Abstract
Introduction:
Stroke is a major cause of disability and the fifth leading cause of death. Currently, the only approved acute medical treatment of ischemic stroke is tissue plasminogen activator (tPA), but its effectiveness is greatly predicated upon early administration of the drug. There is, therefore, an urgent need to find new therapeutic options for acute stroke.
Areas covered:
In this review, we summarize the role of Rho-associated coiled-coil containing kinase (ROCK) and its potential as a therapeutic target in stroke pathophysiology. ROCK is a major regulator of cell contractility, motility, and proliferation. Many of these ROCK-mediated processes in endothelial cells, vascular smooth muscle cells, pericytes, astrocytes, glia, neurons, leukocytes, and platelets are important in stroke pathophysiology, and the inhibition of such processes could improve stroke outcome.
Expert commentary:
ROCK is a potential therapeutic target for cardiovascular disease and ROCK inhibitors have already been approved for human use in Japan and China for the treatment of acute stroke. Further studies are needed to determine the role of ROCK isoforms in the pathophysiology of cerebral ischemia and whether there are further therapeutic benefits with selective ROCK inhibitors.
Keywords: Cerebral ischemia, ischemic stroke, RhoA/Rho-associated coiled-coil containing kinase (ROCK), therapeutic target
1. Introduction
Stroke is a major cause of disability and the fifth-leading cause of death in the USA, producing far-reaching social and economic costs beyond that of the disease itself. An estimated 700,000 ischemic strokes occur each year in the USA, accounting for about $70 billion in costs associated with healthcare services, medications, and loss of work-related wages [1]. Indeed, approximately 30–50% of stroke survivors are functionally disabled and 65% of the total stroke cost is due to long-term care and lost productivity. In addition, the emotional toll on patients and families with devastating stroke cannot be overstated: most elderly people fear disabling stroke more than they fear death [2].
There are two major types of stroke: ischemic stroke and hemorrhagic stroke. Ischemic strokes are caused by obstruction of blood flow that supplies the brain with oxygen-rich blood, accounting for 87% of all strokes. Hemorrhagic strokes, which account for the rest, occur when the artery in the brain leaks blood or ruptures. Transient ischemic attacks, sometimes called ‘mini strokes,’ differ from the other two stroke types because cerebral blood flow is reduced transiently, usually for no more than 5 min, leading to reversible neurological deficits [1].
Ischemic stroke is a multifactorial medical condition with many risk factors, including age, gender, ethnicity, and family history. Modifiable risk factors for stroke include smoking, obesity, excessive alcohol usage, physical inactivity, hypertension, hypercholesterolemia, diabetes, and cardiovascular disorders such as heart failure, heart defect, heart infection, and arrhythmia. Some of these modifiable risks are controllable and deserve special attention in stroke prevention, particularly as a majority of strokes can be prevented [3]. Nevertheless, success with risk factor modification is limited, and there are few effective therapies which can prevent and improve the functional outcome of patients with ischemic stroke.
Presently, the only FDA-approved treatment for ischemic stroke is tissue plasminogen activator (tPA), which is used to recanalize thrombus-occluded blood vessels. Despite its efficacy in thrombolysis, there are two major disadvantages of tPA therapy: a short treatment window of 3 h or up to 4.5 h in certain eligible patients, and potential hemorrhagic transformation. A significant number of stroke victims do not arrive at the hospital in time for tPA administration, and even if they do, it is often difficult to determine prospectively which ischemic strokes will undergo hemorrhagic transformation. Consequently, there is a pressing need to identify new potential therapeutic targets for ischemic stroke for pharmaceutical intervention.
2. Pathophysiology of ischemic stroke
Low respiratory reserves and complete dependence on aerobic metabolism make brain tissue particularly vulnerable to the effects of acute ischemia. Since the brain cannot store energy or use energy sources other than glucose, the brain is virtually dependent on cerebral blood flow for energy metabolism. The presence of a network of collateral arteries in the brain contributes to the spectrum of stroke severity in the affected region. The brain parenchyma undergoes immediate neuronal cell death (infarct core), while other areas of the brain, such as that of the penumbra, may be only partially injured with the potential to recover. The ischemic cascade causes brain damage through the local depletion of oxygen and glucose, leading to a decrease in the production of high-energy phosphate compounds such as adenine triphosphate (ATP). ATP is vital for maintaining the cellular anion and cation gradient through active transport channels; thus, inadequate energy supply can disrupt the careful ionic balance, leading to an efflux of potassium ions in exchange for an influx of sodium, chloride, and calcium ions. The influx of ions is accompanied osmotically by water, resulting in rapid swelling of neurons and glia that causes cytotoxic edema.
The ischemic cascade stimulates the release of excitatory neurotransmitters in the brain, such as glutamate and aspar-tate. Besides the direct neurotoxicity of glutamate in neurons, the activation of ionotropic and metabotropic glutamate receptors leads to further increases of intracellular Ca2+, Na+, and Cl− levels, thereby elevating edema and toxicity. In particular, calcium plays a unique role in the ischemic pathophysiology since it causes several damaging events through the activation of a variety of Ca2+-dependent enzymes, including protein kinase C, myosin light-chain kinase, cyclooxygenase, neuronal nitric oxide synthase, calpain, and various proteases and endonucleases. When reperfusion occurs, oxygen radicals are produced by the cyclooxygenase-dependent conversion of arachidonic acid to prostanoid and by the degradation of hypoxanthine. In addition, the formation of pores in the mitochondrial membrane occurs under ischemic conditions, which leads to a burst of free oxygen radicals and release of proapoptotic molecules such as cytochrome c. Reactive oxygen species are also generated during the inflammatory response after ischemia, which also lead to oxidative stress and damage.
Oxygen radicals serve as important signaling molecules that trigger inflammation and apoptosis. Mechanical or hypoxic damage of the vascular endothelium, toxic damage by inflammatory molecules, free radicals, and especially the destruction of the basal lamina by matrix metalloproteinase can potentially disrupt the blood–brain barrier (BBB). The loss of BBB integrity can lead to vasogenic edema, influx of toxic substances, inflammation, and presumably hemorrhagic complications after stroke. The biphasic opening of BBB after transient ischemic insult can lead to a sustained inflammatory response in the brain. Ischemic injury initially triggers inflammatory cascades in the brain parenchyma. Within minutes of occlusion, there is upregulation of proinflammatory mediators such as platelet-activating factor, tumor necrosis factor (TNF)-α, and interleukin (IL)-1β. These proinflammatory factors induce the expression of adhesion molecules, including inter-cellular adhesion molecule (ICAM)-1, vascular cell adhesion molecule (VCAM)-1, and E-selectin on the vascular endothelium, leading to neutrophil recruitment, attachment, and transmigration from the blood into the brain parenchyma followed by macrophages and monocytes.
Furthermore, the production of toxic mediators by activated inflammatory cells and injured neurons (cytokines, nitric oxide, superoxide anion, and prostanoids) can amplify tissue damage. Neutrophils are the first inflammatory cells to arrive to the ischemic tissue, often within hours after reperfusion, while macrophages and monocytes arrive within a few days. Throughout this inflammatory cascade, cerebral ischemia activates other cells of the neurovascular unit, including microglia and astrocytes, which can amplify inflammation and tissue damage. The functions of inflammatory cells and subsequent neuro-inflammation are a double-edged sword, because of their potential beneficial effects on tissue remodeling [4] (Figure 1). It is, therefore, important to understand each step in the pathophysiology of ischemic stroke in order to develop potential therapeutic targets that can improve stroke outcome.
