Abstract
Bacterial biofilms remain a persistent threat to human health-care due to their role in the development of antimicrobial resistance. To combat multi-drug resistant pathogens, it is crucial to enhance our understanding of not only the regulation of biofilm formation, but also its contribution to bacterial virulence. Iron acquisition lies at the crux of these two subjects. In this review, we discuss the role of iron acquisition in biofilm formation and how hosts impede this mechanism to defend against pathogens. We also discuss recent findings that suggest that biofilm formation can also have the reciprocal effect, influencing siderophore production and iron sequestration.
Keywords: iron acquisition, biofilm, nutritional immunity, siderophore, exopolysaccharides, Pseudomonas aeruginosa
Introduction
Probably due to the common experience of growing lab-adapted strains of bacteria in liquid cultures, we tend to imagine most bacterial growth (both in the environment and during infection) as taking place in a planktonic state, where individual cells interact with each other through quorum sensing systems (although this perception is also inaccurate, as biofilm aggregates frequently exist in liquid cultures as well [Kragh et al., 2018]). In reality, most bacterial growth takes place in the context of biofilms, where structured communities of one or more microbial species are surrounded by extracellular polymeric substances (EPS), including polysaccharides, extracellular DNA, and polypeptides (Friedman and Kolter, 2004; Mulcahy et al., 2008; Flemming and Win-gender, 2010). In Pseudomonas aeruginosa, biofilm development follows a stereotypical course (Sauer et al., 2002; Stoodley et al., 2002). This process is generally broken into five different stages: initial attachment, irreversible attachment and EPS secretion, initial development of biofilm architecture, maturation of biofilm architecture, and “shedding” of single cells from mature biofilms.
Biofilms are ancient (they represent the oldest fossils that have been discovered on the planet [Rasmussen, 2000]) and they form complex, heterologous structures (Bridier et al., 2010). Some even include channels that permit the diffusion of nutrients and oxygen throughout the biofilm, facilitating growth (Kim and Lee, 2016). Several hypotheses have been advanced about the evolutionary origins and advantages of biofilm growth and have suggested that it may have arisen as a mechanism to resist transient shear forces in fluid flow (Rittman, 1982; Peyton and Characklis, 1993; Peyton, 1996), to increase the effective concentration of nutrients and signaling molecules in the proximity of the biofilm (Donlan, 2002), to resist noxious environments (Hall-Stoodley et al., 2004), or even simply to help the bacteria occupy an available area for growth.
Although their evolutionary origins remain speculative, the biomedical consequences of biofilm formation are clear. For several reasons, biofilms dramatically increase resistance to antimicrobials. First, they enrobe the bacteria in EPS, limiting the diffusion of biocides and antibacterials and their access to cells (Mah and O’Toole, 2001; Anderson and O’Toole, 2008). For example, a number of studies have shown a correlation between biofilm viscosity and antimicrobial sensitivity (Stewart, 1996; Gilbert et al., 1998; Wirtanen et al., 1998; Kostenko et al., 2007; Ruhs et al., 2013). Genetic disruption or chemical inhibition of biofilm formation also increases pathogen susceptibility to antimicrobial agents (Rashid et al., 2000; Shih and Huang, 2002; Li and Lee, 2017). Biofilms are also thought to reduce the growth rate of bacterial cells within their matrix, facilitating the appearance of morphologically-distinct cells commonly called ‘persisters’, which show uncommonly high tolerance to insults (Drenkard and Ausubel, 2002; Kester and Fortune, 2014). Phenotypically normal cells can also exhibit diminished antimicrobial sensitivity when their rate of cell division decreases, a phenomenon sometimes called antimicrobial indifference (Jayaraman, 2008). Biofilm formation also promotes the evasion of host immune recognition, phagocytosis, and host bacterial killing (Jensen et al., 1990; Costerton et al., 1999; Leid et al., 2005; Alhede et al., 2014).
As antimicrobial resistance continues to mount, biofilms have become an important therapeutic target in the treatment of infectious diseases. Disruption of established bio-films promotes the removal of bacteria by the immune system and conventional antimicrobials and limits other bio-film-dependent mechanisms of virulence. Small molecules and genetic mutations that compromise biofilm formation have been shown to limit pathogenesis (Hentzer et al., 2003; Cady et al., 2012; Komor et al., 2012; O’Loughlin et al., 2013; Kang and Kirienko, 2017), demonstrating their utility.
Biofilm formation is an integral part of P. aeruginosa infection in mammals, especially in the airways of patients with cystic fibrosis (CF), where this pathogen is a leading cause of death (Singh et al., 2000; Winstanley et al., 2016; Moradali et al., 2017). Despite their obvious value, developing therapeutics that target P. aeruginosa biofilms is challenging. Biofilm formation in P. aeruginosa is particularly complex, involving a variety of redundant regulatory mechanisms (Colvin et al., 2012; Irie et al., 2012). For example, both intracellular and intercellular signaling via secondary messengers such as cyclic diguanylate monophosphate (c-di-GMP) and quorum sensing molecules such as homoserine lactones, are involved in the ‘decision’ to begin the transition to sessile development (Parsek and Greenberg, 2000; Goodman et al., 2004; Camilli and Bassler, 2006; Sakuragi and Kolter, 2007; Petrova and Sauer, 2009; Mikkelsen et al., 2011). As a consequence, various studies have proposed an alternative approach to mitigating biofilm formation: prevent the pathogen from acquiring sufficient iron, and the process will be compromised.
Iron acquisition is necessary for biofilm formation in P. aeruginosa
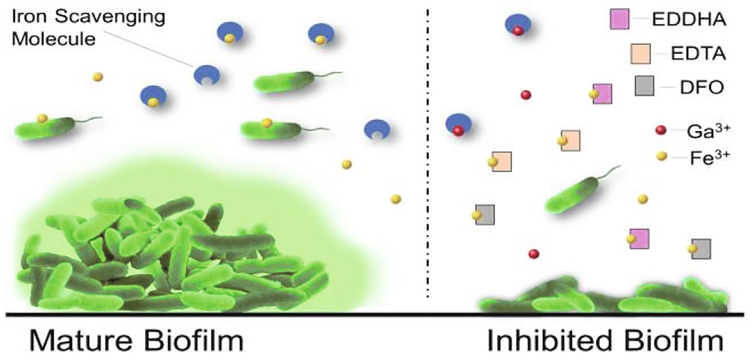
In a seminal study, Singh and colleagues reasoned that if bio-films were such a common occurrence in human infections, the innate immune system should mount some defense against them (Singh et al., 2002). Through careful study they discovered that lactoferrin sequestered iron from the pathogen, causing iron deprivation. This state induced bacterial twitching, preventing the formation of microcolonies. Further works by Greenberg and colleagues showed that pyoverdine (a key siderophore produced by P. aeruginosa, see below) was necessary for the development of biofilms in vitro (Banin et al., 2005, 2006). In contrast, loss of the other major siderophore, pyochelin, had no effect on biofilm development. They also observed that supplementation of the media with ferric citrate (a salt that is actively transported into the bacterium) restored biofilm formation in the absence of pyoverdine, suggesting that iron transportation was the relevant determinant, rather than pyoverdine per se. Based on these observations, a number of studies tested the impact of synthetic iron chelators (such as deferasirox, ethylene diamine tetraacetic acid, ethylene diamine-N,N′-bis(2-hydroxyphenylacetic acid, and others) on P. aeruginosa biofilm formation (Banin et al., 2006; Moreau-Marquis et al., 2009; O’May et al., 2009; Kang and Kirienko, 2017) (Fig. 1). In each case, these compounds disrupted the formation of biofilms.
