Abstract
Medium-chain fatty acids (MCFAs) are mostly generated from dietary triglycerides and can penetrate the blood-brain barrier. Astrocytes in the brain use MCFAs as an alternative energy source. In addition, MCFAs have various regulatory and signaling functions in astrocytes. However, it is unclear how astrocytes sense and take up MCFAs. This study demonstrates that decanoic acid (DA; C10), a saturated MCFA and a ligand of Gαs protein-coupled receptors (Gαs-GPCRs), is a signaling molecule in energy metabolism in primary astrocytes. cAMP synthesis and lactate release were increased via a putative Gαs-GPCR and transmembrane adenylyl cyclase upon short-term treatment with DA. By contrast, monoamine oxidase B-dependent gamma-aminobutyric acid (GABA) synthesis was increased in primary cortical and hypothalamic astrocytes upon long-term treatment with DA. Thus, astrocytes respond to DA by synthesizing cAMP and releasing lactate upon short-term treatment, and by synthesizing and releasing GABA upon long-term treatment, similar to reactive astrocytes. Our data suggest that astrocytes in the brain play crucial roles in lipid-sensing via GPCRs and modulate neuronal metabolism or activity by releasing lactate via astrocyte-neuron lactate shuttle or GABA to influence neighboring neurons.
Keywords: astrocytes, medium-chain fatty acids, cAMP, lactate, decanoic acid, gamma-aminobutyric acid
Graphical Abstract
INTRODUCTION
The major cause of metabolic disorders such as obesity is increased consumption of a high-calorie diet. Chronic intake of a high-fat diet (HFD) increases not only the level of fat in the peripheral system, but also uptake of fatty acids (FAs) into the brain from the plasma [1,2]. Enrichment of saturated FAs causes lipid accumulation in the brain, especially the hypothalamus [3,4,5]. Moreover, free FAs accumulate in astrocytes and neurons in the brain [6,7]. However, it is unclear how FAs accumulate in astrocytes and how free FAs affect the control of energy balance and feeding.
Treatment with long-chain FAs (C13~C21) increases inflammatory responses in cultured astrocytes, despite the fact that these molecules cannot cross the blood-brain barrier (BBB) [8,9,10]. On the other hand, medium-chain FAs (MCFAs; C6~C12), such as octanoic acid (OA; C8) and decanoic acid (DA; C10), can cross the BBB and are rapidly metabolized in the brain [11,12]. These molecules modulate energy metabolism in astrocytes [13]. Along with their role in supplying an energy source, FAs regulate various intracellular signaling pathways and modulate mitochondrial energy production [13]. In particular, FAs are ligands for many G protein-coupled receptors (GPCRs) and members of the free FA receptor (FFAR) family. This family consists of FFAR1/GPR40, FFAR2/GPR43, FFAR3/GPR41, and FFAR4/GPR120, which recognize free FAs [14]. FFAR2 and FFAR3 recognize small-chain FAs (SCFAs; C1~C6). Odorant receptors (ORs), which comprise the largest subfamily of GPCRs, also recognize SCFAs and free FAs [15,16,17]. Among MCFAs, DA increases glycolysis and lactate synthesis in astrocytes [11,18]. MCFAs may have beneficial effects in the brain by activating metabolic shuttle systems that provide an energy source to neighboring neurons in the form of lactate and ketone bodies [18]. Stimulation with MCFAs increases glycolytic activity and consequently induces release of lactate by astrocytes into the extracellular space [19,20,21]. However, little is known about the molecular mechanism by which astrocytes sense free FAs and increase lactate release, and by which GPCRs recognize free FAs in astrocytes.
There are more astrocytes than neurons in most regions of the human brain [22,23]. Astrocytes play a vital role in metabolism by oxidizing FAs to produce ketone bodies, which are an energy source for neurons [24]. In addition, astrocytes regulate energy homeostasis upon short-term exposure to a HFD by metabolizing lipids under physiological conditions [25,26]. However, the roles of astrocytes are altered upon chronic exposure to a HFD [27,28,29]. Hypertrophic and hyperplasic astrocytes, which are conventionally called reactive astrocytes, are observed in the rodent hypothalamus after exposure to a HFD for 24 h or longer [29]. Recent studies suggest that hypertrophic and hyperplasic astrocytes can be divided into two categories, namely, active astrocytes, which express proBDNF, and reactive astrocytes, which express gamma-aminobutyric acid (GABA) [30]. Moreover, proBDNF and GABA have a reciprocal relationship, meaning that aberrant release of GABA might suppress proBDNF expression [30,31]. Astrocytic GABA is synthesized by monoamine oxidase B (MAO-B), which is mainly expressed in astrocytes [32]. MAO-B mediates degradation of the polyamine putrescine, which is a byproduct of toxin degradation, to generate GABA [32,33]. However, it is uncertain whether long-term exposure to MCFAs leads to generation of active or reactive astrocytes.
This study investigated how astrocytes sense free FAs and determined whether astrocytes are active or reactive after short-term and long-term treatment with DA. We treated primary astrocytes with DA and examined whether they sense this MCFA via a GPCR. In addition, we investigated whether long-term exposure to DA increases GABA synthesis via MAO-B, as occurs in reactive astrocytes.
MATERIALS AND METHODS
Culture of primary cortical astrocytes for the cAMP assay
Primary astrocytes were obtained from the cerebral cortex of a E18.5 mouse embryo and cultured as previously described [34], with minor modifications. A total of 1.2×107 mixed glial cells were seeded in a poly-D-lysine-coated T75 flask. After 2 h, the plating medium was replaced by mixed glial cell growth medium, Dulbecco's modified Eagle's medium (DMEM; Thermo Fisher Scientific, MA) supplemented with 10% fetal bovine serum (FBS; Thermo Fisher Scientific, MA) and 1% penicillin/streptomycin). The medium was changed every 3 days for 2 weeks. Thereafter, the mixed glial culture medium, which contained floating primary microglia, was discarded and the T75 flask was shaken at 160 rpm for 1 day in a shaking incubator to completely remove primary microglia. Following treatment with 0.25% trypsin, astrocytes were collected, replated in a T75 flask, and cultured for 4 days to increase their purity. Finally, samples were treated with 0.25% trypsin, and then shaken at 160 rpm for 7 h and lightly tapped to accelerate the isolation of astrocytes. A known number of astrocytes was mixed with fresh culture medium for subsequent experiments.