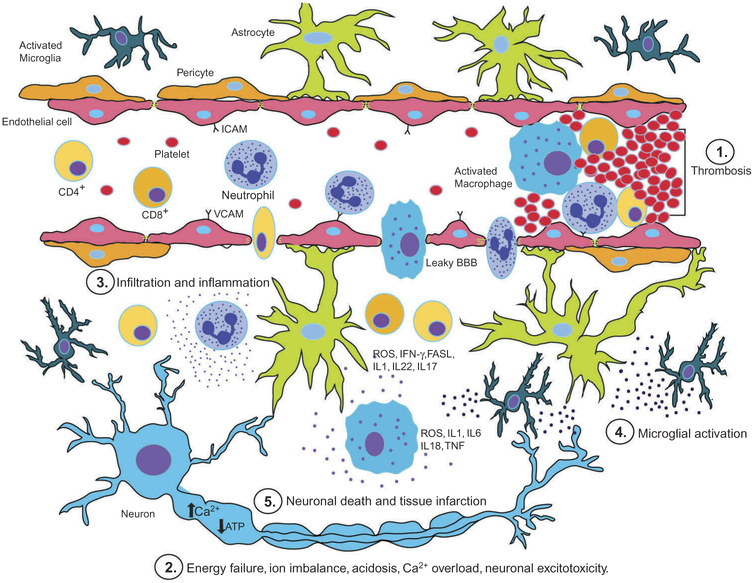
Figure 1.
The diagram outlines the key events that occur after ischemic brain injury following 1. thrombosis, 2. energy failure, imbalance of ion homeostasis, acidosis, intracellular calcium overload, neuronal excitotoxicity, 3. inflammatory cell activation and infiltration, 4. glial cell activation and 5. neuronal death with tissue infarction as a final result.
3. Key features of ROCK
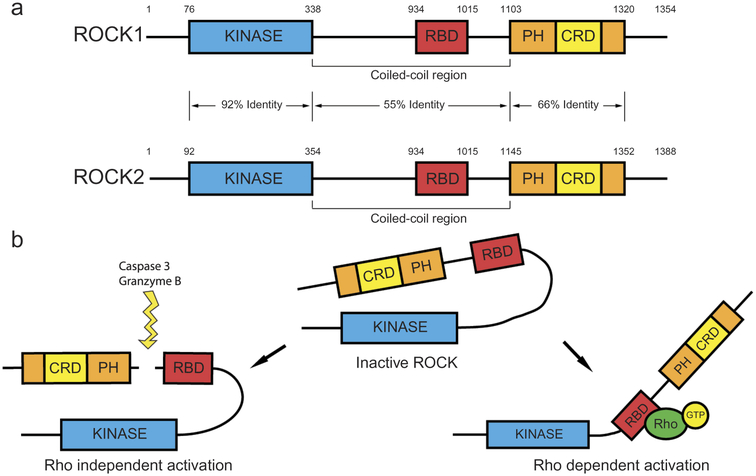
ROCK belongs to the family of serine/threonine kinases and includes two isoforms: ROCK1 and ROCK2 [5]. They were initially discovered as downstream targets of the small GTP-binding protein, RhoA [6,7]. ROCK1 and ROCK2 genes contain 33 exons, which lie on the 18q11 and 2p24 chromosomal regions, respectively [8]. The ROCK proteins are structurally composed of an N-terminal kinase domain followed by a coiled-coil region that contains a Rho-binding domain (RBD). At the carboxyl-terminal region (C-terminal), there is a pleckstrin homology (PH) domain, which flanks a cysteine-rich-region [9,10] (Figure 2(a)). The C-terminal of both isomers, including the RBD and the PH domains, is an auto-inhibitory region that folds back onto the N-terminal region and inhibits their kinase activity under basal conditions [9,11] (Figure 2(b)). In addition, homologous binding of ROCK proteins, which is afforded by their N-terminal kinase domain, increases auto-phosphorylation [12,13], suggesting that dimerization may promote ROCK activity. Overall, the ROCK proteins share 65% amino acid sequence homology, while their kinase domains are 92% similar [6].
Figure 2.
Rho-associated coiled-coil kinase (ROCK) protein structure and activation. (a) The ROCK proteins are composed of an amino terminal domain (N-terminal) followed by a coiled-coil region with Rho binding domain (RBD). The carboxy-terminal (C-terminal) region contains pleckstrin homology domain (PH) and a cysteine-rich region. (b) Inactivation of ROCK by intermolecular association of C-terminal and kinase domain, Rho-GTP dependent activation and conformational change, and Rho independent activation by ROCK cleavage.
The ROCK1 transcript is ubiquitously expressed in all tissue, although with less expression in brain and skeletal muscle, whereas the ROCK2 transcript is present more abundantly in the brain, muscle, heart, lung, and placenta [5,10]. ROCK2 is primarily localized to the cytoplasm [6,7] in association with vimentin and actin stress fibers [14,15]. ROCK2 also localizes to the plasma membrane [16], an association that is afforded by its C-terminal region [14], and the cleavage furrow during late mitosis [17]. In human and mouse skeletal muscle, a unique splice variant of ROCK2 was detected named mROCK2 [18]. This variant contains an insertion of 57 amino acids following the RBD, and is expressed during myogenic differentiation together with ROCK2. While mROCK2 is localized in similar parts of the cell as ROCK2, it is unable to associate with the plasma membrane [18]. The intracellular distribution of ROCK1, however, is not as well established. There is mounting evidence that ROCK1 predominantly associates with the plasma membrane during cell–cell interaction and with adhesion sites and vesicles [19]. ROCK1 additionally localizes to the microtubule-organizing center [20] as well as to the leading and trailing edges of motile cells [21], suggesting its involvement in cell migration.
The Rho GTPases RhoA, RhoB, and RhoC are the most studied ROCK activators. All three Rho proteins in their active GTP-bound state bind to the ROCK RBD domain, opening the ‘clothes pin’ structure and exposing the kinase domain of ROCK, thereby activating ROCK catalytic activity through conformational disinhibition of the C-terminal domain [7,9,22]. Phospholipid binding can also change ROCK conformation, similarly exposing kinase activity that is conducive toward active signaling. ROCK activity can also be changed by modulation or cleavage of the C-terminal domain. For instance, proteolytic cleavage of the inhibitory C-terminal domain by caspase-3 and granzyme B can activate ROCK1 and ROCK2 [10,23,24] (Figure 2(b)). The binding of lipids such as arachidonic acid to the pleckstrin homology domain also promotes ROCK activation in a Rho-independent manner. Other kinases such as polo-like kinase-1 (PLK1) can also regulate ROCK activation in cooperation with RhoA. Furthermore, some GTP-binding proteins such as RhoE can inhibit ROCK1 by binding to sites other than the RBD [25–27]. Both ROCK1 and ROCK2 phosphorylate the consensus sequence Arg/Lys–X–Ser/Thr or Arg/Lys–X–X–Ser/Thr, but other non-consensus phosphorylation sites have been also identified [28,29]. The specificity of ROCK substrates has not been thoroughly analyzed for structural differences, which makes it difficult to draw a distinction between its substrates. At the moment, it appears that the intracellular compartmental localization of ROCK isoforms dictate their function and downstream targets.
4. Functions of ROCKs
4.1. Cytoskeletal reorganization
ROCKs are key regulators of actin cytoskeletal dynamics, phosphorylating diverse downstream targets and affecting numerous intracellular processes that are important for cell contractility, motility, proliferation, and morphology. ROCK1 and ROCK2 represent a divergent signaling hub, whose substrates include myosin light-chain phosphatase (MLCP), Lin-11 Isl-1 Mec-3 (LIM) kinase (LIMK), Ezrin/Radixin/Moesin (ERM), and intermediate filament proteins. As a result, ROCKs are major regulators of smooth muscle contraction, stress fiber formation, microtubule dynamics, intermediate filament assembly, and focal adhesion. The importance of ROCKs is reflected by its central role in several developmental and morphogenesis processes. Global single and double deficiency of ROCK1 and ROCK2 lead to embryonic lethality. Furthermore, deficiency of ROCK1 leads to impaired eyelid closure, blindness, and omphalocele at birth, most likely through a decrease in accumulation of F-actin, whereas deficiency of ROCK2 results in placental insufficiency [25–27,30].
4.2. ROCK and gene expression
There is growing evidence that ROCKs can modulate gene expression by affecting the transcriptional machinery in the nucleus. ROCKs are critical regulators of cytoplasmic-nuclear shifting of profibrotic transcriptional factors such as MRTF-A/B and YAP/TAZ. Indeed, ROCK inhibitors are currently being investigated as potential therapeutic tools for the treatment of fibrosis [31]. As will be discussed in later sections, ROCKs also control transcription factors that play critical roles in inflammation, including NF-κB, IRF4, and IRF7. Both ROCK iso-forms have been detected in the nucleus. ROCK2 can also phosphorylate p300, further enhancing its acetyltransferase activity [32].
