Abstract
Objective
To retrospectively identify risk factors and the prognosis for new-onset atrial fibrillation (AF) after implantation of dual-chamber pacemakers in elderly patients.
Methods
Consecutive patients aged ≥ 65 years who underwent their first implantation of a dual-chamber permanent pacemaker in Beijing Anzhen Hospital from October 2013 to May 2016 were enrolled. Their complete programming and follow-up data were recorded. Follow-up end points included new-onset AF and major adverse cardiovascular and cerebrovascular events.
Results
Altogether, 322 patients were enrolled, with new-onset AF observed in 79 (24.5%) during their follow-up. Multivariable analysis identified four independent predictors of new-onset AF in elderly patients after pacemaker implantation: hypertension (HR = 3.040, 95% CI: 1.09–3.05, P = 0.00); age (HR = 1.966, 95% CI: 1.57–3.68, P = 0.01); left atrial enlargement (HR = 1.645, 95% CI: 1.05–1.25, P = 0.03); high ventricular pacing rate (HR = 1.137, 95% CI: 1.01–1.06, P = 0.01). Univariable analysis indicated that the CHA2DS2-VASc score was also a risk factor for AF (HR = 1.368, 95% CI: 1.178–1.589, P = 0.002), whereas multivariable regression analysis did not. Kaplan–Meier survival analysis showed that the risk for ischemic stroke was significantly higher in the new-onset AF group than in the non-AF group (P < 0.05).
Conclusion
Hypertension, age, left atrial enlargement, and high ventricular pacing rate were independent predictors of new-onset AF in elderly patients after implantation of a permanent pacemaker. New-onset AF increased the risk for ischemic stroke.
Keywords: Atrial fibrillation, Dual-chamber pacemaker, Elderly, Ischemic stroke
1. Introduction
Atrial fibrillation (AF) is one of the most common arrhythmias after pacemaker implantation. It greatly increases the odds of such complications as thromboembolism and heart failure. Previous study had shown that the chance of a person suffering from the AF after implantation of a permanent pacemaker is higher than it is in those without a pacemaker.[1] We detected and diagnosed atrial arrhythmias at an early stage by using a pacemaker to monitor the heart rhythm. In this study, we identified patients who developed new-onset AF after implantation with the help of pacemaker programming and follow-up. We then analyzed the risk factors and prognosis for new-onset AF in patients aged ≥ 65 years after implantation of a dual-chamber pacemaker.
2. Methods
2.1. Patients
We conducted a retrospective analysis of the clinical course and follow-up of 322 patients with sick sinus syndrome (SSS) and/or atrioventricular block (AVB) undergoing their first implantation of a dual-chamber permanent pacemaker in Beijing Anzhen Hospital between October 2013 and May 2016. Inclusion criteria were (1) age ≥ 65 years; and (2) class I and IIA indications for permanent pacemaker implantation. Exclusion criteria included (1) previous electrocardiography (ECG) or Holter monitoring that confirmed preoperative AF or atrial flutter; (2) presence of congenital heart disease, cardiomyopathy, or valvular heart disease; (3) a history of cardiac surgery; (4) moderate to severe anemia, thyroid dysfunction, and/or severe liver and/or kidney dysfunction; (5) acute myocardial infarction; and/or (6) prior cardiac surgery.
2.2. Procedure
Baseline data for the patients were collected, including the patient's name, sex, and age; presence of heart failure; history of hypertension, diabetes, old myocardial infarction, peripheral vascular disease and/or ischemic stroke/transient ischemic attack (TIA); and/or abnormal echocardiographic parameters. Atrial (AP%) and ventricular (VP%) pacing percentages were recorded after 1, 3, 6, and 12 months and then every year thereafter. The risk ratio of the abovementioned risk factors for AF was analyzed.
Each patient underwent preoperative echocardiography, and the results were recorded, including the left atrial diameter, right atrial diameter, left ventricular end-diastolic diameter, left ventricular ejection fraction, mitral valve, tricuspid regurgitation, and pulmonary artery pressure.
