Results of the CheckMate 141 trial are reported, highlighting outcomes among patients who received nivolumab compared with investigator's choice of therapy as first‐line treatment for patients with recurrent or metastatic squamous cell carcinoma of the head and neck after progressing on platinum therapy for locally advanced disease.
Abstract
Nivolumab significantly improved overall survival (OS) vs investigator's choice (IC) of chemotherapy at the primary analysis of randomized, open‐label, phase 3 CheckMate 141 in patients with recurrent or metastatic (R/M) squamous cell carcinoma of the head and neck (SCCHN). Here, we report that OS benefit with nivolumab was maintained at a minimum follow‐up of 11.4 months. Further, OS benefit with nivolumab vs IC was also noted among patients who received first‐line treatment for R/M SCCHN after progressing on platinum therapy for locally advanced disease in the adjuvant or primary (i.e., with radiation) setting.
Introduction
Patients with squamous cell carcinoma of the head and neck (SCCHN) frequently present with advanced disease and receive combined modality therapy [1]. Unfortunately, 10%–15% of patients progress within 6 months of platinum‐based therapy and have a poor prognosis, with no established standard of care [2], [3], [4], [5]. The CheckMate 141 trial investigated nivolumab versus investigator's choice (IC) of therapy in patients with recurrent or metastatic (R/M) SCCHN. Eligible patients had experienced tumor progression or recurrence within 6 months of platinum‐based chemotherapy administered in the locally advanced, recurrent, or metastatic disease setting. Nivolumab significantly extended overall survival (OS) compared with IC (hazard ratio [HR], 0.70; 97.73% confidence interval [CI], 0.51–0.96; p = .01) at primary analysis in the overall study population [6]. Here, we report outcomes among patients who received nivolumab versus IC as first‐line treatment for R/M SCCHN after progressing on platinum therapy for locally advanced disease in the adjuvant or primary (i.e., with radiation) setting, hereafter referred to as first‐line treatment for R/M SCCHN. Updated results with longer follow‐up in the overall population are also reported.
Materials and Methods
In the randomized, open‐label, phase III CheckMate 141 (NCT02105636) trial [6], patients were randomized 2:1 to nivolumab (3 mg/kg every 2 weeks) or IC (methotrexate, docetaxel, or cetuximab). The primary endpoint was OS; additional endpoints included progression‐free survival (PFS), objective response rate (ORR; per Response Evaluation Criteria In Solid Tumors version 1.1), and safety [6]. In the present post hoc analysis, efficacy and safety were assessed in patients receiving nivolumab versus IC as first‐line treatment for R/M SCCHN. Updated results in the overall intent‐to‐treat population, based on a database lock of September 2016, are also reported. Cox proportional hazards models were used to estimate HRs and CIs.
CheckMate 141 was registered with the National Cancer Institute and approved by the institutional board at each participating site. All patients provided informed consent prior to enrollment.
Results
First‐Line Treatment for R/M SCCHN
In all, 78 patients (21.6%) received nivolumab (n = 52) or IC (n = 26) as first‐line treatment for R/M SCCHN. The baseline characteristics of these patients (supplemental online Table 1) were similar to those of the overall population [6].
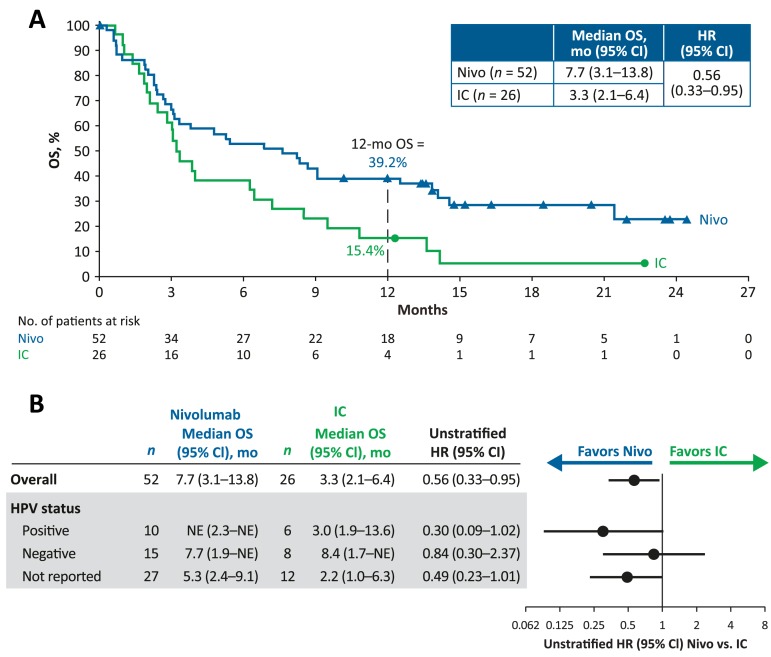
Nivolumab as first‐line treatment improved OS versus IC in patients with R/M SCCHN (median [95% CI], 7.7 [3.1–13.8] vs. 3.3 [2.1–6.4] months; HR [95% CI], 0.56 [0.33–0.95]; Fig. 1). The 12‐month OS rate was 39.2% versus 15.4%, respectively. Median (95% CI) PFS was 2.3 (1.9–3.3) months for nivolumab and 2.3 (1.7–3.2) months for IC; HR, 0.81; 95% CI, 0.48–1.37. The ORR was 19.2% versus 11.5%, respectively; time to response was 2.0 months in both arms (supplemental online Table 2). Grade 3–4 treatment‐related adverse event (TRAE) rates were 27.5% for nivolumab and 32.0% for IC (supplemental online Table 3).