Lactate assay
Lactate release by primary astrocytes was measured using a Lactate Assay Kit (BioVision, Mountain View, CA), according to the manufacturer's instructions. This kit included lactate assay buffer, standard lactate, lactate substrate mix, and lactate enzyme mix. Primary astrocytes were seeded in a 6-well plate (1×106 cells/well) and maintained at 37℃ in a humidified incubator containing 5% CO2 for 2 days. Thereafter, cells were treated with vehicle, 1 mM glucose, various concentrations (10, 30, 100, 300, 600, 1,000, and 1,200 µM) of DA for 1 h, or 100 µM DA for various durations in lactate assay buffer. In some experiments, cells were pretreated with 1 mM SQ22536 and/or 10 µM gallein for 1 h. Lactate release was measured by incubating 50 µl of the supernatant, 2 µl lactate substrate mix, and 2 µl lactate enzyme mix in a 96-well enzyme-linked immunosorbent assay (ELISA) plate for 30 min. The lactate concentration was calculated for the known lactate standard in the kit. The optical density at 450 nm was measured using a VersaMax microplate reader (Molecular Devices, CA).
cAMP ELISA assay
The level of cAMP was quantified using a Direct cAMP ELISA kit with the non-acetylated cAMP standard format (Enzo Life Sciences, NY). A competitive cAMP ELISA was performed according to the manufacturer's protocol. In brief, primary astrocytes were seeded in a 48-well plate (5×104 cells/well) and maintained in a humidified incubator containing 5% CO2 at 37℃ for 2 days. Thereafter, the cells were treated with vehicle, 30 µM forskolin, or various concentrations (10, 30, 100, 300, 600, and 1,000 µM) of DA in Hank's balanced salt solution for 30 min. In some experiments, cells were pretreated with 1 mM SQ22536 for 1 h. cAMP was extracted by lysing the cell pellet in 200 µl of 0.1 M HCl containing 1% Triton X-100 for 10 min followed by centrifugation at 600×g for 2 min to remove cellular debris. To quantify cAMP, 100 µl of the supernatant was analyzed using a Direct cAMP ELISA kit according to the manufacturer's protocol. All incubation steps were performed at room temperature. The optical density at 405 nm was measured using a VersaMax microplate reader.
Culture of primary cortical and hypothalamic astrocytes for immunocytochemistry
Primary astrocytes were obtained from C57BL/6J mouse pups at P0~P2. The cerebral cortex was dissected and the meninges were removed. The entire hypothalamus was dissected, minced, and dissociated into a single-cell suspension via gentle trituration through a Pasteur pipette. Dissociated cells were seeded in a 35 mm plate coated with 0.1 mg/ml poly-D-lysine. Cells were grown in DMEM supplemented with 25 mM glucose, 2 mM glutamine, 10% heat-inactivated horse serum, 10% heat-inactivated FBS, and 1% penicillin-streptomycin. On the third day of culture, cells were washed by repeated pipetting and the medium was replaced to remove debris and other floating cell types. For DA treatment, cells were transferred to a 24-well plate containing coverslips coated with 0.1 mg/ml poly-D-lysine (Sigma-Aldrich, MO) after 11 days in vitro (DIV). The next day, the medium was replaced by DMEM containing 25 mM glucose, 2 mM glutamine, 5% heat-inactivated horse serum, 5% heat-inactivated FBS, 1% penicillin-streptomycin, and 10, 30, or 100 µM DA. Cultured cells were maintained at 37℃ in a humidified incubator containing 5% CO2.
Immunocytochemistry
Primary astrocytes cultured in a 24-well plate were fixed in 4% paraformaldehyde and 0.5% glutaraldehyde (Sigma, G5882-50 ML) for 15 min at room temperature after 13 DIV, and washed with 0.1 M phosphate-buffered saline (PBS) for 5 min. Samples were incubated with 0.1 M PBS containing 0.3% Triton X-100 (Sigma) and 10% donkey serum (Genetex, CA) for 1.5 h to prevent non-specific binding. Cells were incubated with a guinea-pig anti-GABA antibody (ab175; 1:1000; Millipore, MA) and a chicken anti-glial fibrillary acidic protein (GFAP) antibody (ab5541; 1:1000; Millipore) overnight at 4℃. After washing with 0.1 M PBS, cells were incubated with a DyLight 488- or 594-conjugated secondary antibody (1:500; Jackson Laboratory, ME) for 2 h at room temperature. After three rinses with 0.1 M PBS and staining with DAPI (1:3000; Thermo Scientific, MA), coverslips were mounted on Polysine microscopic glass slides (Thermo Scientific, MA). Images were acquired using a Nikon A1R confocal microscope.
Image quantification
Confocal microscopic images were analyzed using ImageJ (NIH). To analyze the fluorescence intensity of GABA labeling in each astrocyte, images were converted to a binary format and each GFAP-positive astrocyte was identified as a region of interest. The mean fluorescence intensity of GABA labeling was measured in each region of interest. Thus, the fluorescence intensity of GABA labeling was only measured in GFAP-positive astrocytes.
RESULTS
DA increases cAMP synthesis via a Gαs-GPCR in primary cortical astrocytes
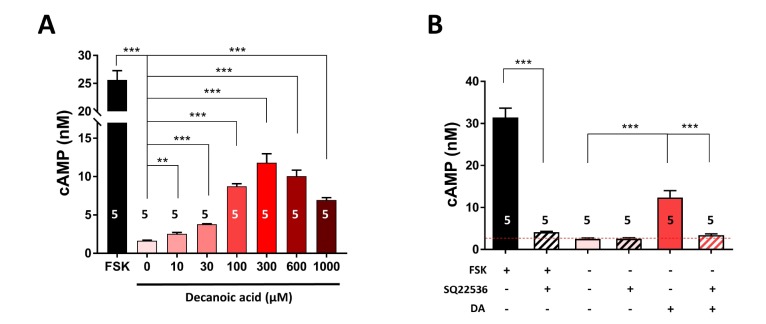
DA is a saturated MCFA that directly stimulates lactate synthesis in astrocytes by modulating their metabolism and supplies an energy source to neighboring neurons via activation of the astrocyteneuron shuttle [12,18]. Bicarbonate-responsive lactate coupling between astrocytes and neurons is reportedly due to enhanced intracellular cAMP formation via activation of soluble adenylyl cyclase (sAC) [35]. We therefore investigated whether cAMP synthesis mediates DA-induced lactate production in astrocytes. To this end, we treated primary cortical astrocytes with different concentrations (0, 10, 30, 100, 300, 600, and 1,000 µM) of DA for 30 min and then measured the intracellular level of cAMP. DA treatment significantly (p<0.001) increased the intracellular cAMP concentration in a dose-dependent manner (Fig. 1A). Similarly, the intracellular cAMP concentration was increased by treatment with forskolin, which activates transmembrane adenylyl cyclase (tmAC) but not sAC (Fig. 1). DA showed agonistic activity for cAMP production and had an EC50 of 82.5 µM. Mammalian sAC is insensitive to G proteins, forskolin, which is a tmAC-specific activator, and SQ22536, which is a tmAC-specific inhibitor [36,37]. By contrast, tmAC is sensitive to Gs-protein of GPCRs, forskolin, and SQ22536. Thus, we examined whether DA-stimulated intracellular cAMP formation is dependent on tmAC. The intracellular cAMP concentration was significantly (p<0.001) higher in forskolin- and DA-treated astrocytes than in untreated astrocytes (Fig. 1B). SQ22536 significantly (p<0.001) attenuated the DA- and forskolin-induced increases in the intracellular cAMP concentration by 90.88% and 94.42%, respectively (Fig. 1B). Collectively, these data suggest that DA induces synthesis of cAMP via coupling of a Gαs-GPCR with tmAC, but not with sAC. These results are consistent with the brain RNA sequencing transcriptome [38,39], which showed that nine tmACs (ADCY1~9) are uniformly expressed in astrocytes, while only one sAC (ADCY10) is extremely lowly expressed. Interestingly, mRNA expression of ADCY2 is highest in mouse and mature human astrocytes.