Fig. 1. Interfering with bacterial iron acquisition inhibits biofilm formation.
Under iron-replete conditions, free iron can be either directly transported into the bacterium by active transport systems or indirectly transported via iron scavenging molecules (e.g., pyoverdine, pyochelin, PQS, etc.). Under these conditions bacteria retain the ability to form mature biofilms. In the presence of iron chelators (such as EDTA, EDDHA, or heterologous siderophores that P. aeruginosa cannot utilize, like deferoxamine), however, iron availability is restricted and biofilm formation is compromised. This indicates that iron uptake is necessary for biofilm formation. Furthermore, certain metals like gallium can compete against iron for bacterial iron-scavenging molecules, preventing iron uptake and inhibiting biofilm formation.
Bacterial iron uptake can also be compromised by gallium, which exists as a trivalent cation with an ionic radius and charge density comparable to iron (III) (Kaneko et al., 2007). Unlike iron, however, gallium is redox inactive, generally precluding it from carrying out the same biochemical functions as iron (Chitambar and Narasimhan, 1991). As such, it is perhaps unsurprising that gallium nitrate killed multi-drug resistant P. aeruginosa, prevented biofilm formation, destroyed established biofilms, and effectively mitigated P. aeruginosa pathogenicity in multiple murine infection models (Kaneko et al., 2007; Banin et al., 2008). It is worth noting that several clinical trials utilizing gallium salts as inhibitors of bacterial infection are currently underway. Since gallium nitrate is already an FDA approved drug (for treatment of hypercalcemia), the pathway to repurposing gallium nitrate as an infection therapeutic is simpler than the development of previously unidentified compounds.
Interestingly, anaerobic growth of P. aeruginosa (such as the conditions believed to exist in the mucosal secretions of cystic fibrosis patients’ airways [Worlitzsch et al., 2002]) is known to stimulate the development of thick, robust bio-films (Yoon et al., 2002). For reasons that remain unclear, growth and biofilm formation under anaerobic conditions demands higher concentrations of ferric iron than otherwise, making P. aeruginosa more susceptible to growth inhibition by chemical chelators (O’May et al., 2009). Interestingly, the most common causative mutation for CF (the ΔF508 mutation, which refers to the loss of the phenylala-nine at position 508 in the CFTR protein) triggers the secretion of excess iron into the extracellular milieu, creating conditions that favor biofilm formation. When P. aeruginosa was grown on a cell line derived from a patient homozygous for the ΔF508 mutation, the increase in extracellular iron caused by this mutation significantly enhanced biofilm production (Moreau-Marquis et al., 2008). Much as in other anaerobic or microaerobic conditions, these conditions showed increased sensitivity to iron chelators, specifically conalbumin, which strongly limited biofilm formation (Bernardini et al., 1993; Hunter et al., 2013).
It should be noted that the phenomena described thus far are likely only to apply to biofilms that are comprised of Pel or Psl. Alginate, the third biofilm polysaccharide produced by P. aeruginosa, is overproduced in mucoid strains isolated from the respiratory tracts of cystic fibrosis patients due to one or more mutations in muc genes (Franklin et al., 2011). Unlike Pel or Psl, alginate is not attached to the cell’s surface, but is secreted into the extracellular milieu. Surprisingly, not only is iron dispensable for alginate biosynthesis, but iron-replete conditions appear to limit alginate production. For example, mucoid strains grown in the presence of iron are unstable and prone to being supplanted by non-mucoid strains (Boyce and Miller, 1980, 1982). In addition, iron starvation can stimulate the appearance of mucoid strains (Terry et al., 1992). However, Vasil and colleagues have noted that at least some mucoid strains of P. aeruginosa appear to have lost the ability to regulate alginate production in iron-replete conditions (Oglesby-Sherrouse et al., 2014).
Nutritional immunity: host inhibition of biofilm formation
The idea of limiting bacterial access to iron is not new. In fact, it could be said that the idea is at least 400 million years old. That is the estimated date of the divergence of the Euteleostomi, amongst most members of which ferritin and transferrin are conserved. In a broader sense, hosts and pathogens compete for several bioavailable transition metals (including iron, copper, zinc, manganese, and molybdenum) (Hood and Skaar, 2012). These metals are required for gene transcription, redox-reactions, and even non-redox, metal-dependent reactions (such as the prolyl hydroxylase domain [PHD]-containing family of proteins that use ferrous iron to split molecular oxygen for protein hydroxylation). The process whereby the host restricts access to these metals is colloquially known as nutritional immunity and has been recently reviewed elsewhere (Palmer and Skaar, 2016; Carver, 2018), so our attention will focus on iron.
To prevent pathogens from acquiring this essential nutrient, hosts withhold intracellular iron using iron-storage proteins such as ferritin or in iron-containing complexes like heme, and restrict extracellular iron availability by secreting iron-sequestering proteins such as transferrin and lactoferrin (Skaar, 2010; Kelson et al., 2013). Transferrin and lactoferrin function similarly to chemical iron chelators; by restricting environmental iron, bacterial biofilm formation is compromised (Fig. 2). For example, apo-transferrin significantly attenuates biofilm formation in Staphylococcus epidermidis and attachment in S. aureus and P. aeruginosa (Ardehali et al., 2002; She et al., 2016). The removal of apotransferrin (or replacing it with iron-saturated transferrin) permits re-establishment of biofilm formation and bacterial adhesion in these systems. Similar results are observed when bacteria are treated with apo-lactoferrin (Singh et al., 2002; Banin et al., 2005; Wakabayashi et al., 2009; Kamiya et al., 2012).
Fig. 2. Hosts and pathogens compete to sequester iron from the environment.
Host cells secrete iron-sequestering proteins such as transferrin and lactoferrin to minimize free extracellular iron. Some bacterial pathogens secrete siderophores to compete against these proteins and scavenge the trace amounts of free iron. Certain siderophores, such as enterobactin (from E. coli and S. typhimurium) and pyoverdine (from P. aeruginosa), can directly remove ferric iron from iron-bound transferrin and lactoferrin, increasing pathogen iron uptake and promoting biofilm formation. To interfere with siderophore activity, certain host cells secrete lipocalin 2 (also known as NGAL) to recognize and bind to siderophores, preventing their function. Production of siderophores that can evade lipocalin 2 has also been linked to pathogenicity.
