple tumors. The addition of 3D ultrasound can enhance the detection of intraductal extension.
Advances in knowledge:
Choice of conservative breast surgery vs mastectomy is still a debate. We used an advanced, contrast-based, application of the mammogram: CESM and a non-invasive 3D breast ultrasound in the assessment of the local extension of the breast cancer regarding size, perifocal stromal infiltration and multiplicity to guide the selection of proper management in proved cases of breast cancer.
INTRODUCTION
In the mean time, there is a trend to perform breast conservative surgery for the early detected breast cancers; however, mastectomy may be a necessity for some patients. The choice of treatment depends on not only on the tumor size relative to the breast size, but also assessment is altered as regards tumor multicentricity/multifocality, and the inability to achieve negative surgical margins.1
Assessment of the stroma adjacent to the breast carcinomas is very important for defining the surgical margins: the presence of chromosomal alteration in the form of loss of heterogeneity on the chromosome 3p in normal terminal ductal lobular units adjacent to early-stage breast carcinomas was found to be associated with increased risk of recurrence of 3.9- to 5.2-fold.2
Contrast-enhanced digital mammography with the injection of a contrast medium could be an alternative for the standard digital mammogram.3 It is a breast imaging modality for analysis of inconclusive findings especially in the dense breast, locoregional staging of breast cancer, and monitoring of the response to neoadjuvant chemotherapy.4 Moreover, it may have a role in detection of ductal carcinoma in situ seen in the form of clustered microcalcifications on the low-dose images.5
Three-dimensional (3D) ultrasound can help in characterization of breast lesions especially architectural distortion,6 and eliminates operator dependence which is the major drawback encountered with two-dimensional (2D) ultrasound.7
3D breast ultrasound can improve the performance of the ultrasound-guided biopsy. Also, it provides a volumetric study of breast tumors, which is important in monitoring response of the tumors to the neoadjuvant chemotherapy.6
This work presented a comparative analysis between the contrast-enhanced spectral mammography (CESM) and the 3D breast ultrasound to assess the intramammary extend of the breast cancer to plan and achieve the suitable surgical approach.
PATIENTS AND METHODS
The study was approved by the ethics committee of the Scientific Research Review Board of the Radiology Department at the main academic research institute in our country.
This is a prospective analytical study that assessed the performance of two advanced breast imaging modalities, namely; the CESM and the 3D breast ultrasound, in the characterization of the extend of the breast cancer regarding: (i) the tumor size, (ii) the multiplicity of the breast tumor, and (iii) the stromal involvement (i.e. free or intraductal extension of the cancer). The post-operative histopathological analysis was the gold standard of reference.
Patients
The current work included 300 breast masses. The study duration was 18 months starting from December 2015 till June 2017.
All of the included patients were presented at a single institute that is special for breast cancer detection, staging, surgery and therapy. Patients were examined in sequence.
Initially, all the patients were subjected to a basic examination of the traditional digital mammogram followed by the 2D ultrasound (2D B mode ultrasound) to detect the breast carcinoma.
Inclusion criteria: malignant breast masses detected in previous traditional mammogram and complementary breast ultrasound and were proved by tissue core biopsy (using a 14 G needle).
Exclusion criteria: (1) histology proved benign breast masses, (2) malignant looking masses (BI-RADS 5) with unavailable pathology report; (3) pregnant patients; (4) contraindication of injection of the contrast media as allergy to contrast or impairment of the renal function.
Methods
CESM was performed using the “Senographe Essential full field digital mammography system” (GE Healthcare, Chalfont St Giles, UK).
First, manual injection of an iodinated contrast agent (iohexol, 300 mg I ml–1) in the antecubital vein was done using a special catheter at a dose of 1.5 ml kg–1, then a considerable compression of the breast is applied, yet not severe enough to occlude blood passage to the breast. Standard mammography is then imaged after a delay of 2 min. Conservative performance of low- and high-energy exposures was taken in each view. Low-energy images are similar to the images in the standard mammography technique. These images were acquired at a range of 26 to 31 peak kilovoltage (kVp) values. On the other side, the range of the kVp values were 45–49 kVp for the high-energy exposures (which is above the k-edge of iodine). Areas of contrast uptake in the breast display enhancement at the high energy images.
Post exposure, weighted logarithmic subtraction of the two images (i.e. low and high energy) was performed to show a contrast enhanced image, where areas of contrast uptake is enhanced over a background suppression of the density of the normal glandular tissue.