4.3. Proliferation
Because ROCKs play crucial roles in cytoskeletal rearrangements and gene expression, they are intimately involved in cell proliferation. ROCK inhibition leads to a delayed S phase by decreasing the translocation of transcriptional factors involved in the G1-S transition. ROCKs also play important roles in the creation of the cleavage furrow and the disassembly of intermediates filament during proliferation [26,27].
4.4. Survival and cell death
The activation of ROCK1 in apoptosis via cleavage of caspase-3 or granzyme B accelerates membrane blebbing and fragmentation of DNA. ROCK-mediated signaling can also promote cell survival during starvation. ROCK1 promotes autophagy and cell survival by phosphorylation of beclin1, although some studies have reported an opposing role of ROCK2 in metabolic regulation by demonstrating that ROCK2 inhibits autophagy. Thus, there is ample evidence to suggest that ROCKs play important roles in cell metabolism and survival [25–27,33]. Further studies, however, are required to elucidate their precise role in these processes.
5. Targeting ROCK in the CNS and ischemic stroke
ROCK inhibitors are beneficial in stroke, spinal cord injury, and in a variety of neurological diseases, including Alzheimer’s and Parkinson’s disease. Several animal studies and autopsy studies in humans have shown that overactivation of the Rho/ROCK pathway plays an important role in the pathophysiology of spinal cord injury [34]. Treatment with nonselective ROCK inhibitors (Y27632 or Fasudil) in mouse models of spinal cord injury was beneficial, promoting axon regeneration and functional recovery [35,36].
As previously mentioned, ROCK can mediate numerous processes in multiple cell types relevant in stroke pathophysiology and recovery, including its roles as a major regulator of cell contractility, motility, and proliferation in cells. Many of these ROCK-regulated processes in the neurovascular unit are important in stroke pathophysiology, and the modification of which can have potential therapeutic effects. In particular, there is some evidence that suggests an important role of ROCK in stroke recovery by modulating neuro-inflammation. In a mouse model of middle cerebral artery occlusion (MCAO), ROCK expression increased by more than twofold in the ischemic region. Furthermore, ROCK inhibitors have been shown to be beneficial in stroke prevention, acute neuroprotection, and chronic stroke recovery by altering inflammation, platelet and endothelial function, smooth muscle contraction, and neuronal regeneration. MCAO models have demonstrated that the inhibition of ROCK, by increasing blood flow, reduces cerebral infarct size by 33% and improves neurologic deficit score by 37% [37]. Chronic treatment with another nonselective ROCK inhibitor, Fasudil, improves learning in rodents after ischemic stroke by reducing inflammation. Acute administration of Fasudil decreased leukocyte recruitment and adhesion to the endothelium after ischemia/reperfusion injury [38]. Interestingly, selective ROCK2 inhibitor KD025 (formerly SLx-2119) also demonstrates dose-dependent reduction of cerebral infarct size and improved outcome after focal cerebral ischemia model in mice, most likely by improving collateral cortical blood flow. KD025 has been shown to be safe and did not elicit significant hypotension when compared with non-selective ROCK inhibitors in aged, diabetic, or female mice [39].
In human studies, inhibition of ROCK by Fasudil leads to significant improvements in both neurological function and clinical outcome in patients after ischemic stroke [40]. In addition, our group has previously shown that ROCK activity in peripheral blood leukocytes is elevated in humans within 48 h of an acute ischemic stroke when compared with healthy controls, but this did not correlate with stroke severity assessed by NIHSS score [37,41]. Such an elevation in leukocytes is consistent with many lines of evidence, suggesting a potential role of ROCK in acute ischemic stroke.
5.1. ROCK and platelets function
The activation of platelets is critical for hemostasis [42], and is the final common pathway in most acute vascular occlusive disorders. RhoA/ROCK pathway plays a multifaceted role in platelet biology. During platelet development, RhoA/ROCK has been proposed as a key regulator of megakaryocyte endomitosis, platelet formation, and platelet release. Deleting RhoA in megakaryocytes leads to abnormally enlarged megakaryocytes that release premature platelets. These platelets are cleared quickly from the circulation, which can lead to macrothrombocytopenia [43].
Platelet activation is a complex process that involves active rearrangement of the actin cytoskeleton, changes in cell shape, secretion of granules, and the retraction of clots, making it likely that RhoA and ROCK play important roles in the regulation of platelet activation. Indeed, studies using megakaryocyte-specific RhoA-deficient mice have shown the importance of RhoA at every critical step of platelet function. For instance, RhoA has been found to mediate platelet shape change, Gα13-mediated GPCR cross-talk activation of integrin αIIbβ3, granule secretion, clot retraction, and thrombus formation and stability. Platelets from RhoA-null mice have impaired shape changes and show mild aggregation deficits that are downstream of Gα13 and Gαq. While RhoA is not required for platelets to spread upon the surface of integrin αIIbβ3 substrate fibrinogen, RhoA is required for integrin activation and granule secretion in response to thrombin and combined ADP and U46619 stimulation, as well as for clot retraction. For instance, megakaryocyte-specific RhoA-deficient mice show macrothrombocytopenia, altered platelet activation, reduced thrombus formation, and prolonged tail-bleeding times [44,45].
Recent studies using ROCK2-deficient platelets partially confirm previous studies using RhoA-deficient platelets, demonstrating that ROCK2-deficient platelets were less responsive to thrombin stimulation in terms of pseudopodia formation, collagen adhesion, and heterotypic aggregation. This corresponded to prolonged bleeding time and increased time to vascular occlusion following vascular injury. When preformed clots from platelet-specific ROCK2-deficient mice were injected into the middle cerebral artery of control mice, clot dissolution and cerebral blood flow recovery occurred more rapidly, leading to decreased cerebral injury and lower neurological deficit scores compared to control mice. Deletion of ROCK2 in platelets, however, had no protective effects in the intra-filament middle cerebral artery occlusion model of stroke, genetic-induced model of atherosclerosis, or carotid artery ligation model of vascular remodeling. These findings indicate that platelet ROCK2 is critical for thrombus formation and stabilization, and is an important pathogenic mediator of thromboembolic stroke [46].
5.2. ROCK in neuro-inflammation
The neuro-inflammatory response is an important process during both acute and late phases of stroke [47]. As previously mentioned, post-ischemic inflammation is a double-edged sword and involves a complex sequence of events. On one hand, as a natural defensive mechanism of the body to protect injured tissue, inflammation helps to isolate the damaged area and promote healing. On the other hand, excessive inflammation can exacerbate ischemic injury. Despite intense investigation, the roles of various types of immune responses in the pathophysiology of ischemic stroke are still not well characterized.
Overactivation of ROCK during acute cerebral ischemia likely contributes to early worsening of cerebral injury, partially by stimulating the inflammatory response. Increased expression of adhesion molecules (i.e. P-selectin and ICAM-1) secondary to the reduction of endothelium-derived NO during ischemic injury is mediated by ROCK, as ROCK is an upstream negative regulator of eNOS. Inhibition of ROCK increases eNOS mRNA stability and expression [48]. Indeed, ROCK inhibitors reduce neutrophil accumulation in the infarct tissue and also reduce infarct volume in animal models of ischemic stroke [49,50]. The activation of ROCK stimulates neutrophil infiltration during vascular inflammation through NADHP oxidase activation and ROS production. Furthermore, in spinal cord injuries, the use of ROCK inhibitors reduces leukocyte infiltration into the spinal cord and promotes neurological recovery [51].