For implantation of the pacemaker and adjustment of its settings, the following steps were undertaken. The atrial electrode lead of the pacemaker was placed in the right atrial appendage and the ventricular lead in the right ventricular apex. The pacing mode was set at DDD, with the lower limit of frequency at 60 beats/min and the upper limit at 150 beats/min. After implantation, the ventricular priority function (“Search AV+, MVP” function of the Vitatiron pacemaker, “VIP” function of the St. Jude Medical pacemaker, “AV Search” function of the Boston Science pacemaker) was activated, and the atrioventricular (AV) interval was extended automatically to minimize ventricular pacing. The atrial high-rate episodes (AHRE) recording function and automatic-mode conversion were activated by convention.
AF is defined as the presence of an AHRE in which > 5 min of a sustained atrial rate equivalent to > 250 (beats per minute bpm) is recorded by the intracavity ECG of the pacemaker, and the pacemaker undertakes simultaneous automatic-mode conversion. Alternatively, ECG or a 24 h Holter monitor records AF during follow-up.
2.3. Statistical analysis
SPSS 22.0 software (IBM Corp., Armonk, NY, USA) was used for statistical analyses. Quantitative data are reported as means ± standard deviation, and enumeration data are expressed with percentages. Quantitative data were compared using a t test, and enumeration data were compared using a χ2 test. Cox multivariable analysis was used to identify risk factors for AF. Kaplan–Meier and log-rank analyses were performed to evaluate the stroke and re-hospitalization rates due to heart failure in patients with new-onset AF. The follow-up ended in October 2017. Follow-up data that did not represent any significant endpoint events were regarded as censored. A value of P < 0.05 was considered to indicate statistical significance.
3. Results
Altogether, 322 patients (166 men, 156 women; age range 65–90 years) were enrolled in this study. The average follow-up time was 30.8 ± 7.9 months (range 17–48 months). Overall, 79 (24.5%) of the 322 patients were found to have new-onset AF at an average follow-up time of 20.6 months (range 3–48 months). Among them, 33 had asymptomatic AF, 75 had paroxysmal AF, and 4 had persistent AF (Table 1).
Table 1. Baseline characteristics of the study population.
| Variables | Total (n = 322) | AF (n = 79) | No AF (n = 243) | P |
| Male | 166 | 42 (53.1%) | 124 (51.0%) | 0.79 |
| Age | 70.8 ± 5.5 | 74.7 ± 5.7 | 69.6 ± 4.8 | 0.00 |
| SSS | 185 | 50 (63.3%) | 135 (55.6%) | 0.24 |
| cAVB | 75 | 22 (27.8%) | 53 (21.8%) | 0.29 |
| Heart failure* | 40 | 12 (15.2%) | 28 (11.5%) | 0.43 |
| Hypertension | 175 | 71 (89.9%) | 104 (42.8%) | 0.00 |
| Diabetes | 83 | 19 (24.1%) | 64 (26.3%) | 0.77 |
| Ischemic stroke/TIA | 47 | 12 (15.2%) | 35 (14.4%) | 0.86 |
| Vascular diseaseΔ | 37 | 12 (15.2%) | 25 (10.3%) | 0.23 |
| LAD, mm | 37.6 ± 3.8 | 41.7 ± 2.9 | 36.3 ± 3.0 | 0.00 |
| LVEDD, mm | 50.1 ± 4.9 | 50.3 ± 5.4 | 49.4 ± 4.8 | 0.24 |
| LVEF% | 56.3 ± 5.4 | 55.5 ± 4.9 | 56.6 ± 5.4 | 0.31 |
| AP% | 40.0 ± 16.4 | 45.0 ± 15.6 | 38.3 ± 16.3 | 0.01 |
| VP% | 48.7 ± 17.1 | 54.9±12.8 | 46.5 ± 17.8 | 0.00 |
| CHA2DS2-VASc score | 3.74 ± 1.42 | 3.27 ± 1.58 | 2.56 ± 1.32 | 0.00 |
Data are presented as means ± SD or n (%). *Patient had been diagnosed with heart failure and maintained treatment when cardiac function during the perioperative period was grade I (New York Hospital Association classification); ΔOld myocardial infarction or peripheral vascular disease. AP%: atrial pacing percentage; cAVB: complete atrioventricular block; LAD: left atrial diameter; LVEDD: left ventricular enddiastolic dimension; LVEF: left ventricular ejection fraction; SSS: sick sinus syndrome; TIA: transient ischemic attack; VP%: ventricular pacing percentage.
