Abstract
Atrial fibrillation (AF) is the most common sustained cardiac arrhythmia in the general population. The Korean Heart Rhythm Society organized a Korean AF Management Guideline Committee and analyzed all available studies regarding the management of AF, including studies on Korean patients. This guideline is based on recent data of the Korean population and the recent guidelines of the European Society of Cardiology, European Association for Cardio-Thoracic Surgery, American Heart Association, and Asia Pacific Heart Rhythm Society. Expert consensus or guidelines for the optimal management of Korean patients with AF were achieved after a systematic review with intensive discussion. This article provides general principles for appropriate risk stratification and selection of anticoagulation therapy in Korean patients with AF. This guideline deals with optimal stroke prevention, screening, rate and rhythm control, risk factor management, and integrated management of AF.
Keywords: Atrial fibrillation, Guideline, Anticoagulants, Therapy
PREAMBLE
This guideline is based on recent data of the Korean population and the recent guidelines.1),2),3),4),5) The level of evidence and strength of the recommendation of particular management options were weighed and graded according to predefined scales as outlined in Tables 1 and 2. The Korea Heart Rhythm Society (KHRS) Committee for Practice Guidelines supervises and coordinates the preparation of a new guideline produced by task forces, expert groups, or consensus panels. The Committee is also responsible for endorsing this guideline.
Table 1. Recommendation classes.
| Classes of recommendations | Definition | Suggested wording to use |
|---|---|---|
| Class I | Evidence and/or general agreement that a given treatment or procedure is beneficial, useful, effective. | Is recommended/is indicated |
| Class II | Conflicting evidence and/or a divergence of opinion about the usefulness/efficacy of the given treatment or procedure. | |
| Class IIa | Weight of evidence/opinion is in favour of usefulness/efficacy. | Should be considered |
| Class IIb | Usefulness/efficacy is less well established by evidence/opinion. | May be considered |
| Class III | Evidence or general agreement that the given treatment or procedure is not useful/effective, and in some cases may be harmful. | Is not recommended |
Table 2. Levels of evidence.
| Levels of evidence | Definition |
|---|---|
| Evidence A | Data derived from multiple RCTs or meta-analyses. |
| Evidence B | Data derived from a single RCT or large non-randomized studies. |
| Evidence C | Consensus of opinion of the experts and/or small studies, retrospective studies, registries. |
RCT = randomized controlled trial.
INTRODUCTION
Atrial fibrillation (AF) is the most common sustained cardiac arrhythmia in the general population.6),7),8),9) AF increases the risk of mortality and morbidity resulting from stroke, congestive heart failure (HF), and impaired quality of life, explaining its enormous socioeconomic and healthcare implications.10) The prevalence of AF has been projected to increase to 12 million people in the USA by the year 2050 and 17.9 million in Europe by the year 2060, with more than half of these patients being ≥80 years of age, leading to substantial public health and economic burdens.11),12),13) Consequently, the healthcare burden associated with AF is growing considerably and is mainly driven by hospitalizations.14),15) As populations continue to age, AF is likely to become a greater public health burden.
EPIDEMIOLOGY OF ATRIAL FIBRILLATION IN KOREA
Many of the risk factors for developing AF also lead to complications related to AF such as stroke and death. The prediction of incident AF has been the focus of reviews, and risk factors have been used to derive clinical risk scores for incident AF.16),17) The largest derivation and validation in an Asian population (including Korea) is the simple C2HEST score: C2: coronary artery disease (CAD)/chronic obstructive pulmonary disease (COPD) (1 point each); H: Hypertension; E: Elderly (Age ≥75, doubled); S: Systolic HF (doubled); T: Thyroid disease (hyperthyroidism).18)
Prevalence and incidence of atrial fibrillation
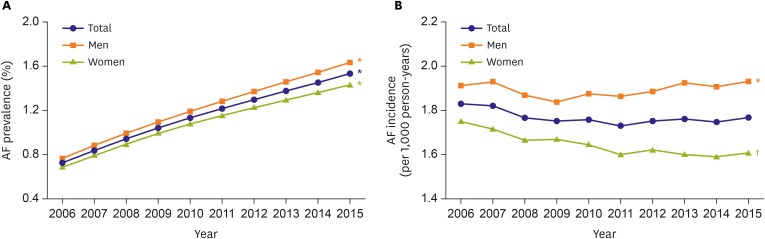
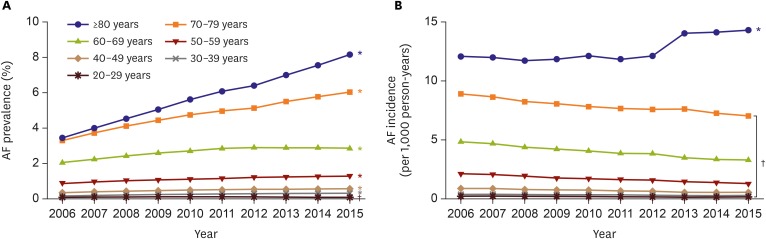
Reliable prevalence and incidence figures are needed for clinicians and policymakers.19) The prevalence of AF progressively increased by 2.10-fold from 0.73% in 2006 to 1.53% in 2015 in Korea. The prevalence was significantly greater in men than in women for all years (Figure 1A). The annual trends of AF incidence were stable with the 10-year overall incidence of 1.77 per 1,000 person-years. The 10-year overall incidence in men was 1.89 per 1,000 person-years, 1.16 times higher than 1.65 per 1,000 person-years in women, and the tendency was consistent over the study period (Figure 1B).20) The prevalence of AF in Korea was similar to the recent prevalence rates of 1.07–1.6% in Asia; 1.07% in 2011 in Taiwan,21) 1.5% in Singapore,22) and 1.6% in 2006 in Japan.23) Across all age groups, the prevalence consistently increased over the study period, except the prevalence among those aged 20–29 years decreased significantly (Figure 2A). The annual AF incidence in subjects aged ≥80 years increased significantly from 12.1 in 2006 to 14.3 per 1,000 person-years in 2015, while the incidences in all other age groups decreased (Figure 2B).20) Patients with regular hospital visits showed a lower prevalence of AF and an increasing trend of the incidence of AF.24),25)
Figure 1. Annual prevalence (A) and incidence (B) of AF, 2006–2015, stratified by sex.
AF = atrial fibrillation.
*p value for increasing trends <0.001. †p value for decreasing trends <0.001.
Figure 2. Annual incidence (A) and prevalence (B) of AF, 2006–2015, stratified by age.
AF = atrial fibrillation.
*p value for increasing trends <0.001. †p value for decreasing trends <0.001.
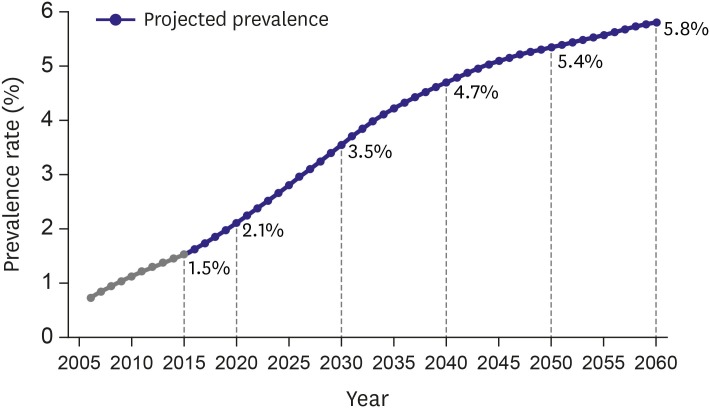
The prevalence of Korean AF is expected to be 5.81% (2,290,591 AF patients) in 2060 (Figure 3), while the prevalence of Taiwan AF is estimated to be 4.01% in 2050.21) Although the prevalence of AF is increasing steeply in Asia, it remains lower in Korea (and many Asian countries) than that of Western populations. Because AF is becoming an important public health burden, regional and socioeconomic inequality of AF patterns and treatment is also important.26) The prevalence of AF differs according to geographical regions and income levels.27)
Figure 3. The projected prevalence of atrial fibrillation.
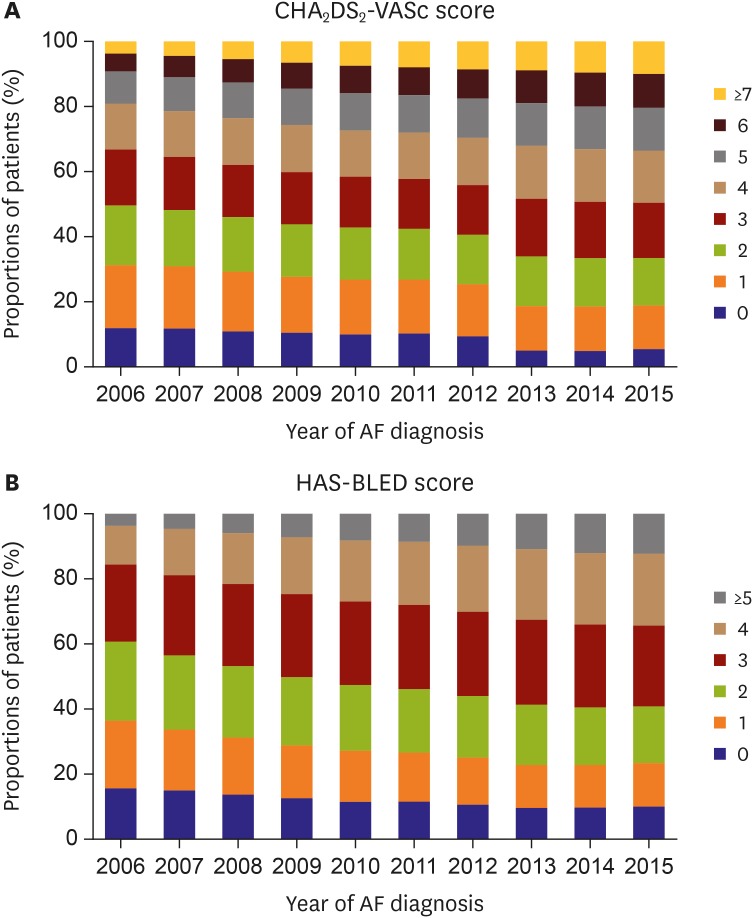
The proportion of patients with a congestive HF, hypertension, age ≥75 (doubled), diabetes mellitus, prior stroke or transient ischemic attack (doubled), vascular disease, age 65–74, female (CHA2DS2-VASc) score ≥2 increased from 68.8% to 81.2% from 2006 to 2015. The proportion of patients with high bleeding risk according to HAS-BLED (hypertension, abnormal renal/liver function [1 point each], stroke, bleeding history or predisposition, labile international normalized ratio [INR], elderly [0.65], drugs/alcohol concomitantly [1 point each]) (score ≥3) increased from 39.3% in 2006 to 59.1% in 2015 (Figure 4).20)
Figure 4. Temporal trends of newly diagnosed AF patient by CHA2DS2-VASc and HAS-BLED scores, 2006–2015.
AF = atrial fibrillation.
Hospital care burden of atrial fibrillation
Overall, hospitalizations for AF increased by 420% from 767 to 3,986 per 1 million Korean population from 2006 to 2015. Most admissions occurred in patients aged ≥70 years, and the most frequent coexisting conditions were hypertension, HF, and COPD. Hospitalizations mainly due to major bleeding and AF control increased, whereas those mainly due to ischemic stroke and myocardial infarction decreased.8),28)
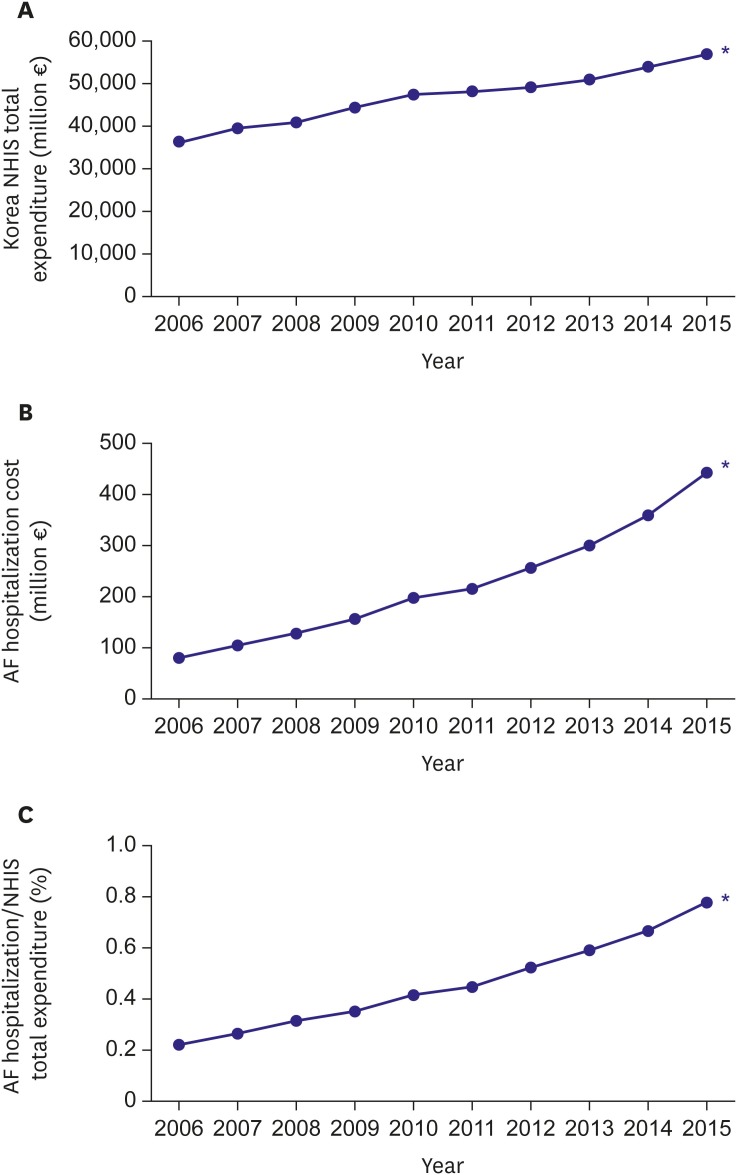
The total cost of care increased even after adjustment for inflation from ₩8.79 billion (€68.4 million) in 2006 to ₩49.8 billion (€388.4 million) in 2015, equivalent to 0.78% of the Korean National Health Insurance Service (NHIS) total expenditure (Figure 5). The total care cost related with AF was estimated as $16–26 billion in the United States, accounting for 1% of the national healthcare budget in the United Kingdom.15),29),30)
Figure 5. Temporal trends of medical costs, 2006–2015. (A) Korean NHIS total expenditures (million €), (B) total AF hospitalization costs (million €), and (C) the proportion of total AF hospitalization costs to Korean NHIS total expenditures (%).
AF = atrial fibrillation; NHIS = National Health Insurance Service.
*p value for trends <0.001.
Prognosis of atrial fibrillation
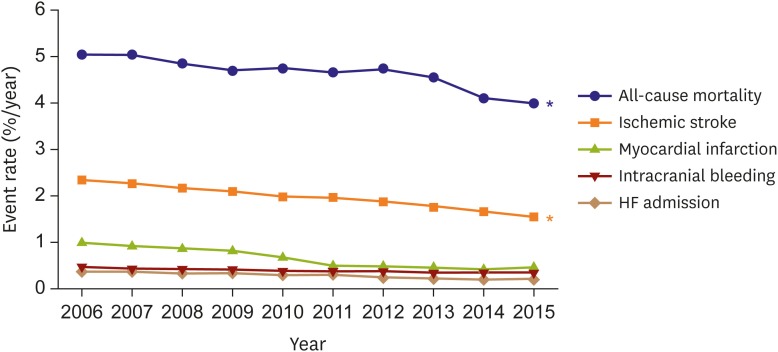
Among prevalent AF patients, annual event rates for all-cause mortality, ischemic stroke, intracranial bleeding, HF admission, and myocardial infarction significantly declined for a decade. In contrast, these events did not change among the non-AF Korean population (Figure 6). Over the last 5 decades, AF-associated mortality decreased by 25% in the Framingham Heart Study.31)
Figure 6. Temporal trends in 1-year adverse event rates of prevalent AF Korean population each year.
AF = atrial fibrillation; HF = heart failure
*p value for trends <0.001.
In Korea, we observed a 20% reduction in mortality over a decade from 5.0%/year in 2006 to 4.0%/year in 2015.20) Overall in-hospital mortality decreased from 7.5% in 2006 to 4.3% in 2015.28) The in-hospital mortality was highest in patients ≥80 years of age (7.7%) and in those with chronic kidney disease (7.4%). Improved survival after AF onset may arise from: 1) earlier detection (lead time) owing to heightened awareness; 2) changed diagnostic criteria (as described above); 3) enhanced surveillance of AF patients; 4) advances in guideline-recommended treatments for AF32) including oral anticoagulation (OAC) therapy to reduce the risk of embolization3); and 5) more aggressive treatment of complications and comorbidities such as hypertension, ischemic heart disease, HF, and hypercholesterolemia.18
Given the high mortality associated with HF33) and stroke,34) the 52% reduction in HF subsequent to AF observed over the study period and the 9% reduction in risk of ischemic stroke is likely to have contributed substantially to the improved survival. Although our results showing a declining associated risk for HF and stroke following AF are in line with those for other Western populations,31) the 1-year rates of HF and stroke are 0.2%/year and 1.8%/year, which are still higher than 0.1%/year and 0.6%/year in an age- and sex-matched non-AF population in 2013.