Fig. 1. DA increases cAMP synthesis via a Gαs-GPCR. (A) The intracellular cAMP concentration was measured in primary astrocytes treated with different concentrations (0, 10, 30, 100, 300, 600, and 1,000 µM) of DA for 30 min. Alternatively, primary astrocytes were treated with 30 µM forskolin (FSK), a tmAC-specific activator, as a positive control. (B) The intracellular cAMP concentration was measured in primary astrocytes treated with 100 µM DA with or without pretreatment with 1 mM SQ22536 for 1 h, which is a tmAC-specific inhibitor. The red dotted line indicates the basal level of lactate release in untreated cells. All data are shown as mean±SEM (n=5) and were analyzed using the Student's t test. *p<0.05, **p<0.01, ***p<0.001.
DA increases lactate release via a Gαs-GPCR in primary cortical astrocytes
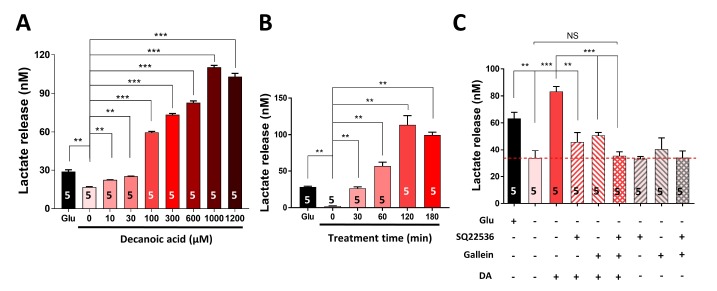
Next, we investigated whether DA stimulates the production and release of lactate via tmAC-mediated cAMP synthesis. To this end, we treated primary cortical astrocytes with different concentrations (0, 10, 30, 100, 300, 600, 1,000, and 1,200 µM) of DA for 1 h and with 100 µM DA for different durations (0, 30, 60, 120, and 180 min). DA treatment significantly (p<0.01) increased lactate release in a dose-dependent (Fig. 2A) and time-dependent (Fig. 2B) manner. The EC50 of DA was 209.2 µM. To determine whether a Gαs-GPCR mediates DA-induced lactate release, we pretreated primary cortical astrocytes with SQ22536, a Gαs-mediated tmAC-specific inhibitor, and/or gallein, a Gβγ signaling inhibitor, and then treated them with DA (Fig. 2C). Pretreatment with SQ22536 and gallein significantly (p<0.001) attenuated the DA-induced increase in lactate release by 75.53% and 65.71%, respectively (Fig. 2C). In addition, pretreatment with both SQ22536 and gallein almost completely (p<0.001) abolished the DA-induced lactate release (96.68%). Taken together, these results suggest that DA increases lactate release via a Gαs-coupled GPCR.
Fig. 2. DA increases lactate release via a Gαs-GPCR. (A, B) Lactate release was measured in primary astrocytes treated with different concentrations (0, 10, 30, 100, 300, 600, 1,000, and 1,200 µM) of DA for 1 h (A) or with 100 µM DA for different durations (0, 30, 60, 120, and 180 min) (B). Glucose (Glu) was used as a positive control. (C) Lactate release was measured in primary astrocytes pretreated with or without SQ22536 (a tmAC inhibitor) and/or gallein (a Gβγ signaling inhibitor) for 1 h and then treated with 100 µM DA. The red dotted line indicates the basal level of lactate release in untreated cells. All data are shown as mean±SEM (n=5) and were analyzed using the Student's t test. *p<0.05, **p<0.01, ***p<0.001, NS, not significant.
DA increases GABA production in primary cortical astrocytes
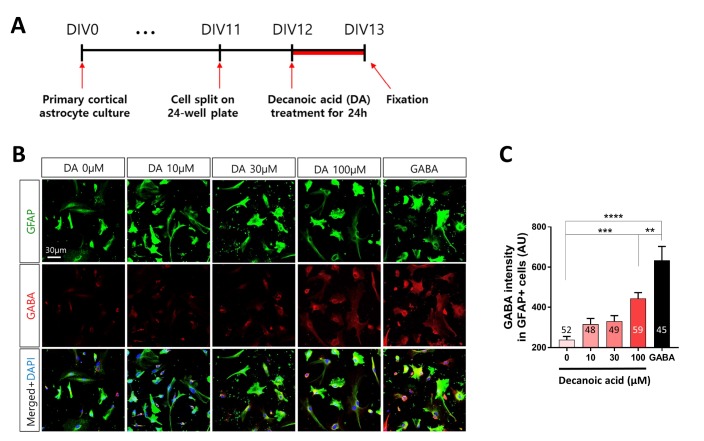
Resting astrocytes transform into reactive astrocytes upon adverse stimulations, such as toxin challenges, which is usually accompanied by neuroinflammation [30]. Continuous and excessive consumption of dietary fats activates the inflammatory response in the hypothalamus, leading to generation of reactive astrocytes [27,29]. Therefore, we tested whether long-term treatment with DA leads to the generation of reactive astrocytes by assessing the level of GABA, which is a marker of reactive astrocytes [29,30], and by co-staining with GFAP, which is a marker of astrocytes. Primary cortical astrocytes were split after 11 DIV and incubated with DA after 12 DIV for 24 h (Fig. 3A). DA treatment increased the GABA level in a dose-dependent manner (Fig. 3B). The GABA level was significantly (p<0.001) increased in cells treated with 100 µM DA (Fig. 3C). Together, these findings indicate that long-term treatment with DA increases the level of GABA in cortical astrocytes in a dose-dependent manner.
Fig. 3. DA increases GABA production in primary cortical astrocytes. (A) Timeline of treatment of primary cortical astrocytes with different concentrations of DA for 24 h followed by immunocytochemistry. (B) Representative fluorescence immunocytochemical images of cells treated with different concentrations (0, 10, 30, and 100 µM) of DA. Cells were treated with 100 µM GABA as a positive control. (C) Fluorescence intensity of GABA labeling in GFAP-positive primary cortical astrocytes. All data are shown as mean±SEM (0 µM, n=52 cells; 10 µM, n=48 cells; 30 µM, n=49 cells; 100 µM, n=59 cells; and GABA, n=45 cells) and were analyzed using Tukey's multiple comparison test. *p<0.05, **p<0.01, ***p<0.001, ****p<0.0001.