Pathogens attempt to overcome iron limitation in at least three ways. First, some pathogens express receptors for lactoferrin or transferrin, in a bid to acquire the proteins and their associated iron (Beddek and Schryvers, 2010; Pogoutse and Moraes, 2017). Second, many human pathogens have heme acquisition pathways comprised of heme-binding receptors and/or even heme-binding molecules called hemophores (Cescau et al., 2007; Huang and Wilks, 2017). Finally, and most commonly, most pathogenic and many non-pathogenic species of bacteria (and fungi) produce small molecule iron chelators called siderophores. These molecules have been evolved to improve the aqueous solubility of iron (III). To facilitate their biological role, these molecules have exceptionally high affinities to ferric iron. This also helps them overcome host iron restriction mechanisms by directly chelating ferric iron from host iron-sequestering proteins (Skaar, 2010) (Fig. 2). For instance, both enterobactin (a high-affinity siderophore produced by a variety of Enterobacteriaceae, including Escherichia coli and Salmonella typhimurium) and pyoverdine (produced by P. aeruginosa) can acquire iron from human iron storage proteins such as transferrin or ferritin (Kvach et al., 1977; Guterman et al., 1978; Carrano and Raymond, 1979; Harris et al., 1979; Tidmarsh et al., 1983; Wolz et al., 1994; Meyer et al., 1996; Xiao and Kisaalita, 1997).
To inhibit siderophore activity, mammalian hosts secrete the siderophore binding protein lipocalin 2 (also known as neutrophil gelatinase-associated lipocalin, or NGAL, to differentiate it from lipocalin 1, which is derived from tears) to recognize and bind to siderophores such as enterobactin (Fig. 2) (Goetz et al., 2002; Flo et al., 2004). Lipocalin 2 is critical for innate immunity, as lipocalin 2-deficient mice exhibit increased bacteremia and sepsis during infection with E. coli (Flo et al., 2004; Berger et al., 2006). In vitro, when bacteria are grown in iron-limited media, lipocalin 2 treatment has a growth-inhibitory and antivirulent effect, which is mitigated by the supplementation of enterobactin or ferrichrome (as a source of iron), suggesting that lipocalin 2 rescues hosts by depriving the pathogen of iron (Flo et al., 2004). However, while lipocalin 2 production has been shown to be an effective host immune response against some pathogens, others (including Klebsiella pneumoniae, Salmonella enterica, and P. aeruginosa) have evolved mechanisms to circumvent this defense. For example, lipocalin 2 does not efficiently bind pyoverdine (Peek et al., 2012), while K. pneumoniae, E. coli, and S. enterica can evade lipocalin 2 by secreting a glycosylated version of enterobactin known as salmochelin (Fischbach et al., 2006). It is worth pointing out that immature or improperly-folded salmochelin molecules can be bound by lipocalin 2 (Valdebenito et al., 2007). K. pneumoniae and Yersinia species also produce a structurally unrelated siderophore called yersiniabactin, which is also not affected by lipocalin 2 (Bachman et al., 2011). Notably, the presence of both enterobactin and yersiniabactin is associated with successful colonization of the respiratory niche by strains of K. pneumoniae (Bachman et al., 2011).
Biofilm formation promotes siderophore production
As noted previously, iron acquisition is necessary for the proper development of biofilms by P. aeruginosa. In many cases, this need is fulfilled by pyoverdine, inextricably linking these two secreted products.
Pyoverdine is arguably the most important siderophore in the in vivo growth and pathogenesis of P. aeruginosa, as demonstrated by the avirulence of pyoverdine-deficient mutants in a variety of infection models (Meyer et al., 1996; Takase et al., 2000; Kirienko et al., 2013; Minandri et al., 2016). Historically, this has been attributed to its ability to also function as a determinant for the activity of the alternate sigma factor PvdS, which controls the expression of several secreted virulence factors, including the translational inhibitor ToxA and the protease PrpL (Lamont et al., 2002). However, more recent data suggest that pyoverdine may also damage host mitochondria by removing iron, triggering mitochondrial turnover (Kirienko et al., 2015; Kang et al., 2018).
Due to its clinical significance, we carried out a high-throughput screen to identify genes necessary for pyoverdine bio-synthesis (Kang and Kirienko, 2017). Surprisingly, this screen yielded many components of biofilm formation, such as Pel exopolysaccharide, flagella, and type IV pili. It is important to note that this phenomenon was observed under iron-replete conditions (as demonstrated by the ability of pyoverdine-deficient mutants to form wild-type levels of biofilm), suggesting that the impairment of biofilm mitigates pyoverdine production. We hypothesize that this phenomenon may have clinical importance in the respiratory tracts of CF patients, where iron concentrations are known to increase (Hunter et al., 2013) and biofilms often appear (Singh et al., 2000; Winstanley et al., 2016; Moradali et al., 2017). In addition, the ability of P. aeruginosa to produce siderophores under iron replete conditions may promote previously unappreciated bacterial proliferation and pathology.
Although the mechanism of biofilm-mediated regulation of pyoverdine remains unclear, there is a strong correlation between biofilm formation and cell aggregation (Visaggio et al., 2015; Kang and Kirienko, 2017). This was initially identified and investigated by Imperi and colleagues, who demonstrated that the exopolysaccharides Pel and Psl were essential for planktonic cell aggregation and pyoverdine production (Visaggio et al., 2015). Artificially inducing cell aggregation, by adding agar to the media for example, was shown to restore pyoverdine in pel psl double mutants (Visaggio et al., 2015). Similarly, we observed that the supplementation of the quorum-sensing molecule Pseudomonas quinolone signal (PQS) to media rapidly stimulated aggregation of planktonic cells, causing high levels of pyoverdine (Kang et al., 2017). The addition of PQS also partially restored pyoverdine production in P. aeruginosa biofilm mutants (Kang et al., 2017). Together, these findings suggest a model where the aggregation of planktonic cells nucleates biofilm formation and induces the production of pyoverdine in a manner that is separate from its regulation by intracellular iron content.
Sequestration of iron by extracellular matrix components
Another intriguing phenomenon that further complicates the relationship between biofilm, iron, and virulence is the discovery that biofilms can store iron. Although iron is essential for most living organisms, it is also quite toxic at high concentrations as it can catalyze the Fenton reaction, which produces reactive oxygen species (ROS). Therefore, bacteria must maintain a delicate balance, acquiring sufficient iron for growth but not enough to allow the wide-spread production of ROS. It now appears that components of the P. aeruginosa biofilm matrix help the bacteria maintain this balance.
Each of the three major polysaccharides produced by P. aeruginosa has a different function during biofilm formation. Psl is produced in both planktonic and biofilm cells. In planktonic cells, Psl promotes cell surface attachment, the initial step of biofilm formation (Ma et al., 2006; Vogeleer et al., 2014). Once cells have attached, Psl exopolysaccharide facilitates biofilm maturation by promoting cell-cell interactions within the extracellular matrix, anchoring the cells to the biofilm (Ma et al., 2006, 2009). In contrast, Pel exopolysaccharide does not play an important role in cell attachment; instead, Pel significantly contributes to biofilm growth by promoting cell-cell interactions necessary for cell aggregation (Colvin et al., 2011). The importance of this function varies across P. aeruginosa strains (Colvin et al., 2012). For instance, P. aeruginosa PAO1, unlike PA14, primarily utilizes Psl, while Pel is dispensable for biofilm formation (Colvin et al., 2011). Interestingly, this mirrors the importance of this exopolysaccharide on pyoverdine production: while pel mutants display attenuated biofilm formation in PA14, they will produce wild-type levels of pyoverdine in PAO1 (Kang and Kirienko, 2017).