SCREENING FOR ATRIAL FIBRILLATION
The early detection of asymptomatic AF could prevent associated ischemic stroke associated by instituting appropriate anticoagulation.35),36) AF first diagnosed at the event of stroke comprise nearly 10% of total ischemic stroke cases. The incidence of screen-detected AF strongly depends on the population screened and screening duration/intensity.37)
Screening for atrial fibrillation by 12-lead electrocardiography
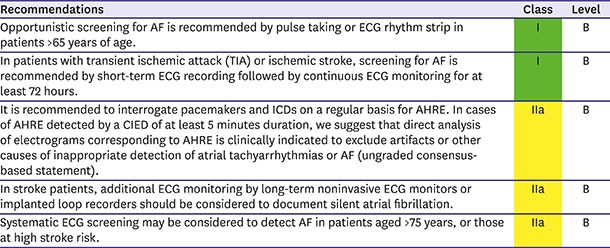
To diagnose AF, its documentation on electrocardiogram (ECG) is mandatory. As the misdiagnosis of AF could cause unnecessary risks and costs for patient management, confirming the diagnosis on ECG is essential. The American College of Cardiology/American Heart Association/Heart Rhythm Society guideline of the management of AF recommends the ECG documentation of AF as a class I indication.2) Moreover, AF is frequently asymptomatic, especially in older people.38) As such, symptom-driven ECG has a substantial limitation for detecting AF. One study revealed that 161 of 476 individuals with new subclinical AF were at an increased risk of cardiovascular and all-cause mortality compared to patients with typical symptoms after the adjustment for age and stroke risk score.39) Another study showed that a single-point screening of a general population ≥ 65 years of age detects subclinical AF in 1.4% of cases and that AF is almost always persistent.40) In the prospective EURObservational Research Programme registry, mortality at 1 year was more than 2-fold higher in asymptomatic than symptomatic patients (9.4% vs. 4.2%, p<0.001) independent of age and comorbidities.41) In the Belgrade AF study including consecutive first-diagnosed AF patients, 10-year survival free of ischemic stroke or AF progression was worse in patients with an asymptomatic presentation.42) The SAFE study43) showed that targeted and total population screening for subclinical AF seems cost-effective in people aged ≥65 years, and similar results were repeatedly reported using intermittent ECG screening in different populations.44),45) A systemic review showed screening of elderly people revealed a prevalence of 2.3% for persistent AF using short-term ECG monitoring or ECG after pulse palpation.40) These findings encourage the further evaluation of systematic AF screening programs in elderly or increased risk populations, such as stoke survivors or patients with intracardiac devices.
Extended-term screening for atrial fibrillation
As elderly populations continue increasing, the incidence of subclinical AF is also increasing.46) Furthermore, due to recent advances in new technologies, underdiagnosed AF could be detected. Stepwise screening with 12-lead ECG and handheld ECG recordings increased the rate of diagnosis of asymptomatic paroxysmal AF in unselected residents of Halmstad, Sweden aged 75–76 years.47) Long-term monitoring with implantable or wearable devices like smartphones or smart watches has been validated for the detection of short-term asymptomatic AF.
In the REHEARSE-AF study, self-screening using a handheld ECG device once or twice weekly demonstrated a hazard ratio of 3.9 for the detection of AF at 12 months compared with routine care.48) A nongovernmental organization–led community-based screening program based around community centers demonstrated that the prevalence of AF was 2.3% and newly diagnosed AF was 0.69% with a mean CHA2DS2-VASc score of 3.9±1.5.49) The ASSERT-II study investigated the prevalence of subclinical AF among 256 patients with an average left atrial volume of 76.5 mL using implantable loop recorders, and subclinical AF lasting ≥ 5 minutes was detected among 34.4% of patients per year over a mean follow-up of 16 months.50) From these data, the AF-SCREEN group, an international collaboration including more than 100 physicians, nurses, allied health professionals, health economists, and patient advocates, endorsed the use of routine screening of at-risk populations.35)
Screening of patients with intracardiac device or previous stroke
A cardiac-implanted electronic device (CIED) could continuously monitor atrial rhythm and detect atrial high-rate episodes (AHRE). However, AHRE has been not used to detect AF. A minimum 5-minute AHRE duration had clinical relevance in the MOST study.51) Alternative arbitrary or data-derived AHRE burden cut points ranging from 5 minutes to 24 hours have been explored over the subsequent 10 years.52) The ASSERT study indicated that stroke risk was increased only in patients with AHRE ≥24 hours.53) The stroke risk in AHRE patients seemed lower than that in patients with diagnosed AF,54) and strokes often occur without AHRE being detected within 30 days before the event.55),56),57),58) Patients with CIED should be regularly screened for AHRE, while those with AHRE should undergo further assessments for stroke risk factors and overt AF, including ECG monitoring.
Stroke is the first manifestation of AF in >25% of AF-related stroke cases.59) In the Swedish registry of ischemic strokes, approximately 9% were associated with subclinical AF and 20% with undertreated AF,59),60) whereas in a global registry, 10% were caused by previously unknown AF.61) Sequential ECG monitoring detected AF in 23.7% of stroke survivors62) and in 11.5% in different meta-analyses of prospective observational studies or randomized controlled trials (RCTs),50) with variations depending on optimal timing, methods, and duration of monitoring for the detection of AF. Cryptogenic stroke defined as the cause of ischemic stroke remains uncertain despite a complete diagnostic evaluation.63) AF detection is not uncommon in unselected stroke patients (hazard ratio [HR], 6.2; 95% confidence interval [CI], 4.4–8.3),64) but is more likely in patients with cryptogenic stroke with implantable loop recorders or who have undergone prolonged ECG monitoring.64),65) Accordingly, prolonged ECG monitoring seems reasonable in all survivors of ischemic stroke without overt AF.
Summary of recommendations for AF screening
DETECTION AND MANAGEMENT OF RISK FACTORS AND CONCOMITANT CARDIOVASCULAR DISEASE
Several concomitant conditions are closely related to AF development, recurrence, and complications. The prevention, detection, and treatment of these conditions are essential to preventing AF and reducing its burden. AF independently increases all-cause mortality, and only 1 in 10 deaths in AF patient are related to stroke, while >7 in 10 are cardiovascular.66) Hence, cardiovascular and comorbidity risk management is essential as part of the holistic or integrated care of AF management to reduce deaths and hospitalisations.66)
HF and AF coexist in many patients and can exacerbate each other. HF is a risk factor of AF (HR, 1.43; 95% CI, 0.85–2.40).67),68) The principal of AF management in HF patients does not differ from that in patients without HF, and these efforts should be performed regardless of left ventricular ejection fraction (LVEF).69) Appropriate OAC therapy by patient stroke risk is crucial and optimal HF therapy by guideline is also important.69) Angiotensin-converting enzyme inhibitors (ACEIs)/angiotensin receptor blockers (ARBs) with a beta-blocker or eplerenone reduced the risk of new-onset AF in patients with reduced LVEF HF patients.70),71),72),73),74) According to recently published data in the CASTLE-AF trial, catheter ablation of AF reduced the risk of all-cause death (47%) and cardiovascular death (51%) in patients with HF and reduced LVEF.75) Catheter ablation of AF in HF patients could be a treatment option for improvement outcomes in selected patients.
Hypertension is a risk factor of AF development (HR, 1.32; 95% CI, 1.08–1.60) and a risk factor of stroke and bleeding in AF patients. Good blood pressure control should be considered part of the optimal care of AF patients.68),76),77) Several previous reports suggested that ACEIs or ARBs had a beneficial effect on new-onset AF and the prevention of AF recurrence.70),71),72),78),79),80),81),82)
Diabetes is a commonly prevalent comorbidity with AF sharing common risk factors.83),84),85),86) Diabetes is a risk factor of AF (HR, 1.25; 95% CI, 0.98–1.60) and a risk factor of stroke in AF patients, with no profound differences between type I and type II diabetes.68),87),88) Although there is no evidence that intensive glycemic control does not reduce AF development, diabetes severity is associated with an increased risk of AF development (e.g. diabetic retinopathy).87),89)
INTEGRATED TREATMENTS FOR ATRIAL FIBRILLATION PATIENTS
One important issue for implementing integrated care management of AF is how to get people to remember the components of such an approach. The latter should streamline the holistic management pathway whether in primary care, hospitals and even understanding by patient.
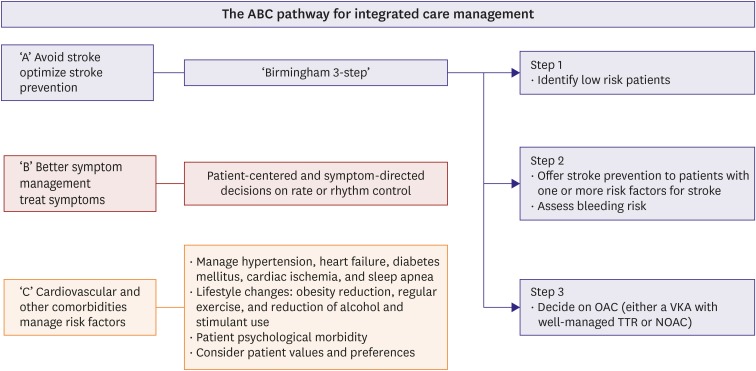
Use of the ABC pathway of integrated care management is suggested as follows: 1) ‘A’ Avoid stroke with Anticoagulation; 2) ‘B’ Better symptom management (i.e. patient-centered, symptom directed decisions on rate vs. rhythm control); 3) ‘C’ Cardiovascular and comorbidity management, including lifestyle factors (Figure 7).90) Application of the simple ABC pathway allows the streamlining of integrated care for AF patients in a holistic manner and has been reported to be associated with a lower risk of adverse outcomes, including recent data in a Korean cohort.66) An integrated approach for AF management is the basis of consistent guideline-based treatment of AF and such guideline adherence helps improve patient outcomes.91),92),93),94)
Figure 7. The basic concept of acute and chronic management of AF. Modified from Lip et al.5).
AF = atrial fibrillation; NOAC = non-vitamin K antagonist oral anticoagulant; OAC = oral anticoagulation therapy; TTR = time in therapeutic range.
To accurately assess the effect of AF on cardiovascular disease, it is necessary to refer to a cardiologist after an initial diagnosis, especially if acute treatment is required, as follows: 1) unstable vital signs including uncontrollably fast heart rate; 2) symptomatic bradycardia despite reduction or stopping of nodal blocker; 3) ongoing or severe angina with reduced left ventricular function; and 4) transient ischemic attack, stroke, or thromboembolic events.
Components of integrated care
Integrated AF care include patient's active participation, multidisciplinary approach technology use, and all treatment approach (Table 3).
Table 3. Integrated AF treatment.
| Patient's active participation | Multidisciplinary approach | Technology use | All treatment approach |
|---|---|---|---|
| • Patients oriented treatment | • Physicians (primary care physicians, cardiologists, cardiovascular surgeons, arrhythmia specialists, and stroke specialists) and allied health professionals | • Technical support for free communication among team members | • Life style modification |
| • Patient education by specialists | • Good communication and education between the patient and the physician | • Checklist and communication tools | • Anticoagulation |
| • Encouragement for autonomy, self-management | • Monitoring tool on therapy adherence and effectiveness | • Rate control | |
| • Proper control on related comorbidities | • Rhythm control by AADs | ||
| • Patient's active participation on decision marking | • Catheter ablation and surgical interventions (ablation, LAA occluder, AF surgery, etc.) |
AAD = antiarrhythmic drug; AF = atrial fibrillation; LAA = left atrial appendage.
Active patient participation
Chronic diseases such as AF can be expected to have a better long-term therapeutic effect if the patient is well aware of the disease and his or her responsibility in the treatment process.95) Patient-oriented treatment, including the involvement of patients in the decision-making stage, can increase compliance and respect individual preferences, requirements, and autonomy.96) However, the awareness rate of AF was <10% in 2017, which was conducted by the KHRS (release in press conference). Therefore, the KHRS is making diverse efforts to increase awareness of the general public through information campaigns, including risk factor information, recognition, treatment, and self-management of the disease. Self-management includes adapting to the treatment process, changing lifestyles, such as smoking cessation and weight control, and requires patients to be aware of the treatment method and goal.97),98)
Multidisciplinary approach
A multidisciplinary approach involving primary care physicians, cardiologists, cardiovascular surgeons, arrhythmia specialists, and stroke specialists who first encounter the patient can help the patient actively participate in treatment. By engaging the patient in the stage, the patient can adhere to the treatment, which enhances its effect.99),100) Thus, a multidisciplinary approach to AF involves not only specialized medical knowledge but also good communication and education between the patient and physician.
Technological use for smooth communication among medical staff
For the integrated treatment of AF, it is essential to communicate and exchange smoothly among members. This requires technical support for free communication between patients and physicians, primary care physicians, and arrhythmia specialists. Digital programs and smartphone apps can help with this process.101) One pilot study using a smartphone App shows how this can be operationalized.102) Because Korea has high rate of smartphone use, a smartphone App can potentially be used for the management of AF.
All treatments for atrial fibrillation
The ABC pathway described above includes proactive assessment and management of cardiovascular disease and risk factors (cardiovascular and comorbidity risk reduction).90) To this end, the active management of related diseases such as obesity, hypertension, sleep apnea and diabetes should be performed, and lifestyle corrections such as smoking, drinking, and exercise should be corrected.90),103),104)
Diagnostic approach to atrial fibrillation
Integrated assessment of patients with atrial fibrillation
A review of the history of systemic embolism including cardiac infarction and symptoms of AF and causes should be performed. The possible causes of correction should be assessed through interviews regarding lifestyle habits such as diabetes, hypertension, COPD, obesity, and sleep apnea; underlying diseases such as hyperthyroidism; and drinking or smoking.103),104),105),106),107),108),109),110) An analysis of standardized Korean NHIS screening data showed that increased blood pressure and fasting blood sugar alone increased the incidence of AF in pre-hypertensive and pre-diabetic patients.103) Even in an analysis of Asian patients with relatively low degree of body mass index, the incidence of AF increased and the prognosis was poor when obesity was comorbid.104),111) A 12-lead ECG should be used to evaluate the presence of cardiac conduction disturbances, ischemic heart disease, and structural heart disease. Transthoracic echocardiography should be performed on all patients to determine the treatment strategy for AF.
Additional diagnostic methods for patients with atrial fibrillation
Twenty-four-hour Holter monitoring is useful for evaluating heart rate and the relationship between symptoms and AF. In particular, information about heart rate during exercise or activity provided by 24-hour Holter monitoring can be used to determine if the goal of heart rate modulation through drug therapy has been achieved. Transesophageal echocardiography (TEE) is useful for evaluating left atrial function and screening for thrombus in the left atrium. Therefore, the evaluation of intracardiac thrombi through TEE is essential in patients who are undergoing invasive sinus rhythm conversion or radiofrequency ablation.112),113)
Follow-up of patients with atrial fibrillation
Most AF patients require periodic follow-up for continuous optimal treatment. Follow-up can be performed by primary care physicians, cardiologists, or arrhythmia specialists. Follow-up of the treatment plan, continued patient participation, and any needed treatment modifications are necessary. The treatment of AF involves prognosis-related treatment (anticoagulant therapy and treatment of cardiovascular disease) and symptom-related treatment (heart rate or cardiac rhythm control).90),114) In addition, if AF is partially recurrent, if the overall frequency, duration of AF decrease, and clinical symptoms are controlled, it is considered successful. The management of diseases (obesity, hypertension, HF, diabetes mellitus, sleep apnea) related to AF should be provided continuously,103),106),111),115),116) while lifestyle factors such as smoking and drinking should be monitored in an integrated manner.117),118)
STROKE PREVENTION THERAPY IN ATRIAL FIBRILLATION PATIENTS
Prediction of stroke risk
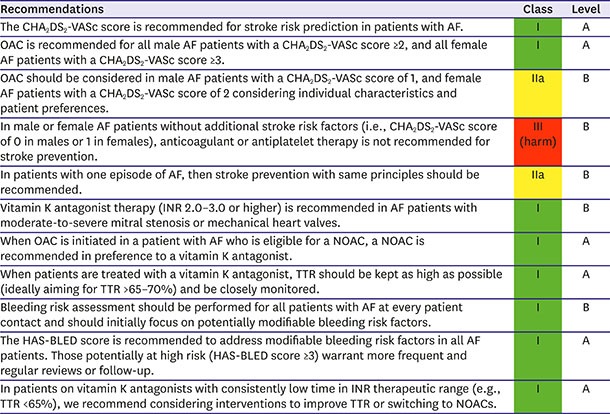
Stroke prevention is the principal management priority in patients with AF. Compared to control or placebo, OAC therapy reduces the risk of stroke by 64% and the risk of death by 26%119) but also increases bleeding risk, which can be fatal. As non-vitamin K oral anticoagulants (NOAC) showed improved efficacy and safety compared with warfarin, the threshold for initiating OAC therapy decreased from an annual stroke rate of 1.7% with vitamin K antagonists to 0.9% with NOAC.120)
The CHA2DS2-VASc score is now used in most guidelines for stroke prevention in patients with AF.2),3),121) The adjusted incidence rates (per 100 person-years) of ischemic stroke were 3.79 in Korea, being 0.26 in low-risk patients (CHA2DS2-VASc score 0 [male] or 1 [female]), 1.18 in intermediate-risk patients (CHA2DS2-VASc score 1 [male]), and 5.30 in high-risk patients (CHA2DS2-VASc ≥2). The incidence rates of patients with a CHA2DS2-VASc score of 1 (male), 2, 3, 4, 5, 6, and 7 or more were 1.04, 1.91, 2.54, 4.72, 5.79, 8.36, and 8.82, respectively (Table 4).9)
Table 4. Ischemic stroke or composite thromboembolism endpoint/100 years at risk in relation to CHA2DS2-VASc scores in Korean patients without anticoagulation throughout follow-up4) .
| CHA2DS2-VASc score | Number of patients | Ischemic stroke | Ischemic stroke/systemic embolism | ||
|---|---|---|---|---|---|
| Unadjusted | Adjusted for aspirin* | Unadjusted | Adjusted for aspirin* | ||
| 0 (male) or 1 (female) | 860 | 0.23 | 0.26 | 0.26 | 0.29 |
| 1 (male) | 550 | 1.04 | 1.18 | 1.20 | 1.35 |
| 2 | 975 | 1.91 | 2.21 | 2.04 | 2.35 |
| 3 | 911 | 2.54 | 2.88 | 2.67 | 3.04 |
| 4 | 836 | 4.72 | 5.34 | 5.10 | 5.76 |
| 5 | 770 | 5.79 | 6.54 | 5.98 | 6.76 |
| 6 | 513 | 8.36 | 9.50 | 8.61 | 9.77 |
| 7 or more | 440 | 8.82 | 9.97 | 9.03 | 10.21 |
| Total | 5,855 | 3.32 | 3.79 | 3.49 | 3.98 |
TE = thromboembolic event.