DA increases GABA production via MAO-B in primary cortical astrocytes
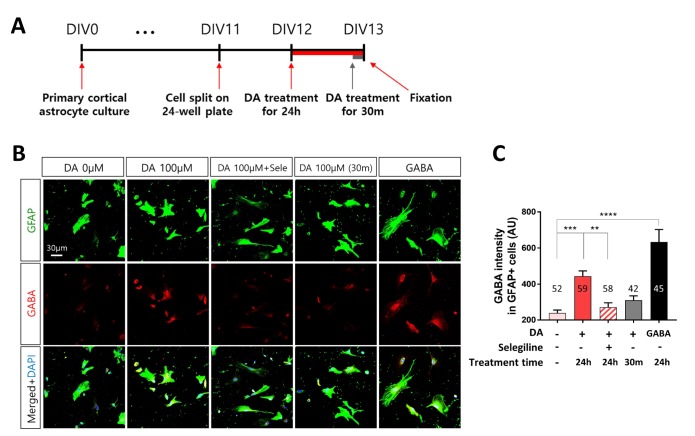
MAO-B generated from putrescine mediates GABA production in reactive astrocytes under pathological conditions [40]. We investigated whether MAO-B mediates DA-induced GABA production. To this end, we treated primary cortical astrocytes with DA and selegiline, which is a selective MAO-B inhibitor (Fig. 4A). Long-term treatment with DA for 24 h increased GABA production in cortical astrocytes, and this effect was significantly (p<0.01) attenuated by selegiline. However, short-term treatment with DA for 30 min did not significantly increase GABA production in cortical astrocytes (Fig. 4B, C). Taken together, long-term treatment with DA leads to generation of reactive astrocytes that produce GABA in a MAO-B-dependent manner, while short-term treatment with DA does not.
Fig. 4. DA increases GABA production via MAO-B in primary cortical astrocytes. (A) Timeline of treatment of primary cortical astrocytes with DA for 24 h or 30 min followed by immunocytochemistry. (B) Representative fluorescence immunocytochemical images of cells treated with 100 µM DA for different durations. Cells were treated with 100 µM GABA as a positive control. (C) Fluorescence intensity of GABA labeling in GFAP-positive primary cortical astrocytes. All data are shown as mean±SEM (0 µM, n=52 cells; 100 µM for 24 h, n=59 cells; 100 µM for 24 h with selegiline, n=58 cells; 100 µM for 30 min, n=42 cells; and GABA, n=45 cells) and were analyzed using Tukey's multiple comparison test. *p<0.05, **p<0.01, ***p<0.001, ****p<0.0001.
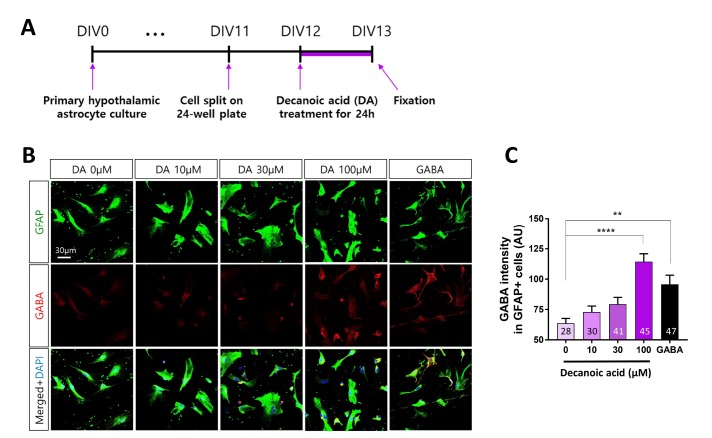
DA increases GABA production in primary hypothalamic astrocytes
The hypothalamus is the primary center of metabolic control [41] and contains hypertrophic and hyperplasic astrocytes after long-term feeding of a HFD [29,42,43]. Moreover, the BBB is highly fenestrated in the hypothalamus, which enables astrocytes to sense and rapidly respond to circulating nutrients such as free FAs derived from dietary lipids and hormones [4]. Therefore, we investigated whether long-term treatment with DA increases the GABA level in hypothalamic astrocytes, as observed in cortical astrocytes. Similar to cortical astrocytes, hypothalamic astrocytes were split after 11 DIV and incubated with DA after 12 DIV for 24 h (Fig. 5A). DA treatment increased the GABA level in a dosedependent manner (Fig. 5B). The GABA level was significantly (p<0.0001) increased by treatment with 100 µM DA for 24 h (Fig. 5C). Together, these findings indicate that long-term treatment with DA increases the GABA level in hypothalamic astrocytes in a dose-dependent manner.
Fig. 5. DA increases GABA production in primary hypothalamic astrocytes. (A) Timeline of treatment of primary hypothalamic astrocytes with different concentrations of DA for 24 h followed by immunocytochemistry. (B) Representative fluorescence immunocytochemical images of cells treated with different concentrations (0, 10, 30, and 100 µM) of DA. Cells were treated with 100 µM GABA as a positive control. (C) Fluorescence intensity of GABA labeling in GFAP-positive primary hypothalamic astrocytes. All data are shown as mean±SEM (0 µM, n=28 cells; 10 µM, n=30 cells; 30 µM, n=41 cells; 100 µM, n=45 cells; and GABA, n=47 cells) and were analyzed using Tukey's multiple comparison test. *p<0.05, **p<0.01, ***p<0.001, ****p<0.0001.
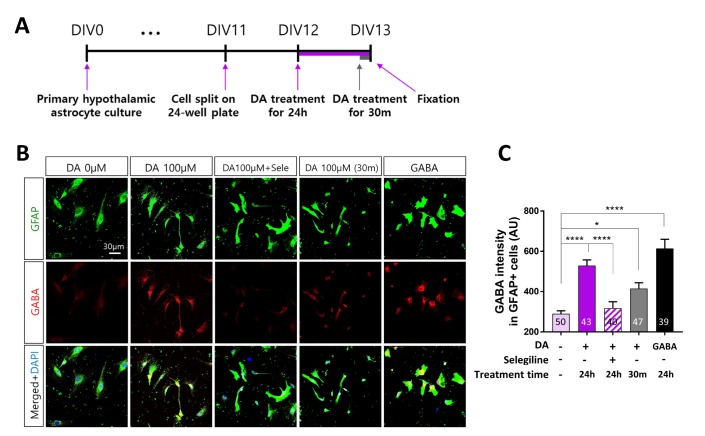
DA increases GABA production via MAO-B in primary hypothalamic astrocytes
We investigated whether MAO-B mediates DA-induced GABA production in hypothalamic astrocytes, as observed in cortical astrocytes (Fig. 6A). Selegiline significantly (p<0.0001) attenuated the increase in GABA production induced by treatment with DA for 24 h in hypothalamic astrocytes (Fig. 6B, C). In contrast with cortical astrocytes, treatment with DA for 30 min significantly (p<0.05) increased the GABA level in hypothalamic astrocytes (Fig. 6B, C). These results suggest that the effects of DA on MAO-B-dependent GABA production are more acute in hypothalamic astrocytes than in cortical astrocytes.