Increasing evidence suggests that T lymphocytes also play an important role in mediating post-stroke inflammation [52]. Cytotoxic T cells (CD8+) induce tissue damage by releasing cytotoxic granules or through TNF receptor-mediated cytotoxicity, and is a process further regulated by CD4 + T-helper 1 (Th1) and CD4 + T-helper 2(Th2) cells. Th1 cell secretion of proinflammatory cytokines is strongly associated with neuro-degenerative diseases [53]. Th2 cells downregulate the activity of Th1 cells, and studies have shown their beneficial anti-inflammatory response in promoting stroke recovery with improved clinical outcomes after ischemic stroke by upregulating anti-apoptotic factors such as Bcl-2 and Bcl-xL [54,55]. Another study examined Th17 and Treg, also subsets of T cells, and concluded that they are pro- and anti-inflammatory, respectively, and thereby promote and inhibit tissue damage [56]. Several studies have also shown that Tregs can reduce cerebral infarct size post ischemic injury [56,57]. T cell lineage differentiation or polarization is mediated by cytokines. IFN-γ activates Th1 pathways by upregulating transcription factors such as STAT1. STAT1 activates T-box expressed in T cells (T-bet), which is a master transcriptional activator for Th1 differentiation. In contrast, lineage differentiation to the Th2 phenotype requires binding of IL4 to its receptor, which triggers activation of STAT6. The activation of STAT6 upregulates GATA-binding protein 3 (GATA-3), which potently activates Th2, producing Th2 cytokines IL4 and IL5 and inhibiting Th1 cytokine IFN-γ [58]. Activation of Th17 and IL17 production is regulated by activation of the retinoic acid-related orphan receptor (ROR)γt transcription factor. Production of Treg subset-specific cytokine IL17 is regulated by activation of the Foxp3 transcription factor.
The role of Rho-kinase (ROCK) and its downstream effectors in T cell function is not well known. However, it is likely that ROCKs can mediate T cell activation by upregulating certain transcription factors that affect the actin cytoskeleton, which has been demonstrated by the inhibition of ROCK with Y-27632 in T cells [59,60]. Indeed, inhibition of ROCK with Y-27632 in T cells leads to inhibition of actomyosin polymerization, reduced T cell proliferation, and decreased T cell receptor activation [61]. In addition, ROCK activity is important for T cell polarization and trans-endothelial migration, both important elements in T cell infiltration into the brain after ischemia [62]. ROCK also activates the transcription factor nuclear factor-κB (NF-κB), which is a potent activator of T cell function [60]. Moreover, stimulation of TCR activates RhoA, which leads to activation of the p38 mitogen-activated protein kinase (p38MAPK), which in turn induces upregulation of IL4, IL10 and IFN-γ production [63,64]. ROCK2 phosphorylates IRF4, a transcription factor that is required for production of IL17, IL21, and differentiation of Th17 T cells [65].
Stroke survivors have more than twice the risk of developing dementia compared with people who never had stroke [66]. A recent study has shown that B-lymphocytes may contribute to dementia in stroke patients. B cell density and IgG antibody levels in post-mortem brain tissues from patients who experienced dementia after stroke were significantly higher when compared with that of age-matched controls who had not been diagnosed with stroke or dementia [67]. However, the involvement of ROCKs in B cell function remains largely unknown. Some reports have indicated that ROCKs regulate cytoskeletal remodeling in B cells, and that antigen internalization is greatly dependent on ROCK1 [68]. ROCKs may also be involved in B-cell migration, as ROCK inhibitors alter B cell chemotaxis and adhesion [69].
Microglial cells are resident brain macrophages, which play an important role in ischemic stroke. Microglial proliferation peaks at 2–3 days after brain ischemic injury but remain for several weeks, producing proinflammatory cytokines which can have detrimental effects on brain tissue following ischemia [57]. ROCK activation in microglia stimulates cytokine production. The ROCK inhibitor, Fasudil, has protective effects on hippocampal neurons following hypoxia/reoxygenation injury by suppressing microglial secretion of proinflammatory factors [70]. It is difficult to distinguish between microglial cells and blood-derived macrophages that infiltrate brain tissues 2–7 days after stroke. One of the most commonly expressed chemokines in the CNS during inflammation is monocyte chemoattractant protein-1 (MCP-1, CCL2). MCP-1 is involved in BBB opening through the recruitment of monocytes and macrophages and can activate lymphocytes in the brain during neuropathological states. Bone marrow-derived ROCK1-deficient macrophages have decreased chemotaxis to MCP-1/CCL2. Inhibition of ROCK by Y-27632 attenuates TNF-α-mediated monocyte migration, and leads to the induction of MCP-1 via the p38MAPK pathway [71,72]. Recent studies suggest that the ROCK signaling plays a critical role in macrophage polarization into M1 and M2 subtypes in aged-related macular degeneration. ROCK2 inhibition upregulates the accumulation of protective M2 macrophages without affecting macrophage recruitment [73]. Furthermore, Fasudil shifts inflammatory M1 to anti-inflammatory M2 macrophages, thus attenuating experimental autoimmune encephalomyelitis. Targeting ROCK signaling in microglia and blood-borne macrophage plasticity opens up new possibilities for ROCK inhibitors in post-ischemic stroke treatment and long-term recovery [74]. Indeed, ROCK inhibitors have shown beneficial effects in many autoimmune diseases, such as rheumatoid arthritis, systemic lupus erythematosus, scleroderma, and giant cell arthritis [65,75–77].
5.3. ROCK and BBB
ROCKs are important proteins regulating the BBB due to its actin cytoskeletal and proinflammatory effects. For instance, RhoA/ROCK activation leads to contraction and increased permeability in response to inflammatory stimuli. The RhoA/ROCK pathway is upregulated by chemokine-like MCP-1/CCL2 through G-protein receptors, inducing occludin, claudin-5, and ZO-1 Ser/Thr-phosphorylation and relocalization from tight junctions (TJ), ultimately increasing barrier permeability [78]. Furthermore, adhesion molecules such as ICAM-1 and VCAM-1 were shown, in response to lymphocyte/monocyte adhesion, to transduce signals in rat brain endothelial cell lines, including the activation of the RhoA/ROCK pathway; leading to increased infiltration of inflammatory cells in the CNS. In addition, ROCK signaling is important in astrocyte-secreted sonic hedgehog in neurogenesis and angiogenesis after oxygen glucose deprivation [79].
5.4. ROCK inhibition in neuroprotection and neuroregeneration
The restorative capacity of adult brain is very limited after stroke or in neurodegenerative diseases. RhoA/ROCK/LIMK is a key inhibitory pathway in axonal growth [80]. Besides inhibition of axonal growth, ROCK can also be deleterious for neuronal survival. Because of these highly important roles of ROCK in neuronal fate, Fasudil has shown potential neuroprotective effects that can trigger neurogenesis and an increase in neuron survivability after oxygen glucose deprivation, axotomy, serum deprivation, or 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP)-induced lesions (a rodent model of Parkinson’s disease) [81,82]. This effect was partially mediated through astrocyte-secreted G-CSF [83]. ROCK2 is strongly expressed in the brain and spinal cord as well as its downstream signaling molecule LIMK1. Inhibition of ROCK2 leads to neuronal survival and axonal stability during regeneration, whereas inhibition of the LIMK1/cofilin pathway only led to neurite outgrowth and regeneration, but did not affect neuronal survivability [33,81]. These findings suggest novel therapeutic applications of selective ROCK inhibitors for stroke recovery, preventing neurodegeneration and stimulating neuroregeneration in various neurological disorders.
6. Development of selective ROCK inhibitors as a treatment for stroke
Despite evidence of increased ROCK activity in a variety of pathological conditions, including ischemic stroke, little is known about the molecular mechanisms leading to increased ROCK activity or their downstream targets. Determining the precise role of ROCK in ischemic stroke and stroke recovery is limited by nonselective pharmacological inhibitors, since they cannot discriminate between the two ROCK isoforms. Widely used nonselective ROCK inhibitors, Fasudil and Y-27632, target ROCK’s ATP-dependent kinase domain and are equipotent in inhibiting both isoforms. Consequently, genetic approaches with tissue-specific targeting of ROCK isoforms offer the greatest likelihood of success in dissecting the pathophysiological role of ROCK1 and ROCK2 in ischemic stroke. Another important limitation for the use of ROCK inhibitors is that at higher concentrations they inhibit other serine-threonine kinases such as PKA and PKC. The hydroxyl form of Fasudil, Hydroxyfasudil, has the same inhibitory activity but has a much longer half-life (30 min versus 5 h). Due to these limitations and the nonselectivity of ROCK inhibitors, Fasudil is the only ROCK inhibitor approved for human use in Japan and China, but not in the USA or Europe. In Japan and China, Fasudil has been used for the prevention and treatment of cerebral ischemia, as well as for vasospasm following subarachnoid hemorrhage [84]. However, since its approval in 1995, several adverse effects such as hypotension, intracranial hemorrhage, and abnormal hepatic and renal function have been observed.