*Adjustment made for exposure to aspirin treatment, assuming that aspirin provides a 19% reduction in TE risk, to give an indication of ‘untreated’ rates.
The more recent focus of stroke prevention in patients with non-valvular AF has shifted away from predicting “high-risk” patients toward initially identifying patients at a “truly low risk” of ischemic stroke in whom OAC has no net clinical benefit.122),123),124),125) Korean patients who were categorized as “low risk” by the CHA2DS2-VASc score (i.e. score 0 in males or 1 in females) consistently had an event rate of <1%/year.9) CHA2DS2-VASc had the best sensitivity (98.8% vs. 85.7% in CHADS2 and 74.8% in the ATRIA study) and negative predictive value (98.8% vs. 95.3% for CHADS2 and 93.7% for the ATRIA study) for the prediction of stroke incidence and was best for predicting the absence of ischemic stroke during 5 years of follow-up (odds ratio, 16.4; 95% CI, 8.8–30.8).125) However, the CHA2DS2-VASc score had lower net reclassification improvement and c-index suggesting that it is specifically better to discriminate true low-risk AF for stroke.125),126)
Individual stroke risk factors: sex, age, and hypertension
On multivariate analysis, significant associations between CHA2DS2-VASc risk factors and ischemic stroke were observed. The significance of vascular disease or diabetes mellitus were attenuated after multivariate adjustment, and female sex (HR, 0.73; 95% CI, 0.64–0.84) had a lower risk of ischemic stroke than male sex in Korean NHIS sample cohort (Table 5).9),126) Coronary and peripheral artery disease have been reported to be important independent risks for stroke in AF.127),128) Several cohort studies have shown that female sex is a risk factor for stroke, although this is dependent on age and the presence of other non-sex risk factors.129),130),131),132),133) Recently, female sex was suggested as a risk modifier for stroke in patients with AF, rather than a risk factor.134) Several Asian cohort studies from Hong Kong,135) China,136) Taiwan,127) and Japan137) have suggested that female sex was not an independent risk factor for ischemic stroke, again suggesting some potential ethnic differences in the risk of stroke between Asian and non-Asian populations. Consistent with previous Asian studies, female sex was not a risk factor for stroke in a Korean cohort; instead, it was associated with a lower stroke risk of ischemic stroke than male sex. Other risk factors in our population such as older age, previous stroke or TIA history, HF, and hypertension remained independent stroke risk factors consistent with findings in western cohorts.9)
Table 5. Associations between baseline factors and ischemic stroke in patients without anticoagulant treatment4) .
| Ischemic stroke | ||||||
|---|---|---|---|---|---|---|
| Number with event | Univariable | Multivariable | ||||
| HR | 95% CI | HR | 95% CI | |||
| Age (years) | ||||||
| <65 | 161/2,594 | Ref | Ref | |||
| 65–74 | 320/1,700 | 3.44 | 2.84–4.16 | 2.11 | 1.73–2.58 | |
| >75 | 338/1,561 | 4.70 | 3.90–5.68 | 3.11 | 2.51–3.85 | |
| Women | 385/2,835 | 0.94 | 0.82–1.07 | 0.75 | 0.63–0.86 | |
| Ischemic stroke/TIA | 411/1,433 | 3.81 | 3.32–4.37 | 2.58 | 2.23–2.97 | |
| Atherosclerotic disease | ||||||
| Myocardial infarction | 139/764 | 1.49 | 1.24–1.79 | 0.97 | 0.81–1.17 | |
| Peripheral arterial disease | 113/611 | 1.45 | 1.19–1.76 | 0.95 | 0.78–1.17 | |
| Vascular disease* | 221/1,206 | 1.54 | 1.32–1.80 | 0.98 | 0.84–1.15 | |
| HF | 380/1,869 | 2.16 | 1.88–2.48 | 1.23 | 1.06–1.42 | |
| Hypertension | 750/4,422 | 4.04 | 3.16–5.17 | 1.85 | 1.43–2.40 | |
| Diabetes | 216/1,168 | 1.53 | 1.31–1.79 | 1.13 | 0.96–1.32 | |
| ESRD | 23/89 | 2.36 | 1.56–3.57 | 2.03 | 1.33–3.09 | |
| COPD | 134/673 | 1.72 | 1.43–2.07 | 1.13 | 0.94–1.37 | |
| Aspirin use | 505/2,636 | 1.93 | 1.68–2.23 | 1.30 | 1.12–1.50 | |
CI = confidence interval; COPD = chronic obstructive pulmonary disease; ESRD = end-stage renal disease; HF = heart failure; HR = hazard ratio; TIA = transient ischemic attack.
*Vascular disease includes previous myocardial infarction, peripheral arterial disease, or aortic plaque.
Older age is the most important predictor of ischemic stroke in Korean and Taiwan patients with AF.138),139) Patients aged 65–74 years without other risk factors showed a significantly higher risk of stroke than those with one risk factor other than age. Lowering the current age threshold (age ≥65 years) in the CHA2DS2-VASc score to age ≥55 years might be appropriate among Asian patients with AF.139) Two recent Korean studies suggested that blood pressure should be controlled more strictly in AF patients.77),103)
Recommended anticoagulation for Korean patients.
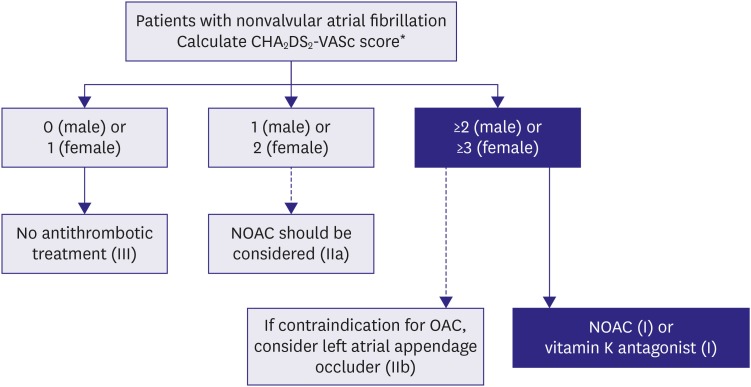
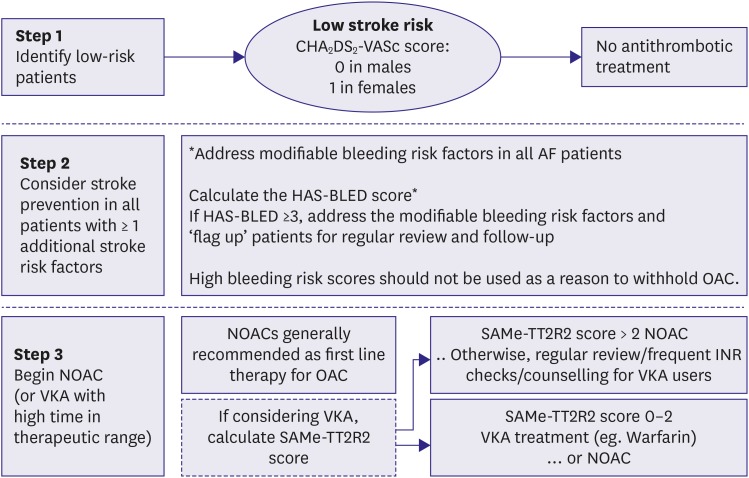
The performance of CHA2DS2-VASc score in Korean populations is comparable with that seen in Western populations. For patients with AF without valvular heart disease, including those with paroxysmal AF, who are at low risk of stroke (e.g., CHA2DS2-VASc score of 0 in males or 1 in females), we suggest no antithrombotic therapy (class III). The next step is to consider stroke prevention (i.e., OAC therapy) for patients with 1 or more non-sex CHA2DS2-VASc stroke risk factors. For patients with a single non-sex CHA2DS2-VASc stroke risk factor, we suggest OAC rather than no therapy, aspirin, or combination therapy with aspirin and clopidogrel (class IIa); and for those at high risk of stroke (e.g., CHA2DS2 ≥2 in males or ≥3 in females), we recommend OAC rather than no therapy, aspirin, or combination therapy with aspirin and clopidogrel (class I) (Figure 8).
Figure 8. Stroke prevention strategy in patients with AF.
AF = atrial fibrillation; HF = heart failure; NOAC = non-vitamin K oral anticoagulant; OAC = oral anticoagulation.
*CHA2DS2-VASs score: a congestive HF, hypertension, age ≥75 (doubled), diabetes mellitus, prior stroke or transient ischemic attack (doubled), vascular disease, age 65–74, female.
Where we recommend or suggest in favor of OAC, we suggest using a NOAC rather than adjusted-dose vitamin K antagonist therapy. With the latter, it is important to aim for good quality anticoagulation control with a time in therapeutic range (TTR) >70%. Attention to modifiable bleeding risk factors (e.g., uncontrolled blood pressure [BP], labile INRs, concomitant use of aspirin or nonsteroidal anti-inflammatory drugs (NSAIDs) in an anticoagulated patient, alcohol excess) should be made at each patient contact, and the HAS-BLED score used to assess the risk of bleeding whereby ‘high risk’ patients (score ≥3) should be reviewed and followed up more frequently. While NOACs are increasingly the preferred option, warfarin is still widely used and the SAMe-TT2R2 score (which has been validated even in Asian cohorts)140) can help identify patients less likely to do well on warfarin, so as to arrange more frequent INR checks, education and counselling – or to consider a NOAC instead of warfarin (Figure 9).5)
Figure 9. Practical management algorithm in light of the 2018 American College of Chest Physicians guidelines, which are evidence based and GRADE.
AF = atrial fibrillation; INR = international normalized ratio; NOAC = non-vitamin K antagonist oral anticoagulant; OAC = oral anticoagulation therapy.
*HAS-BLED: hypertension, abnormal renal/liver function (1 point each), stroke, bleeding history or predisposition, labile INR, elderly (0.65), drugs/alcohol concomitantly (1 point each).
Dynamic adjustment of stroke and bleeding risk
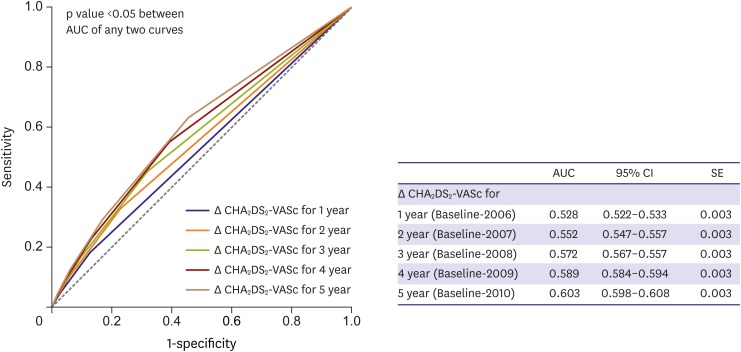
Many clinical variables of stroke and bleeding risk score have “dynamic” variation through follow-up.141),142),143) Age increases annually in all patients, and incident hypertension, diabetes mellitus, vascular disease, congestive HF, and prior stroke or transient ischemic attack may become evident in some patients. These dynamic changes in risk factors may increase the CHA2DS2-VASc score, stroke risk category, and absolute ischemic stroke rate. Despite using only baseline CHA2DS2-VASc score to predict the risk of ischemic stroke in AF patients, a time-dependent CHA2DS2-VASc score and “delta CHA2DS2-VASc score” (follow-up minus baseline) improved the prediction of ischemic stroke in Korean and Taiwan AF populations (Figure 10).141),142)
Figure 10. Receiver operating characteristic curves of Δ CHA2DS2-VASc score for predicting ischemic stroke during the entire second follow-up period.
AUC = area under the curve; CI = confidence interval; SE = standard error.
Recommended non-vitamin K oral anticoagulants dose regimen for Korean patients
The benefits of NOAC was more profound in Asian population than non-Asian population.144) Based on the standard dose group, NOAC was more effective (OR of stroke/systemic embolism, 0.65 vs. 0.85; p interaction=0.045) and safer (OR for major bleeding, 0.57 vs. 0.89; p interaction=0.046) in Asians than non-Asians. Among high-risk Asian AF population, dabigatran, rivaroxaban, and apixaban demonstrated similar risk of ischemic stroke and lower risk of intracerebral hemorrhage compared with warfarin. All-cause death was significantly lower only with dabigatran and apixaban, whereas not with rivaroxaban in a Korean study.145) However, rivaroxaban also decreased all-cause death in a different Korean study.146)
Elderly patients with AF (such as those aged ≥80 years) and patients with impaired renal function were included in the landmark NOAC trials, but these important subgroups comprised only a small proportion of the patient populations. For dabigatran, reduction of the daily recommended dose to 110 mg twice daily (b.i.d.) is indicated for patients aged ≥80 years. This dose can be reduced to 110 mg b.i.d. if the patient is aged 75–79 years and has other comorbidities that could affect bleeding risk such as previous gastritis, peptic ulcer disease, and moderate renal impairment. Indeed, label (or guideline) – adherent use of dabigatran is clearly associated with better outcomes for stroke, major bleeding, and mortality.147),148) Insufficient published data for apixaban, edoxaban, and rivaroxaban indicate that further work is needed to clarify the bleeding risks of NOAC in the elderly.
For Korean patients with AF, a reduced dosing regimen for dabigatran (110 mg b.i.d.) in patients aged ≥75 years or who have an estimated glomerular filtration rate (eGFR) of 30–50 mL/min; rivaroxaban (15 mg once daily [q.d.]) in patients aged ≥80 years or with an eGFR of 15–49 mL/min; apixaban (2.5 mg b.i.d.) if 2 of the 3 following criteria are present: age ≥80 years or an eGFR 15–29 mL/min or body weight ≤60 kg; or edoxaban (30 mg q.d.) if eGFR is 15–50 mL/min are recommended (Table 6).
Table 6. Dose reduction of NOACs281) .
| Drug | Dose reduction criteria | Dose |
|---|---|---|
| Dabigatran | Creatinine clearance 30–50 mL/min | Dabigatran 110 mg b.i.d. |
| P-glycoprotein inhibitors* | ||
| Clopidogrel, aspirin, NSAIDs | ||
| Increased bleeding risk† | ||
| Age 75 years or more | ||
| Rivaroxaban | Age 80 years or more | Rivaroxaban 15 mg q.d. |
| Creatinine clearance 15–50 mL/min‡ | ||
| Apixaban | At least 2: 1) age 80 years or more, 2) body weight 60 kg or less, 3) creatinine ≥1.5 mg/dL | Apixaban 2.5 mg b.i.d. |
| Edoxaban | P-glycoprotein inhibitors* | Edoxaban 30 mg q.d. |
| Body weight 60 kg or less | ||
| Creatinine clearance 15–50 mL/min‡ |
b.i.d., bis in die (twice a day or twice daily); NOAC, non-vitamin K antagonist oral anticoagulant; NSAID = nonsteroidal anti-inflammatory drug; q.d., quaque die (once a day or once daily).
*P-glycoprotein inhibitors: amiodarone, verapamil, dronedarone, etc.; †Increased bleeding risk: coagulopathy, thrombocytopenia, platelet dysfunction, recent major trauma or biopsy, infective endocarditis; ‡Should be used with caution in patients with significant renal impairment (creatinine clearance 15–29 mL/min).