For image analysis “Image Diagnostic Mammography Workstation” (Genenral Electric Healthcare, NY) was used. The extension of the breast cancer was assessed via tracing of the abnormal contrast uptake in the subtraction post contrast images.
Ultrasound
Ultrasound was performed by two different methods of scanning:
(i) 2D B-mode ultrasound:
2D B-mode ultrasound was performed using Philips iU22 xMatrix ultrasound system with L 12–5 transducer.
(ii) 3D ultrasound:
Free-hand ultrasound was performed twice by experienced radiologists using a Philips iU22 xMatrix ultrasound system.
First, we used linear 12–5 probe where we manually sweep the lesion for surface rendering. Then, if the contrast between lesion and the surrounding breast tissue is poor, 3D static volume contrast imaging optimize the contours of the detected breast mass. After that, MPR images and volume acquisition were performed using X6-1 pure wave xMatrix transducer. Lesions were viewed in the axial, sagittal and most importantly coronal planes. The 3D volume data set displayed in a multiplanar view measures the long axes distances in all three perpendicular planes.
Image analysis was done by five radiologists/readers; two for the contrast based mammogram (of 35 and 20 years experience), two for the performance and the interpretation of the 3D breast ultrasound (of 25 and 20 years experience) and a fifth reader (of 30 years experience) to settle the decision in cases that showed mismatching results between the contrast enhanced mammogram and the 3D ultrasound.
The standard of reference
The used standard of reference was the complete pathologic specimens removed at surgery.
The surgically removed malignant breast lesions were measured in millimeters using a calibrated ruler, and then were fixed in formalin.
Statistical analysis
Discrete data are presented as mean ± one standard deviation. Categorical data are presented as percentages.
Differences between means were analyzed using paired Student’s t-test. Correlation studies were carried out using Pearson’s correlation coefficient (r). The lesion size was compared to pathology directly using the Student’s paired t-test and then was analyzed to show the degree of agreement between the different imaging modalities and the pathology within ± 5%. Different test results were compared to pathology to categorize true-positive, false-positive, true-negative and false-negative categories.
Sensitivity, specificity, positive predictive value, negative predictive value, false positive rate, false negative rate and accuracy were calculated.
The level of statistical significance (p-value) for all tests was set at 0.05.
RESULTS
In the current work, there were 300 patients with proved breast carcinomas.
The age of the patients ranged between 24 and 70 years, with the mean age 53.98 ± 10.45 years. Out of these, 270 (90%) patients presented with a symptomatic breast mass for the first time, 6 (2%) presented with a recurrent breast mass, another 6 (2%) for post-lumpectomy follow-up, and 18 (6%) patients were asymptotic and presented for screening
Invasive ductal carcinoma were diagnosed in 193 (64.3%) cases; grade 2–3, invasive ductal carcinoma with intraductal carcinoma in situ component seen in 53 cases (17.7), 45 (15%) were diagnosed as mixed invasive ductal plus lobular carcinoma and 4 (13.3%) were micropapillary carcinoma and another 5 were mucinous carcinomas (16.7).
Size (of the largest mass in case of multiple lesions), multiplicity, and associate stromal involvement were evaluated for the included breast cancer.
Lesion size
The largest dimension of the lesion was measured in cm by each modality and compared to the largest dimension of the pathology specimen.
The sizes of the included cancers were first measured at digital mammogram and 2D breast ultrasound prior and in separate session to the measurements reported at the CESM and the 3D breast ultrasound.
Table 1 showed the statistical comparison of the lesion size by either CESM or 3D ultrasound of the breast or their combination vs pathology.
Table 1. .
Breast cancer size (max. dimension) as detected by either the traditional digital mammogram and 2D breast ultrasound, CESM, 3D ultrasound and all modalities compared to pathology
| Image modality | Size in cm | Pathology | p-value | r | ||
| Mean | SD | Mean | SD | |||
| DM + 2D ultrasound | 3.93 | 1.78 | 3.80 | 2.05 | 0.872 | 0.78 |
| CESM | 4.41 | 2.11 | 3.80 | 2.05 | 0.237 | 0.77 |
| 3D ultrasound | 3.50 | 1.72 | 3.80 | 2.05 | 0.429 | 0.78 |
| All techniques | 4.17 | 1.83 | 3.80 | 2.05 | 0.440 | 0.80 |
2D, two-dimensional; 3D, three-dimensional; CESM, contrast-enhanced spectral mammography; DM, digital mammography; r, Pearson’s correlation coefficient; SD, standard deviation.