Currently, there are several ROCK inhibitors undergoing clinical trials. Y39983/RKI983, AR12286, and AMA0076 are in phase II for ocular hypertension and glaucoma. For hypertension and glaucoma treatment, INS117548 is in a phase I clinical trial. BA210 is in a phase II clinical trial for spinal cord injury, and SAR407899 is in phase II for treatment of erectile dysfunction [85–87]. In addition, many of the so-called ‘pleiotropic’ effects of statins or HMG-CoA reductase inhibitors could be mediated by ROCK inhibition [88,89]. In cultured cells and animals, inhibition of the Rho/ROCK pathway by statins exerts an anti-atherogenic effect that is independent of cholesterol reduction. In animals, however, the required dose of statins to inhibit the pathway appears to be far higher than that used by clinicians for lipid lowering, raising legitimate concerns about the relevance of these experimental findings. Consequently, the clinical benefits that are derived from the inhibitory effect of ROCK by statins remain to be seen.
An intriguing result of large clinical trials with statins is the reduction in ischemic stroke [90]. In human studies of older ischemic patients who were not taking statins at the time of admission, discharge statin therapy was associated with a lower risk of major adverse cardiovascular events, and the patients had nearly a month more home-time during a 2-year post-hospitalization period [88]. Large, placebo-controlled, and randomized trials show that statins reduce cerebrovascular events in primary and secondary prevention [91] (4S Group, 1994; CARE Investigators [92]; LIPID Study Group, 1998 [93]; WOSCOPS Group, 1998 [94]; HPS Collaborative Group, 2004 [95]). Despite the fact that cohort studies show no association between serum cholesterol concentrations and stroke, and that non-statin lipid-lowering intervention studies did not demonstrate the same level of stroke protection, these trials demonstrated that statin treatment reduces the incidence of stroke by 25–30%, most likely through non-cholesterol-dependent effects [96,97]. Despite these studies, the precise potential use of statin pleiotropy on the Rho/ROCK pathway in the treatment and prevention of stroke remains to be further elucidated.
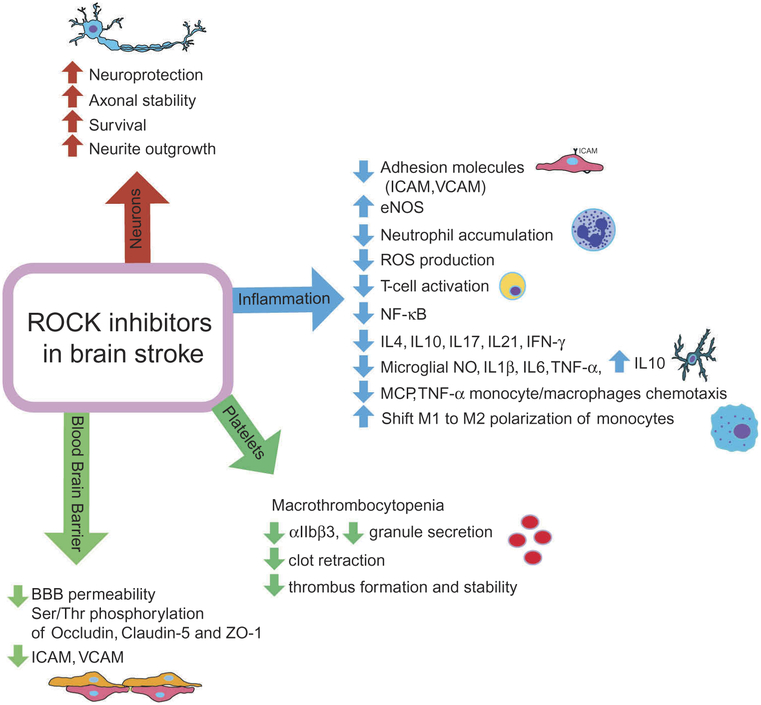
There are a number of other drugs that could also potentially inhibit the RhoA/ROCK pathway, including nitrogen-containing bisphosphonates, calcium channels blockers, and drugs targeting the renin-angiotensin system [98,99]. Nonetheless, due to the importance of ROCK in various aspects of vascular function and the inflammatory cascade, the development of selective ROCK inhibitors has garnered significant interest from the pharmaceutical industry as a potential therapy for ischemic stroke (Figure 3).
Figure 3.
Potential therapeutic benefits of ROCK inhibitors during different pathophysiological events in acute ischemic stroke.
7. Expert commentary
ROCK has been identified as a potential therapeutic target for ischemic stroke. Indeed, some of the non-cholesterol lowering benefits of statins may be due to ROCK inhibition by statins. Because ROCK is a critical regulator of the actin cytoskeleton, deletion or suppression of ROCK activity below basal levels may lead to cellular toxicity and untoward side effects. Thus, identifying individuals who are at risk for ischemic stroke and have elevated ROCK activity may provide greater precision and a wider therapeutic window regarding who may benefit from ROCK inhibition. Furthermore, understanding the patho-physiological role of ROCK isoforms in ischemic stroke may lead to the development of isoform-specific ROCK inhibitors. Because of the amino acid sequence homology of the kinase domains between ROCK isoforms, this may be difficult if the target is their kinase domains. Nevertheless, understanding the function of ROCK1 and ROCK2 in the pathogenesis of ischemic stroke may help determine whether selective ROCK inhibitors could be clinically useful.
8. Five-year view
More and more ROCK inhibitors will be developed within the next few years. Some will be selective for either ROCK1 or ROCK2, while others will be nonselective. The key question in the development of ROCK inhibitors as a therapy for acute ischemic stroke is who would be the most likely to benefit. It is reasonable to assume that individuals with elevated ROCK activity and who are at risk for ischemic stroke would be the mostly likely population selected for a randomized trial to test the benefits of acute ROCK inhibition in stroke evolution. Alternatively, genetic association studies may uncover patient populations where polymorphisms of ROCK genes may be associated with increased stroke risk. Indeed, we have previously shown that ROCK1 polymorphism was associated with increased risk of stroke in the Women’s Genome Health Study [100]. The ability to predict who would benefit from ROCK inhibitors among all patients with ischemic stroke will greatly help define their clinical use.
Key issues.
ROCK inhibition by Fasudil leads to greater improvements in both neurological function and clinical outcomes in patients with ischemic stroke.
ROCK has been proposed as a key regulator of megakaryocyte endomitosis, platelet formation, and platelet release.
ROCK2 is critical for platelets activation, thrombus formation, and thrombus stabilization.
ROCK inhibitors reduce activation and acumulation of inflammatory cells in the brain after reperfusion, and reduce infarct volume in animal models of ischemic stroke.
ROCK inhibitors have been shown to exert neuroprotective effects following hypoxia/reoxygenation injury by preventing neurodegeneration and stimulating neuroregeneration.
ROCK signaling may play a critical role in macrophage polarization.
RhoA/ROCK pathway inhibitors may help attenuate blood brain barrier permeability after ischemic stroke.
Determining the precise role and potential use of ROCK in stroke is limited by selectivity of pharmacological inhibitors. There is a potential need for the development of safe and selective ROCK inhibitors.
Acknowledgments
Funding
This paper was supported by National Institutes of Health grants (HL052233 and NS070001).
Footnotes
Declaration of interest
JK Liao is a consultant for Celegene, Pliant & Kadmon. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
References
Papers of special note have been highlighted as either of interest (•) or of considerable interest (••) to readers.