Using the abovementioned clinical characteristics of the patients, a univariable analysis was performed using the Forward LR analysis method. It concluded that six factors (i.e., age, hypertension, atrial and ventricular pacing rates, left atrial diameter, CHA2DS2-VASc score) were risk factors for AF. A multivariable regression analysis using a Cox regression model then showed that hypertension, age, left atrial diameter enlargement, and high ventricular pacing rate were independent risk factors for new-onset AF in patients aged ≥ 65 years after implantation of a permanent pacemaker (Table 2). The results of the univariable analysis indicated that CHA2DS2-VASc score was a significant factor [hazard ratio (HR) = 1.368, 95% confidence interval (CI): 1.178–1.589, P = 0.002], but the results of multivariable regression analysis did not consider it an independent risk factor for AF.
Table 2. Results of stepwise Cox regression analysis.
| Factors | HR | 95% CI | P |
| Hypertension | 3.040 | 1.09–3.05 | 0.00 |
| Age | 1.966 | 1.57–3.68 | 0.01 |
| LAD | 1.645 | 1.05–1.25 | 0.03 |
| VP% | 1.137 | 1.01–1.06 | 0.01 |
CI: confidence interval; HR: hazard ratio.
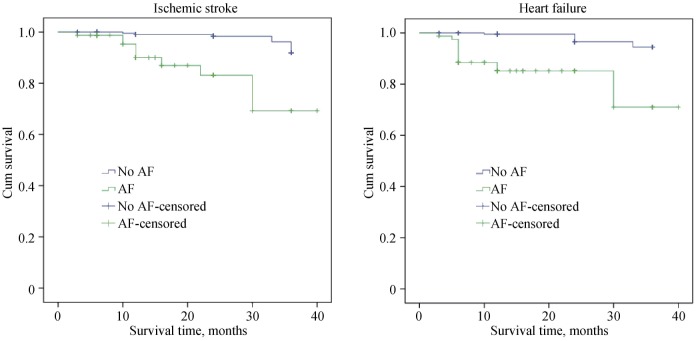
An analysis regarding prognosis was carried out on the patients with new-onset AF. During the follow-up period, 18 patients had been hospitalized because of heart failure. Among them, 11 had developed new-onset AF. Moreover, 15 patients suffered from new-onset ischemic stroke, among whom 9 developed new-onset AF. Results of the Kaplan– Meier survival analysis and the log-rank analysis showed that the risks for ischemic stroke and hospitalization due to heart failure were significantly higher in those with new-onset AF than in those without it (P < 0.05, Figure 1).
Figure 1. The distribution of major cardiac and brain adverse events in the population was studied.
4. Discussion
Cardiac pacing is the only effective treatment for symptomatic bradycardia. Implantation of a pacemaker reduces symptoms caused by an insufficient blood supply to the vital organs such as the heart and brain, thereby improving patients' quality of life, sometimes even saving a life. Pacemakers, however, alter cardiac electrophysiology and hemodynamics, possibly contributing to the occurrence of AF. From the perspective of the mechanism of AF, a structural anomaly of the heart or cardiac muscle is the pathology responsible for the onset of AF. Slow intra-atrial conduction, anisotropic conduction, and an increased refractory period of dispersion comprise the electrophysiological basis of AF. Alteration of atrial electrical and ion channel expression and dysfunction caused by a pacemaker could contribute to the development of AF.[2]
A slight difference in morbidity was observed for a large sample of clinical trials. It was due to differences in the enrollment of the target population, the pacing mode used, and the definition and diagnostic criteria for AF. In the Pacemaker Selection in the Elderly (PASE) trial,[3] the incidence of AF was 18% during an 18-month follow-up. In the Mode Selection Trial (MOST),[4] 15.8% of 2010 patients were diagnosed with AF within the first year after pacemaker implantation. When the follow-up was extended to 33 months, the AF incidence was 22.5%. A recent single-center Canadian study[5] reported that, among 445 patients who had undergone pacemaker implantation, 55.3% developed AF within 6 months. Similar to the numbers in these large-scale studies, our research, with an average follow-up of 30.8 ± 7.9 months, showed that 24.5% of pacemaker-embedded patients aged ≥ 65 years developed AF.