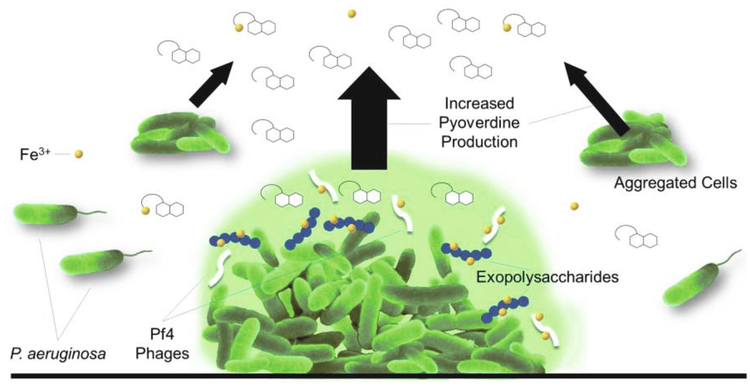
A recent study by Ma and colleagues demonstrated that all three of these exopolysaccharides can sequester free environmental iron (Fig. 3). In brief, alginate binds ferric iron, Pel binds ferrous iron, and Psl binds to both (Yu et al., 2016). Importantly, P. aeruginosa is capable of utilizing iron bound to Psl to support its growth during in iron-limiting environments (Yu et al., 2016).
Fig. 3. P. aeruginosa biofilm can store excess iron.
In P. aeruginosa biofilms, secreted exopolysaccharides and Pf4 bacteriophages can bind iron, sequestering it in the extracellular matrix. Alginate and Pf4 can sequester ferric iron, Pel exopolysaccharide can sequester ferrous iron, and Psl exopolysaccharide can sequester both. In the case of Psl, P. aeruginosa can utilize iron-bound Psl for growth and bio-film formation under conditions of iron restriction. Furthermore, aggregated cells (in both planktonic and sessile states) exhibit increased pyoverdine production, suggesting another regulatory mechanism for iron uptake.
This ability to sequester iron is not unique to polysaccha-rides from P. aeruginosa. Exopolysaccharides from Xanthomonas campestris, Paracoccus zeaxanthinifaciens, and Klebsiella oxytoca have also been shown to bind iron (Baldi et al., 2009; Moppert et al., 2009; Javvadi et al., 2018). Like Psl, cyclic β-(1,2) glucans from X. campestris can store iron that is utilized by the bacteria to support growth under iron-restricted conditions (Javvadi et al., 2018). This phenomenon is wide-spread enough that exopolysaccharides are being considered as potential substrates of heavy metal bioremediation due to their ability to bind various metals (De Philippis et al., 2011; Gupta and Diwan, 2017; Mohite et al., 2017).
Another component of the P. aeruginosa biofilm matrix that is capable of sequestering iron is the filamentous bacteriophage Pf4 (Fig. 3). The Pf4 prophage within the P. aeruginosa genome is highly expressed in biofilm cells, resulting in orders of magnitude greater phage production in biofilms than planktonic cell cultures (Whiteley et al., 2001; Webb et al., 2004). Pf4 activity is necessary for normal bio-film development and maturation, as well as pathogen virulence (Rice et al., 2009). Phage activity also triggers death of P. aeruginosa cells in CF infection isolates (Webb et al., 2003; Kirov et al., 2007), and has been posited to drive P. aeruginosa to a mucoid state (Miller and Rubero, 1984; Hoiby et al., 2001). Pf4 bacteriophage in P. aeruginosa biofilms can also directly bind to ferric iron, as demonstrated by Raman-binding analysis and the induction of phage cross-linking in the presence of ferric iron (Penner et al., 2016). This iron-chelating activity gives P. aeruginosa an advantage during polymicrobial interactions. For instance, P. aeruginosa inhibits Aspergillus fumigatus biofilm formation via Pf4-mediated iron sequestration (Ferreira et al., 2015; Penner et al., 2016). Pf4 bacteriophage can inhibit A. fumigatus biofilms even in the absence of live P. aeruginosa, but this inhibition is deterred by supplementation of ferric iron (Penner et al., 2016). Although Pf4 functions as an important component of the P. aeruginosa biofilm matrix that can sequester ferric iron, it is currently unknown whether P. aeruginosa can utilize iron-bound Pf4 as a source of iron, either directly or indirectly.
Conclusion
Because both host and pathogens require iron for essential cellular processes, iron homeostasis has become a widely studied topic in microbial pathogenesis and immunology. Iron metabolism is involved in many facets of biofilm biology, necessitating the development of pathogen-targeted systems to prevent iron acquisition (e.g., transferrin, lactoferrin, siderophore-binding proteins, etc.) As biofilms enhance bacterial resistance to antimicrobial treatment and facilitate evasion of host immune recognition, they continue to represent an important subject of research.
Based on the competition for iron between hosts and pathogens, many proposals have been made to use a variety of synthetic chelators or gallium to compromise bacterial iron acquisition. However, more recent findings suggest that this approach may be more complicated than initially believed. For example, many biofilm-producing bacterial species grow in polymicrobial communities. It is yet unclear whether disrupting the production of biofilm by a single pathogen in such a community will be clinically beneficial. It may, in fact, be detrimental for host health. For example, iron chelators may affect P. aeruginosa, but leave S. aureus biofilm production functional, creating an environment where the pathogen can flourish without a competitor for resources. Clearly, a better understanding of host-pathogen, pathogen-pathogen, and other more complex relationships is necessary before we can accurately predict the consequences of tampering with bacterial biofilms or iron acquisition pathways in the context of infection.
Acknowledgements
Pseudomonas and pyoverdine work in the authors’ lab was funded by the following grants to NVK: Welch Foundation Research Grant C-1930 and National Institutes of Health Grant K22 AI110552. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript. We would like to apologize to authors who have published on this research topic and whose work was not cited due to space constraints.