There was no significant difference between the sizes measured by CESM and 3D ultrasound and the pathological size.
Correlation studies using Pearson’s correlation coefficient showed that both breast imaging modalities achieved good correlation with the size measured by pathology, however, none of them could achieve excellent or linear correlation. The highest correlation was shown by 3D ultrasound (p value = 0.43).
Size agreement
Table 2 showed the agreement of the CESM and the 3D breast ultrasound with the pathology within 5% range. CESM showed highest accuracy (32.7%) followed by the 3D ultrasound (24.7%).
Table 2. .
Size agreement between the traditional sonomammogram (i.e. DM + 2D ultrasound), CESM and 3D breast ultrasound vs pathology within ± 5% range
| DM + 2D | CESM | 3D ultrasound | All techniques | |||||
| n | % | n | % | n | % | n | % | |
| Agreed | 30 | 10 | 98 | 32.7 | 74 | 24.7 | 70 | 23.3 |
| Overestimated | 162 | 54 | 130 | 43.3 | 96 | 32 | 122 | 40.7 |
| Underestimated | 108 | 36 | 72 | 24 | 130 | 43.3 | 108 | 36 |
2D, two-dimensional; 3D, three-dimensional; CESM, contrast-enhanced spectral mammography; DM, digital mammography; n, number.
CESM showed tendency to size overestimation (43.3%), while 3D ultrasound showed tendency to underestimation (43.3%).
We combined the assessment of all modalities regarding the size agreement, we considered—to minimize the risk of positive (involved) surgical margins—the value of the greatest dimension. At this stage, the accuracy of the measured size was only 23.3%.
Multiplicity
Table 3 showed the sensitivity and the specificity for the detection of the tumor multiplicity as compared to pathology. CESM was the most accurate modality (94%) in this situation (Figure 1). The performance of the traditional DM supported by the 2D breast ultrasound was better than the solo performance of the 3D breast ultrasound with an accuracy value 88% compared to 84% for the latter modality.
Table 3. .
The statistical indices in evaluating the satellite foci and multiplicity of the included breast cancers by single breast imaging modality (DM + 2D ultrasound; CESM; 3D ultrasound) and all techniques as compared to the pathology
| DM + 2D ultrasound | CESM | 3D ultrasound | All techniques | |
| True-positive | 48 | 54 | 24 | 54 |
| True-negative | 216 | 228 | 228 | 210 |
| False-positive | 24 | 12 | 12 | 30 |
| False-negative | 12 | 6 | 36 | 6 |
| Sensitivity | 80% | 90% | 40% | 90% |
| Specificity | 90% | 95% | 95% | 88% |
| Positive-predictive value | 67% | 82% | 67% | 64% |
| Negative-predictive value | 95% | 97% | 86% | 97% |
| False-positive rate | 10% | 5% | 5% | 13% |
| False-negative rate | 20% | 10% | 60% | 10% |
| Accuracy | 88% | 94% | 84% | 88% |
2D, two-dimensional; 3D, three-dimensional; CESM,contrast-enhancedspectral mammography
Figure 1.
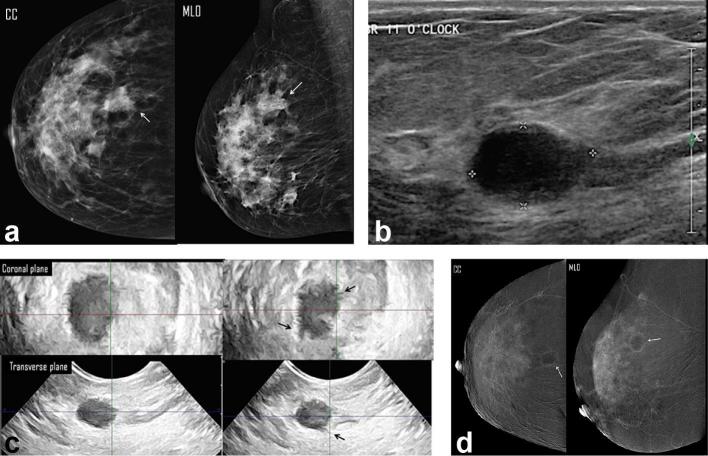
A 66-year-old female presented with right breast lump. (a) Digital mammogram MLO & CC views showed an upper outer spiculated mass associate with overlying focal dermal dimpling (white arrow) of max. dimension of 3.98 cm (overestimated length) and a nearby evident two ill-defined nodules (b) Right breast, two-dimensional ultrasound images showed three ill-defined solid masses at 12 clock; the dominant one measured 2.3 cm in max. dimension. (c) Three-dimensional breast ultrasound: coronal (left image) and transverse (right image) planes: the dominant mass was the most accessible for examination; it measured 3.5 cm, in max. dimension. No intraductal component. (d) CESM revealed an upper outer quadrant enhancing spiculated mass measured 3.5 cm in max. dimension. There were surrounding multiple enhancing smaller masses (white arrows) and foci (arrow heads). Histopathology: multifocal IDC, with max. dimension of 3.5 cm and free adherent stroma. CESM was the modality that showed accurate assessment of the multiplicity of the breast cancer. CC, craniocaudal; CESM, contrast-enhanced spectral mammography; IDC, invasive ductal carcinoma; MLO, medio lateral oblique.