Figure 1.
Survival among patients randomized to nivolumab or IC as first‐line treatment for R/M SCCHN after progressing on or after platinum therapy (within 6 months) in the adjuvant or primary (i.e., with radiation) setting for locally advanced disease: Kaplan‐Meier plot of OS (A) and treatment effect on OS (B) among patients randomized to nivolumab or IC as first‐line treatment for R/M SCCHN after progressing on or after platinum therapy.
Abbreviations: CI, confidence interval; HPV, human papillomavirus; HR, hazard ratio; IC, investigator's choice; mo, months; NE, not estimable; Nivo, nivolumab; OS, overall survival; R/M, recurrent or metastatic; SCCHN, squamous cell carcinoma of the head and neck.
One‐Year Follow‐Up in the Overall Intent‐to‐Treat Population
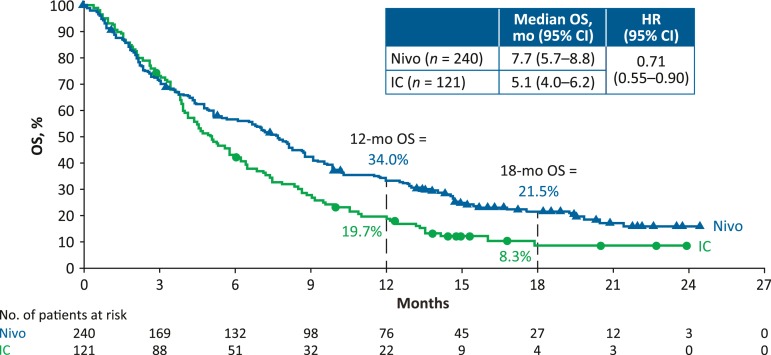
With a minimum follow‐up of 11.4 months, 16/240 patients (7%) in the nivolumab arm and 1/121 patients (1%) in the IC arm in the intent‐to‐treat population were still on treatment (supplemental online Fig. 1). Median (range) duration of therapy was 1.9 (0–24+) months for nivolumab and 1.9 (0–12+) months for IC. Nivolumab continued to improve OS versus IC (Fig. 2), with the 18‐month OS rate nearly tripled (21.5% vs. 8.3%). OS among subgroups was generally consistent with overall treatment effect (supplemental online Fig. 2). Median (95% CI) PFS was 2.0 (1.9–2.1) months for nivolumab and 2.3 (2.0–3.1) months for IC; HR, 0.87; 95% CI, 0.69–1.11. ORR did not change from the initial analysis [6]; six patients in the nivolumab arm and one patient in the IC arm had a complete response and were alive at last follow‐up. As of database lock, three patients were off‐study and four patients still on‐study had not progressed. Median (range) time to response was 2.1 (1.8–7.4) months for nivolumab versus 2.0 (1.9–4.6) months for IC. Median (range) duration of response was 9.7 (2.8–20.3+) months versus 4.0 (1.5+ to 8.5+) months, respectively.
Figure 2.
Kaplan‐Meier plot of OS in the overall intent‐to‐treat population.
Abbreviations: CI, confidence interval; HR, hazard ratio; IC, investigator's choice; mo, months; Nivo, nivolumab; OS, overall survival.
TRAEs in the overall treated population in the 1‐year follow‐up were consistent with the initial analysis; longer follow‐up identified no new safety signals. Grade 3–4 TRAE rates were 15.3% for nivolumab versus 36.0% for IC (supplemental online Table 4). Select endocrine TRAEs were more frequent with nivolumab than with IC; none was grade 3–4. Skin‐related TRAEs were the most common select TRAEs in both treatment arms.