References
- Alhede M, Bjarnsholt T, Givskov M, and Alhede M 2014. Pseudomonas aeruginosa biofilms: mechanisms of immune evasion. Adv. Appl. Microbiol 86, 1–40. [DOI] [PubMed] [Google Scholar]
- Anderson GG and O’Toole GA 2008. Innate and induced resistance mechanisms of bacterial biofilms. Curr. Top. Microbiol. Immunol 322, 85–105. [DOI] [PubMed] [Google Scholar]
- Ardehali R, Shi L, Janatova J, Mohammad SF, and Burns GL 2002. The effect of apo-transferrin on bacterial adhesion to bio-materials. Artif. Organs 26, 512–520. [DOI] [PubMed] [Google Scholar]
- Bachman MA, Oyler JE, Burns SH, Caza M, Lepine F, Dozois CM, and Weiser JN 2011. Klebsiella pneumoniae yersiniabactin promotes respiratory tract infection through evasion of lipocalin 2. Infect. Immun 79, 3309–3316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baldi F, Marchetto D, Battistel D, Daniele S, Faleri C, De Castro C, and Lanzetta R 2009. Iron-binding characterization and polysaccharide production by Klebsiella oxytoca strain isolated from mine acid drainage. J. Appl. Microbiol 107, 1241–1250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banin E, Brady KM, and Greenberg EP 2006. Chelator-induced dispersal and killing of Pseudomonas aeruginosa cells in a bio-film. Appl. Environ. Microbiol 72, 2064–2069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banin E, Lozinski A, Brady KM, Berenshtein E, Butterfield PW, Moshe M, Chevion M, and Greenberg EP 2008. The potential of desferrioxamine-gallium as an anti-Pseudomonas therapeutic agent. Proc. Natl. Acad. Sci. USA 105, 16761–16766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banin E, Vasil ML, and Greenberg EP 2005. Iron and Pseudo-monas aeruginosa biofilm formation. Proc. Natl. Acad. Sci. USA 102, 11076–11081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beddek AJ and Schryvers AB 2010. The lactoferrin receptor complex in Gram negative bacteria. Biometals 23, 377–386. [DOI] [PubMed] [Google Scholar]
- Berger T, Togawa A, Duncan GS, Elia AJ, You-Ten A, Wake-ham A, Fong HE, Cheung CC, and Mak TW 2006. Lipocalin 2-deficient mice exhibit increased sensitivity to Escherichia coli infection but not to ischemia-reperfusion injury. Proc. Natl. Acad. Sci. USA 103, 1834–1839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernardini ML, Sanna MG, Fontaine A, and Sansonetti PJ 1993. OmpC is involved in invasion of epithelial cells by Shigella flexneri. Infect. Immun 61, 3625–3635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyce JR and Miller RV 1980. Effects of cations on stability of cystic fibrosis associated mucoid Pseudomonas. Lancet 2, 268–269. [DOI] [PubMed] [Google Scholar]
- Boyce JR and Miller RV 1982. Selection of nonmucoid derivatives of mucoid Pseudomonas aeruginosa is strongly influenced by the level of iron in the culture medium. Infect. Immun 37, 695–701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bridier A, Dubois-Brissonnet F, Boubetra A, Thomas V, and Briandet R 2010. The biofilm architecture of sixty opportunistic pathogens deciphered using a high throughput CLSM method. J. Microbiol. Methods 82, 64–70. [DOI] [PubMed] [Google Scholar]
- Cady NC, McKean KA, Behnke J, Kubec R, Mosier AP, Kasper SH, Burz DS, and Musah RA 2012. Inhibition of bio-film formation, quorum sensing and infection in Pseudomonas aeruginosa by natural products-inspired organosulfur compounds. PLoS One 7, e38492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Camilli A and Bassler BL 2006. Bacterial small-molecule signaling pathways. Science 311, 1113–1116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carrano CJ and Raymond KN 1979. Ferric ion sequestering agents. 2. Kinetics and mechanism of iron removal from transferrin by enterobactin and synthetic tricatechols. J. Am. Chem. Soc 101, 5401–5404. [Google Scholar]
- Carver PL 2018. The battle for iron between humans and microbes. Curr. Med. Chem 25, 85–96. [DOI] [PubMed] [Google Scholar]
- Cescau S, Cwerman H, Letoffe S, Delepelaire P, Wandersman C, and Biville F 2007. Heme acquisition by hemophores. Bio-metals 20, 603–613. [DOI] [PubMed] [Google Scholar]
- Chitambar CR and Narasimhan J 1991. Targeting iron-depen dent DNA synthesis with gallium and transferrin-gallium. Patho-biology 59, 3–10. [DOI] [PubMed] [Google Scholar]
- Colvin KM, Gordon VD, Murakami K, Borlee BR, Wozniak DJ, Wong GC, and Parsek MR 2011. The pel polysaccharide can serve a structural and protective role in the biofilm matrix of Pseudomonas aeruginosa. PLoS Pathog 7, e1001264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colvin KM, Irie Y, Tart CS, Urbano R, Whitney JC, Ryder C, Howell PL, Wozniak DJ, and Parsek MR 2012. The Pel and Psl polysaccharides provide Pseudomonas aeruginosa structural redundancy within the biofilm matrix. Environ. Microbiol 14, 1913–1928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costerton JW, Stewart PS, and Greenberg EP 1999. Bacterial biofilms: a common cause of persistent infections. Science 284, 1318–1322. [DOI] [PubMed] [Google Scholar]
- De Philippis R, Colica G, and Micheletti E 2011. Exopolysaccharide-producing cyanobacteria in heavy metal removal from water: molecular basis and practical applicability of the biosorption process. Appl. Microbiol. Biotechnol 92, 697–708. [DOI] [PubMed] [Google Scholar]
- Donlan RM 2002. Biofilms: microbial life on surfaces. Emerg. Infect. Dis 8, 881–890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drenkard E and Ausubel FM 2002. Pseudomonas biofilm formation and antibiotic resistance are linked to phenotypic variation. Nature 416, 740–743. [DOI] [PubMed] [Google Scholar]
- Ferreira JA, Penner JC, Moss RB, Haagensen JA, Clemons KV, Spormann AM, Nazik H, Cohen K, Banaei N, Carolino E, et al. 2015. Inhibition of Aspergillus fumigatus and its biofilm by Pseudomonas aeruginosa is dependent on the source, phenotype and growth conditions of the bacterium. PLoS One 10, e0134692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischbach MA, Lin H, Zhou L, Yu Y, Abergel RJ, Liu DR, Raymond KN, Wanner BL, Strong RK, Walsh CT, et al. 2006. The pathogen-associated iroA gene cluster mediates bacterial evasion of lipocalin 2. Proc. Natl. Acad. Sci. USA 103, 16502–16507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flemming HC and Wingender J 2010. The biofilm matrix. Nat. Rev. Microbiol 8, 623–633. [DOI] [PubMed] [Google Scholar]
- Flo TH, Smith KD, Sato S, Rodriguez DJ, Holmes MA, Strong RK, Akira S, and Aderem A 2004. Lipocalin 2 mediates an innate immune response to bacterial infection by sequestrating iron. Nature 432, 917–921. [DOI] [PubMed] [Google Scholar]