Hypertension is the most significant risk factor for AF following pacemaker implantation (HR = 3.040). Hypertension causes AF via various sophisticated mechanisms. It is often associated with the development of ventricular hypertrophy and diastolic dysfunction, which gradually increase left ventricular end-diastolic pressure, leading to increased left atrial pressure. The end results are atrial enlargement and fibrosis.
Aging is also an independent factor for AF development.[6] Aging leads to increased myocardial and adipose tissue, reduced sinus node function, and slow conduction. The left atrium is the target organ for hypertension, heart failure, and coronary heart disease. Left atrial enlargement becomes an independent risk factor for AF. Studies have shown that, with each 5-mm increase in the left atrial diameter, the risk of atrial fibrillation increases by 39%.[7] The present research did not show that an increased CHA2DS2-VASc score was an independent risk factor for new-onset AF, possibly because the CHA2DS2-VASc score includes only general risk factors that do not contribute distinctively to the disease.
Saitoh, et al.[8] found that, in AF patients without coronary heart disease, fibrosis and lipomatosis of sinoatrial nodal cells were seen and that these cells were significantly decreased. It has been suggested that sinoatrial node dysfunction prompts the occurrence of AF. However, the possibility that patients with SSS have already developed cardiomyopathy could not be excluded. In SSS patients, there are fewer than usual electrical impulses generated from the sinus node to the atrium because of the sinus dysfunction, causing irregular extopic excitations and reentries. Hence, those patients are more likely to develop atrial arrhythmias along with the SSS. At the same time, bradycardia prolongs the atrial period of vulnerability and increases dispersion of the atrial refractory period. Once the premature atrial contraction (long-cycle/short-cycle phenomenon) occurs, excitation of atrial cells tends to appear during the vulnerable period of the atrium or during the window period of reentry. Anisotropic conduction becomes more severe especially when atrial cells are damaged. All of these factors trigger the occurrence of AF. However, the impact of the atrial pacing rate on AF remains controversial.[9],[10]
The large-scale, prospective, randomized, controlled study SAFARI[11] showed that dynamic atrial overdrive therapy can significantly reduce the AF burden. Another study of 301 patients[12] with SSS (ages 70–90 years) showed that a high atrial pacing rate after pacemaker implantation could reduce the incidence of AF. In addition, a meta-analysis evaluating the incidence of AF after cardiac surgery showed that an atrial pacing rate slightly higher than the patients' own heart rate could reduce the incidence of AF by 37%.[13] Others, however, have reached different conclusions. In a meta-analysis, Elkayam, et al.[14] showed that a higher atrial pacing rate could strongly increase the odds of pacemaker implantation-related AF, and Li's research[15] showed that AP% ≥ 60% was an independent risk factor for pacemaker implantation-related AF in SSS patients. In our study, the univariable analysis showed that the impact of AP% on AF was significant, although the multivariable regression analysis did not indicate that AP% was an independent risk factor. On the one hand, atrial pacing stabilizes the atrial conducting pathway by inhibiting atrial premature beats, erasing the long interval after atrial premature beats and reducing the atrial refractory period dispersion. On the other hand, it causes non-physiological electro-conducting order, prolongs the duration of atrial stimulus (causing desynchronized contraction of both atrium, delayed left atrial contraction, decreased left ventricular filling and increased left atrial pressure), leading to a greater chance of developing AF.[16] A sick sinus prompts the development of AF itself because of some pathophysiological changes. How much the electrophysiological change caused by atrial pacing contributes to the occurrence of AF is still under debate.
Minimizing ventricular pacing has been widely accepted. A high ventricular pacing rate is significantly associated with the occurrence of AF. Studies, including MOST and MINERVA, have confirmed that the higher the percentage of ventricular pacing, the greater is the incidence of AF.[17],[18] The SAVE PACE study[19] compared the effects of a high ventricular pacing rate versus a minimal ventricular pacing rate. Altogether, 1065 SSS cases were randomly assigned to either a minimal ventricular pacing group or a fixed short AV-interval group. The ventricular pacing percentage of the latter group was up to 99%, whereas it was only 9% in the former group whose incidence of AF was significantly lower than it was in the fixed AV-interval group. Theoretically, complete AV block is expected to lead to a high rate of ventricular pacing, but this study did not find that complete AV block was a risk factor for AF. This outcome might be related to the duration of AVB. The follow-up showed that most of the third-degree AVB occurred discontinuously, with a normal AV interval or second-degree AVB seen in between. In addition, a substantial number of elderly patients suffered from delayed AV conduction together with SSS. Even after the AV interval of their pacemaker was prolonged, AV conduction by the heart itself was still too little and ventricular pacing was still too much. In this research, the high rate of ventricular pacing was an independent risk factor for AF, whereas there was no significant difference in the incidence of AF between the SSS and third-degree AVB groups. It is thus highly significant if the AV interval is optimized to encourage conduction by the heart itself, therefore minimizing the ventricular pacing rate after pacemaker implantation.