Stromal involvement
Table 4 showed the sensitivity and the specificity of CESM and 3D ultrasound in the detection of peritumoral stromal involvement as compared to pathology. The highest accuracy was reported by the 3D ultrasound (98%), (Figures 2 and 3). CESM was the least accurate with a value of 60% but the specificity was 100%. Combining the performance of both techniques had yielded a comparable accuracy value to that elicited by the individual performance of the 3D breast ultrasound (98%).
Table 4. .
The statistical indices in evaluating the stromal involvement by either DM + 2D ultrasound; CESM; 3D ultrasound and all techniques compared to the pathology
| DM + 2D ultrasound | CESM | 3D ultrasound | All techniques | |
| True-positive | 240 | 144 | 258 | 264 |
| True-negative | 30 | 36 | 36 | 30 |
| False-positive | 6 | 0 | 0 | 6 |
| False-negative | 24 | 120 | 6 | 0 |
| Sensitivity | 91% | 55% | 98% | 100% |
| Specificity | 83% | 100% | 100% | 83% |
| Positive-predictive value | 98% | 100% | 100% | 98% |
| Negative-predictive value | 56% | 23% | 86% | 100% |
| False-positive rate | 17% | 0% | 0% | 17% |
| False-negative rate | 9% | 45% | 2% | 0% |
| Accuracy | 90% | 60% | 98% | 98% |
2D, two-dimensional; 3D, three-dimensional; CESM,contrast-enhancedspectral mammography; DM,digital mammography.
Figure 2.
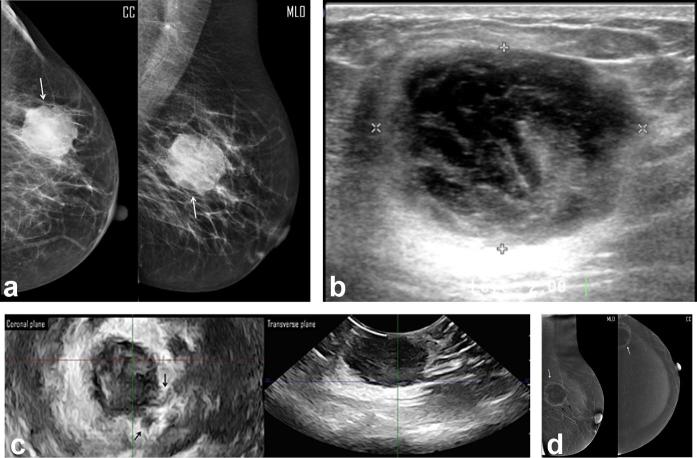
A 44-year-old female presented by right breast lump. (a) Digital mammogram MLO & CC views showed heterogeneously dense breasts (ACR “c”), and an upper outer mass (white arrow) of irregular margin, not easily distinguished regarding the type of the breast density, approx. measured 3.4 cm in max. dimension. (b) Two-dimensional breast ultrasound showed complex cystic mass with posterolateral irregular thick walls and measured 1.74 cm in max. dimension. (c) Three-dimensional breast ultrasound: coronal (upper row) and transverse (lower row) planes: coronal planes revealed an indistinct margin of the mass with tiny speculations extending into the surrounding stroma. Also the transverse plane confirmed the suggestion of associated an intraductal extension (black arrows). The measured maximum dimension was 1.87 cm. (d) CESM revealed an upper outer quadrant mass with lucent center and thick non-uniform marginal contrast uptake (white arrows). No extensions into the surrounding stroma. The max. dimension measured was 2.1 cm. Note the moderate background enhancement of the breast parenchyma. Histopathology: single mass, IDC with max. dimension of 2 cm and positive surrounding stroma for cancer cell extension. CC, craniocaudal;CESM, contrast-enhanced spectral mammography; IDC, invasiveductal carcinoma; MLO, mediolateraloblique.