Low anticoagulation rate
The OAC rate of total AF in the Korean nationwide cohort remains very low at about 18%, while the usage rate of aspirin exceeds 35%.8),25) However, the OAC rate of Korean AF was similar to the recent OAC rates of Taiwan nationwide cohort. Li et al.149) reported that the overall guideline adherence rate was only 13% and even lower among patients with a high CHA2DS2-VASc score in this non-selected nationwide AF registry. Moreover, guideline-adherent antithrombotic management was associated with a 38% lower risk of mortality.
The analysis of a prospective multicenter study performed in tertiary hospitals in Korea (COmparison study of Drugs for symptom control and complication prEvention of Atrial Fibrillation [CODE-AF] registry) showed the optimistic future of stroke prevention in patients with AF. The current OAC rate of AF patients with high stroke risk (CHA2DS2-VASc score ≥2) was about 83%.150) Consistently, in patients with at least 3-year regular hospital visit, OAC prescription rate was higher than nationwide AF registry and tertiary hospital-based registry. It was increased from 34.7% to 50.6%, whereas antiplatelet prescription decreased gradually from 48.2% to 31.5% between 2008 and 2015.151)
However, the recent improvement of OAC rate in tertiary hospitals in Korea is related with increased NOAC use because it was >50%, whereas that of warfarin was still low at 20%. Among AF patients with a high stroke risk and at least 3-year regular hospital visits, OAC utilization was lower in the suburban/rural regions than that observed in the urban regions (48.2% vs. 51.8%, respectively; p<0.001).27)
BLEEDING RISK
Risk factors for bleeding with non-vitamin K antagonist oral anticoagulant, vitamin K antagonist, and antiplatelet therapy
Bleeding risk varies from person to person depending on their pre-existing comorbidities, current antithrombotic regimen and adherence, concomitant medication, and lifestyle choices. Many of these factors cannot be altered but some are modifiable or potentially modifiable.5),152)
• BP control: Good control of BP is vital to reduce the risk of stroke and is essential to decrease the risk of bleeding (particularly intracranial haemorrhage) on antithrombotic therapy.
• Anticoagulation control: Among patients receiving vitamin K antagonist, maintenance of an INR in the therapeutic range (2.0–3.0) is essential. The proportion of TTR should be at least 65% but the ultimate aim/target should be 100%.
• Concomitant medication predisposing to bleeding: Nonessential use of concomitant antiplatelet drugs and NSAIDs should be avoided since these medications increase the risk of bleeding in patients receiving OACs.
• Alcohol intake: Excessive alcohol intake (chronic or binge drinking) increases the risk of bleeding predominantly due to the risk of trauma, but in chronic alcohol abuse through poor medication adherence, hepatic and variceal disease.
• Lifestyle factors: Avoidance of work and/or leisure activities that have the potential to cause serious trauma should be advised.
• Bridging periods off anticoagulation: Interruption of OAC should be avoided to reduce stroke risk since the majority of cardiovascular procedures (e.g., pacemaker implantation or percutaneous coronary intervention [PCI]) can be safely performed on OAC. Bridging (i.e., stopping OAC and providing anticoagulation cover with heparin) should be used in patients with mechanical heart valves but does not appear to be otherwise advantageous.153),154)
• Appropriate choice of OAC: Choice of OAC should be made on an individual basis after stroke and bleeding risk assessment, discussion with the patient and adherence to the prescribing label.
• Falls risk and cognitive impairment: The benefits of ischemic stroke reduction generally outweigh the risk of harm from serious bleeding with OAC use. One estimate was that the patient would need to fall 295 times per year for the risk from falls to outweigh the benefits of stroke reduction.155)
• Reversal of biochemical anomalies: Patients with anemia or reduced platelet count or impaired hepatic/renal function should be investigated and proactively managed.
Bleeding risk assessment
Attention to modifiable bleeding risks are important. However, only relying on this to assess bleeding risk is an inferior strategy to a formal bleeding assessment score, as shown in independent cohorts even from Asia.156),157),158)
There are multiple bleeding risk scores that have been proposed for bleeding risk stratification, with the HEMORR2HAGES (hepatic or renal disease, ethanol abuse, malignancy, older, reduced platelet count/function, hypertension, anemia, genetic factors, excessive fall risk, and stroke), HAS-BLED (hypertension, abnormal renal/liver function [1 point each], stroke, bleeding history or predisposition, labile INR, elderly [0.65], drugs/alcohol concomitantly [1 point each]), ATRIA, ORBIT, and ABC-bleeding scores that have been derived and validated in AF populations.159)
The simple HAS-BLED score has been shown to be similar or outperform older bleeding scores, as well as more simple bleeding scores that include fewer clinical parameters. A high bleeding risk score is not a reason to withhold OAC, as the net clinical benefit is even greater in those patients with high bleeding risk.
Recommendations for stroke prevention in patients with AF
LEFT ATRIAL APPENDAGE OCCLUSION AND EXCLUSION
Left atrial appendage occlusion devices
Transcatheter left atrial appendage (LAA) occlusion or percutaneous LAA ligation has been performed since LAA was proven to be the major source of thrombus formation in patients with non-valvular AF. Two RCTs (PROTECT AF and PREVAIL) have directly compared the LAA occlusion using a Watchman device® with vitamin K antagonist, and these data suggested that LAA occlusion was non-inferior to vitamin K antagonist for the prevention of stroke in AF patients with a moderate stroke risk.160),161),162),163) Recently published longer-term follow-ups of RCTs have demonstrated that the LAA occlusion might reduce the risk of thromboembolic stroke compared with vitamin K antagonist.164) A high implantation success rate (98%) with an acceptable procedure-related complication rate of 4% at 30 days was reported in a large recent European registry.165),166) However, these data were in contrast with the analyses from insurance databases and systematic reviews that claimed higher serious complications related with the implantation procedure, possibly identifying a certain degree of reporting bias. There is also uncertainty how a LAA occlusion would compare against a NOAC. Therefore, the Korean AF guideline recommends that AF patients with contraindications for long-term OAC therapy, a recurrent thromboembolic event, or a high risk of stroke despite OAC therapy may be considered to have LAA occlusion for stroke prevention purposes (class IIb).
Left atrial appendage occlusion or exclusion
Surgical LAA occlusion or exclusion in conjunction with cardiac surgery has been performed with multiple techniques for many decades. The feasibility and safety of surgical LAA occlusion/exclusion were proven in various observational studies.167) However, limited controlled trial data have been published. The role of concomitant AF surgery and LAA occlusion has been evaluated and reported in an RCT in 2015 without showing a clear benefit of LAA exclusion for stroke prevention in the subgroup undergoing AF surgery. A large RCT is currently underway.168) Therefore, the Korean AF guideline recommends that patients with AF undergoing cardiac surgery may benefit from surgical occlusion or the exclusion of LAA for stroke prevention (class IIb). Patients undergoing thoracoscopic AF surgery may benefit from surgical occlusion or the exclusion of LAA for stroke prevention (class IIb).
RATE CONTROL
Heart rate control is a substantial part of the treatment of patients with AF despite the remarkable advancement of pharmacologic and non-pharmacologic rhythm control management. An adequately and appropriately controlled ventricular rate can reduce or eliminate symptoms, improve hemodynamics, and prevent tachycardia-induced cardiomyopathy.
Rate control can be achieved with beta-blockers, non-dihydropyridine calcium channel blockers, digoxin, or combination therapy. Certain antiarrhythmic agents including amiodarone and sotalol also have rate-controlling effects, but they should be reserved for patients requiring rhythm control therapy. When considering which drug to use, clinicians should consider the patient's symptoms, hemodynamic status, presence of HF, and precipitating factors for AF. Medications for acute and long-term rate control are presented in Table 7.
Table 7. Rate control therapy in patients with AF25) .
| Drug | Acute rate control (IV) | Long-term rate control (PO) | Adverse effect | Comments | |
|---|---|---|---|---|---|
| Beta-blockers | |||||
| Bisoprolol | Not available | 1.25–10 mg q.d. or split | Bradycardia, AV block, and hypotension. | Bronchospasm is rare. In cases of asthma, recommend beta-1 selective agents. Contra-indicated in acute HF and a history of severe bronchospasm. | |
| Carvedilol | Not available | 3.125–25 mg b.i.d. | Lethargy, headache, peripheral edema, upper respiratory tract symptoms, gastrointestinal upset, and dizziness. | ||
| 8–64 mg q.d. (ER) | |||||
| Metoprolol | Not available | 12.5–100 mg b.i.d. | |||
| 25–200 mg q.d. (ER) | |||||
| Nebivolol | Not available | 1.25–10 mg q.d. or split | |||
| Esmolol | 500 mcg/kg IV bolus over 1 minutes, then 50–250 mcg/kg/min | ||||
| Calcium-channel blockers | |||||
| Diltiazem | 0.25 mg/kg IV bolus over 2 minutes, then 5–15 mg/h | 60–120 mg t.i.d. | Bradycardia, AV block, and hypotension. | Use with caution in combination with beta-blockers. Reduce dose with hepatic impairment and start with smaller dose in renal impairment. Contra-indicated in left ventricular failure with pulmonary congestion or LVEF <40%. | |
| 90–360 mg q.d. (ER) | Dizziness, malaise, lethargy, headache, hot flushes, gastrointestinal upset, and edema. | ||||
| Verapamil | 0.075–0.15 mg/kg IV bolus over 2 minutes, then 5 mcg/kg/min | 40–120 mg t.i.d. | |||
| 120–480 mg q.d. (ER) | |||||
| Cardiac glycosides | |||||
| Digoxin | 0.25 mg IV with repeated dosing to a maximum of 0.75–1.5 mg over 24 hours | 0.0625–0.25 mg q.d. | Gastrointestinal upset, dizziness, blurred vision, headache, and rash. In toxic states (serum levels >2 ng/mL), digoxin is proarrhythmic and can aggravate HF, particularly with coexistent hypokalemia. | High plasma levels associated with increased risk of death. Check renal function before starting and adapt dose in patients with CKD. Contra-indicated in patients with accessory pathways, ventricular tachycardia and hypertrophic cardiomyopathy with outflow tract obstruction. | |
| Specific indications | |||||
| Amiodarone | 300 mg IV over 1 hour, then 10–50 mg/hr over 24 hours (preferably via central venous catheter). | 100–200 mg q.d. | Hypotension, bradycardia, nausea, QT prolongation, pulmonary toxicity, skin discoloration, thyroid dysfunction, corneal deposits and cutaneous reaction with extravasation. | Suggested as adjunctive therapy in patients where heart rate control cannot be achieved using combination therapy. | |
AF = atrial fibrillation; AV = atrioventricular; b.i.d. = twice a day or twice daily; CKD = chronic kidney disease; ER = extended release; HF = heart failure; IV = intravenous; LVEF = left ventricular ejection fraction; PO = per os; q.d. = once a day or once daily; t.i.d. = 3 times a day.
Acute rate control
In patients with new-onset AF, heart rate control is often needed to control symptoms. Clinicians should identify causes of increased heart rate, such as infection, anemia, and thyrotoxicosis. Beta-blockers and non-dihydropyridine calcium channel blockers (diltiazem/verapamil) are preferred for acute rate control because of their rapid action and effectiveness at high sympathetic tone.169),170),171),172),173),174),175) Lenient rate control (heart rate <110/min) is sufficient in most cases.
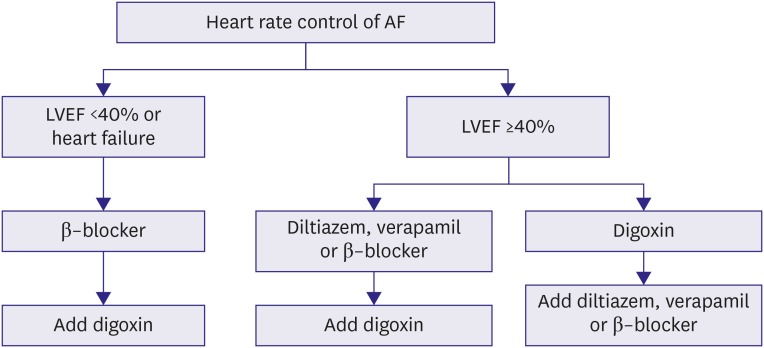
In patients with congestive HF or left ventricular dysfunction, beta-blockers, digoxin, or their combination should be used because diltiazem and verapamil have negative inotropic effects in those with an LVEF <40%.176),177) In patients with hemodynamic instability or severely reduced ejection fraction (EF), intravenous amiodarone would be an option.178),179) Urgent electrical cardioversion should be considered in hemodynamically unstable patients despite thromboembolic risk unless they are first anticoagulated (Figure 11).
Figure 11. AF rate control management.
AF = atrial fibrillation; LVEF = left ventricular ejection fraction.
Long-term rate control
Beta-blockers are most commonly used to achieve long-term rate control, followed by non-dihydropyridine calcium channel blockers (diltiazem/verapamil), digoxin, and amiodarone. Physicians should evaluate the patient's comorbidities, such as HF, asthma, or COPD, to ensure appropriate drug selection.32)
In patients with left ventricular dysfunction (EF <40%), beta-blockers, digoxin. or their combination are preferred.116) However, beta-blockers should be avoided in patients with asthma or COPD. Beta-blockers help rate control but may not have prognostic benefit in HF.73) Lenient rate control (heart rate <110/min) is usually acceptable regardless of HF status, but stricter rate control is required if symptoms remain uncontrolled (Figure 11).180)
Atrioventricular (AV) nodal ablation consisting of permanent pacemaker implantation could be an option in selected patients with a rapid ventricular rate refractory to medical therapy. However, AV nodal ablation is usually reserved for the elderly because of their life-long pacemaker dependency.
Recommendations for rate control in patients with AF

RHYTHM CONTROL
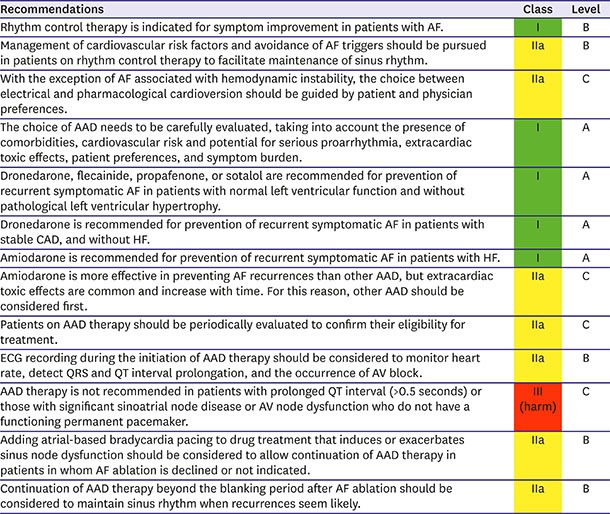
The purpose of rhythm control management is to improve hemodynamic instability and AF-related symptoms for restoring and maintaining sinus rhythm.181) The restoration and maintenance of sinus rhythm after AAD treatment are more effective than those after placebo treatment.3),182),183),184) However, it remains inconclusive whether superior rhythm control management improves prognosis in anticoagulated patients with AF.181),182),185),186),187),188),189),190) Additional invasive ablation therapy has been developed for and applied in medically refractory AF patients,191),192),193) and prospective large-scale trials (CODE-AF, EAST-AFNET, and CABANA) attributed quality of life and prognosis improvements to the beneficial effect of rhythm control strategies in patients with AF.194),195),196)
Acute rhythm control strategy
Electrical direct current cardioversion is the only rapid and effective procedure to restore sinus rhythm in hemodynamically unstable AF patients.197),198),199) Electrical cardioversion was safely conducted in sedated or anesthetized AF patients with intravenous midazolam or propofol; when used, vital signs, especially O2 saturation, should be monitored.200) During the post-cardioversion period, the skin to which the patch is attached and serial ECG should be monitored for burns or severe bradycardia.197),198)
Pretreatment with flecainide,183) propafenone,201) amiodarone,202),203) and sotalol202) (not beta-blocker, verapamil,204),205),206) or digoxin207),208)) could improve the efficacy of restoration and maintenance of sinus rhythm during the post-cardioversion period. AADs for pharmacological cardioversion are presented in Table 8. Proper anticoagulation is needed in AF patients prior to electrical cardioversion209),210),211),212) because anticoagulation dramatically reduced the risk of embolic stroke.213),214) AF patients planned to undergo electrical cardioversion should be anticoagulated from 3 weeks before to 4 weeks after unless permanent anticoagulation is indicated. A recent NOAC trial demonstrated the efficacy of preventing the occurrence of embolic stroke in AF patients subjected to electrical cardioversion.
Table 8. AADs for pharmacological cardioversion25) .
| Drug | Route | Dosage | Risks |
|---|---|---|---|
| Amiodarone | Oral | Oral 600–800 mg daily in divided doses to a total load of up to 10 g, then 200 mg q.d. as maintenance | Gastrointestinal upset, constipation, bradycardia/AV block, hypotension, QT prolongation, torsades de pointes (rare), phlebitis (IV), increased INR |
| IV | 150 mg over 10 minutes, then 1 mg/min for 6 hours, then 0.5 mg/min for 18 hours or change to oral dosing | ||
| Flecainide | Oral | 200–300 mg | Atrial flutter with 1:1 AV conduction, ventricular proarrhythmia, hypotension |
| Propafenone | Oral | 450–600 mg | Atrial flutter with 1:1 AV conduction, ventricular proarrhythmia, hypotension |
AAD = antiarrhythmic drug; AV = atrioventricular; INR = international normalized ratio; IV = intravenous; q.d. = once a day or once daily.