- Franklin MJ, Nivens DE, Weadge JT, and Howell PL 2011. Biosynthesis of the Pseudomonas aeruginosa extracellular polysaccharides, alginate, Pel, and Psl. Front. Microbiol 2, 167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedman L and Kolter R 2004. Two genetic loci produce distinct carbohydrate-rich structural components of the Pseudomonas aeruginosa biofilm matrix. J. Bacteriol 186, 4457–4465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilbert P, Jones MV, Allison DG, Heys S, Maira T, and Wood P 1998. The use of poloxamer hydrogels for the assessment of biofilm susceptibility towards biocide treatments. J. Appl. Microbiol 85, 985–990. [DOI] [PubMed] [Google Scholar]
- Goetz DH, Holmes MA, Borregaard N, Bluhm ME, Raymond KN, and Strong RK 2002. The neutrophil lipocalin NGAL is a bacteriostatic agent that interferes with siderophore-mediated iron acquisition. Mol. Cell 10, 1033–1043. [DOI] [PubMed] [Google Scholar]
- Goodman AL, Kulasekara B, Rietsch A, Boyd D, Smith RS, and Lory S 2004. A signaling network reciprocally regulates genes associated with acute infection and chronic persistence in Pseudomonas aeruginosa. Dev. Cell 7, 745–754. [DOI] [PubMed] [Google Scholar]
- Gupta P and Diwan B 2017. Bacterial exopolysaccharide mediated heavy metal removal: A Review on biosynthesis, mechanism and remediation strategies. Biotechnol. Rep. (Amst) 13, 58–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guterman SK, Morris PM, and Tannenberg WJ 1978. Feasibility of enterochelin as an iron-chelating drug: studies with human serum and a mouse model system. Gen. Pharmacol 9, 123–127. [DOI] [PubMed] [Google Scholar]
- Hall-Stoodley L, Costerton JW, and Stoodley P 2004. Bacterial biofilms: from the natural environment to infectious diseases. Nat. Rev. Microbiol 2, 95–108. [DOI] [PubMed] [Google Scholar]
- Harris WR, Carrano CJ, and Raymond KN 1979. Isolation, characterization, and formation constants of ferric aerobactin. J. Am. Chem. Soc 101, 2722–2727. [Google Scholar]
- Hentzer M, Wu H, Andersen JB, Riedel K, Rasmussen TB, Bagge N, Kumar N, Schembri MA, Song Z, Kristoffersen P, et al. 2003. Attenuation of Pseudomonas aeruginosa virulence by quorum sensing inhibitors. EMBO J 22, 3803–3815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoiby N, Krogh Johansen H, Moser C, Song Z, Ciofu O, and Kharazmi A 2001. Pseudomonas aeruginosa and the in vitro and in vivo biofilm mode of growth. Microbes Infect 3, 23–35. [DOI] [PubMed] [Google Scholar]
- Hood MI and Skaar EP 2012. Nutritional immunity: transition metals at the pathogen-host interface. Nat. Rev. Microbiol 10, 525–537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang W and Wilks A 2017. Extracellular heme uptake and the challenge of bacterial cell membranes. Annu. Rev. Biochem 86, 799–823. [DOI] [PubMed] [Google Scholar]
- Hunter RC, Asfour F, Dingemans J, Osuna BL, Samad T, Malfroot A, Cornelis P, and Newman DK 2013. Ferrous iron is a significant component of bioavailable iron in cystic fibrosis airways. MBio 4, e00557–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Irie Y, Borlee BR, O’Connor JR, Hill PJ, Harwood CS, Wozniak DJ, and Parsek MR 2012. Self-produced exopolysaccha-ride is a signal that stimulates biofilm formation in Pseudomonas aeruginosa. Proc. Natl. Acad. Sci. USA 109, 20632–20636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Javvadi S, Pandey SS, Mishra A, Pradhan BB, and Chatterjee S 2018. Bacterial cyclic β-(1,2)-glucans sequester iron to protect against iron-induced toxicity. EMBO Rep 19, 172–186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jayaraman R 2008. Bacterial persistence: some new insights into an old phenomenon. J. Biosci 33, 795–805. [DOI] [PubMed] [Google Scholar]
- Jensen ET, Kharazmi A, Lam K, Costerton JW, and Hoiby N 1990. Human polymorphonuclear leukocyte response to Pseudomonas aeruginosa grown in biofilms. Infect. Immun 58, 2383–2385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamiya H, Ehara T, and Matsumoto T 2012. Inhibitory effects of lactoferrin on biofilm formation in clinical isolates of Pseudomonas aeruginosa. J. Infect. Chemother 18, 47–52. [DOI] [PubMed] [Google Scholar]
- Kaneko Y, Thoendel M, Olakanmi O, Britigan BE, and Singh PK 2007. The transition metal gallium disrupts Pseudomonas aeruginosa iron metabolism and has antimicrobial and anti-biofilm activity. J. Clin. Invest 117, 877–888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang D, Kirienko DR, Webster P, Fisher AL, and Kirienko NV 2018. Pyoverdine, a siderophore from Pseudomonas aeruginosa, translocates into C. elegans, removes iron, and activates a distinct host response. Virulence 9, 804–817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang D and Kirienko NV 2017. High-throughput genetic screen reveals that early attachment and biofilm formation are necessary for full pyoverdine production by Pseudomonas aeruginosa. Front. Microbiol 8, 1707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang D, Turner KE, and Kirienko NV 2017. PqsA promotes pyoverdine production via biofilm formation. Pathogens 7, 3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelson AB, Carnevali M, and Truong-Le V 2013. Gallium-based anti-infectives: targeting microbial iron-uptake mechanisms. Curr. Opin. Pharmacol 13, 707–716. [DOI] [PubMed] [Google Scholar]
- Kester JC and Fortune SM 2014. Persisters and beyond: mechanisms of phenotypic drug resistance and drug tolerance in bacteria. Crit. Rev. Biochem. Mol. Biol 49, 91–101. [DOI] [PubMed] [Google Scholar]
- Kim SK and Lee JH 2016. Biofilm dispersion in Pseudomonas aeruginosa. J. Microbiol 54, 71–85. [DOI] [PubMed] [Google Scholar]
- Kirienko NV, Ausubel FM, and Ruvkun G 2015. Mitophagy confers resistance to siderophore-mediated killing by Pseudo-monas aeruginosa. Proc. Natl. Acad. Sci. USA 112, 1821–1826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirienko NV, Kirienko DR, Larkins-Ford J, Wählby C, Ruvkun G, and Ausubel FM 2013. Pseudomonas aeruginosa disrupts Caenorhabditis elegans iron homeostasis, causing a hypoxic response and death. Cell Host Microbe 13, 406–416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirov SM, Webb JS, O’May CY, Reid DW, Woo JK, Rice SA, and Kjelleberg S 2007. Biofilm differentiation and dispersal in mucoid Pseudomonas aeruginosa isolates from patients with cystic fibrosis. Microbiology 153, 3264–3274. [DOI] [PubMed] [Google Scholar]
- Komor U, Bielecki P, Loessner H, Rohde M, Wolf K, West-phal K, Weiss S, and Haussler S 2012. Biofilm formation by Pseudomonas aeruginosa in solid murine tumors - a novel model system. Microbes Infect 14, 951–958. [DOI] [PubMed] [Google Scholar]
- Kostenko V, Ceri H, and Martinuzzi RJ 2007. Increased tolerance of Staphylococcus aureus to vancomycin in viscous media. FEMS Immun. Med. Microbiol 51, 277–288. [DOI] [PubMed] [Google Scholar]