Figure 3.
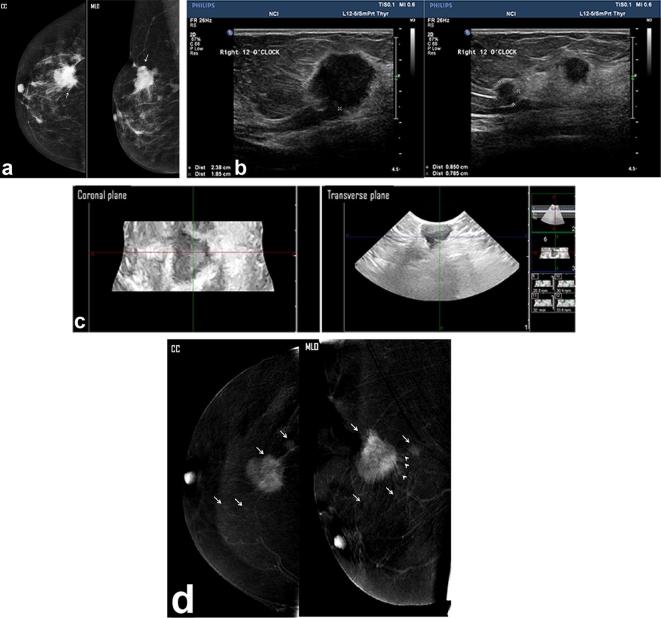
A left breast lump detected in a diagnostic mammogram of a 39-year-old patient. (a) Two views digital mammogram MLO & CC showed an upper outer ill-defined mass (white arrow), mammogram overestimated the tumor size of a measured max. dimension of 5.2 cm. (b) Two- dimensional breast ultrasound showed complex cystic mass with intracystic solid components and measured in max. dimension, 3.67 cm. (c) Three- dimensional breast ultrasound: coronal (left image) and transverse (right image) planes: an intraductal component could be noted more clarified in the coronal plane (black arrows). The measured maximum dimension was 4 cm. (d) CESM revealed an upper outer quadrant mass that showed rim enhancement (white arrows). Minimal tumor extension into the surrounding stroma was noted (peri-tumoral enhancing streaks—arrows). The max. dimension measured was 4.16 cm. Histopathology: single mass, IDC with max. dimension of 4 cm and positive stromal involvement of intraductal component. Both CESM and 3D ultrasound estimated accurate mass size and positive intraductal tumor extension.
Characterization of the disease extend in breast cancer require combined assessment by both CESM and 3D breast ultrasound; both modalities showed identical specificity in the assessment of the tumor multiplicity (95%) and peritumoral stromal involvement (100%), yet CESM was superior in size estimation (Figures 1–3) with agreement percentage of 32.7%.
DISCUSSION
Amongst the important things for applying primary systemic treatment and/or breast conserving surgery is the size of the breast tumor.8 Also, management options divert in concern of the tumor multiplicity which is a very important item in the choice of the appropriate type of surgery.9
The microenvironment of the tissues changes dramatically during tumor formation, this take place in the form the increased number of fibroblast and myofibroblasts, the infiltration of the lymphocytes, angiogenesis, and remodeling of the extracellular matrix that is adjacent to the cancer cells.10
Intraductal tumor extension is an important concern in patients whom are candidates for conservative breast surgeries.11
This study included 300 female patients with histopathological proven breast cancer. The most common pathological diagnosis of the included carcinomas was the invasive ductal carcinoma, Grade 2–3 that was encountered in 64.3% of cases (n = 193).
Up to our knowledge, there was neither a similar sized study nor similar study design in the earlier in the literatures. In the current work, we analyzed the diagnostic performance of the CESM and the 3D breast ultrasound in the assessment of the breast cancer extend with respect to the size of the breast cancer, the tumor multiplicity, and the surrounding stromal involvement of intraductal extension of the cancer.