Fig. 6. DA increases GABA production via MAO-B in primary hypothalamic astrocytes. (A) Timeline of treatment of primary hypothalamic astrocytes with DA for 24 h or 30 min followed by immunocytochemistry. (B) Representative fluorescence immunocytochemical images of cells treated with 100 µM DA with different durations. Cells were treated with 100 µM GABA as a positive control. (C) Fluorescence intensity of GABA labeling in GFAP-positive primary hypothalamic astrocytes. All data are shown as mean±SEM (0 µM, n=50 cells; 100 µM for 24 h, n=43 cells; 100 µM for 24 h with selegiline, n=40 cells; 100 µM for 30 min, n=47 cells; and GABA, n=39 cells) and were analyzed using Tukey's multiple comparison test. *p<0.05, **p<0.01, ***p<0.001, ****p<0.0001.
DISCUSSION
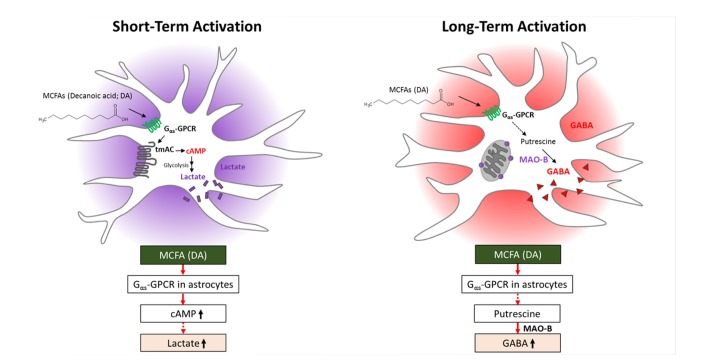
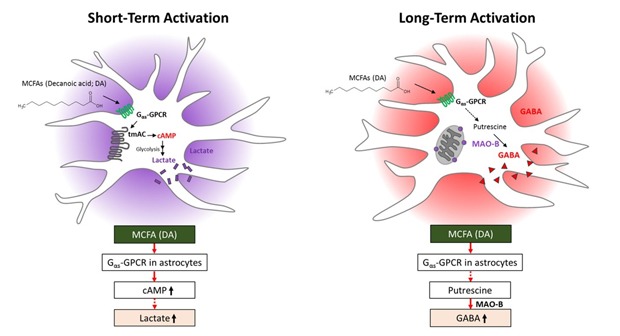
This study demonstrates for the first time that short-term treatment with DA (C10), a saturated MCFA, increases cAMP formation and lactate release in primary astrocytes via a signaling pathway coupled to a Gαs-GPCR and tmAC (Fig. 7). Astrocytes may play a critical role by supplying lactate as an energy source to neighboring neurons. By contrast, long-term treatment with DA induces synthesis of GABA via MAO-B by changing the functional characteristics of astrocytes (Fig. 7). Accumulation of MAO-B-dependent GABA in reactive astrocytes is expected to inhibit neurons. Our study reveals a novel molecular mechanism by which astrocytes recognize free MCFAs and reports the effects of short- and long-term treatment with DA. This unique MCFA-GPCR interaction in astrocytes may provide novel insights into the functional basis for control of the energy balance and feeding in the brain.
Fig. 7. Effects of short- and long-term treatment with DA on astrocytes.
MCFAs are an important source of ketogenesis to form ketone bodies, which travel to the brain and are used as an alternative energy source by neurons when the blood glucose level is severely reduced during fasting [44]. Whereas hepatic ketogenesis has been well-elucidated, ketogenesis via oxidation of free FAs also occurs in astrocytes in the brain [45]. Although MCFAs are substrates for ketogenesis in astrocytes [45], their cellular signaling functions are mostly unknown. This study reveals that DA has a novel functional role as a signaling molecule by interacting with a GPCR (Figs. 1, 2). While long-term treatment with DA induced synthesis of GABA via MAO-B, short-term treatment with DA induced lactate release via a Gαs-GPCR-tmAC-cAMP pathway. This MCFA-GPCR interaction provides novel insights into the functional basis for how astrocytes sense free FAs in the brain. FAs are ligands for many GPCRs. For example, members of the FFAR family recognize free FAs. This family consists of FFAR1/GPR40 and FFAR4/GPR120, which are activated by medium- and long-chain free FAs, respectively, and FFAR3/GPR41 and FFAR2/GPR43, which are activated by short-chain free FAs [14]. In addition, a recent study revealed that ORs recognize SCFAs and MCFAs in many non-olfactory tissues [17]. Representative examples are Olfr78 [15], Olfr544 [46], and Olfr15 [47,48], which recognize SCFAs, dicarboxylic MCFAs, and MCFAs, respectively. A further study is required to identify the GPCR that recognizes free MCFAs in astrocytes.
The physiological concentration of MCFAs in the brain and blood required to stimulate astrocytes is an important parameter. MCFAs move rapidly and readily from blood to the brain via the BBB, especially in the hypothalamic region where the BBB is highly fenestrated [11]. MCFAs, particularly OA and DA, elicit beneficial effects on children who suffer from seizures, and consequently such children have been traditionally treated with a medium-chain triglyceride (MCT) diet [49]. OA and DA are detected in plasma at various time points over 24 h when children are fed a MCT diet. In addition, OA and DA are detected at concentrations of 0.104~2.1 mM and 12~250 µM, respectively, in plasma of mice fed a MCT diet for 4 days [12]. Consistently, the EC50 of DA was 82.5 and 209.2 µM for cAMP production and lactate release, respectively (Figs. 1, 2). Taken together, these data suggest that treatment with ~100~200 µM DA is sufficient to stimulate astrocytes in vivo.
The accumulation of cytosolic GABA suggests that long-term treatment with DA triggers an increase in putrescine, which is the major precursor of GABA. How does DA trigger an increase in putrescine? A clue might lie in the findings that DA activated a Gαs-GPCR and increased cAMP synthesis in astrocytes. cAMP has been suggested to induce autophagy via beclin 1, which is a mammalian ortholog of yeast autophagy-related gene 6 [50]. Furthermore, treatment with the cell-permeable cAMP analog 8-CPT-cAMP increases the levels of LC3-II and beclin 1 in adipocytederived mesenchymal stem cells [50]. Therefore, an increase in cAMP upon DA treatment may activate autophagy, which allows the orderly degradation and recycling of cellular components in astrocytes [51]. The endocytosed DA-Gαs-GPCR complex might be degraded to increase the levels of L-arginine and L-ornithine, which are a source of putrescine [52]. Putrescine may then be further degraded via MAO-B to produce GABA, a molecular marker of reactive astrocytes [30,32]. These possibilities require further investigations.