- 1.Mozaffarian D, Benjamin EJ, Go AS et al. Heart disease and stroke statistics-2016 update: A report from the American Heart Association. Circulation. 2016;133(4):e38–360. [DOI] [PubMed] [Google Scholar]
- 2.Solomon NA, Glick HA, Russo CJ, et al. Patient preferences for stroke outcomes. Stroke. 1994. September;25(9):1721–1725. [DOI] [PubMed] [Google Scholar]
- 3.Benjamin EJ, Blaha MJ, Chiuve SE, et al. Heart disease and stroke statistics-2017 update: a report from the American Heart Association. Circulation. 2017. March 07;135(10):e146–e603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.del Zoppo GJ. The neurovascular unit in the setting of stroke. J Intern Med. 2010. February;267(2):156–171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nakagawa O, Fujisawa K, Ishizaki T, et al. ROCK-I and ROCK-II, two isoforms of Rho-associated coiled-coil forming protein serine/threonine kinase in mice. FEBS Lett. 1996. August 26;392(2):189–193. [DOI] [PubMed] [Google Scholar]
- 6.Leung T, Manser E, Tan L, et al. A novel serine/threonine kinase binding the Ras-related RhoA GTPase which translocates the kinase to peripheral membranes. J Biol Chem. 1995. December 8;270(49):29051–29054. [DOI] [PubMed] [Google Scholar]
- 7.Matsui T, Amano M, Yamamoto T, et al. Rho-associated kinase, a novel serine/threonine kinase, as a putative target for small GTP binding protein Rho. EMBO J. 1996. May 1;15(9):2208–2216. [PMC free article] [PubMed] [Google Scholar]
- 8.Takahashi K, Sasaki T, Mammoto A, et al. Interaction of radixin with Rho small G protein GDP/GTP exchange protein Dbl. Oncogene. 1998. June 25;16(25):3279–3284. [DOI] [PubMed] [Google Scholar]
- 9.Ishizaki T, Maekawa M, Fujisawa K, et al. The small GTP-binding protein Rho binds to and activates a 160 kDa Ser/Thr protein kinase homologous to myotonic dystrophy kinase. EMBO J. 1996. April 15;15(8):1885–1893. [PMC free article] [PubMed] [Google Scholar]
- 10.Leung T, Chen XQ, Manser E, et al. The p160 RhoA-binding kinase ROK alpha is a member of a kinase family and is involved in the reorganization of the cytoskeleton. Mol Cell Biol. 1996. October;16(10):5313–5327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Amano M, Chihara K, Nakamura N, et al. The COOH terminus of Rho-kinase negatively regulates rho-kinase activity. J Biol Chem. 1999. November 5;274(45):32418–32424. [DOI] [PubMed] [Google Scholar]
- 12.Garg R, Riento K, Keep N, et al. N-terminus-mediated dimerization of ROCK-I is required for RhoE binding and actin reorganization. Biochem J. 2008. April 15;411(2):407–414. [DOI] [PubMed] [Google Scholar]
- 13.Jacobs M, Hayakawa K, Swenson L, et al. The structure of dimeric ROCK I reveals the mechanism for ligand selectivity. J Biol Chem. 2006. January 6;281(1):260–268. [DOI] [PubMed] [Google Scholar]
- 14.Sin WC, Chen XQ, Leung T, et al. RhoA-binding kinase alpha translocation is facilitated by the collapse of the vimentin intermediate filament network. Mol Cell Biol. 1998. November;18(11):6325–6339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chen XQ, Tan I, Ng CH, et al. Characterization of RhoA-binding kinase ROKalpha implication of the pleckstrin homology domain in ROKalpha function using region-specific antibodies. J Biol Chem. 2002. April 12;277(15):12680–12688. [DOI] [PubMed] [Google Scholar]
- 16.Kimura K, Fukata Y, Matsuoka Y, et al. Regulation of the association of adducin with actin filaments by Rho-associated kinase (Rhokinase) and myosin phosphatase. J Biol Chem. 1998. March 6;273(10):5542–5548. [DOI] [PubMed] [Google Scholar]
- 17.Inada H, Togashi H, Nakamura Y, et al. Balance between activities of Rho kinase and type 1 protein phosphatase modulates turnover of phosphorylation and dynamics of desmin/vimentin filaments. J Biol Chem. 1999. December 3;274(49):34932–34939. [DOI] [PubMed] [Google Scholar]
- 18.Pelosi M, Marampon F, Zani BM, et al. ROCK2 and its alternatively spliced isoform ROCK2m positively control the maturation of the myogenic program. Mol Cell Biol. 2007. September;27(17):6163–6176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Smith AL, Dohn MR, Brown MV, et al. Association of Rho-associated protein kinase 1 with E-cadherin complexes is mediated by p120-catenin. Mol Biol Cell. 2012. January;23(1):99–110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chevrier V, Piel M, Collomb N, et al. The Rho-associated protein kinase p160ROCK is required for centrosome positioning. J Cell Biol. 2002. May 27;157(5):807–817. . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Farber MJ, Rizaldy R, Hildebrand JD. Shroom2 regulates contractility to control endothelial morphogenesis. Mol Biol Cell. 2011. March 15;22(6):795–805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Boureux A, Vignal E, Faure S, et al. Evolution of the Rho family of ras-like GTPases in eukaryotes. Mol Biol Evol. 2007. January;24(1):203–216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Coleman ML, Sahai EA, Yeo M, et al. Membrane blebbing during apoptosis results from caspase-mediated activation of ROCK I. Nat Cell Biol. 2001. April;3(4):339–345. [DOI] [PubMed] [Google Scholar]
- 24.Sebbagh M, Renvoize C, Hamelin J, et al. Caspase-3-mediated cleavage of ROCK I induces MLC phosphorylation and apoptotic membrane blebbing. Nat Cell Biol. 2001. April;3(4):346–352. [DOI] [PubMed] [Google Scholar]
- 25.Amano M, Nakayama M, Kaibuchi K. Rho-kinase/ROCK: a key regulator of the cytoskeleton and cell polarity. Cytoskeleton (Hoboken, NJ). 2010. September;67(9):545–554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Schofield AV, Bernard O. Rho-associated coiled-coil kinase (ROCK) signaling and disease. Crit Rev Biochem Mol Biol. 2013. Jul-Aug;48(4):301–316. [DOI] [PubMed] [Google Scholar]
- 27.Julian L, Olson MF. Rho-associated coiled-coil containing kinases (ROCK): structure, regulation, and functions. Small GTPases. 2014;5: e29846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kang JH, Jiang Y, Toita R, et al. Phosphorylation of Rho-associated kinase (Rho-kinase/ROCK/ROK) substrates by protein kinases A andC. Biochimie. 2007. January;89(1):39–47. [DOI] [PubMed] [Google Scholar]
- 29.Riou P, Kjaer S, Garg R, et al. 14-3-3 proteins interact with a hybrid prenyl-phosphorylation motif to inhibit G proteins. Cell. 2013. April 25;153(3):640–653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Thumkeo D, Watanabe S, Narumiya S. Physiological roles of Rho and Rho effectors in mammals. Eur J Cell Biol. 2013. Oct-Nov;92(10–11):303–315. [DOI] [PubMed] [Google Scholar]
- 31.Knipe RS, Tager AM, Liao JK. The Rho kinases: critical mediators of multiple profibrotic processes and rational targets for new therapies for pulmonary fibrosis. Pharmacol Rev. 2015;67(1):103–117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tanaka T, Nishimura D, Wu RC, et al. Nuclear Rho kinase, ROCK2, targets p300 acetyltransferase. J Biol Chem. 2006. June 2;281(22):15320–15329. [DOI] [PubMed] [Google Scholar]
- 33.Koch JC, Tonges L, Barski E, et al. ROCK2 is a major regulator of axonal degeneration, neuronal death and axonal regeneration in the CNS. Cell Death Dis. 2014;5:e1225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Forgione N, Fehlings MG. Rho-ROCK inhibition in the treatment of spinal cord injury. World Neurosurg. 2014. Sep-Oct;82(3–4):e535–e539. [DOI] [PubMed] [Google Scholar]
- 35.Fournier AE, Takizawa BT, Strittmatter SM. Rho kinase inhibition enhances axonal regeneration in the injured CNS. J Neuroscience. 2003. February 15;23(4):1416–1423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Boato F, Hendrix S, Huelsenbeck SC, et al. C3 peptide enhances recovery from spinal cord injury by improved regenerative growth of descending fiber tracts. J Cell Sci. 2010. May 15;123(Pt 10):1652–1662. [DOI] [PubMed] [Google Scholar]
- ••37.Rikitake Y, Kim HH, Huang Z, et al. Inhibition of Rho kinase (ROCK) leads to increased cerebral blood flow and stroke protection. Stroke. 2005. October;36(10):2251–2257.Publication that builds foundation for potential use of ROCK inhibitors in cerebral ischemia.