One of the main prognostic indicators in evaluating cases with breast cancer is the size of the cancer. The accurate prediction of the tumor size—at the stage of radiologic diagnosis—is very important for the appropriate selection of the plan of the management.
In this study, the largest dimension of the breast cancer was measured by each of the included breast imaging modalities and was compared to the largest dimension measured at the pathology specimen. The diagnostic accuracy for the combined performance of both imaging modalities was measured.
Since the traditional digital mammogram combined with 2D breast ultrasound (DM + 2D ultrasound) is the baseline investigation employed in our institute (National Cancer institute - Cairo University), as well as many others worldwide; we thought it would be useful to assess its diagnostic accuracy as well in characterization of the breast cancer extend, either on individual basis or in combination with the advanced mammogram and ultrasound applications (i.e. CESM and 3D breast ultrasound).
We found that the most accurate modality for the assessment of the size of the breast cancer was CESM followed by 3D ultrasound with no significant size difference between them and the size measured at the pathology specimen. On the other hand, DM & 2D ultrasound were less accurate and showed significant size difference as compared with the dimension of the cancer size seen at the pathology.
However, all imaging modalities employed showed good correlation with the size of the cancer measured by the pathology. 3D ultrasound showed the highest correlation. DM + 2D ultrasound also showed good correlation with the pathological size similar to 3D ultrasound. The correlation showed tendency to improvement with the addition of CESM and 3D ultrasound to the baseline of DM + 2D ultrasound.
We measured the size agreement—in the 5% range—of the included imaging modalities with that measured at the pathological specimen. CESM showed the highest accuracy. Combining different imaging modalities did not yield an increase in the accuracy of the measured size.
Our results are keeping with Fallenberg et al12 that showed CESM to be more accurate than DM with better correlation with the pathology size. However, in contrast to their study, our results found DM to overestimate the size of the breast cancer.
Our results also agree with Jochelson et al13 and Lobbes et al14 that showed good correlation between the size measured by the CESM and that measured by the pathology.
Blum et al15 assessed the performance of the CESM and showed similar results, they stated that CESM was more accurate than 2D ultrasound in the assessment of the size. Similar to their study, ours showed that CESM had a tendency to overestimate the size of the breast disease, while 2D ultrasound showed a tendency to underestimate.
Other studies by Gruber et al8 and Pritt et al16 also reported significant value of underestimation of the tumor size by the 2D ultrasound. The underestimation of the tumor size by 2D ultrasound may be due to the fact that often the largest dimension of the lesion cannot be measured, especially, if we consider that many cancers are vertical (taller-than-wide) lesions. These lesions are characterized by posterior acoustic shadowing which obscures the posterior border of the lesion, often making measurement of the longest axis difficult.
Keeping with our results, Luczyńska et al17 showed similar results where they mentioned an overestimation of the tumor size with DM & CESM. However, in their study, the size difference was smaller for the CESM.
Concerning freehand 3D ultrasound, there are few studies that had discussed its performance in the pre-operative measurement of the tumor size. Tamaki et al18 reported good correlation with the size measured by the breast 3D ultrasound and the pathological size. Also, Cho et al19 mentioned that a more accurate size of the breast tumor is to be measured with 3D ultrasound than 2D ultrasound.
Surgical options divert among breast cancers whether it is unifocal, multifocal or multicentric. Presence of tumor satellites distant or within the tumor vicinity (provided the size of the breast itself) could alter the management from breast conservative surgeries to radical mastectomy.
In our study, we—pre-operatively—evaluated the multiplicity of the included breast cancers. CESM showed the highest accuracy, followed by DM + 2D ultrasound.
The lowest accuracy in the assessment of multiple masses was found in the 3D ultrasound. This may be explained by the fact that the freehand 3D ultrasound does not scan the whole breast. That is to say, it is more beneficial in the tumor characterization than in its detection. This problem can be overcome, if we used the automated 3D ultrasound that allows scanning of the whole beast not just the cancer of concern.
On combining the performance of the different breast imaging modalities included, the detection of tumor multiplicity was equal by the all techniques to the performance of DM + 2D ultrasound with an accuracy of 88%. CESM alone reported the highest accuracy value of 94%.