What is the relevance of MAO-B-dependent GABA production? MAO-B mediates astrocytic GABA production [32]. We previously demonstrated that astrocytic GABA is released tonically by reactive astrocytes in the hippocampus and is responsible for memory impairment in a mouse model of Alzheimer's disease [33]. Excessive production of GABA upon long-term exposure to DA may inhibit neighboring neurons, especially in the hypothalamus, where the neural circuits responsible for food intake and energy expenditure exist. The roles of astrocytic GABA produced upon long-term exposure to MCFAs in hypothalamic functions, especially food intake and energy expenditure, should be investigated in the future. MAO-B also mediates H2O2 production [32,53]. Reactive astrocytes produce large amounts of reactive oxygen species (ROS), which are chemically reactive chemical species containing oxygen such as H2O2 [54], in a MAO-B-dependent manner [55,56]. ROS produced by oxidation of monoamines, such as acetylputrescine [30], induce hypertrophy of astrocytic processes and increase cell proliferation [55,57,58]. Long-term exposure to DA may induce excessive ROS production in astrocytes. These exciting possibilities require future investigation.
Our findings suggest that astrocytes play crucial roles in lipidsensing in the brain and modulate metabolism in nearby neurons by releasing lactate and/or GABA. We propose that modulation of lactate and GABA production mediated by a GPCR and MAO-B is a new approach to control energy metabolism in astrocytes.
ACKNOWLEDGEMENTS
This research was supported by the Brain Research Program through the NRF funded by the Ministry of Science and ICT (2018M3C7A1056682) and by the DGIST R&D Program of the Ministry of Science, ICT, and Future Planning (18-BD-06, 18-BT-02).
References
- 1.Wang SW, Wang M, Grossman BM, Martin RJ. Effects of dietary fat on food intake and brain uptake and oxidation of fatty acids. Physiol Behav. 1994;56:517–522. doi: 10.1016/0031-9384(94)90295-x. [DOI] [PubMed] [Google Scholar]
- 2.Karmi A, Iozzo P, Viljanen A, Hirvonen J, Fielding BA, Virtanen K, Oikonen V, Kemppainen J, Viljanen T, Guiducci L, Haaparanta-Solin M, Någren K, Solin O, Nuutila P. Increased brain fatty acid uptake in metabolic syndrome. Diabetes. 2010;59:2171–2177. doi: 10.2337/db09-0138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Giles C, Takechi R, Mellett NA, Meikle PJ, Dhaliwal S, Mamo JC. The effects of long-term saturated fat enriched diets on the brain lipidome. PLoS One. 2016;11:e0166964. doi: 10.1371/journal.pone.0166964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Borg ML, Omran SF, Weir J, Meikle PJ, Watt MJ. Consumption of a high-fat diet, but not regular endurance exercise training, regulates hypothalamic lipid accumulation in mice. J Physiol. 2012;590:4377–4389. doi: 10.1113/jphysiol.2012.233288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Posey KA, Clegg DJ, Printz RL, Byun J, Morton GJ, Vivekanandan-Giri A, Pennathur S, Baskin DG, Heinecke JW, Woods SC, Schwartz MW, Niswender KD. Hypothalamic proinflammatory lipid accumulation, inflammation, and insulin resistance in rats fed a high-fat diet. Am J Physiol Endocrinol Metab. 2009;296:E1003–E1012. doi: 10.1152/ajpendo.90377.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bourre JM, Pascal G, Durand G, Masson M, Dumont O, Piciotti M. Alterations in the fatty acid composition of rat brain cells (neurons, astrocytes, and oligodendrocytes) and of subcellular fractions (myelin and synaptosomes) induced by a diet devoid of n-3 fatty acids. J Neurochem. 1984;43:342–348. doi: 10.1111/j.1471-4159.1984.tb00906.x. [DOI] [PubMed] [Google Scholar]
- 7.Bernoud N, Fenart L, Bénistant C, Pageaux JF, Dehouck MP, Molière P, Lagarde M, Cecchelli R, Lecerf J. Astrocytes are mainly responsible for the polyunsaturated fatty acid enrichment in blood-brain barrier endothelial cells in vitro. J Lipid Res. 1998;39:1816–1824. [PubMed] [Google Scholar]
- 8.Gupta S, Knight AG, Gupta S, Keller JN, Bruce-Keller AJ. Saturated long-chain fatty acids activate inflammatory signaling in astrocytes. J Neurochem. 2012;120:1060–1071. doi: 10.1111/j.1471-4159.2012.07660.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tsuji A. Small molecular drug transfer across the blood-brain barrier via carrier-mediated transport systems. NeuroRx. 2005;2:54–62. doi: 10.1602/neurorx.2.1.54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Vijay N, Morris ME. Role of monocarboxylate transporters in drug delivery to the brain. Curr Pharm Des. 2014;20:1487–1498. doi: 10.2174/13816128113199990462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ebert D, Haller RG, Walton ME. Energy contribution of octanoate to intact rat brain metabolism measured by 13C nuclear magnetic resonance spectroscopy. J Neurosci. 2003;23:5928–5935. doi: 10.1523/JNEUROSCI.23-13-05928.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dean HG, Bonser JC, Gent JP. HPLC analysis of brain and plasma for octanoic and decanoic acids. Clin Chem. 1989;35:1945–1948. [PubMed] [Google Scholar]
- 13.Schönfeld P, Wojtczak L. Short- and medium-chain fatty acids in energy metabolism: the cellular perspective. J Lipid Res. 2016;57:943–954. doi: 10.1194/jlr.R067629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hara T, Kashihara D, Ichimura A, Kimura I, Tsujimoto G, Hirasawa A. Role of free fatty acid receptors in the regulation of energy metabolism. Biochim Biophys Acta. 2014;1841:1292–1300. doi: 10.1016/j.bbalip.2014.06.002. [DOI] [PubMed] [Google Scholar]