Discussion
Consistent with outcomes in the overall patient population of CheckMate 141, nivolumab as first‐line treatment improved OS and ORR compared with IC in patients with R/M SCCHN. PFS was similar with nivolumab versus IC, as were rates of high‐grade TRAEs. Nivolumab is the only agent to significantly improve survival at primary analysis in a randomized phase III trial for platinum‐refractory R/M SCCHN. With a minimum follow‐up of 11.4 months in the present analysis, efficacy and safety in the overall intent‐to‐treat population were similar to results at earlier time points [6].
The current standard of care for first‐line treatment of platinum‐eligible R/M SCCHN is the EXTREME regimen; however, patients with platinum‐refractory SCCHN were not included in the EXTREME trial. Patients eligible for CheckMate 141, who were platinum‐refractory due to progression within 6 months of treatment in the primary setting, are generally not candidates for platinum‐containing therapies such as EXTREME [7]. Their treatment options are limited to methotrexate, taxanes, or cetuximab—the IC options in the CheckMate 141 trial. Nivolumab as first‐line treatment for R/M SCCHN resulted in a 12‐month OS of 39% in patients with platinum‐refractory disease. Furthermore, quality‐of‐life benefits were observed with nivolumab versus IC in CheckMate 141 [8].
Conclusion
Although these data represent a small subgroup of patients, the results support the use of nivolumab as first‐line therapy for patients with R/M SCCHN who progressed within 6 months of platinum‐based therapy in the adjuvant or primary setting. CheckMate 714 (NCT02823574) is an ongoing, randomized, double‐blind, phase II study designed to evaluate the clinical benefit of adding anti–CTLA‐4 targeted therapy (ipilimumab) to nivolumab for patients with platinum‐refractory or platinum‐eligible R/M SCCHN [9]. Nivolumab plus ipilimumab is being evaluated in CheckMate 651 as first‐line therapy for platinum‐eligible R/M disease versus EXTREME (NCT02741570) [10].
See http://www.TheOncologist.com for supplemental material available online.
Supplementary Material
Acknowledgments
We thank the patients and their families, as well as the clinical study teams, for making this study possible. This study was sponsored by Bristol‐Myers Squibb (Princeton, NJ) and Ono Pharmaceutical Company Ltd. (Osaka, Japan). Professional medical writing assistance was provided by Virginie Adam, Ph.D., and Beth Burke, Ph.D., C.M.P.P., of Evidence Scientific Solutions, and was funded by Bristol‐Myers Squibb.
Footnotes
For Further Reading: Jessica M. Moskovitz, Jennifer Moy, Tanguy Y. Seiwert et al. Immunotherapy for Head and Neck Squamous Cell Carcinoma: A Review of Current and Emerging Therapeutic Options. The Oncologist 2017;22:680–693.
Implications for Practice: This review article summarizes recently developed agents that harness the immune system to fight head and neck squamous cell carcinoma. A brief review of the immune system and its role in cancer development is included. Recently completed and emerging therapeutic trials centering on the immune system and head and neck cancer are reviewed.
Disclosures
Maura L. Gillison: Amgen, AstraZeneca, Bristol‐Myers Squibb, Celgene, GlaxoSmithKline, Lilly, Merck (C/A, H, travel arrangements), AstraZeneca, Bristol‐Myers Squibb, Kyowa, Merck (RF); George Blumenschein, Jr.: Bristol‐Myers Squibb, Bayer, Clovis, Merck, Celgene, AbbVie, ARIAD (C/A), Bristol‐Myers Squibb, Novartis, Xcovery, AstraZeneca, Adaptimmune, Immatics, Macrogenetics, Bayer, Merck, Celgene, Genentech, GlaxoSmithKline (RF), UT MD Anderson Cancer Center (E), AbbVie, Merck (SAB); Jerome Fayette: Bristol‐Myers Squibb (SAB), Merck (H); Joel Guigay: Bristol‐Myers Squibb, Merck KGaA, AstraZeneca, Innate Pharma (C/A), Bristol‐Myers Squibb, Merck KGaA, Novartis (RF); Lisa Licitra: Eisai, Merck Sharp & Dohme, Merck Serono, Boehringer Ingelheim, Novartis, AstraZeneca, Roche (RF [to institution]), Eisai, Brisol‐Myers Squibb, Merck Sharp & Dohme, Merck Serono, Boehringer Ingelheim, Novartis, AstraZeneca, Roche, Bayer, Debiopharm, Sobi (C/A); Kevin J. Harrington: Amgen, AstraZeneca, Bristol‐Myers Squibb, Merck, Merck Sharp & Dohme (C/A), Amgen, AstraZeneca, Bristol‐Myers Squibb, Merck, Merck Sharp & Dohme, Pfizer (H, SAB), AstraZeneca, Merck Sharp & Dohme (RF); Stefan Kasper: Bristol‐Myers Squibb (C/A, H, SAB); Everett E. Vokes: Bristol‐Myers Squibb (C/A, RF); Caroline Even: Bristol‐Myers Squibb, Merck Sharp & Dohme, Merck, Innate Pharma, AstraZeneca (C/A); Naomi Kiyota: Ono Pharmaceutical Co., Ltd (C/A), Ono Pharmaceutical Co., Ltd, AstraZeneca (RF), Ono Pharmaceutical Co., Ltd, Eisai, Bristol‐Meyers Squibb, Bayer (H); Makoto Tahara: Merck Sharp & Dohme, Bayer, Eisai, Otsuka, AstraZeneca, Pfizer, Ono Pharmaceutical Co., Ltd, Bristol‐Myers Squibb (C/A), Novartis, Nao, Boehringer Ingelheim, Eisai, AstraZeneca, Pfizer, Ono Pharmaceutical Co., Ltd, Bristol‐Myers Squibb (RF); Manish Monga: Bristol‐Myers Squibb (E); Robert L. Ferris: AstraZeneca/MedImmune, Bristol‐Myers Squibb, Celgene, Merck, Pfizer (SAB), AstraZeneca/MedImmune, Bristol‐Myers Squibb, Celgene (RF). The other authors indicated no financial relationships.