- 38.Wang QM, Stalker TJ, Gong Y, et al. Inhibition of Rho-kinase attenuates endothelial-leukocyte interaction during ischemia-reperfusion injury. Vasc Med. 2012. December;17(6):379–385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hyun LJ, Zheng Y, von Bornstadt D, et al. Selective ROCK2 inhibition in focal cerebral ischemia. Ann Clin Translational Neurol. 2014. January;1(1):2–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- •• 40.Shibuya M, Hirai S, Seto M, et al. Effects of fasudil in acute ischemic stroke: results of a prospective placebo-controlled double-blind trial. J Neurol Sci. 2005. November 15;238(1–2):31–39. PubMed PMID: .Very important publication from 2005 showing beneficial effect of fasudin in ischemic stroke.
- • 41.Feske SK, Sorond FA, Henderson GV, et al. Increased leukocyte ROCK activity in patients after acute ischemic stroke. Brain Res. 2009. February 27;1257:89–93.Publication from our group showing elevated ROCK in peripheral leukocytes in humans within 48 h of an acute ischemic stroke that did not correlate with stroke severity assessed by NIHSS score.
- 42.Stoll G, Kleinschnitz C, Nieswandt B. Molecular mechanisms of thrombus formation in ischemic stroke: novel insights and targets for treatment. Blood. 2008. November 1;112(9):3555–3562. [DOI] [PubMed] [Google Scholar]
- •• 43.Suzuki A, Shin JW, Wang Y, et al. RhoA is essential for maintaining normal megakaryocyte ploidy and platelet generation [Research Support, N.I.H., Extramural]. PloS One. 2013;8(7):e69315.Publication outlining importance of RhoA in normal megakaryocyte function and platelet aggregation.
- •• 44.Pleines I, Hagedorn I, Gupta S, et al. Megakaryocyte-specific RhoA deficiency causes macrothrombocytopenia and defective platelet activation in hemostasis and thrombosis. Blood. 2012. January 26;119 (4):1054–1063.Important finding showing essential role of RhoA in clot retraction resulted in prolonged tail bleeding and impaired hemostasis.
- 45.Klages B, Brandt U, Simon MI, et al. Activation of G12/G13 results in shape change and Rho/Rho-kinase-mediated myosin light chain phosphorylation in mouse platelets. J Cell Biol. 1999. February 22;144(4):745–754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sladojevic N, Oh GT, Kim HH, et al. Decreased thromboembolic stroke but not atherosclerosis or vascular remodeling in mice with ROCK2-deficient platelets. Cardiovasc Res. 2017. April 14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Magnus T, Wiendl H, Kleinschnitz C. Immune mechanisms of stroke. Curr Opin Neurol. 2012. June;25(3):334–340. [DOI] [PubMed] [Google Scholar]
- 48.Laufs U, Liao JK. Post-transcriptional regulation of endothelial nitric oxide synthase mRNA stability by Rho GTPase. J Biol Chem. 1998. September 11;273(37):24266–24271. [DOI] [PubMed] [Google Scholar]
- 49.Satoh S, Kobayashi T, Hitomi A, et al. Inhibition of neutrophil migration by a protein kinase inhibitor for the treatment of ischemic brain infarction. Jpn J Pharmacol. 1999. May;80(1):41–48. [DOI] [PubMed] [Google Scholar]
- 50.Satoh S, Utsunomiya T, Tsurui K, et al. Pharmacological profile of hydroxy fasudil as a selective rho kinase inhibitor on ischemic brain damage. Life Sci. 2001. August 10;69(12):1441–1453. [DOI] [PubMed] [Google Scholar]
- 51.Hara M, Takayasu M, Watanabe K, et al. Protein kinase inhibition by fasudil hydrochloride promotes neurological recovery after spinal cord injury in rats. J Neurosurg. 2000. July;93(1 Suppl):94–101. [DOI] [PubMed] [Google Scholar]
- 52.Liu Z, Fan Y, Won SJ, et al. Chronic treatment with minocycline preserves adult new neurons and reduces functional impairment after focal cerebral ischemia. Stroke. 2007. January;38(1):146–152. [DOI] [PubMed] [Google Scholar]
- 53.Brecht S, Schwarze K, Waetzig V, et al. Changes in peptidyl-prolyl cis/trans isomerase activity and FK506 binding protein expression following neuroprotection by FK506 in the ischemic rat brain. Neuroscience. 2003;120(4):1037–1048. [DOI] [PubMed] [Google Scholar]
- 54.Wei Y, Yemisci M, Kim HH, et al. Fingolimod provides long-term protection in rodent models of cerebral ischemia. Ann Neurol. 2011. January;69(1):119–129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Kipnis J, Yoles E, Porat Z, et al. T cell immunity to copolymer 1 confers neuroprotection on the damaged optic nerve: possible therapy for optic neuropathies. Proc Natl Acad Sci U S A. 2000. June 20;97(13):7446–7451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Ochs HD, Oukka M, Torgerson TR. TH17 cells and regulatory T cells in primary immunodeficiency diseases. J Allergy Clin Immunol. 2009. May;123(5):977–983; quiz 984–985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Jin R, Yang G, Li G. Inflammatory mechanisms in ischemic stroke: role of inflammatory cells. J Leukoc Biol. 2010. May;87(5):779–789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Kim ES, Kim RS, Ren RF, et al. Transforming growth factor-beta inhibits apoptosis induced by beta-amyloid peptide fragment 25–35 in cultured neuronal cells. Brain Res Mol Brain Res. 1998. November 20;62(2):122–130. [DOI] [PubMed] [Google Scholar]
- 59.Nakagawa M, Higuchi I, Yoshidome H, et al. Familial facioscapulohumeral muscular dystrophy: phenotypic diversity and genetic abnormality. Acta Neurol Scand. 1996. Feb-Mar;93(2–3):189–192. [DOI] [PubMed] [Google Scholar]
- 60.Shimada H, Rajagopalan LE. Rho kinase-2 activation in human endothelial cells drives lysophosphatidic acid-mediated expression of cell adhesion molecules via NF-kappaB p65. J Biol Chem. 2010. April 23;285(17):12536–12542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Caplan S, Zeliger S, Wang L, et al. Cell-surface-expressed T-cell antigen-receptor zeta chain is associated with the cytoskeleton. Proc Natl Acad Sci U S A. 1995. May 23;92(11):4768–4772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- • 62.Heasman SJ, Ridley AJ. Multiple roles for RhoA during T cell transendothelial migration. Small GTPases. 2010. November;1(3):174–179.Reference targeting importance of ROCK activity in T cell polarization and transendothelial migration after brain ischemia.
- 63.Teramoto H, Salem P, Robbins KC, et al. Tyrosine phosphorylation of the vav proto-oncogene product links FcepsilonRI to the Rac1-JNK pathway. J Biol Chem. 1997. April 18;272(16):10751–10755. [DOI] [PubMed] [Google Scholar]
- 64.Perona R, Montaner S, Saniger L, et al. Activation of the nuclear factor-kappaB by Rho, CDC42, and Rac-1 proteins. Genes Dev. 1997. February 15;11(4):463–475. [DOI] [PubMed] [Google Scholar]
- •• 65.Biswas PS, Gupta S, Chang E, et al. Phosphorylation of IRF4 by ROCK2 regulates IL-17 and IL-21 production and the development of autoimmunity in mice. J Clin Invest. 2010. September;120(9):3280–3295.Publication from our group showing that ROCK2 phosphory-late IRF4, transcription factor that is absolutely required for production of IL17, IL21 and the differentiation of Th17 T cells.