- Kragh KN, Alhede M, Rybtke M, Stavnsberg C, Jensen PO, Tolker-Nielsen T, Whiteley M, and Bjarnsholt T 2018. Inoculation method could impact the outcome of microbiological experiments. Appl. Environ. Microbiol 84, e02264–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kvach JT, Wiles TI, Mellencamp MW, and Kochan I 1977. Use of transferrin-iron enterobactin complexes as the source of iron by serum-exposed bacteria. Infect. Immun 18, 439–445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamont IL, Beare PA, Ochsner U, Vasil AI, and Vasil ML 2002. Siderophore-mediated signaling regulates virulence factor production in Pseudomonas aeruginosa. Proc. Natl. Acad. Sci. USA 99, 7072–7077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leid JG, Willson CJ, Shirtliff ME, Hassett DJ, Parsek MR, and Jeffers AK 2005. The exopolysaccharide alginate protects Pseudomonas aeruginosa biofilm bacteria from IFN-gamma-mediated macrophage killing. J. Immun 175, 7512–7518. [DOI] [PubMed] [Google Scholar]
- Li XH and Lee JH 2017. Antibiofilm agents: A new perspective for antimicrobial strategy. J. Microbiol 55, 753–766. [DOI] [PubMed] [Google Scholar]
- Ma L, Conover M, Lu H, Parsek MR, Bayles K, and Wozniak DJ 2009. Assembly and development of the Pseudomonas aeruginosa biofilm matrix. PLoS Pathog 5, e1000354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma L, Jackson KD, Landry RM, Parsek MR, and Wozniak DJ 2006. Analysis of Pseudomonas aeruginosa conditional psl variants reveals roles for the psl polysaccharide in adhesion and maintaining biofilm structure postattachment. J. Bacteriol 188, 8213–8221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mah TF and O’Toole GA 2001. Mechanisms of biofilm resistance to antimicrobial agents. Trends Microbiol 9, 34–39. [DOI] [PubMed] [Google Scholar]
- Meyer JM, Neely A, Stintzi A, Georges C, and Holder IA 1996. Pyoverdin is essential for virulence of Pseudomonas aeruginosa. Infect. Immun 64, 518–523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mikkelsen H, Sivaneson M, and Filloux A 2011. Key two-component regulatory systems that control biofilm formation in Pseudomonas aeruginosa. Environ. Microbiol 13, 1666–1681. [DOI] [PubMed] [Google Scholar]
- Miller RV and Rubero VJ 1984. Mucoid conversion by phages of Pseudomonas aeruginosa strains from patients with cystic fibrosis. J. Clin. Microbiol 19, 717–719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minandri F, Imperi F, Frangipani E, Bonchi C, Visaggio D, Facchini M, Pasquali P, Bragonzi A, and Visca P 2016. Role of iron uptake systems in Pseudomonas aeruginosa virulence and airway infection. Infect. Immun 84, 2324–2335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohite BV, Koli SH, Narkhede CP, Patil SN, and Patil SV 2017. Prospective of microbial exopolysaccharide for heavy metal exclusion. Appl. Biochem. Biotechnol 183, 582–600. [DOI] [PubMed] [Google Scholar]
- Moppert X, Le Costaouec T, Raguenes G, Courtois A, SimonColin C, Crassous P, Costa B, and Guezennec J 2009. Investigations into the uptake of copper, iron and selenium by a highly sulphated bacterial exopolysaccharide isolated from microbial mats. J. Ind. Microbiol. Biotechnol 36, 599–604. [DOI] [PubMed] [Google Scholar]
- Moradali MF, Ghods S, and Rehm BH 2017. Pseudomonas aeruginosa lifestyle: A paradigm for adaptation, survival, and persistence. Front. Cell. Infect. Microbiol 7, 39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moreau-Marquis S, Bomberger JM, Anderson GG, Swiatecka-Urban A, Ye S, O’Toole GA, and Stanton BA 2008. The ΔF508-CFTR mutation results in increased biofilm formation by Pseudomonas aeruginosa by increasing iron availability. Am. J. Physiol. Lung Cell. Mol. Physiol 295, L25–L37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moreau-Marquis S, O’Toole GA, and Stanton BA 2009. Tobra mycin and FDA-approved iron chelators eliminate Pseudomonas aeruginosa biofilms on cystic fibrosis cells. Am. J. Respir. Cell Mol. Biol 41, 305–313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mulcahy H, Charron-Mazenod L, and Lewenza S 2008. Extra-cellular DNA chelates cations and induces antibiotic resistance in Pseudomonas aeruginosa biofilms. PLoS Pathog 4, e1000213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Loughlin CT, Miller LC, Siryaporn A, Drescher K, Semmelhack MF, and Bassler BL 2013. A quorum-sensing inhibitor blocks Pseudomonas aeruginosa virulence and biofilm formation. Proc. Natl. Acad. Sci. USA 110, 17981–17986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’May CY, Sanderson K, Roddam LF, Kirov SM, and Reid DW 2009. Iron-binding compounds impair Pseudomonas aeruginosa biofilm formation, especially under anaerobic conditions. J. Med. Microbiol 58, 765–773. [DOI] [PubMed] [Google Scholar]
- Oglesby-Sherrouse AG, Djapgne L, Nguyen AT, Vasil AI, and Vasil ML 2014. The complex interplay of iron, biofilm formation, and mucoidy affecting antimicrobial resistance of Pseudo-monas aeruginosa. Pathog. Dis 70, 307–320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palmer LD and Skaar EP 2016. Transition metals and virulence in bacteria. Annu. Rev. Genet 50, 67–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parsek MR and Greenberg EP 2000. Acyl-homoserine lactone quorum sensing in Gram-negative bacteria: a signaling mechanism involved in associations with higher organisms. Proc. Natl. Acad. Sci. USA 97, 8789–8793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peek ME, Bhatnagar A, McCarty NA, and Zughaier SM 2012. Pyoverdine, the major siderophore in Pseudomonas aeruginosa, evades NGAL recognition. Interdiscip. Perspect. Infect. Dis 2012, 843509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Penner JC, Ferreira JA, Secor PR, Sweere JM, Birukova MK, Joubert LM, Haagensen JA, Garcia O, Malkovskiy AV, Kaber G, et al. 2016. Pf4 bacteriophage produced by Pseudo-monas aeruginosa inhibits Aspergillus fumigatus metabolism via iron sequestration. Microbiology 162, 1583–1594. [DOI] [PubMed] [Google Scholar]
- Petrova OE and Sauer K 2009. A novel signaling network essential for regulating Pseudomonas aeruginosa biofilm development. PLoS Pathog 5, e1000668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peyton BM 1996. Effects of shear stress and substrate loading rate on Pseudomonas aeruginosa biofilm thickness and density. Wat. Res 30, 29–36. [Google Scholar]
- Peyton BM and Characklis WG 1993. A statistical analysis of the effect of substrate utilization and shear stress on the kinetics of biofilm detachment. Biotechnol. Bioeng 41, 728–735. [DOI] [PubMed] [Google Scholar]
- Pogoutse AK and Moraes TF 2017. Iron acquisition through the bacterial transferrin receptor. Crit. Rev. Biochem. Mol. Biol 52, 314–326. [DOI] [PubMed] [Google Scholar]