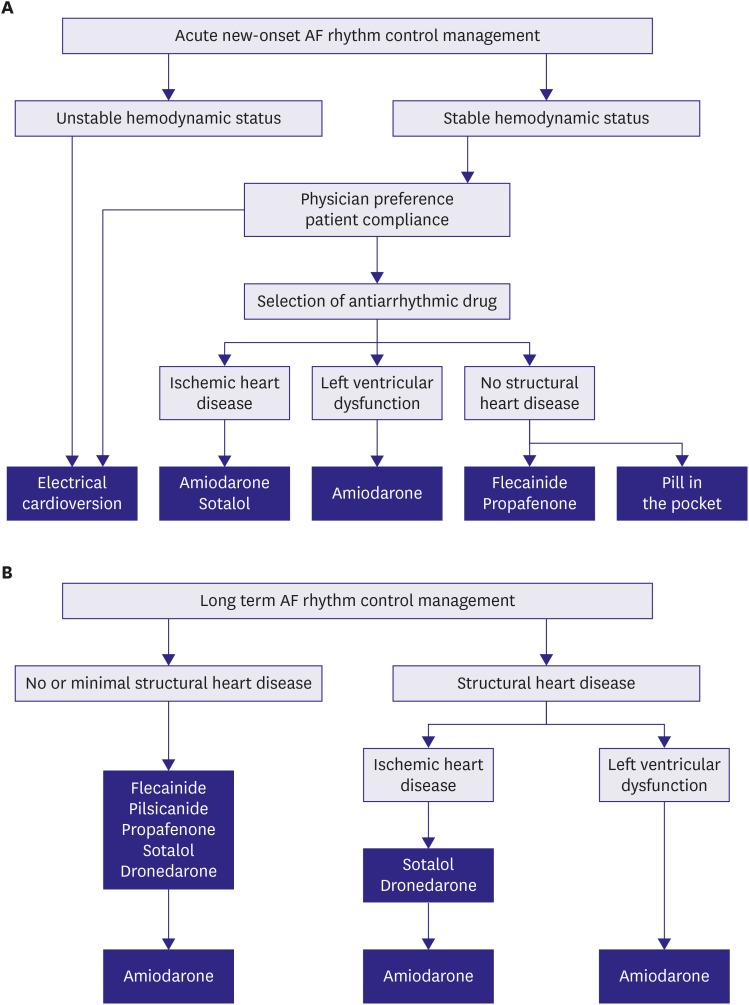
A meta-analysis demonstrated that AADs could also efficiently restore and maintain sinus rhythm as rhythm control management.181),215),216),217),218),219),220),221),222),223),224),225) In AF patients with stable hemodynamic status, prescription AADs could be the main option (easily available) in general practice without sedation or starvation during pretreatment compared with electrical cardioversion. Flecainide and propafenone are the most common AADs for acute rhythm management,222),223),224),225),226),227) but they are relatively contraindicated in AF patients without structural heart disease. Amiodarone could be prescribed to AF patients with structural heart disease and reduce the heart by >10–12 beats per minute within intravenous infusion after 8–12 hours.216) Both amiodarone and flecainide are more efficient at restoring sinus rhythm than sotalol.220),221),228),229) A single oral dose of flecainide 200–300 mg or propafenone 450–600 mg could be taken to control paroxysmal AF-related symptoms and restore sinus rhythm out of the hospital in experienced AF patients as confirmed in previous hospitalization (Figure 12A).230),231)
Figure 12. AF rhythm control management. (A) acute onset AF and (B) long-term AF.
AF = atrial fibrillation.
Long-term rhythm control strategy
Physician preference and the improvement of AF-related symptoms, drug compliance, pro-arrhythmic side effects, and extra-cardiac toxicities should be considered in long-term rhythm control management. AADs for the maintenance of sinus rhythm are presented in Table 9. In addition, lifestyle modifications and well-controlled cardiovascular disease could be additionally beneficial for preventing AF recurrence and maintaining sinus rhythm in patients treated with AADs during long-term rhythm control management.232)
Table 9. Oral AADs used to maintain sinus rhythm in patients with AF25) .
| Drug | Dose | Contraindications and precautions | Warning signs warranting discontinuation |
|---|---|---|---|
| Amiodarone | 400–600 mg daily in divided doses for 2–4 weeks; maintenance typically 100–200 mg q.d. | Caution: SA or AV node dysfunction, infranodal conduction disease, prolonged QT interval, liver disease, lung disease | QT prolongation >500 ms |
| Inhibits most CYPs and P-glycoprotein: increases warfarin, statins, and digoxin concentration | |||
| Dronedarone | 400 mg b.i.d. | Contraindication: NYHA class III or IV HF, permanent AF, concomitant therapy with QT-prolonging drugs or powerful CYP3A4 inhibitors (e.g., verapamil, diltiazem, azole antifungal agents), CrCl <30 mL/min. | QT prolongation >500 ms |
| Caution: liver disease inhibits CYP3A, CYP2D6, P-glycoprotein: increases concentration of digitalis, beta-blockers, and of some statins. | |||
| Flecainide | 50–200 mg b.i.d. (usually 50–100 mg b.i.d.) | Contraindication: CAD, HF, CrCl <50 mL/min | QRS duration increases >25% above baseline |
| Caution: SA or AV node dysfunction, infranodal conduction disease, atrial flutter, liver disease. | |||
| CYP2D6 inhibitors (e.g., quinidine, fluoxetine, tricyclics) increase plasma concentration. | |||
| Pilsicainide | 50 mg t.i.d. | Contraindicated: IHD, reduced LVEF. | QRS duration increases >25% above baseline |
| Caution: SA or AV node dysfunction, infranodal conduction disease, atrial flutter, renal impairment | |||
| Propafenone | Immediate release: 150–300 mg t.i.d. | Contraindication: IHD, reduced LVEF | QRS duration increases >25% above baseline |
| Caution: SA or AV node dysfunction, infranodal conduction disease, atrial flutter, liver disease, renal impairment, asthma | |||
| Extended release: 225–425 mg b.i.d. (usually 225–325 mg b.i.d.) | CYP2D6 inhibitors (e.g. quinidine, fluoxetine, tricyclics) increase plasma concentration | ||
| Increases concentration of digitalis and warfarin | |||
| Sotalol | 40–160 mg b.i.d. | Contraindication: HF, significant LV hypertrophy, prolonged QT interval, hypokalemia, hypomagnesemia, asthma, CrCl <50 mL/min | QT interval >500 ms, QT prolongation by >60 ms upon therapy initiation |
AAD = antiarrhythmic drug; AF = atrial fibrillation; AV = atrioventricular; b.i.d. = twice a day or twice daily; CAD = coronary artery disease; CrCl, creatinine clearance; CYP2D6 = cytochrome P450 2D6; CYP3A4 = cytochrome P450 3A4; HF = heart failure; IHD = ischemic heart disease; LVEF = left ventricular ejection fraction; NYHA = New York Heart Association; q.d. = once a day or once daily; SA = sinoatrial.
AAD safety must be considered in terms of pro-arrhythmic side effects and extra-cardiac toxicities. Flecainide, propafenone, and pilsicainide are indicated to control rhythm in AF patients without structural heart disease but are contraindicated in AF patients with ischemic heart disease or with left ventricular dysfunction due to poor prognosis.233),234) Extra-cardiac toxicity is rarely reported.235),236) Flecainide, propafenone, and pilsicainide should be prescribed with an AV nodal blocker for the prevention of use dependency (increased ventricular rate in atrial flutter).237) Propafenone has a beta-blocker effect and CYP2D2 as a substrate (7% of the general population has no CYP2D2). In a recent meta-analysis, class IC AAD use was not significantly correlated with all-cause mortality compared with class IA AAD and class III sotalol uses.184) Class IC AADs also significantly reduced the recurrence of AF and had efficacy similar to dronedarone and sotalol but inferior to amiodarone (Figure 12B).238),239)
Amiodarone can be prescribed in patients with left ventricular dysfunction.182),240) QT interval and U wave should be monitored to prevent torsade de pointes.241) In particular, long-term amiodarone therapy may have extra-cardiac toxicity in the liver, thyroid, lung, skin, and cornea. Therefore, amiodarone should be replaced by an alternative AAD if any side effects or toxicities appear during long-term therapy.242) Dronedarone reduces the heart rate, maintains sinus rhythm, and reduces cardiovascular mortality and hospitalization in paroxysmal or persistent AF patients.243),244) However, dronedarone increased the mortality rate in decompensated HF.245),246) Sotalol showed inferior efficacy to amiodarone221) and similar efficacy to propafenone for maintaining sinus rhythm.247) Sotalol could effectively suppress the re-entry mechanism. Sotalol may be the first choice for long-term rhythm control management in AF patients with ischemic heart disease. However, sotalol is prone to inducing QT prolongation, and caution is needed in females, renal impairment and if left ventricular hypertrophy is present.238)
Recommendations for rhythm control strategies in patients with AF
Anticoagulation in patients who undergo cardioversion
The periprocedural risk of thromboembolic events during cardioversion can be substantially reduced by adequate anticoagulation.213) In patients with AF or an atrial flutter ≥48 hours or with an unknown duration, OAC with vitamin K antagonist (INR, 2.0–3.0) is recommended for at least 3 weeks before electrical or pharmacological cardioversion and 4 weeks afterward regardless of CHA2DS2-VASc score.3) If early cardioversion is attempted, TEE should be performed to exclude the presence of left atrial thrombus.248) After 4 weeks, long-term anticoagulation is decided based on each patient's risk of stroke using CHA2DS2-VASc score. Patients with a CHA2DS2-VASc score ≥2 require long-term use of OAC irrespective of the cardioversion results. Subgroup analyses of phase 3 clinical trials249),250),251) and recent prospective RCTs of rivaroxaban,252) edoxaban,253) and apixaban254) have demonstrated that electrical cardioversion in patients treated with a NOAC for ≥3 weeks had similar periprocedural thromboembolic and bleeding risks to those treated with warfarin. Therefore, anticoagulation using NOAC can be an alternative to vitamin K antagonist in patients who undergo cardioversion. Because routine coagulation tests do not accurately measure the anticoagulation effects of NOACs, if patient adherence to the NOAC regimen is uncertain, TEE can be performed prior to cardioversion.255) No RCTs have compared anticoagulation strategies in patients with AF or an atrial flutter ≤48 hours. It is common practice to perform cardioversion after a single dose of unfractionated heparin (UFH) or low molecular weight heparin (LMWH) without TEE. Although data are limited, it is reasonable to administer a single dose of NOAC ≥4 hours before cardioversion instead of UFH or LMWH. Importantly, TEE or anticoagulation ≥3 weeks before cardioversion can be considered in patients with a high stroke risk or an AF duration ≤48 hours.
Recommendations for stroke prevention in patients undergoing cardioversion

ANTICOAGULATION IN SPECIFIC CONDITIONS
Atrial fibrillation patients undergoing percutaneous coronary intervention
Antithrombotic regimen
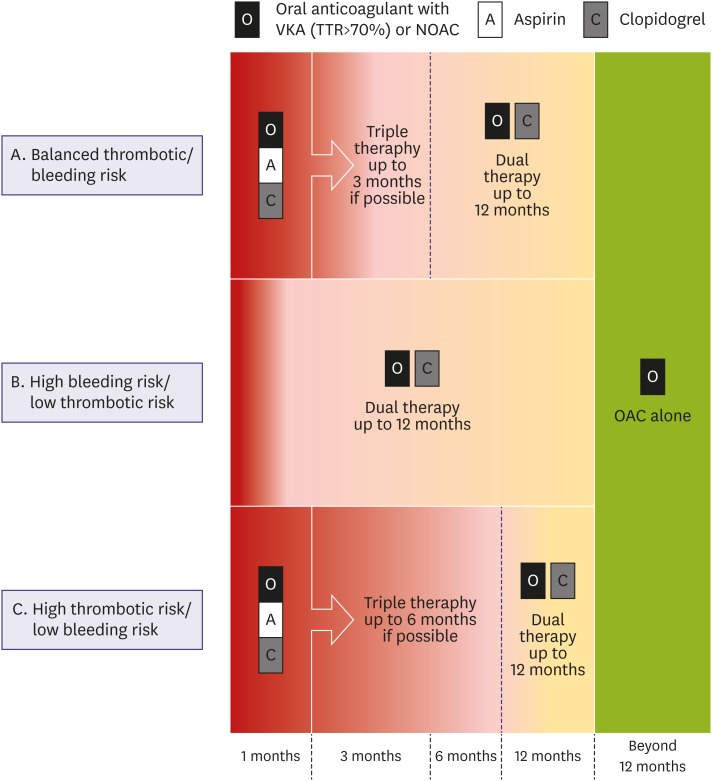
An estimated 5–15% of AF patients may undergo PCI in the future.3) However, it is very challenging to choose optimal antithrombotic regimens for AF patients treated with PCI.256) Dual antiplatelet therapy (DAPT) with aspirin and a P2Y12 inhibitor (clopidogrel) is essential for patients treated with PCI to prevent stent thrombosis.257) On the other hand, OAC is imperative to preventing stroke in AF patients.258) Thus, theoretically, triple therapy, combining all drugs including DAPT and OAC, may be a reasonable choice as an initial antithrombotic regimen. However, prolonged triple therapy has been associated with an increased risk of bleeding and even mortality.259),260) In a large Korean observational study, triple therapy with aspirin, clopidogrel, and warfarin was associated with a 4.5-fold increased risk of major bleeding compared to DAPT.261)
Recent well-designed RCTs suggested dual therapy with a single antiplatelet agent and an OAC might be safer and show similar efficacy to triple therapy for preventing ischemic/thromboembolic events.259),262),263),264) Dual therapy with clopidogrel and NOAC was suggested to be a safe initial alternative regimen to triple therapy.262),263) However, in patients with high ischemic event risk, short-term triple therapy still seems warranted.3) Thus, we recommend the following (Figure 13). First, as initial antithrombotic treatment, triple therapy should be used for as short a duration as possible unless patients are at high risk of ischemic events. Second, dual therapy should be continued after the cessation of the triple therapy until 12 months after PCI. Third, dual therapy with clopidogrel and NOAC could be considered as an alternative initial antithrombotic regimen in patients with a high risk of bleeding (e.g. HAS-BLED ≥3). Fourth, monotherapy with OAC should be considered 12 months after PCI. Meanwhile, the safety of potent P2Y12 inhibitors (prasugrel, ticagrelor) has not been well evaluated in this population. Clopidogrel is the first recommended P2Y12 inhibitor. We discuss the evidence of dual therapy and monotherapy below.
Figure 13. Suggested algorithm for antithrombotic therapy in patients undergoing PCI.
NOAC = non-vitamin K antagonist oral anticoagulant; OAC = oral anticoagulant; PCI = percutaneous coronary intervention; TTR = time in therapeutic range.
Dual therapy with clopidogrel and warfarin
In an RCT (WOEST trial), dual therapy with clopidogrel and warfarin reduced the bleeding risk by 64% and adverse cardiac events by 40% compared with triple therapy with aspirin, clopidogrel, and warfarin.259) Although this study was underpowered to compare ischemic/thromboembolic events, it has great implications for demonstrating the safety of dual therapy with clopidogrel and warfarin compared to that of triple therapy.
Dual therapy with clopidogrel and non-vitamin K oral anticoagulants
The efficacy and safety of dual therapy with clopidogrel and NOAC have been demonstrated in RCTs. In the PIONEER AF-PCI trial, dual therapy with a fixed dose of rivaroxaban 15 mg and a P2Y12 inhibitor (mostly clopidogrel) was compared with triple therapies with very-low-dose rivaroxaban (2.5 mg b.i.d.) or warfarin.263) In that study, the two rivaroxaban arms reduced the risk of clinically significant bleeding compared with triple therapy with warfarin, while the ischemic/thrombotic events were comparable. The results should be interpreted carefully. The trial design is complex (DAPT duration varied) and underpowered for the comparison of the ischemic events. Rivaroxaban 2.5 mg has not been evaluated for stroke prevention in AF patients.
The RE-DUAL PCI trial compared dual therapy with two doses of dabigatran (110 or 150 mg b.i.d.) combined with a P2Y12 inhibitor (clopidogrel in 88%, ticagrelor in 12%) and triple therapy consisting of aspirin, a P2Y12 inhibitor (clopidogrel in 92%, ticagrelor in 8%), and warfarin.262) The dabigatran arms were associated with a reduced risk of major or clinically relevant nonmajor bleeding (dabigatran 110 mg: HR, 0.52; dabigatran 150 mg: HR, 0.72) compared to triple therapy with warfarin, while the risk of ischemic/thromboembolic events was comparable. This trial was also underpowered to compare the individual ischemic/thromboembolic events, although as an adequately powered secondary efficacy composite outcome, the combined dabigatran arms were no significantly different to the warfarin-based arm.264) Both used dosages of dabigatran that have been proven to prevent stroke in AF patients.265)
The AUGUSTUS and ENTRUST-AF trials are the ongoing RCTs of apixaban and edoxaban, respectively.266),267) The AUGUSTUS trial in particular will present the efficacy and safety of many different regimens of antithrombotic therapy including triple therapy with on-label dosages of NOAC.