Dromain et al20 compared the performance of the DM if combined with CESM to that of the DM, in one hand and to the performance of the DM if combined with 2D ultrasound, on the other hand. The sensitivity of the CESM combined with DM to detect multiplicity of the breast tumors was 93 vs 78% for the DM if used alone. The specificity was equal in both performances. There was no significant improvement in the sensitivity and the specificity between the use of the CESM in combination with DM and the DM if combined with the 2D ultrasound. However, our study reported a higher sensitivity for the CESM than for the combined performance of the DM with the 2D ultrasound in the detection of multiple tumor masses. The sensitivity of the CESM measured was 90 vs 80% for DM if combined with the 2D ultrasound.
Our results are also keeping with Jochelson et al13, Fallenberg et al12 and Luczyńska et al17 that reported higher sensitivity of the CESM than the DM in detecting multiple breast lesions. Luczyńska et al17 reported a CESM accuracy of 80%, while our study reported an accuracy value of 94%.
The presence of free or intraductal extension of the diagnosed breast cancer affects greatly the risk of local recurrence. Up to our knowledge, there are no published studies—that discussed the role of different breast imaging modalities—in this issue had been added to the body of the literature.
However, a study that was performed by Tamaki et al18 had given a hint about the sensitivity of the 3D breast ultrasound in detecting intraductal spread and that it was 79% and specificity was 100%. Our study reported sensitivity of 100% and specificity of 73%.
In our study, the 3D ultrasound technique showed the highest sensitivity while both the CESM and the 3D ultrasound showed specificity of 100%, but the accuracy of their performance was higher in case of the 3D US (98%).
However, the impact of these findings has to be verified in the future long term follow up studies to determine whether the stromal involvement is a significant risk factor for local tumor recurrence.
Conclusion
CESM is a recommend baseline investigation in patients with cancer breast to increase the accuracy of size measurement and the detection of satellite foci of the tumor. The addition of 3D ultrasound can enhance the detection of intraductal extension and stromal affection.
Contributor Information
Maha Hussien Helal, Email: dr.mahahelal@yahoo.com.
Sahar Mahmoud Mansour, Email: sahar_mnsr@yahoo.com.
Lamia Adel Salaleldin, Email: lamiaadel73@hotmail.com.
Basma Mohamed Alkalaawy, Email: basmaalkalaawy@hotmal.com.
Dorria Saleh Salem, Email: dorriasalem@yahoo.com.
Nadia Mahmoud Mokhtar, Email: nadiamokhtar@hotmail.com.
REFERENCES
- 1.Senkus E, Kyriakides S, Penault-Llorca F, Poortmans P, Thompson A, Zackrisson S, et al. Primary breast cancer: ESMO clinical practice guidelines for diagnosis, treatment and follow-up. Ann Oncol 2013; 24: (Suppl 6): vi7–vi23. doi: 10.1093/annonc/mdt284 [DOI] [PubMed] [Google Scholar]
- 2.Rummel S, Valente AL, Kane JL, Shriver CD, Ellsworth RE. Genomic (in)stability of the breast tumor microenvironment. Mol Cancer Res 2012; 10: 1526–31. doi: 10.1158/1541-7786.MCR-12-0425 [DOI] [PubMed] [Google Scholar]
- 3.Dromain C, Balleyguier C, Muller S, Mathieu MC, Rochard F, Opolon P, et al. Evaluation of tumor angiogenesis of breast carcinoma using contrast-enhanced digital mammography. AJR Am J Roentgenol 2006; 187: W528–W537. doi: 10.2214/AJR.05.1944 [DOI] [PubMed] [Google Scholar]
- 4.Lobbes MB, Smidt ML, Houwers J, Tjan-Heijnen VC, Wildberger JE. Contrast enhanced mammography: techniques, current results, and potential indications. Clin Radiol 2013; 68: 935–44. doi: 10.1016/j.crad.2013.04.009 [DOI] [PubMed] [Google Scholar]
- 5.Lalji U, Lobbes M. Contrast-enhanced dual-energy mammography: a promising new imaging tool in breast cancer detection. Womens Health 2014; 10: 289–98. doi: 10.2217/WHE.14.18 [DOI] [PubMed] [Google Scholar]
- 6.Athanasiou A, Tardivon A, Ollivier L, Thibault F, El Khoury C, Neuenschwander S. How to optimize breast ultrasound. Eur J Radiol 2009; 69: 6–13. doi: 10.1016/j.ejrad.2008.07.034 [DOI] [PubMed] [Google Scholar]