- Rashid MH, Rumbaugh K, Passador L, Davies DG, Hamood AN, Iglewski BH, and Kornberg A 2000. Polyphosphate kinase is essential for biofilm development, quorum sensing, and virulence of Pseudomonas aeruginosa. Proc. Natl. Acad. Sci. USA 97, 9636–9641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rasmussen B 2000. Filamentous microfossils in a 3,235-million-year-old volcanogenic massive sulphide deposit. Nature 405, 676–679. [DOI] [PubMed] [Google Scholar]
- Rice SA, Tan CH, Mikkelsen PJ, Kung V, Woo J, Tay M, Hauser A, McDougald D, Webb JS, and Kjelleberg S 2009. The biofilm life cycle and virulence of Pseudomonas aeruginosa are dependent on a filamentous prophage. ISME J 3, 271–282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rittman BE 1982. The effect of shear stress on biofilm loss rate. Biotechnol. Bioeng 24, 501–506. [DOI] [PubMed] [Google Scholar]
- Ruhs PA, Boni L, Fuller GG, Inglis RF, and Fischer P 2013. In situ quantification of the interfacial rheological response of bacterial biofilms to environmental stimuli. PLoS One 8, e78524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakuragi Y and Kolter R 2007. Quorum-sensing regulation of the biofilm matrix genes (pel) of Pseudomonas aeruginosa. J. Bacteriol 189, 5383–5386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sauer K, Camper AK, Ehrlich GD, Costerton JW, and Davies DG 2002. Pseudomonas aeruginosa displays multiple pheno- types during development as a biofilm. J. Bacteriol 184, 1140–1154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- She P, Chen L, Qi Y, Xu H, Liu Y, Wang Y, Luo Z, and Wu Y 2016. Effects of human serum and apo-transferrin on Staphylococcus epidermidis RP62A biofilm formation. Microbiologyopen 5, 957–966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shih PC and Huang CT 2002. Effects of quorum-sensing deficiency on Pseudomonas aeruginosa biofilm formation and antibiotic resistance. J. Antimicrob. Chemother 49, 309–314. [DOI] [PubMed] [Google Scholar]
- Singh PK, Parsek MR, Greenberg EP, and Welsh MJ 2002. A component of innate immunity prevents bacterial biofilm development. Nature 417, 552–555. [DOI] [PubMed] [Google Scholar]
- Singh PK, Schaefer AL, Parsek MR, Moninger TO, Welsh MJ, and Greenberg EP 2000. Quorum-sensing signals indicate that cystic fibrosis lungs are infected with bacterial biofilms. Nature 407, 762–764. [DOI] [PubMed] [Google Scholar]
- Skaar EP 2010. The battle for iron between bacterial pathogens and their vertebrate hosts. PLoS Pathog 6, e1000949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stewart PS 1996. Theoretical aspects of antibiotic diffusion into microbial biofilms. Antimicrob. Agents Chemother 40, 2517–2522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoodley P, Sauer K, Davies DG, and Costerton JW 2002. Bio-films as complex differentiated communities. Annu. Rev. Micro-biol 56, 187–209. [DOI] [PubMed] [Google Scholar]
- Takase H, Nitanai H, Hoshino K, and Otani T 2000. Impact of siderophore production on Pseudomonas aeruginosa infections in immunosuppressed mice. Infect. Immun 68, 1834–1839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terry JM, Pina SE, and Mattingly SJ 1992. Role of energy metabolism in conversion of nonmucoid Pseudomonas aeruginosa to the mucoid phenotype. Infect. Immun 60, 1329–1335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tidmarsh GF, Klebba PE, and Rosenberg LT 1983. Rapid release of iron from ferritin by siderophores. J. Inorg. Biochem 18, 161–168. [DOI] [PubMed] [Google Scholar]
- Valdebenito M, Muller SI, and Hantke K 2007. Special conditions allow binding of the siderophore salmochelin to siderocalin (NGAL-lipocalin). FEMS Microbiol. Lett 277, 182–187. [DOI] [PubMed] [Google Scholar]
- Visaggio D, Pasqua M, Bonchi C, Kaever V, Visca P, and Imperi F 2015. Cell aggregation promotes pyoverdine-dependent iron uptake and virulence in Pseudomonas aeruginosa. Front. Microbiol 6, 902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vogeleer P, Tremblay YD, Mafu AA, Jacques M, and Harel J 2014. Life on the outside: role of biofilms in environmental persistence of Shiga-toxin producing Escherichia coli. Front. Micro-biol 5, 317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wakabayashi H, Yamauchi K, Kobayashi T, Yaeshima T, Iwatsuki K, and Yoshie H 2009. Inhibitory effects of lactoferrin on growth and biofilm formation of Porphyromonas gingivalis and Prevotella intermedia. Antimicrob. Agents Chemother 53, 3308–3316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Webb JS, Lau M, and Kjelleberg S 2004. Bacteriophage and phenotypic variation in Pseudomonas aeruginosa biofilm development. J. Bacteriol 186, 8066–8073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Webb JS, Thompson LS, James S, Charlton T, Tolker-Nielsen T, Koch B, Givskov M, and Kjelleberg S 2003. Cell death in Pseudomonas aeruginosa biofilm development. J. Bacteriol 185, 4585–4592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whiteley M, Bangera MG, Bumgarner RE, Parsek MR, Teitzel GM, Lory S, and Greenberg EP 2001. Gene expression in Pseudomonas aeruginosa biofilms. Nature 413, 860–864. [DOI] [PubMed] [Google Scholar]
- Winstanley C, O’Brien S, and Brockhurst MA 2016. Pseudo-monas aeruginosa evolutionary adaptation and diversification in cystic fibrosis chronic lung infections. Trends Microbiol 24, 327–337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wirtanen G, Salo S, Allison DG, Mattila-Sandholm T, and Gil-bert P 1998. Performance evaluation of disinfectant formulations using poloxamer-hydrogel biofilm-constructs. J. Appl. Microbiol 85, 965–971. [DOI] [PubMed] [Google Scholar]
- Wolz C, Hohloch K, Ocaktan A, Poole K, Evans RW, Rochel N, Albrecht-Gary AM, Abdallah MA, and Döring G 1994. Iron release from transferrin by pyoverdin and elastase from Pseudomonas aeruginosa. Infect. Immun 62, 4021–4027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Worlitzsch D, Tarran R, Ulrich M, Schwab U, Cekici A, Meyer KC, Birrer P, Bellon G, Berger J, Weiss T, et al. 2002. Effects of reduced mucus oxygen concentration in airway Pseudomonas infections of cystic fibrosis patients. J. Clin. Invest 109, 317–325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao R and Kisaalita WS 1997. Iron acquisition from transferrin and lactoferrin by Pseudomonas aeruginosa pyoverdin. Micro-biology 143 (Pt 7), 2509–2515. [DOI] [PubMed] [Google Scholar]
- Yoon SS, Hennigan RF, Hilliard GM, Ochsner UA, Parvatiyar K, Kamani MC, Allen HL, DeKievit TR, Gardner PR, Schwab U, et al. 2002. Pseudomonas aeruginosa anaerobic respiration in biofilms: relationships to cystic fibrosis pathogenesis. Dev. Cell 3, 593–603. [DOI] [PubMed] [Google Scholar]
- Yu S, Wei Q, Zhao T, Guo Y, and Ma LZ 2016. A survival strategy for Pseudomonas aeruginosa that uses exopolysaccharides to sequester and store iron to stimulate Psl-dependent biofilm formation. Appl. Environ. Microbiol 82, 6403–6413. [DOI] [PMC free article] [PubMed] [Google Scholar]