During the long-term follow-up, it was found that new-onset AF after pacemaker implantation might increase the risk of heart dysfunction. In addition, the risk for ischemic stroke in patients with AF detected by pacemakers was significantly higher than it was in patients without AF. At present, the mechanisms and consequences of ischemic stroke caused by AF detected by clinical examination are clear. For pacemaker-detected AF, however, there are still no adequate clinical trials that have explored the contribution of AF to ischemic stroke. Also, clinical research has provided no evidence to prove that anticoagulation can improve the prognosis of AF detected by pacemakers.[20] Larger-scale clinical trials are thus needed.
The aim of this study was to identify risk factors associated with pacemaker implantation-related AF in elderly patients. There were some limitations, however, in our design: Some of the patients might have had asymptomatic AF prior to implantation but were included in the group observation because of the lack of clinical evidence of AF. In addition, the pacemaker itself improved AF monitoring and significantly increased the detection of asymptomatic AF.[21] These conditions might have allowed us to overestimate the incidence of new-onset AF after pacemaker implantation. AF has gained an increasing amount of clinical attention. We could benefit patients by fully assessing their preoperative risk factors, taking advantage of pacemaker monitoring for AF, and strengthening postoperative anti-arrhythmia management.
Acknowledgments
There was no conflict of interests to be declared. This study was supported by the Project of Cardiovascular Disease in Precise Medicine of Beijing laboratory and the Special Funding for Personnel Training in Beijing Geriatric Hospital.
References
- 1.Nielsen JC. Mortality and incidence of atrial fibrillation in paced patients. J Cardiovasc Electrophysiol. 2002;13:17–22. doi: 10.1111/j.1540-8167.2002.tb01948.x. [DOI] [PubMed] [Google Scholar]
- 2.Wang M, Siu GW, Lee KL, et al. Effects of right low septalvs right atrial appendage pacing on atrial mechanical function and dyssynchrony in patients with sinus node dysfunction and pamxysmal atrial fibrillation. Europace. 2011;13:1268–1274. doi: 10.1093/europace/eur110. [DOI] [PubMed] [Google Scholar]
- 3.Stambler BS, Ellenbogen KA, Orav EJ, et al. Predictors and clinical impact of atrial fibrillation after pacemaker implantation in elderly patients treated with dual chamber versus ventricular pacing. Pacing Clin Electrophysiol. 2003;26:2000–2007. doi: 10.1046/j.1460-9592.2003.00309.x. [DOI] [PubMed] [Google Scholar]
- 4.Fleischmann KE, Orav EJ, Lamas GA, et al. Atrial fibrillation and quality of life after pacemaker implantation for sick sinus syndrome: data from the Mode Selection Trial (MOST) Am Heart J. 2009;158:78–83. doi: 10.1016/j.ahj.2009.02.023. [DOI] [PubMed] [Google Scholar]
- 5.Healey JS, Martin JL, Duncan A, et al. Pacemaker-detected atrial fibrillation in patients with pacemakers: prevalence, predictors, and current use of oral anticoagulation. Can J Cardiol. 2013;29:224–228. doi: 10.1016/j.cjca.2012.08.019. [DOI] [PubMed] [Google Scholar]
- 6.Hu DY, Sun YH. Etiology and epidemiology of atrial fibrillation. Chinese Journal of Practical Internal Medicine. 2006;25:163–164. [Google Scholar]
- 7.Benjamin EJ, Levy D, Vaziri SM, et al. Independent risk factors for Atrial Fibrillation in a population-based cohort. JAMA. 1994;271:840–844. [PubMed] [Google Scholar]