(C/A) Consulting/advisory relationship; (RF) Research funding; (E) Employment; (ET) Expert testimony; (H) Honoraria received; (OI) Ownership interests; (IP) Intellectual property rights/inventor/patent holder; (SAB) Scientific advisory board
References
- 1.National Comprehensive Cancer Network. NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines®) Head and Neck Cancers. Available at https://www.nccn.org/professionals/physician_gls/default.aspx. Accessed May 8, 2017.
- 2. Zenda S, Onozawa Y, Boku N et al. Single‐agent docetaxel in patients with platinum‐refractory metastatic or recurrent squamous cell carcinoma of the head and neck (SCCHN). Jpn J Clin Oncol 2007;37:477–481. [DOI] [PubMed] [Google Scholar]
- 3. Machiels JP, Subramanian S, Ruzsa A et al. Zalutumumab plus best supportive care versus best supportive care alone in patients with recurrent or metastatic squamous‐cell carcinoma of the head and neck after failure of platinum‐based chemotherapy: An open‐label, randomised phase 3 trial. Lancet Oncol 2011;12:333–343. [DOI] [PubMed] [Google Scholar]
- 4. de Andrade DA, Machiels JP. Treatment options for patients with recurrent or metastatic squamous cell carcinoma of the head and neck, who progress after platinum‐based chemotherapy. Curr Opin Oncol 2012;24:211–217. [DOI] [PubMed] [Google Scholar]
- 5. Nguyen‐Tan PF, Zhang Q, Ang KK et al. Randomized phase III trial to test accelerated versus standard fractionation in combination with concurrent cisplatin for head and neck carcinomas in the Radiation Therapy Oncology Group 0129 trial: Long‐term report of efficacy and toxicity. J Clin Oncol 2014;32:3858–3866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Ferris RL, Blumenschein G Jr, Fayette J et al. Nivolumab for recurrent squamous‐cell carcinoma of the head and neck. N Engl J Med 2016;375:1856–1867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Vermorken JB, Mesia R, Rivera F et al. Platinum‐based chemotherapy plus cetuximab in head and neck cancer. N Engl J Med 2008;359:1116–1127. [DOI] [PubMed] [Google Scholar]
- 8. Harrington KJ, Ferris RL, Blumenschein G Jr et al. Nivolumab versus standard, single‐agent therapy of investigator's choice in recurrent or metastatic squamous cell carcinoma of the head and neck (CheckMate 141): Health‐related quality‐of‐life results from a randomised, phase 3 trial. Lancet Oncol 2017;18:1104–1115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Haddad R, Gillison M, Ferris RL et al. Double‐blind, two‐arm, phase 2 study of nivolumab (nivo) in combination with ipilimumab (ipi) versus nivo and ipi‐placebo (PBO) as first‐line (1L) therapy in patients (pts) with recurrent or metastatic squamous cell carcinoma of the head and neck (R/M SCCHN)—CheckMate 714. Ann Oncol 2016;27(suppl 6):1017TiPa. [Google Scholar]
- 10. Argiris A, Gillison M, Ferris RL et al. A randomized, open‐label, phase 3 study of nivolumab in combination with ipilimumab vs extreme regimen (cetuximab + cisplatin/carboplatin + fluorouracil) as first‐line therapy in patients with recurrent or metastatic squamous cell carcinoma of the head and neck‐CheckMate 651. Ann Oncol 2016;27(suppl 6):1016TiPa. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.