- 7.Watermann DO, Földi M, Hanjalic-Beck A, Hasenburg A, Lüghausen A, Prömpeler H, et al. Three-dimensional ultrasound for the assessment of breast lesions. Ultrasound Obstet Gynecol 2005; 25: 592–8. doi: 10.1002/uog.1909 [DOI] [PubMed] [Google Scholar]
- 8.Gruber IV, Rueckert M, Kagan KO, Staebler A, Siegmann KC, Hartkopf A, et al. Measurement of tumour size with mammography, sonography and magnetic resonance imaging as compared to histological tumour size in primary breast cancer. BMC Cancer 2013; 13: 328. doi: 10.1186/1471-2407-13-328 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Helal MH, Mansour SM, Zaglol M, Salaleldin LA, Nada OM, Haggag MA. Staging of breast cancer and the advanced applications of digital mammogram: what the physician needs to know? Br J Radiol 2017; 90: 20160717. doi: 10.1259/bjr.20160717 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hu M, Polyak K. Molecular characterisation of the tumour microenvironment in breast cancer. Eur J Cancer 2008; 44: 2760–5. doi: 10.1016/j.ejca.2008.09.038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ohtake T, Abe R, Kimijima I, Fukushima T, Tsuchiya A, Hoshi K, et al. Intraductal extension of primary invasive breast carcinoma treated by breast-conservative surgery. Computer graphic three-dimensional reconstruction of the mammary duct-lobular systems. Cancer 1995; 76: 32–45. doi: [DOI] [PubMed] [Google Scholar]
- 12.Fallenberg EM, Dromain C, Diekmann F, Engelken F, Krohn M, Singh JM, et al. Contrast-enhanced spectral mammography versus MRI: Initial results in the detection of breast cancer and assessment of tumour size. Eur Radiol 2014; 24: 256–64. doi: 10.1007/s00330-013-3007-7 [DOI] [PubMed] [Google Scholar]
- 13.Jochelson MS, Dershaw DD, Sung JS, Heerdt AS, Thornton C, Moskowitz CS, et al. Bilateral contrast-enhanced dual-energy digital mammography: feasibility and comparison with conventional digital mammography and MR imaging in women with known breast carcinoma. Radiology 2013; 266: 743–51. doi: 10.1148/radiol.12121084 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lobbes MB, Lalji UC, Nelemans PJ, Houben I, Smidt ML, Heuts E, et al. The quality of tumor size assessment by contrast-enhanced spectral mammography and the benefit of additional breast MRI. J Cancer 2015; 6: 144–50. doi: 10.7150/jca.10705 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Blum KS, Rubbert C, Mathys B, Antoch G, Mohrmann S, Obenauer S. Use of contrast-enhanced spectral mammography for intramammary cancer staging: preliminary results. Acad Radiol 2014; 21: 1363–9. doi: 10.1016/j.acra.2014.06.012 [DOI] [PubMed] [Google Scholar]
- 16.Pritt B, Ashikaga T, Oppenheimer RG, Weaver DL. Influence of breast cancer histology on the relationship between ultrasound and pathology tumor size measurements. Mod Pathol 2004; 17: 905–10. doi: 10.1038/modpathol.3800138 [DOI] [PubMed] [Google Scholar]
- 17.Luczyńska E, Heinze-Paluchowska S, Dyczek S, Blecharz P, Rys J, Reinfuss M. Contrast-enhanced spectral mammography: comparison with conventional mammography and histopathology in 152 women. Korean J Radiol 2014; 15: 689–96. doi: 10.3348/kjr.2014.15.6.689 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tamaki Y, Akashi-Tanaka S, Ishida T, Uematsu T, Sawai Y, Kusama M, et al. 3D imaging of intraductal spread of breast cancer and its clinical application for navigation surgery. Breast Cancer 2002; 9: 289–95. doi: 10.1007/BF02967606 [DOI] [PubMed] [Google Scholar]
- 19.Cho KR, Seo BK, Lee JY, Pisano ED, Je BK, Lee JY, et al. A comparative study of 2D and 3D ultrasonography for evaluation of solid breast masses. Eur J Radiol 2005; 54: 365–70. doi: 10.1016/j.ejrad.2004.07.006 [DOI] [PubMed] [Google Scholar]
- 20.Dromain C, Thibault F, Diekmann F, Fallenberg EM, Jong RA, Koomen M, et al. Dual-energy contrast-enhanced digital mammography: initial clinical results of a multireader, multicase study. Breast Cancer Res 2012; 14: R94. doi: 10.1186/bcr3210 [DOI] [PMC free article] [PubMed] [Google Scholar]