- 8.Saitoh S. The histopathological substratum for atrial fibrillation in man. Acta Pathol Jpn. 1982;32:183–191. doi: 10.1111/j.1440-1827.1982.tb02040.x. [DOI] [PubMed] [Google Scholar]
- 9.Nielsen JC, Thomsen PE, Højberg S, et al. A comparison of single-lead atrial pacing with dual-chamber pacing in sick sinus syndrome. Eur Heart J. 2011;32:686–696. doi: 10.1093/eurheartj/ehr022. [DOI] [PubMed] [Google Scholar]
- 10.Ogawa H, Ishikawa T, Matsushita K, et al. Effects of right atrial pacing preference in prevention of paroxysmal atrial fibrillation: Atrial Pacing Preference study (APP study) Circ J. 2008;72:700–704. doi: 10.1253/circj.72.700. [DOI] [PubMed] [Google Scholar]
- 11.Gold MR, Adler S, Fauchier L, et al. Impact of atrial prevention pacing on atrial fibrillation burden: primary results of the Study of Atrial Fibrillation Reduction (SAFARI) trial. Heart Rhythm. 2009;6:295–301. doi: 10.1016/j.hrthm.2008.11.033. [DOI] [PubMed] [Google Scholar]
- 12.Peng H, Shen LH, Ma WY. Analysis of the influence factors of atrial pacing ratio on atrial fibrillation after pacemaker operation in elderly patients. Chinese Journal of Multiple Organ Diseases in the Elderly. 2015;14:607–611. [Google Scholar]
- 13.Crystal E, Garfinkle MS, Connolly SS, et al. Interventions for preventing post-operative trial fibrillation in patients undergoing heart surgery. Cochrane Database Syst Rev. 2004;4:CD003611. doi: 10.1002/14651858.CD003611.pub2. [DOI] [PubMed] [Google Scholar]
- 14.Elkayam LU, Koehler JL, Sheldon TJ, et al. The influence of atrial and ventricular pacing on the incidence of atrial Fibrillation: a meta-analysis. PACE. 2011;34:1593–1599. doi: 10.1111/j.1540-8159.2011.03192.x. [DOI] [PubMed] [Google Scholar]
- 15.Li TF, Ren XJ. The effect of atrial pacing ratio on atrial fibrillation after pacemaker operation in patients with sick sinus syndrome. Journal of Cardiovascular and Pulmonary Diseases. 2015;34:752–756. [Google Scholar]
- 16.Yasuoka Y, Abe H, Umekawa S, et al. Interatrial septum pacing decreases atrial dyssynchrony on strain rate imaging compared with right atrial appendage pacing. Pacing Clin Electrophysiol. 2011;34:370–376. doi: 10.1111/j.1540-8159.2010.02976.x. [DOI] [PubMed] [Google Scholar]
- 17.Glotzer TV, Hellkamp AS, Zimmerman J, et al. Atrial high rate episodes detected by pacemaker diagnostics predict death and stroke: report of the Atrial Diagnostics Ancillary Study of the Mode Selection Trial (MOST) Circulation. 2003;107:1614–1619. doi: 10.1161/01.CIR.0000057981.70380.45. [DOI] [PubMed] [Google Scholar]
- 18.Boriani G, Tukkie R, Manolis AS, et al. Atrial antitachycardia pacing and managed Ventricularpacing in bradycardia patients with paroxysmal or persistent atrial tachyarrhythmias: the MINERVA randomized multicentre international trial. Eur Heart J. 2014;35:2352–2362. doi: 10.1093/eurheartj/ehu165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sweeney MO, Bank AJ, Nsah E, et al. Minimizing ventricular pacing to reduce atrial fibrillation in sinus-node disease. N Engl J Med. 2007;357:1000–1008. doi: 10.1056/NEJMoa071880. [DOI] [PubMed] [Google Scholar]
- 20.Merinopoulos I, Raphael CE. Device-identified atrial fibrillation at pacing clinics. Should it guide anticoagulation? Int J Cardiol. 2016;207:378–381. doi: 10.1016/j.ijcard.2016.01.040. [DOI] [PubMed] [Google Scholar]
- 21.Quirino G, Barold SS, Giammaria M, et al. Diagnosis of paroxysmal atrial fibrillation in patients with implanted pacemakers: relation-ship to symptoms and other variables. Pacing Clin Electrophysiol. 2009;32:91–98. doi: 10.1111/j.1540-8159.2009.02181.x. [DOI] [PubMed] [Google Scholar]