Monotherapy with oral anticoagulation
In a nationwide observational study of 8,700 AF patients with a history of PCI ≥1 year prior, the addition of the antiplatelet agent to warfarin was not associated with a reduced risk of ischemic/thromboembolic events but significantly increased bleeding risk.268) The efficacy and safety of NOAC monotherapy in patients with stable coronary artery disease has not been well evaluated. However, global guidelines recommend the use of OAC monotherapy in AF patients with stable coronary artery disease.3),255),269)
Anticoagulation in patients who undergo catheter ablation of atrial fibrillation
Since catheter ablation of AF carries a risk of periprocedural thromboembolic complications, anticoagulation is indicated before, during, and after the procedure irrespective of the patient's CHA2DS2-VASc score. AF ablation under uninterrupted vitamin K antagonist use is recommended based on previous studies showing that this strategy was associated with better safety and efficacy outcomes.270) Meta-analysis or registry data on periprocedural anticoagulation using dabigatran,271) apixaban,272) rivaroxaban,273) and edoxaban274) demonstrated similar thromboembolic and bleeding events compared with uninterrupted vitamin K antagonist. In addition, recent RCTs with rivaroxaban,275) dabigatran,276) and apixaban277) also showed that patients on uninterrupted NOACs had similar or even better major bleeding rates than those on vitamin K antagonist. Therefore, anticoagulation with NOAC can be an alternative to vitamin K antagonists in patients who undergo catheter ablation of AF. During the ablation, the intravenous administration of heparin is recommended to maintain a target activated clotting time ≥300 seconds.270) NOACs can be re-administered 3–5 hours after the procedure once adequate hemostasis is achieved. Anticoagulation should be continued for at least 2 months after ablation regardless of the patient's stroke risk or procedure results due to a thrombogenic state following ablation. After 2 months, long-term anticoagulation should be decided based on each individual patient's risk of stroke independent of the procedure's success.
Anticoagulation therapy and renal function
Patients with AF and moderate to severe renal dysfunction are at an increased risk of simultaneous ischemic stroke and major bleeding.278),279),280),281) Therefore, the use of OACs in AF patients with renal dysfunction is troublesome. In AF patients with moderate to severe renal dysfunction and a CHA2DS2-VASc score ≥2, the use of OACs was approved as beneficial for lowering the event rate of ischemic stroke despite the mildly increased risk of bleeding.282) In a meta-analysis, NOACs were better than warfarin for reducing the risk of stroke/systemic embolism as well as major bleeding in AF patients with mild to moderate renal dysfunction.283),284) During NOAC use, renal function should be monitored carefully.285)
In patients with moderate renal dysfunction, NOAC dose should be reduced. Dabigatran should be reduced to 110 mg b.i.d. in patients with creatinine clearance of 30–50 mL/min.265) Rivaroxaban should be reduced to 15 mg q.d. in patients with creatinine clearance of 15–50 mL/min.286) Apixaban should be reduced to 2.5 mg b.i.d. in patients with serum creatinine ≥1.5 mg/dL and age ≥80 years or body weight ≤60 kg.287) Edoxaban should be reduced to 30 mg q.d. in patients with creatinine clearance of 15–50 mL/min.288) Table 3 shows the recommended dose reduction of NOAC in Korea.
In patients with severe renal dysfunction (creatinine clearance <15 mL/min), NOAC use is not recommended. OAC use may be inappropriate in patients with renal dysfunction who are on dialysis, although the data are weak and often do not consider TTR.289),290),291),292) However, if TTR is >70%, warfarin may have some benefits even in dialysis patients.293)
However, apixaban has United States Federal Drug Administration (FDA)-approved indication for use in hemodialysis patients. Based on data from the apixaban package insert, patients with AF may receive full-dose therapy (5 mg b.i.d.) while on hemodialysis as long as they are <80 years of age and weigh > 60 kg in the United States. If a patient is on dialysis and is >80 years of age or weighs <60 kg, then the dose is reduced to 2.5 mg b.i.d. A recent study in end stage renal failure showed no significant differences between anticoagulants in the risks of stroke or systemic embolism; however, Apixaban was associated with a 28% lower risk of major bleeding than warfarin.294) The results of the current analysis are in contradiction to bleeding-related morbidity and mortality attributed to dabigatran and rivaroxaban in a previous analysis of hemodialysis patients, suggesting that the increased bleeding risk in end-stage kidney disease is not a drug class effect for all DOACs. No prospective study has examined OAC therapy in patients with renal transplantation. Nonetheless, OAC doses should be adjusted according to the creatinine clearance of the donor's kidney.
In patients with a creatinine clearance ≥ 95 mL/min, dabigatran 150 mg b.i.d., rivaroxaban 20 mg q.d., and apixaban 5 mg b.i.d. are recommended. Edoxaban 60 mg q.d. is not recommended according to FDA approval. Recent studies showed that edoxaban was effective overall, but low-dose edoxaban had lower relative effectiveness for preventing stroke or systemic embolism than with warfarin at higher creatinine clearance levels (>95 mL/min) in Korean AF patients.295),296) Similar data was observed for CrCl >95 mL/min with apixaban and rivaroxaban, but not dabigatran.293)
Anticoagulation therapy in elderly patients
Increasing age is a risk factor for simultaneous stroke and major bleeding in patients with AF.297),298),299) The elderly population is fragile and prone to falls. Nonetheless, OAC use is recommended in elderly AF patients because of the high benefit/risk ratio.300) Recent Asian data showed that Among patients with AF ≥90 years of age, warfarin was associated with a lower risk of ischemic stroke and positive net clinical benefit. Compared with warfarin, NOACs were associated with a lower risk of intracranial haemorrhage. Thus, OACs may still be considered as thromboprophylaxis for elderly patients, with NOACs being the more favorable choice.301),302)
All 4 NOACs in the elderly population are approved as beneficial for the risk reduction of intracranial bleeding compared with warfarin.265),286),287),288) In the ARISTOTLE trial, apixaban 5 mg b.i.d. reduced major bleeding in patients 65–74 years of age and ≥75 years of age.303) In the ENGAGE-TIMI trial, edoxaban 60 mg q.d. decreased the risk of major bleeding in patients <75 years of age but had similar risks in patients >75 years of age compared with warfarin.288) However, dabigatran showed a significant age–treatment interaction in the RELY trial. Dabigatran 150 mg b.i.d. reduced major bleeding in patients <75 years of age but an increased risk of major bleeding in patients >75 years of age compared with warfarin.265) In the ROCKET-AF trial, rivaroxaban demonstrated a similar risk of major bleeding as warfarin and no age-treatment interaction.286) However, the J-ROCKET study and many real-world data suggested that low-dose rivaroxaban might be safe and effective in elderly Asian AF patients with relatively low body weight.145),304),305) Therefore, the Korean AF guideline recommends low-dose rivaroxaban (15 mg q.d.) in patients aged ≥80 years.306)
CONCLUSIONS
For AF detection, ECG screening is necessary, especially in stroke survivors and the elderly. Integrated AF management, including active patient participation, a multidisciplinary approach, and technology use, is recommended from diagnosis to treatment and systematic follow-up. Such a holistic approach (the ABC pathway) can improve treatment outcomes, by considering lifestyle modifications, OAC, rate control, rhythm control by AADs, catheter ablation, and surgical intervention. Patient awareness of the disease, education and engagement with management decisions are important.
The Korean versions of separate guidelines for OAC, rate and rhythm control, risk factor management, and integrated treatment of AF patients are available in the Korean Journal of Medicine (http://ekjm.org/) or the home page of the Korean Heart Rhythm Society (http://k-hrs.org/intro.asp).
ACKNOWLEDGEMENTS
We thank professor Gregory YH Lip (University of Liverpool, UK) for comments.
Footnotes
Funding: This study was supported by a research grant from the Korean Healthcare Technology R&D project funded by the Ministry of Health & Welfare (HI15C1200).
Conflict of Interest: The authors declare no financial conflicts of interest.
- Conceptualization: Joung B, Lee KH, Kim TH, Choi EK, Lim WH, Kang KW, Shim J, Lim HE, Park J, Lee SR, Lee YS.
- Data curation: Lim WH, Kang KW, Shim J, Lim HE, Park J, Lee SR, Lee YS.
- Formal analysis: Joung B, Lee JM.
- Funding acquisition: Joung B.
- Methodology: Joung B.
- Project administration: Joung B.
- Resources: Joung B.
- Software: Joung B.
- Supervision: Joung B.
- Validation: Joung B.
- Writing - original draft: Joung B, Lee JM, Lee KH, Kim TH, Choi EK, Lim WH, Kang KW, Shim J, Lim HE, Park J, Lee SR, Lee YS.
- Writing - review & editing: Joung B.
References
- 1.Jones C, Pollit V, Fitzmaurice D, Cowan C Guideline Development Group. The management of atrial fibrillation: summary of updated NICE guidance. BMJ. 2014;348:g3655. doi: 10.1136/bmj.g3655. [DOI] [PubMed] [Google Scholar]
- 2.January CT, Wann LS, Alpert JS, et al. 2014 AHA/ACC/HRS guideline for the management of patients with atrial fibrillation: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines and the Heart Rhythm Society. J Am Coll Cardiol. 2014;64:e1–e76. doi: 10.1016/j.jacc.2014.03.022. [DOI] [PubMed] [Google Scholar]
- 3.Kirchhof P, Benussi S, Kotecha D, et al. 2016 ESC Guidelines for the management of atrial fibrillation developed in collaboration with EACTS. Eur Heart J. 2016;37:2893–2962. doi: 10.1093/eurheartj/ehw210. [DOI] [PubMed] [Google Scholar]
- 4.Chiang CE, Okumura K, Zhang S, et al. 2017 consensus of the Asia Pacific Heart Rhythm Society on stroke prevention in atrial fibrillation. J Arrhythm. 2017;33:345–367. doi: 10.1016/j.joa.2017.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lip GY, Banerjee A, Boriani G, et al. Antithrombotic therapy for atrial fibrillation: CHEST guideline and expert panel report. Chest. 2018:[Epub ahead of print]. doi: 10.1016/j.chest.2018.07.040. [DOI] [PubMed] [Google Scholar]
- 6.Benjamin EJ, Wolf PA, D'Agostino RB, Silbershatz H, Kannel WB, Levy D. Impact of atrial fibrillation on the risk of death: the Framingham Heart Study. Circulation. 1998;98:946–952. doi: 10.1161/01.cir.98.10.946. [DOI] [PubMed] [Google Scholar]
- 7.January CT, Wann LS, Alpert JS, et al. 2014 AHA/ACC/HRS guideline for the management of patients with atrial fibrillation: a report of the American College of Cardiology/American Heart Association Task Force on practice guidelines and the Heart Rhythm Society. Circulation. 2014;130:e199–e267. doi: 10.1161/CIR.0000000000000041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lee H, Kim TH, Baek YS, et al. The trends of atrial fibrillation-related hospital visit and cost, treatment pattern and mortality in Korea: 10-year nationwide sample cohort data. Korean Circ J. 2017;47:56–64. doi: 10.4070/kcj.2016.0045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kim TH, Yang PS, Uhm JS, et al. CHA2DS2-VASc score (congestive heart failure, hypertension, age ≥75 [doubled], diabetes mellitus, prior stroke or transient ischemic attack [doubled], vascular disease, age 65–74, female) for stroke in Asian patients with atrial fibrillation: a Korean nationwide sample cohort study. Stroke. 2017;48:1524–1530. doi: 10.1161/STROKEAHA.117.016926. [DOI] [PubMed] [Google Scholar]
- 10.Stewart S, Hart CL, Hole DJ, McMurray JJ. A population-based study of the long-term risks associated with atrial fibrillation: 20-year follow-up of the Renfrew/Paisley study. Am J Med. 2002;113:359–364. doi: 10.1016/s0002-9343(02)01236-6. [DOI] [PubMed] [Google Scholar]
- 11.Go AS, Hylek EM, Phillips KA, et al. Prevalence of diagnosed atrial fibrillation in adults: national implications for rhythm management and stroke prevention: the AnTicoagulation and Risk Factors in Atrial Fibrillation (ATRIA) Study. JAMA. 2001;285:2370–2375. doi: 10.1001/jama.285.18.2370. [DOI] [PubMed] [Google Scholar]
- 12.Miyasaka Y, Barnes ME, Gersh BJ, et al. Secular trends in incidence of atrial fibrillation in Olmsted County, Minnesota, 1980 to 2000, and implications on the projections for future prevalence. Circulation. 2006;114:119–125. doi: 10.1161/CIRCULATIONAHA.105.595140. [DOI] [PubMed] [Google Scholar]
- 13.Krijthe BP, Kunst A, Benjamin EJ, et al. Projections on the number of individuals with atrial fibrillation in the European Union, from 2000 to 2060. Eur Heart J. 2013;34:2746–2751. doi: 10.1093/eurheartj/eht280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Coyne KS, Paramore C, Grandy S, Mercader M, Reynolds M, Zimetbaum P. Assessing the direct costs of treating nonvalvular atrial fibrillation in the United States. Value Health. 2006;9:348–356. doi: 10.1111/j.1524-4733.2006.00124.x. [DOI] [PubMed] [Google Scholar]
- 15.Stewart S, Murphy NF, Walker A, McGuire A, McMurray JJ. Cost of an emerging epidemic: an economic analysis of atrial fibrillation in the UK. Heart. 2004;90:286–292. doi: 10.1136/hrt.2002.008748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Li Y, Pastori D, Guo Y, Wang Y, Lip GY. Risk factors for new-onset atrial fibrillation: a focus on Asian populations. Int J Cardiol. 2018;261:92–98. doi: 10.1016/j.ijcard.2018.02.051. [DOI] [PubMed] [Google Scholar]
- 17.Allan V, Honarbakhsh S, Casas JP, et al. Are cardiovascular risk factors also associated with the incidence of atrial fibrillation? A systematic review and field synopsis of 23 factors in 32 population-based cohorts of 20 million participants. Thromb Haemost. 2017;117:837–850. doi: 10.1160/TH16-11-0825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Li YG, Pastori D, Farcomeni A, et al. A simple clinical risk score (C2HEST) for predicting incident atrial fibrillation in Asian subjects: derivation in 471,446 Chinese subjects, with internal validation and external application in 451,199 Korean subjects. Chest. 2018:[Epub ahead of print]. doi: 10.1016/j.chest.2018.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kannel WB, Wolf PA, Benjamin EJ, Levy D. Prevalence, incidence, prognosis, and predisposing conditions for atrial fibrillation: population-based estimates. Am J Cardiol. 1998;82:2N–9N. doi: 10.1016/s0002-9149(98)00583-9. [DOI] [PubMed] [Google Scholar]
- 20.Kim D, Yang PS, Jang E, et al. 10-year nationwide trends of the incidence, prevalence, and adverse outcomes of non-valvular atrial fibrillation nationwide health insurance data covering the entire Korean population. Am Heart J. 2018;202:20–26. doi: 10.1016/j.ahj.2018.04.017. [DOI] [PubMed] [Google Scholar]
- 21.Chao TF, Liu CJ, Tuan TC, et al. Lifetime risks, projected numbers, and adverse outcomes in Asian patients with atrial fibrillation: a report from the Taiwan nationwide AF cohort study. Chest. 2018;153:453–466. doi: 10.1016/j.chest.2017.10.001. [DOI] [PubMed] [Google Scholar]
- 22.Yap KB, Ng TP, Ong HY. Low prevalence of atrial fibrillation in community-dwelling Chinese aged 55 years or older in Singapore: a population-based study. J Electrocardiol. 2008;41:94–98. doi: 10.1016/j.jelectrocard.2007.03.012. [DOI] [PubMed] [Google Scholar]