- 15.Pluznick JL, Protzko RJ, Gevorgyan H, Peterlin Z, Sipos A, Han J, Brunet I, Wan LX, Rey F, Wang T, Firestein SJ, Yanagisawa M, Gordon JI, Eichmann A, Peti-Peterdi J, Caplan MJ. Olfactory receptor responding to gut microbiota-derived signals plays a role in renin secretion and blood pressure regulation. Proc Natl Acad Sci U S A. 2013;110:4410–4415. doi: 10.1073/pnas.1215927110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pluznick J. A novel SCFA receptor, the microbiota, and blood pressure regulation. Gut Microbes. 2014;5:202–207. doi: 10.4161/gmic.27492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kang N, Koo J. Olfactory receptors in non-chemosensory tissues. BMB Rep. 2012;45:612–622. doi: 10.5483/BMBRep.2012.45.11.232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Thevenet J, De Marchi U, Domingo JS, Christinat N, Bultot L, Lefebvre G, Sakamoto K, Descombes P, Masoodi M, Wiederkehr A. Medium-chain fatty acids inhibit mitochondrial metabolism in astrocytes promoting astrocyteneuron lactate and ketone body shuttle systems. FASEB J. 2016;30:1913–1926. doi: 10.1096/fj.201500182. [DOI] [PubMed] [Google Scholar]
- 19.Pellerin L, Magistretti PJ. Glutamate uptake into astrocytes stimulates aerobic glycolysis: a mechanism coupling neuronal activity to glucose utilization. Proc Natl Acad Sci U S A. 1994;91:10625–10629. doi: 10.1073/pnas.91.22.10625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Itoh Y, Esaki T, Shimoji K, Cook M, Law MJ, Kaufman E, Sokoloff L. Dichloroacetate effects on glucose and lactate oxidation by neurons and astroglia in vitro and on glucose utilization by brain in vivo. Proc Natl Acad Sci U S A. 2003;100:4879–4884. doi: 10.1073/pnas.0831078100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Herrero-Mendez A, Almeida A, Fernández E, Maestre C, Moncada S, Bolaños JP. The bioenergetic and antioxidant status of neurons is controlled by continuous degradation of a key glycolytic enzyme by APC/C-Cdh1. Nat Cell Biol. 2009;11:747–752. doi: 10.1038/ncb1881. [DOI] [PubMed] [Google Scholar]
- 22.Pakkenberg B, Gundersen HJ. Total number of neurons and glial cells in human brain nuclei estimated by the disector and the fractionator. J Microsc. 1988;150:1–20. doi: 10.1111/j.1365-2818.1988.tb04582.x. [DOI] [PubMed] [Google Scholar]
- 23.Sherwood CC, Stimpson CD, Raghanti MA, Wildman DE, Uddin M, Grossman LI, Goodman M, Redmond JC, Bonar CJ, Erwin JM, Hof PR. Evolution of increased glianeuron ratios in the human frontal cortex. Proc Natl Acad Sci U S A. 2006;103:13606–13611. doi: 10.1073/pnas.0605843103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Blázquez C, Sánchez C, Velasco G, Guzmán M. Role of carnitine palmitoyltransferase I in the control of ketogenesis in primary cultures of rat astrocytes. J Neurochem. 1998;71:1597–1606. doi: 10.1046/j.1471-4159.1998.71041597.x. [DOI] [PubMed] [Google Scholar]
- 25.Buckman LB, Thompson MM, Lippert RN, Blackwell TS, Yull FE, Ellacott KL. Evidence for a novel functional role of astrocytes in the acute homeostatic response to high-fat diet intake in mice. Mol Metab. 2014;4:58–63. doi: 10.1016/j.molmet.2014.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Argente-Arizón P, Freire-Regatillo A, Argente J, Chowen JA. Role of non-neuronal cells in body weight and appetite control. Front Endocrinol (Lausanne) 2015;6:42. doi: 10.3389/fendo.2015.00042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.García-Cáceres C, Fuente-Martín E, Argente J, Chowen JA. Emerging role of glial cells in the control of body weight. Mol Metab. 2012;1:37–46. doi: 10.1016/j.molmet.2012.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Nutritional value of medium chain triglycerides. Nutr Rev. 1968;26:352. [PubMed] [Google Scholar]
- 29.Thaler JP, Yi CX, Schur EA, Guyenet SJ, Hwang BH, Dietrich MO, Zhao X, Sarruf DA, Izgur V, Maravilla KR, Nguyen HT, Fischer JD, Matsen ME, Wisse BE, Morton GJ, Horvath TL, Baskin DG, Tschöp MH, Schwartz MW. Obesity is associated with hypothalamic injury in rodents and humans. J Clin Invest. 2012;122:153–162. doi: 10.1172/JCI59660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chun H, An H, Lim J, Woo J, Lee J, Ryu H, Lee CJ. Astrocytic proBDNF and tonic GABA distinguish active versus reactive astrocytes in hippocampus. Exp Neurobiol. 2018;27:155–170. doi: 10.5607/en.2018.27.3.155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Marmigère F, Rage F, Tapia-Arancibia L. GABA-glutamate interaction in the control of BDNF expression in hypothalamic neurons. Neurochem Int. 2003;42:353–358. doi: 10.1016/s0197-0186(02)00100-6. [DOI] [PubMed] [Google Scholar]
- 32.Yoon BE, Woo J, Chun YE, Chun H, Jo S, Bae JY, An H, Min JO, Oh SJ, Han KS, Kim HY, Kim T, Kim YS, Bae YC, Lee CJ. Glial GABA, synthesized by monoamine oxidase B, mediates tonic inhibition. J Physiol. 2014;592:4951–4968. doi: 10.1113/jphysiol.2014.278754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Jo S, Yarishkin O, Hwang YJ, Chun YE, Park M, Woo DH, Bae JY, Kim T, Lee J, Chun H, Park HJ, Lee DY, Hong J, Kim HY, Oh SJ, Park SJ, Lee H, Yoon BE, Kim Y, Jeong Y, Shim I, Bae YC, Cho J, Kowall NW, Ryu H, Hwang E, Kim D, Lee CJ. GABA from reactive astrocytes impairs memory in mouse models of Alzheimer’s disease. Nat Med. 2014;20:886–896. doi: 10.1038/nm.3639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tamashiro TT, Dalgard CL, Byrnes KR. Primary microglia isolation from mixed glial cell cultures of neonatal rat brain tissue. J Vis Exp. 2012:e3814. doi: 10.3791/3814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Choi HB, Gordon GR, Zhou N, Tai C, Rungta RL, Martinez J, Milner TA, Ryu JK, McLarnon JG, Tresguerres M, Levin LR, Buck J, MacVicar BA. Metabolic communication between astrocytes and neurons via bicarbonate-responsive soluble adenylyl cyclase. Neuron. 2012;75:1094–1104. doi: 10.1016/j.neuron.2012.08.