- 66.Allan LM, Rowan EN, Firbank MJ, et al. Long term incidence of dementia, predictors of mortality and pathological diagnosis in older stroke survivors. Brain. 2011. December;134(Pt 12):3716–3727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Doyle KP, Quach LN, Sole M. B-lymphocyte-mediated delayed cognitive impairment following stroke. J Neurosci. 2015. February 4;35(5):2133–2145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Natkanski E, Lee WY, Mistry B, et al. B cells use mechanical energy to discriminate antigen affinities. Science (New York, NY). 2013. June 28;340(6140):1587–1590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.McDonald DA, Shi C, Shenkar R, et al. Fasudil decreases lesion burden in a murine model of cerebral cavernous malformation disease. Stroke. 2012. February;43(2):571–574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Ding J, Li QY, Wang X, et al. Fasudil protects hippocampal neurons against hypoxia-reoxygenation injury by suppressing microglial inflammatory responses in mice. J Neurochem. 2010. September;114(6):1619–1629. [DOI] [PubMed] [Google Scholar]
- 71.Wang HW, Liu PY, Oyama N, et al. Deficiency of ROCK1 in bone marrow-derived cells protects against atherosclerosis in LDLR−/−mice. FASEB J. 2008. October;22(10):3561–3570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Matoba K, Kawanami D, Ishizawa S, et al. Rho-kinase mediates TNF-alpha-induced MCP-1 expression via p38 MAPK signaling pathway in mesangial cells. Biochem Biophys Res Commun. 2010. November 26;402(4):725–730. [DOI] [PubMed] [Google Scholar]
- • 73.Zandi S, Nakao S, Chun KH, et al. ROCK-isoform-specific polarization of macrophages associated with age-related macular degeneration. Cell Rep. 2015. February 24;10(7):1173–1186.Recently published paper showing that ROCK signaling is playing critical role in macrophage polarization in aged-related macular degeneration.
- 74.Barakat R, Redzic Z. The role of activated microglia and resident macrophages in the neurovascular unit during cerebral ischemia: is the jury still out? Med Princ Pract. 2015. August 19;25:3–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Isgro J, Gupta S, Jacek E, et al. Enhanced rho-associated protein kinase activation in patients with systemic lupus erythematosus. Arthritis Rheum. 2013. June;65(6):1592–1602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Zanin-Zhorov A, Weiss JM, Nyuydzefe MS, et al. Selective oral ROCK2 inhibitor down-regulates IL-21 and IL-17 secretion in human T cells via STAT3-dependent mechanism. Proc Natl Acad Sci U S A. 2014. November 25;111(47):16814–16819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Lally L, Pernis A, Narula N, et al. Increased rho kinase activity in temporal artery biopsies from patients with giant cell arteritis. Rheumatology (Oxford, England). 2015. March;54(3):554–558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Stamatovic SM, Keep RF, Kunkel SL, et al. Potential role of MCP-1 in endothelial cell tight junction ‘opening’: signaling via Rho and Rho kinase. J Cell Sci. 2003. November 15;116(Pt 22):4615–4628. [DOI] [PubMed] [Google Scholar]
- 79.He QW, Xia YP, Chen SC, et al. Astrocyte-derived sonic hedgehog contributes to angiogenesis in brain microvascular endothelial cells via RhoA/ROCK pathway after oxygen-glucose deprivation. Mol Neurobiol. 2013. June;47(3):976–987. [DOI] [PubMed] [Google Scholar]
- 80.Tonges L, Koch JC, Bahr M, et al. ROCKing regeneration: rho kinase inhibition as molecular target for neurorestoration. Front Mol Neurosci. 2011;4:39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Tonges L, Frank T, Tatenhorst L, et al. Inhibition of rho kinase enhances survival of dopaminergic neurons and attenuates axonal loss in a mouse model of Parkinson’s disease. Brain. 2012. November;135 (Pt 11):3355–3370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Yamashita K, Kotani Y, Nakajima Y, et al. Fasudil, a Rho kinase (ROCK) inhibitor, protects against ischemic neuronal damage in vitro and in vivo by acting directly on neurons. Brain Res. 2007. June;18(1154):215–224. [DOI] [PubMed] [Google Scholar]
- 83.Ding J, Yu JZ, Li QY, et al. Rho kinase inhibitor Fasudil induces neuroprotection and neurogenesis partially through astrocyte-derived G-CSF. Brain Behav Immun. 2009. November;23(8):1083–1088. [DOI] [PubMed] [Google Scholar]
- 84.Shimokawa H, Takeshita A. Rho-kinase is an important therapeutic target in cardiovascular medicine. Arterioscler Thromb Vasc Biol. 2005. September;25(9):1767–1775. [DOI] [PubMed] [Google Scholar]
- 85.Lohn M, Plettenburg O, Ivashchenko Y, et al. Pharmacological characterization of SAR407899, a novel rho-kinase inhibitor. Hypertension. 2009. September;54(3):676–683. [DOI] [PubMed] [Google Scholar]
- 86.Williams RD, Novack GD, van Haarlem T, et al. Ocular hypotensive effect of the Rho kinase inhibitor AR-12286 in patients with glaucoma and ocular hypertension. Am J Ophthalmol. 2011. November;152(5):834–841.e1. [DOI] [PubMed] [Google Scholar]
- 87.Chen J, Runyan SA, Robinson MR. Novel ocular antihypertensive compounds in clinical trials. Clin Ophthalmol. 2011;5:667–677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.O’Brien EC, Greiner MA, Xian Y, et al. Clinical effectiveness of statin therapy after ischemic stroke: primary results from the statin therapeutic area of the patient-centered research into outcomes stroke patients prefer and effectiveness research (PROSPER) study. Circulation. 2015. October 13;132(15):1404–1413. [DOI] [PubMed] [Google Scholar]
- 89.Oesterle A, Laufs U, Liao JK. Pleiotropic effects of statins on the cardiovascular system. Circ Res. 2017. January 06;120(1):229–243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Crouse JR 3rd, Byington RP, Furberg CD. HMG-CoA reductase inhibitor therapy and stroke risk reduction: an analysis of clinical trials data. Atherosclerosis. 1998. May;138(1):11–24. [DOI] [PubMed] [Google Scholar]
- 91.Pedersen TR, Olsson AG, Faergeman O, et al. Lipoprotein changes and reduction in the incidence of major coronary heart disease events in the Scandinavian Simvastatin Survival Study (4S). 1998 Atheroscler Suppl. 2004. October;5(3):99–106. [DOI] [PubMed] [Google Scholar]
- 92.Sacks FM, Pfeffer MA, Moye LA, et al. The effect of pravastatin on coronary events after myocardial infarction in patients with average cholesterol levels. Cholesterol and recurrent events trial investigators. N Engl J Med. 1996. October 3;335(14):1001–1009. [DOI] [PubMed] [Google Scholar]
- 93.The Long-Term Intervention with Pravastatin in Ischaemic Disease (LIPID) Study Group. Prevention of cardiovascular events and death with pravastatin in patients with coronary heart disease and a broad range of initial cholesterol levels. N Engl J Med. 1998. November 5;339(19):1349–1357. [DOI] [PubMed] [Google Scholar]
- 94.West of Scotland Coronary Prevention Study Group. Influence of pravastatin and plasma lipids on clinical events in the West of Scotland Coronary Prevention Study (WOSCOPS). Circulation. 1998. April 21;97(15):1440–1445. [DOI] [PubMed] [Google Scholar]
- 95.Sever PS, Dahlof B, Poulter NR, et al. Prevention of coronary and stroke events with atorvastatin in hypertensive patients who have average or lower-than-average cholesterol concentrations, in the Anglo-Scandinavian Cardiac Outcomes Trial–Lipid Lowering Arm (ASCOT-LLA): a multicentre randomised controlled trial. Drugs. 2004;64(Suppl 2):43–60. [DOI] [PubMed] [Google Scholar]
- 96.Amarenco P, Lavallee P, Touboul PJ. Stroke prevention, blood cholesterol, and statins. Lancet Neurol. 2004. May;3(5):271–278. [DOI] [PubMed] [Google Scholar]
- 97.Law MR, Wald NJ, Rudnicka AR. Quantifying effect of statins on low density lipoprotein cholesterol, ischaemic heart disease, and stroke: systematic review and meta-analysis. BMJ (Clinical Research Ed). 2003. June 28;326(7404):1423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Russell RG. Bisphosphonates: the first 40 years. Bone. 2011. July;49(1):2–19. [DOI] [PubMed] [Google Scholar]
- 99.Hata T, Soga J, Hidaka T, et al. Calcium channel blocker and Rho-associated kinase activity in patients with hypertension. J Hypertens. 2011. February;29(2):373–379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Zee RYL, Wang QM, Chasman DI, et al. Gene variations of ROCKs and risk of ischaemic stroke: the Women’s Genome Health Study. Clin Sci. 2014. June;126(12):829–835. [DOI] [PMC free article] [PubMed] [Google Scholar]