- 23.Iguchi Y, Kimura K, Aoki J, et al. Prevalence of atrial fibrillation in community-dwelling Japanese aged 40 years or older in Japan: analysis of 41,436 non-employee residents in Kurashiki-city. Circ J. 2008;72:909–913. doi: 10.1253/circj.72.909. [DOI] [PubMed] [Google Scholar]
- 24.Lee SR, Choi EK, Han KD, Cha MJ, Oh S. Trends in the incidence and prevalence of atrial fibrillation and estimated thromboembolic risk using the CHA2DS2-VASc score in the entire Korean population. Int J Cardiol. 2017;236:226–231. doi: 10.1016/j.ijcard.2017.02.039. [DOI] [PubMed] [Google Scholar]
- 25.Yang PS, Ryu S, Kim D, et al. Variations of prevalence and incidence of atrial fibrillation and oral anticoagulation rate according to different analysis approaches. Sci Rep. 2018;8:6856. doi: 10.1038/s41598-018-25111-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yang PS, Joung B. Regional and socioeconomic inequality of atrial fibrillation with regular hospital visit. Korean Circ J. 2018;48:635–636. doi: 10.4070/kcj.2018.0116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lee SR, Choi EK, Han K, Cha MJ, Oh S. Prevalence of non-valvular atrial fibrillation based on geographical distribution and socioeconomic status in the entire Korean population. Korean Circ J. 2018;48:622–634. doi: 10.4070/kcj.2017.0362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kim D, Yang PS, Jang E, et al. Increasing trends in hospital care burden of atrial fibrillation in Korea, 2006 through 2015. Heart. 2018:[Epub ahead of print]. doi: 10.1136/heartjnl-2017-312930. [DOI] [PubMed] [Google Scholar]
- 29.Kim MH, Johnston SS, Chu BC, Dalal MR, Schulman KL. Estimation of total incremental health care costs in patients with atrial fibrillation in the United States. Circ Cardiovasc Qual Outcomes. 2011;4:313–320. doi: 10.1161/CIRCOUTCOMES.110.958165. [DOI] [PubMed] [Google Scholar]
- 30.Lee WC, Lamas GA, Balu S, Spalding J, Wang Q, Pashos CL. Direct treatment cost of atrial fibrillation in the elderly American population: a Medicare perspective. J Med Econ. 2008;11:281–298. doi: 10.3111/13696990802063425. [DOI] [PubMed] [Google Scholar]
- 31.Chugh SS, Jui J, Gunson K, et al. Current burden of sudden cardiac death: multiple source surveillance versus retrospective death certificate-based review in a large U.S. community. J Am Coll Cardiol. 2004;44:1268–1275. doi: 10.1016/j.jacc.2004.06.029. [DOI] [PubMed] [Google Scholar]
- 32.Van Gelder IC, Rienstra M, Crijns HJ, Olshansky B. Rate control in atrial fibrillation. Lancet. 2016;388:818–828. doi: 10.1016/S0140-6736(16)31258-2. [DOI] [PubMed] [Google Scholar]
- 33.Schmidt M, Ulrichsen SP, Pedersen L, Bøtker HE, Nielsen JC, Sørensen HT. 30-year nationwide trends in incidence of atrial fibrillation in Denmark and associated 5-year risk of heart failure, stroke, and death. Int J Cardiol. 2016;225:30–36. doi: 10.1016/j.ijcard.2016.09.071. [DOI] [PubMed] [Google Scholar]
- 34.Schmidt M, Jacobsen JB, Johnsen SP, Bøtker HE, Sørensen HT. Eighteen-year trends in stroke mortality and the prognostic influence of comorbidity. Neurology. 2014;82:340–350. doi: 10.1212/WNL.0000000000000062. [DOI] [PubMed] [Google Scholar]
- 35.Freedman B, Camm J, Calkins H, et al. Screening for atrial fibrillation: a report of the AF-SCREEN international collaboration. Circulation. 2017;135:1851–1867. doi: 10.1161/CIRCULATIONAHA.116.026693. [DOI] [PubMed] [Google Scholar]
- 36.Mairesse GH, Moran P, Van Gelder IC, et al. Screening for atrial fibrillation: a European Heart Rhythm Association (EHRA) consensus document endorsed by the Heart Rhythm Society (HRS), Asia Pacific Heart Rhythm Society (APHRS), and Sociedad Latinoamericana de Estimulación Cardíaca y Electrofisiología (SOLAECE) Europace. 2017;19:1589–1623. doi: 10.1093/europace/eux177. [DOI] [PubMed] [Google Scholar]
- 37.Arya A, Piorkowski C, Sommer P, Kottkamp H, Hindricks G. Clinical implications of various follow up strategies after catheter ablation of atrial fibrillation. Pacing Clin Electrophysiol. 2007;30:458–462. doi: 10.1111/j.1540-8159.2007.00693.x. [DOI] [PubMed] [Google Scholar]
- 38.Davis RC, Hobbs FD, Kenkre JE, et al. Prevalence of atrial fibrillation in the general population and in high-risk groups: the ECHOES study. Europace. 2012;14:1553–1559. doi: 10.1093/europace/eus087. [DOI] [PubMed] [Google Scholar]
- 39.Siontis KC, Gersh BJ, Killian JM, et al. Typical, atypical, and asymptomatic presentations of new-onset atrial fibrillation in the community: characteristics and prognostic implications. Heart Rhythm. 2016;13:1418–1424. doi: 10.1016/j.hrthm.2016.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lowres N, Neubeck L, Redfern J, Freedman SB. Screening to identify unknown atrial fibrillation. A systematic review. Thromb Haemost. 2013;110:213–222. doi: 10.1160/TH13-02-0165. [DOI] [PubMed] [Google Scholar]
- 41.Boriani G, Laroche C, Diemberger I, et al. Asymptomatic atrial fibrillation: clinical correlates, management, and outcomes in the EORP-AF Pilot General Registry. Am J Med. 2015;128:509–518.e2. doi: 10.1016/j.amjmed.2014.11.026. [DOI] [PubMed] [Google Scholar]
- 42.Potpara TS, Polovina MM, Marinkovic JM, Lip GY. A comparison of clinical characteristics and long-term prognosis in asymptomatic and symptomatic patients with first-diagnosed atrial fibrillation: the Belgrade Atrial Fibrillation Study. Int J Cardiol. 2013;168:4744–4749. doi: 10.1016/j.ijcard.2013.07.234. [DOI] [PubMed] [Google Scholar]
- 43.Hobbs FD, Fitzmaurice DA, Mant J, et al. A randomised controlled trial and cost-effectiveness study of systematic screening (targeted and total population screening) versus routine practice for the detection of atrial fibrillation in people aged 65 and over. The SAFE study. Health Technol Assess. 2005;9:iii–iv. doi: 10.3310/hta9400. [DOI] [PubMed] [Google Scholar]
- 44.Aronsson M, Svennberg E, Rosenqvist M, et al. Cost-effectiveness of mass screening for untreated atrial fibrillation using intermittent ECG recording. Europace. 2015;17:1023–1029. doi: 10.1093/europace/euv083. [DOI] [PubMed] [Google Scholar]
- 45.Levin LA, Husberg M, Sobocinski PD, et al. A cost-effectiveness analysis of screening for silent atrial fibrillation after ischaemic stroke. Europace. 2015;17:207–214. doi: 10.1093/europace/euu213. [DOI] [PubMed] [Google Scholar]
- 46.Son MK, Lim NK, Park HY. Trend of prevalence of atrial fibrillation and use of oral anticoagulation therapy in patients with atrial fibrillation in South Korea (2002–2013) J Epidemiol. 2018;28:81–87. doi: 10.2188/jea.JE20160149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Engdahl J, Andersson L, Mirskaya M, Rosenqvist M. Stepwise screening of atrial fibrillation in a 75-year-old population: implications for stroke prevention. Circulation. 2013;127:930–937. doi: 10.1161/CIRCULATIONAHA.112.126656. [DOI] [PubMed] [Google Scholar]
- 48.Halcox JP, Wareham K, Cardew A, et al. Assessment of Remote Heart Rhythm Sampling Using the AliveCor Heart Monitor to Screen for Atrial Fibrillation: the REHEARSE-AF Study. Circulation. 2017;136:1784–1794. doi: 10.1161/CIRCULATIONAHA.117.030583. [DOI] [PubMed] [Google Scholar]
- 49.Chan NY, Choy CC, Chan CK, Siu CW. Effectiveness of a nongovernmental organization-led large-scale community atrial fibrillation screening program using the smartphone electrocardiogram: an observational cohort study. Heart Rhythm. 2018;15:1306–1311. doi: 10.1016/j.hrthm.2018.06.006. [DOI] [PubMed] [Google Scholar]
- 50.Healey JS, Alings M, Ha A, et al. Subclinical atrial fibrillation in older patients. Circulation. 2017;136:1276–1283. doi: 10.1161/CIRCULATIONAHA.117.028845. [DOI] [PubMed] [Google Scholar]
- 51.Glotzer TV, Hellkamp AS, Zimmerman J, et al. Atrial high rate episodes detected by pacemaker diagnostics predict death and stroke: report of the Atrial Diagnostics Ancillary Study of the MOde Selection Trial (MOST) Circulation. 2003;107:1614–1619. doi: 10.1161/01.CIR.0000057981.70380.45. [DOI] [PubMed] [Google Scholar]
- 52.Healey JS, Connolly SJ, Gold MR, et al. Subclinical atrial fibrillation and the risk of stroke. N Engl J Med. 2012;366:120–129. doi: 10.1056/NEJMoa1105575. [DOI] [PubMed] [Google Scholar]
- 53.Van Gelder IC, Healey JS, Crijns HJ, et al. Duration of device-detected subclinical atrial fibrillation and occurrence of stroke in ASSERT. Eur Heart J. 2017;38:1339–1344. doi: 10.1093/eurheartj/ehx042. [DOI] [PubMed] [Google Scholar]
- 54.Hindricks G, Pokushalov E, Urban L, et al. Performance of a new leadless implantable cardiac monitor in detecting and quantifying atrial fibrillation: results of the XPECT trial. Circ Arrhythm Electrophysiol. 2010;3:141–147. doi: 10.1161/CIRCEP.109.877852. [DOI] [PubMed] [Google Scholar]
- 55.Brambatti M, Connolly SJ, Gold MR, et al. Temporal relationship between subclinical atrial fibrillation and embolic events. Circulation. 2014;129:2094–2099. doi: 10.1161/CIRCULATIONAHA.113.007825. [DOI] [PubMed] [Google Scholar]
- 56.Glotzer TV, Daoud EG, Wyse DG, et al. The relationship between daily atrial tachyarrhythmia burden from implantable device diagnostics and stroke risk: the TRENDS study. Circ Arrhythm Electrophysiol. 2009;2:474–480. doi: 10.1161/CIRCEP.109.849638. [DOI] [PubMed] [Google Scholar]
- 57.Santini M, Gasparini M, Landolina M, et al. Device-detected atrial tachyarrhythmias predict adverse outcome in real-world patients with implantable biventricular defibrillators. J Am Coll Cardiol. 2011;57:167–172. doi: 10.1016/j.jacc.2010.08.624. [DOI] [PubMed] [Google Scholar]
- 58.Boriani G, Glotzer TV, Santini M, et al. Device-detected atrial fibrillation and risk for stroke: an analysis of >10,000 patients from the SOS AF project (Stroke preventiOn Strategies based on Atrial Fibrillation information from implanted devices) Eur Heart J. 2014;35:508–516. doi: 10.1093/eurheartj/eht491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Freedman B, Potpara TS, Lip GY. Stroke prevention in atrial fibrillation. Lancet. 2016;388:806–817. doi: 10.1016/S0140-6736(16)31257-0. [DOI] [PubMed] [Google Scholar]
- 60.Friberg L, Rosenqvist M, Lindgren A, Terént A, Norrving B, Asplund K. High prevalence of atrial fibrillation among patients with ischemic stroke. Stroke. 2014;45:2599–2605. doi: 10.1161/STROKEAHA.114.006070. [DOI] [PubMed] [Google Scholar]
- 61.Perera KS, Vanassche T, Bosch J, et al. Global survey of the frequency of atrial fibrillation-associated stroke: embolic stroke of undetermined source global registry. Stroke. 2016;47:2197–2202. doi: 10.1161/STROKEAHA.116.013378. [DOI] [PubMed] [Google Scholar]
- 62.Sposato LA, Cipriano LE, Saposnik G, Ruíz Vargas E, Riccio PM, Hachinski V. Diagnosis of atrial fibrillation after stroke and transient ischaemic attack: a systematic review and meta-analysis. Lancet Neurol. 2015;14:377–387. doi: 10.1016/S1474-4422(15)70027-X. [DOI] [PubMed] [Google Scholar]
- 63.Adams HP, Jr, Bendixen BH, Kappelle LJ, et al. Classification of subtype of acute ischemic stroke. Definitions for use in a multicenter clinical trial. TOAST. Trial of Org 10172 in Acute Stroke Treatment. Stroke. 1993;24:35–41. doi: 10.1161/01.str.24.1.35. [DOI] [PubMed] [Google Scholar]
- 64.Gladstone DJ, Spring M, Dorian P, et al. Atrial fibrillation in patients with cryptogenic stroke. N Engl J Med. 2014;370:2467–2477. doi: 10.1056/NEJMoa1311376. [DOI] [PubMed] [Google Scholar]
- 65.Thijs VN, Brachmann J, Morillo CA, et al. Predictors for atrial fibrillation detection after cryptogenic stroke: results from CRYSTAL AF. Neurology. 2016;86:261–269. doi: 10.1212/WNL.0000000000002282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Proietti M, Romiti GF, Olshansky B, Lane DA, Lip GY. Improved outcomes by integrated care of anticoagulated patients with atrial fibrillation using the simple ABC (Atrial Fibrillation Better Care) pathway. Am J Med. 2018:[Epub ahead of print]. doi: 10.1016/j.amjmed.2018.06.012. [DOI] [PubMed] [Google Scholar]
- 67.Guha K, McDonagh T. Heart failure epidemiology: European perspective. Curr Cardiol Rev. 2013;9:123–127. doi: 10.2174/1573403X11309020005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Schnabel RB, Yin X, Gona P, et al. 50 year trends in atrial fibrillation prevalence, incidence, risk factors, and mortality in the Framingham Heart Study: a cohort study. Lancet. 2015;386:154–162. doi: 10.1016/S0140-6736(14)61774-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Lip GY, Heinzel FR, Gaita F, et al. European Heart Rhythm Association/Heart Failure Association joint consensus document on arrhythmias in heart failure, endorsed by the Heart Rhythm Society and the Asia Pacific Heart Rhythm Society. Europace. 2016;18:12–36. doi: 10.1093/europace/euv191. [DOI] [PubMed] [Google Scholar]
- 70.Schneider MP, Hua TA, Böhm M, Wachtell K, Kjeldsen SE, Schmieder RE. Prevention of atrial fibrillation by Renin-Angiotensin system inhibition a meta-analysis. J Am Coll Cardiol. 2010;55:2299–2307. doi: 10.1016/j.jacc.2010.01.043. [DOI] [PubMed] [Google Scholar]
- 71.Healey JS, Baranchuk A, Crystal E, et al. Prevention of atrial fibrillation with angiotensin-converting enzyme inhibitors and angiotensin receptor blockers: a meta-analysis. J Am Coll Cardiol. 2005;45:1832–1839. doi: 10.1016/j.jacc.2004.11.070. [DOI] [PubMed] [Google Scholar]
- 72.Jibrini MB, Molnar J, Arora RR. Prevention of atrial fibrillation by way of abrogation of the renin-angiotensin system: a systematic review and meta-analysis. Am J Ther. 2008;15:36–43. doi: 10.1097/MJT.0b013e31804beb59. [DOI] [PubMed] [Google Scholar]
- 73.Kotecha D, Holmes J, Krum H, et al. Efficacy of β blockers in patients with heart failure plus atrial fibrillation: an individual-patient data meta-analysis. Lancet. 2014;384:2235–2243. doi: 10.1016/S0140-6736(14)61373-8. [DOI] [PubMed] [Google Scholar]
- 74.Swedberg K, Zannad F, McMurray JJ, et al. Eplerenone and atrial fibrillation in mild systolic heart failure: results from the EMPHASIS-HF (Eplerenone in Mild Patients Hospitalization And SurvIval Study in Heart Failure) study. J Am Coll Cardiol. 2012;59:1598–1603. doi: 10.1016/j.jacc.2011.11.063. [DOI] [PubMed] [Google Scholar]
- 75.Marrouche NF, Brachmann J, Andresen D, et al. Catheter ablation for atrial fibrillation with heart failure. N Engl J Med. 2018;378:417–427. doi: 10.1056/NEJMoa1707855. [DOI] [PubMed] [Google Scholar]
- 76.Manolis AJ, Rosei EA, Coca A, et al. Hypertension and atrial fibrillation: diagnostic approach, prevention and treatment. Position paper of the Working Group ‘Hypertension Arrhythmias and Thrombosis’ of the European Society of Hypertension. J Hypertens. 2012;30:239–252. doi: 10.1097/HJH.0b013e32834f03bf. [DOI] [PubMed] [Google Scholar]
- 77.Kim D, Yang PS, Kim TH, et al. Ideal blood pressure in patients with atrial fibrillation. J Am Coll Cardiol. 2018;72:1233–1245. doi: 10.1016/j.jacc.2018.05.076. [DOI] [PubMed] [Google Scholar]
- 78.Marott SC, Nielsen SF, Benn M, Nordestgaard BG. Antihypertensive treatment and risk of atrial fibrillation: a nationwide study. Eur Heart J. 2014;35:1205–1214. doi: 10.1093/eurheartj/eht507. [DOI] [PubMed] [Google Scholar]
- 79.Wachtell K, Lehto M, Gerdts E, et al. Angiotensin II receptor blockade reduces new-onset atrial fibrillation and subsequent stroke compared to atenolol: the Losartan Intervention For End Point Reduction in Hypertension (LIFE) study. J Am Coll Cardiol. 2005;45:712–719. doi: 10.1016/j.jacc.2004.10.068. [DOI] [PubMed] [Google Scholar]