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Buck J, Sinclair ML, Schapal L, Cann MJ, Levin LR. Cytosolic adenylyl cyclase defines a unique signaling molecule in mammals. Proc Natl Acad Sci U S A. 1999;96:79–84. doi: 10.1073/pnas.96.1.79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bitterman JL, Ramos-Espiritu L, Diaz A, Levin LR, Buck J. Pharmacological distinction between soluble and transmembrane adenylyl cyclases. J Pharmacol Exp Ther. 2013;347:589–598. doi: 10.1124/jpet.113.208496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zhang Y, Chen K, Sloan SA, Bennett ML, Scholze AR, O’Keeffe S, Phatnani HP, Guarnieri P, Caneda C, Ruderisch N, Deng S, Liddelow SA, Zhang C, Daneman R, Maniatis T, Barres BA, Wu JQ. An RNA-sequencing transcriptome and splicing database of glia, neurons, and vascular cells of the cerebral cortex. J Neurosci. 2014;34:11929–11947. doi: 10.1523/JNEUROSCI.1860-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zhang Y, Sloan SA, Clarke LE, Caneda C, Plaza CA, Blumenthal PD, Vogel H, Steinberg GK, Edwards MS, Li G, Duncan JA, 3rd, Cheshier SH, Shuer LM, Chang EF, Grant GA, Gephart MG, Barres BA. Purification and characterization of progenitor and mature human astrocytes reveals transcriptional and functional differences with mouse. Neuron. 2016;89:37–53. doi: 10.1016/j.neuron.2015.11.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Yoon BE, Woo J, Lee CJ. Astrocytes as GABA-ergic and GABA-ceptive cells. Neurochem Res. 2012;37:2474–2479. doi: 10.1007/s11064-012-0808-z. [DOI] [PubMed] [Google Scholar]
- 41.Balland E, Dam J, Langlet F, Caron E, Steculorum S, Messina A, Rasika S, Falluel-Morel A, Anouar Y, Dehouck B, Trinquet E, Jockers R, Bouret SG, Prévot V. Hypothalamic tanycytes are an ERK-gated conduit for leptin into the brain. Cell Metab. 2014;19:293–301. doi: 10.1016/j.cmet.2013.12.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Valdearcos M, Robblee MM, Benjamin DI, Nomura DK, Xu AW, Koliwad SK. Microglia dictate the impact of saturated fat consumption on hypothalamic inflammation and neuronal function. Cell Rep. 2014;9:2124–2138. doi: 10.1016/j.celrep.2014.11.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Buckman LB, Thompson MM, Moreno HN, Ellacott KL. Regional astrogliosis in the mouse hypothalamus in response to obesity. J Comp Neurol. 2013;521:1322–1333. doi: 10.1002/cne.23233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hawkins RA, Biebuyck JF. Ketone bodies are selectively used by individual brain regions. Science. 1979;205:325–327. doi: 10.1126/science.451608. [DOI] [PubMed] [Google Scholar]
- 45.Auestad N, Korsak RA, Morrow JW, Edmond J. Fatty acid oxidation and ketogenesis by astrocytes in primary culture. J Neurochem. 1991;56:1376–1386. doi: 10.1111/j.1471-4159.1991.tb11435.x. [DOI] [PubMed] [Google Scholar]
- 46.Kang N, Bahk YY, Lee N, Jae Y, Cho YH, Ku CR, Byun Y, Lee EJ, Kim MS, Koo J. Olfactory receptor Olfr544 responding to azelaic acid regulates glucagon secretion in α-cells of mouse pancreatic islets. Biochem Biophys Res Commun. 2015;460:616–621. doi: 10.1016/j.bbrc.2015.03.078. [DOI] [PubMed] [Google Scholar]
- 47.Munakata Y, Yamada T, Imai J, Takahashi K, Tsukita S, Shirai Y, Kodama S, Asai Y, Sugisawa T, Chiba Y, Kaneko K, Uno K, Sawada S, Hatakeyama H, Kanzaki M, Miyazaki JI, Oka Y, Katagiri H. Olfactory receptors are expressed in pancreatic β-cells and promote glucose-stimulated insulin secretion. Sci Rep. 2018;8:1499. doi: 10.1038/s41598-018-19765-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Leem J, Shim HM, Cho H, Park JH. Octanoic acid potentiates glucose-stimulated insulin secretion and expression of glucokinase through the olfactory receptor in pancreatic β-cells. Biochem Biophys Res Commun. 2018;503:278–284. doi: 10.1016/j.bbrc.2018.06.015. [DOI] [PubMed] [Google Scholar]
- 49.Bach AC, Babayan VK. Medium-chain triglycerides: an update. Am J Clin Nutr. 1982;36:950–962. doi: 10.1093/ajcn/36.5.950. [DOI] [PubMed] [Google Scholar]
- 50.Ugland H, Naderi S, Brech A, Collas P, Blomhoff HK. cAMP induces autophagy via a novel pathway involving ERK, cyclin E and Beclin 1. Autophagy. 2011;7:1199–1211. doi: 10.4161/auto.7.10.16649. [DOI] [PubMed] [Google Scholar]
- 51.Mizushima N, Komatsu M. Autophagy: renovation of cells and tissues. Cell. 2011;147:728–741. doi: 10.1016/j.cell.2011.10.026. [DOI] [PubMed] [Google Scholar]
- 52.Reitzer L, Schneider BL. Metabolic context and possible physiological themes of sigma(54)-dependent genes in Escherichia coli. Microbiol Mol Biol Rev. 2001;65:422–444. doi: 10.1128/MMBR.65.3.422-444.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Mallajosyula JK, Kaur D, Chinta SJ, Rajagopalan S, Rane A, Nicholls DG, Di Monte DA, Macarthur H, Andersen JK. MAO-B elevation in mouse brain astrocytes results in Parkinson’s pathology. PLoS One. 2008;3:e1616. doi: 10.1371/journal.pone.0001616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Hayyan M, Hashim MA, AlNashef IM. Superoxide ion: generation and chemical implications. Chem Rev. 2016;116:3029–3085. doi: 10.1021/acs.chemrev.5b00407. [DOI] [PubMed] [Google Scholar]
- 55.Borroni E, Bohrmann B, Grueninger F, Prinssen E, Nave S, Loetscher H, Chinta SJ, Rajagopalan S, Rane A, Siddiqui A, Ellenbroek B, Messer J, Pähler A, Andersen JK, Wyler R, Cesura AM. Sembragiline: a novel, selective monoamine oxidase type b inhibitor for the treatment of Alzheimer’s disease. J Pharmacol Exp Ther. 2017;362:413–423. doi: 10.1124/jpet.117.241653. [DOI] [PubMed] [Google Scholar]
- 56.Pizzinat N, Copin N, Vindis C, Parini A, Cambon C. Reactive oxygen species production by monoamine oxidases in intact cells. Naunyn Schmiedebergs Arch Pharmacol. 1999;359:428–431. doi: 10.1007/pl00005371. [DOI] [PubMed] [Google Scholar]
- 57.de Pablo Y, Nilsson M, Pekna M, Pekny M. Intermediate filaments are important for astrocyte response to oxidative stress induced by oxygen-glucose deprivation and reperfusion. Histochem Cell Biol. 2013;140:81–91. doi: 10.1007/s00418-013-1110-0. [DOI] [PubMed] [Google Scholar]
- 58.Sauer H, Wartenberg M, Hescheler J. Reactive oxygen species as intracellular messengers during cell growth and differentiation. Cell Physiol Biochem. 2001;11:173–186. doi: 10.1159/000047804. [DOI] [PubMed] [Google Scholar]