- 80.Madrid AH, Bueno MG, Rebollo JM, et al. Use of irbesartan to maintain sinus rhythm in patients with long-lasting persistent atrial fibrillation: a prospective and randomized study. Circulation. 2002;106:331–336. doi: 10.1161/01.cir.0000022665.18619.83. [DOI] [PubMed] [Google Scholar]
- 81.Ueng KC, Tsai TP, Yu WC, et al. Use of enalapril to facilitate sinus rhythm maintenance after external cardioversion of long-standing persistent atrial fibrillation. Results of a prospective and controlled study. Eur Heart J. 2003;24:2090–2098. doi: 10.1016/j.ehj.2003.08.014. [DOI] [PubMed] [Google Scholar]
- 82.Anand K, Mooss AN, Hee TT, Mohiuddin SM. Meta-analysis: inhibition of renin-angiotensin system prevents new-onset atrial fibrillation. Am Heart J. 2006;152:217–222. doi: 10.1016/j.ahj.2006.01.007. [DOI] [PubMed] [Google Scholar]
- 83.Du X, Ninomiya T, de Galan B, et al. Risks of cardiovascular events and effects of routine blood pressure lowering among patients with type 2 diabetes and atrial fibrillation: results of the ADVANCE study. Eur Heart J. 2009;30:1128–1135. doi: 10.1093/eurheartj/ehp055. [DOI] [PubMed] [Google Scholar]
- 84.Schoen T, Pradhan AD, Albert CM, Conen D. Type 2 diabetes mellitus and risk of incident atrial fibrillation in women. J Am Coll Cardiol. 2012;60:1421–1428. doi: 10.1016/j.jacc.2012.06.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Rizzo MR, Sasso FC, Marfella R, et al. Autonomic dysfunction is associated with brief episodes of atrial fibrillation in type 2 diabetes. J Diabetes Complications. 2015;29:88–92. doi: 10.1016/j.jdiacomp.2014.09.002. [DOI] [PubMed] [Google Scholar]
- 86.Ziolo MT, Mohler PJ. Defining the role of oxidative stress in atrial fibrillation and diabetes. J Cardiovasc Electrophysiol. 2015;26:223–225. doi: 10.1111/jce.12560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Fatemi O, Yuriditsky E, Tsioufis C, et al. Impact of intensive glycemic control on the incidence of atrial fibrillation and associated cardiovascular outcomes in patients with type 2 diabetes mellitus (from the Action to Control Cardiovascular Risk in Diabetes Study) Am J Cardiol. 2014;114:1217–1222. doi: 10.1016/j.amjcard.2014.07.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Fangel MV, Nielsen PB, Larsen TB, et al. Type 1 versus type 2 diabetes and thromboembolic risk in patients with atrial fibrillation: a Danish nationwide cohort study. Int J Cardiol. 2018;268:137–142. doi: 10.1016/j.ijcard.2018.05.037. [DOI] [PubMed] [Google Scholar]
- 89.Lee SR, Choi EK, Rhee TM, et al. Evaluation of the association between diabetic retinopathy and the incidence of atrial fibrillation: a nationwide population-based study. Int J Cardiol. 2016;223:953–957. doi: 10.1016/j.ijcard.2016.08.296. [DOI] [PubMed] [Google Scholar]
- 90.Lip GY. The ABC pathway: an integrated approach to improve AF management. Nat Rev Cardiol. 2017;14:627–628. doi: 10.1038/nrcardio.2017.153. [DOI] [PubMed] [Google Scholar]
- 91.Mazurek M, Shantsila E, Lane DA, Wolff A, Proietti M, Lip GY. Guideline-adherent antithrombotic treatment improves outcomes in patients with atrial fibrillation: insights from the community-based Darlington atrial fibrillation registry. Mayo Clin Proc. 2017;92:1203–1213. doi: 10.1016/j.mayocp.2017.05.023. [DOI] [PubMed] [Google Scholar]
- 92.Proietti M, Nobili A, Raparelli V, et al. Adherence to antithrombotic therapy guidelines improves mortality among elderly patients with atrial fibrillation: insights from the REPOSI study. Clin Res Cardiol. 2016;105:912–920. doi: 10.1007/s00392-016-0999-4. [DOI] [PubMed] [Google Scholar]
- 93.Ancedy Y, Lecoq C, Saint Etienne C, et al. Antithrombotic management in patients with atrial fibrillation undergoing coronary stent implantation: what is the impact of guideline adherence? Int J Cardiol. 2016;203:987–994. doi: 10.1016/j.ijcard.2015.11.090. [DOI] [PubMed] [Google Scholar]
- 94.Gorin L, Fauchier L, Nonin E, Charbonnier B, Babuty D, Lip GY. Prognosis and guideline-adherent antithrombotic treatment in patients with atrial fibrillation and atrial flutter: implications of undertreatment and overtreatment in real-life clinical practice; the Loire Valley Atrial Fibrillation Project. Chest. 2011;140:911–917. doi: 10.1378/chest.10-2436. [DOI] [PubMed] [Google Scholar]
- 95.Nuño R, Coleman K, Bengoa R, Sauto R. Integrated care for chronic conditions: the contribution of the ICCC Framework. Health Policy. 2012;105:55–64. doi: 10.1016/j.healthpol.2011.10.006. [DOI] [PubMed] [Google Scholar]
- 96.Bodenheimer T, Wagner EH, Grumbach K. Improving primary care for patients with chronic illness. JAMA. 2002;288:1775–1779. doi: 10.1001/jama.288.14.1775. [DOI] [PubMed] [Google Scholar]
- 97.Lorig KR, Holman H. Self-management education: history, definition, outcomes, and mechanisms. Ann Behav Med. 2003;26:1–7. doi: 10.1207/S15324796ABM2601_01. [DOI] [PubMed] [Google Scholar]
- 98.Alonso-Coello P, Montori VM, Solà I, et al. Values and preferences in oral anticoagulation in patients with atrial fibrillation, physicians' and patients' perspectives: protocol for a two-phase study. BMC Health Serv Res. 2008;8:221. doi: 10.1186/1472-6963-8-221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Seaburg L, Hess EP, Coylewright M, Ting HH, McLeod CJ, Montori VM. Shared decision making in atrial fibrillation: where we are and where we should be going. Circulation. 2014;129:704–710. doi: 10.1161/CIRCULATIONAHA.113.004498. [DOI] [PubMed] [Google Scholar]
- 100.Stiggelbout AM, Van der Weijden T, De Wit MP, et al. Shared decision making: really putting patients at the centre of healthcare. BMJ. 2012;344:e256. doi: 10.1136/bmj.e256. [DOI] [PubMed] [Google Scholar]
- 101.Hendriks JM, de Wit R, Vrijhoef HJ, Tieleman RG, Crijns HJ. An integrated chronic care program for patients with atrial fibrillation: study protocol and methodology for an ongoing prospective randomised controlled trial. Int J Nurs Stud. 2010;47:1310–1316. doi: 10.1016/j.ijnurstu.2009.12.017. [DOI] [PubMed] [Google Scholar]
- 102.Guo Y, Chen Y, Lane DA, Liu L, Wang Y, Lip GY. Mobile health technology for atrial fibrillation management integrating decision support, education, and patient involvement: mAF App Trial. Am J Med. 2017;130:1388–1396.e6. doi: 10.1016/j.amjmed.2017.07.003. [DOI] [PubMed] [Google Scholar]
- 103.Lee SS, Ae Kong K, Kim D, et al. Clinical implication of an impaired fasting glucose and prehypertension related to new onset atrial fibrillation in a healthy Asian population without underlying disease: a nationwide cohort study in Korea. Eur Heart J. 2017;38:2599–2607. doi: 10.1093/eurheartj/ehx316. [DOI] [PubMed] [Google Scholar]
- 104.Baek YS, Yang PS, Kim TH, et al. Associations of abdominal obesity and new-onset atrial fibrillation in the general population. J Am Heart Assoc. 2017;6:6. doi: 10.1161/JAHA.116.004705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Benjamin EJ, Levy D, Vaziri SM, D'Agostino RB, Belanger AJ, Wolf PA. Independent risk factors for atrial fibrillation in a population-based cohort. The Framingham Heart Study. JAMA. 1994;271:840–844. [PubMed] [Google Scholar]
- 106.Wang TJ, Parise H, Levy D, et al. Obesity and the risk of new-onset atrial fibrillation. JAMA. 2004;292:2471–2477. doi: 10.1001/jama.292.20.2471. [DOI] [PubMed] [Google Scholar]
- 107.Gami AS, Hodge DO, Herges RM, et al. Obstructive sleep apnea, obesity, and the risk of incident atrial fibrillation. J Am Coll Cardiol. 2007;49:565–571. doi: 10.1016/j.jacc.2006.08.060. [DOI] [PubMed] [Google Scholar]
- 108.Heeringa J, Kors JA, Hofman A, van Rooij FJ, Witteman JC. Cigarette smoking and risk of atrial fibrillation: the Rotterdam Study. Am Heart J. 2008;156:1163–1169. doi: 10.1016/j.ahj.2008.08.003. [DOI] [PubMed] [Google Scholar]
- 109.Conen D, Tedrow UB, Cook NR, Moorthy MV, Buring JE, Albert CM. Alcohol consumption and risk of incident atrial fibrillation in women. JAMA. 2008;300:2489–2496. doi: 10.1001/jama.2008.755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Roh SY, Choi JI, Lee JY, et al. Catheter ablation of atrial fibrillation in patients with chronic lung disease. Circ Arrhythm Electrophysiol. 2011;4:815–822. doi: 10.1161/CIRCEP.110.960435. [DOI] [PubMed] [Google Scholar]
- 111.Kong KA, Park J, Hong SH, Hong YS, Sung YA, Lee H. Associations between body mass index and mortality or cardiovascular events in a general Korean population. PLoS One. 2017;12:e0185024. doi: 10.1371/journal.pone.0185024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Manning WJ, Weintraub RM, Waksmonski CA, et al. Accuracy of transesophageal echocardiography for identifying left atrial thrombi. A prospective, intraoperative study. Ann Intern Med. 1995;123:817–822. doi: 10.7326/0003-4819-123-11-199512010-00001. [DOI] [PubMed] [Google Scholar]
- 113.Hwang JJ, Chen JJ, Lin SC, et al. Diagnostic accuracy of transesophageal echocardiography for detecting left atrial thrombi in patients with rheumatic heart disease having undergone mitral valve operations. Am J Cardiol. 1993;72:677–681. doi: 10.1016/0002-9149(93)90884-f. [DOI] [PubMed] [Google Scholar]
- 114.Lip G, Freedman B, De Caterina R, Potpara TS. Stroke prevention in atrial fibrillation: past, present and future. Comparing the guidelines and practical decision-making. Thromb Haemost. 2017;117:1230–1239. doi: 10.1160/TH16-11-0876. [DOI] [PubMed] [Google Scholar]
- 115.Kim TH, Shim CY, Park JH, et al. Left ventricular diastolic dysfunction is associated with atrial remodeling and risk or presence of stroke in patients with paroxysmal atrial fibrillation. J Cardiol. 2016;68:104–109. doi: 10.1016/j.jjcc.2015.10.008. [DOI] [PubMed] [Google Scholar]
- 116.Yu HT, Yang PS, Lee H, et al. Outcomes of rate-control treatment in patients with atrial fibrillation and heart failure: a nationwide cohort study. Circ J. 2018;82:652–658. doi: 10.1253/circj.CJ-17-0669. [DOI] [PubMed] [Google Scholar]
- 117.Nieuwlaat R, Olsson SB, Lip GY, et al. Guideline-adherent antithrombotic treatment is associated with improved outcomes compared with undertreatment in high-risk patients with atrial fibrillation. Am Heart J. 2007;153:1006–1012. doi: 10.1016/j.ahj.2007.03.008. [DOI] [PubMed] [Google Scholar]
- 118.Stock S, Pitcavage JM, Simic D, et al. Chronic care model strategies in the United States and Germany deliver patient-centered, high-quality diabetes care. Health Aff (Millwood) 2014;33:1540–1548. doi: 10.1377/hlthaff.2014.0428. [DOI] [PubMed] [Google Scholar]
- 119.Hart RG, Pearce LA, Aguilar MI. Meta-analysis: antithrombotic therapy to prevent stroke in patients who have nonvalvular atrial fibrillation. Ann Intern Med. 2007;146:857–867. doi: 10.7326/0003-4819-146-12-200706190-00007. [DOI] [PubMed] [Google Scholar]
- 120.Eckman MH, Singer DE, Rosand J, Greenberg SM. Moving the tipping point: the decision to anticoagulate patients with atrial fibrillation. Circ Cardiovasc Qual Outcomes. 2011;4:14–21. doi: 10.1161/CIRCOUTCOMES.110.958108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Chiang CE, Wang TD, Li YH, et al. 2010 guidelines of the Taiwan Society of Cardiology for the management of hypertension. J Formos Med Assoc. 2010;109:740–773. doi: 10.1016/S0929-6646(10)60120-9. [DOI] [PubMed] [Google Scholar]
- 122.Olesen JB, Torp-Pedersen C, Hansen ML, Lip GY. The value of the CHA2DS2-VASc score for refining stroke risk stratification in patients with atrial fibrillation with a CHADS2 score 0–1: a nationwide cohort study. Thromb Haemost. 2012;107:1172–1179. doi: 10.1160/TH12-03-0175. [DOI] [PubMed] [Google Scholar]
- 123.Coppens M, Eikelboom JW, Hart RG, et al. The CHA2DS2-VASc score identifies those patients with atrial fibrillation and a CHADS2 score of 1 who are unlikely to benefit from oral anticoagulant therapy. Eur Heart J. 2013;34:170–176. doi: 10.1093/eurheartj/ehs314. [DOI] [PubMed] [Google Scholar]
- 124.Chao TF, Liu CJ, Wang KL, et al. Using the CHA2DS2-VASc score for refining stroke risk stratification in ‘low-risk’ Asian patients with atrial fibrillation. J Am Coll Cardiol. 2014;64:1658–1665. doi: 10.1016/j.jacc.2014.06.1203. [DOI] [PubMed] [Google Scholar]
- 125.Kim TH, Yang PS, Kim D, et al. CHA2DS2-VASc score for identifying truly low-risk atrial fibrillation for stroke: a Korean nationwide cohort study. Stroke. 2017;48:2984–2990. doi: 10.1161/STROKEAHA.117.018551. [DOI] [PubMed] [Google Scholar]
- 126.Kang SH, Choi EK, Han KD, et al. Risk of ischemic stroke in patients with non-valvular atrial fibrillation not receiving oral anticoagulants: Korean nationwide population-based study. Circ J. 2017;81:1158–1164. doi: 10.1253/circj.CJ-16-1267. [DOI] [PubMed] [Google Scholar]
- 127.Lin LY, Lee CH, Yu CC, et al. Risk factors and incidence of ischemic stroke in Taiwanese with nonvalvular atrial fibrillation-- a nation wide database analysis. Atherosclerosis. 2011;217:292–295. doi: 10.1016/j.atherosclerosis.2011.03.033. [DOI] [PubMed] [Google Scholar]
- 128.Steensig K, Olesen KK, Thim T, et al. Coronary artery disease is independent risk factor for stroke among patients with atrial fibrillation. J Am Coll Cardiol. 2018:[Epub ahead of print]. doi: 10.1016/j.jacc.2018.08.1046. [DOI] [PubMed] [Google Scholar]
- 129.Wang TJ, Massaro JM, Levy D, et al. A risk score for predicting stroke or death in individuals with new-onset atrial fibrillation in the community: the Framingham Heart Study. JAMA. 2003;290:1049–1056. doi: 10.1001/jama.290.8.1049. [DOI] [PubMed] [Google Scholar]
- 130.Fang MC, Singer DE, Chang Y, et al. Gender differences in the risk of ischemic stroke and peripheral embolism in atrial fibrillation: the AnTicoagulation and Risk factors In Atrial fibrillation (ATRIA) study. Circulation. 2005;112:1687–1691. doi: 10.1161/CIRCULATIONAHA.105.553438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Avgil Tsadok M, Jackevicius CA, Rahme E, Humphries KH, Behlouli H, Pilote L. Sex differences in stroke risk among older patients with recently diagnosed atrial fibrillation. JAMA. 2012;307:1952–1958. doi: 10.1001/jama.2012.3490. [DOI] [PubMed] [Google Scholar]
- 132.Friberg L, Benson L, Rosenqvist M, Lip GY. Assessment of female sex as a risk factor in atrial fibrillation in Sweden: nationwide retrospective cohort study. BMJ. 2012;344:e3522. doi: 10.1136/bmj.e3522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Mikkelsen AP, Lindhardsen J, Lip GY, Gislason GH, Torp-Pedersen C, Olesen JB. Female sex as a risk factor for stroke in atrial fibrillation: a nationwide cohort study. J Thromb Haemost. 2012;10:1745–1751. doi: 10.1111/j.1538-7836.2012.04853.x. [DOI] [PubMed] [Google Scholar]
- 134.Nielsen PB, Skjøth F, Overvad TF, Larsen TB, Lip GY. Female sex is a risk modifier rather than a risk factor for stroke in atrial fibrillation: should we use a CHA2DS2-VA score rather than CHA2DS2-VASc? Circulation. 2018;137:832–840. doi: 10.1161/CIRCULATIONAHA.117.029081. [DOI] [PubMed] [Google Scholar]
- 135.Siu CW, Lip GY, Lam KF, Tse HF. Risk of stroke and intracranial hemorrhage in 9727 Chinese with atrial fibrillation in Hong Kong. Heart Rhythm. 2014;11:1401–1408. doi: 10.1016/j.hrthm.2014.04.021. [DOI] [PubMed] [Google Scholar]
- 136.Guo Y, Apostolakis S, Blann AD, et al. Validation of contemporary stroke and bleeding risk stratification scores in non-anticoagulated Chinese patients with atrial fibrillation. Int J Cardiol. 2013;168:904–909. doi: 10.1016/j.ijcard.2012.10.052. [DOI] [PubMed] [Google Scholar]
- 137.Tomita H, Okumura K, Inoue H, et al. Validation of risk scoring system excluding female sex from CHA2DS2-VASc in Japanese patients with nonvalvular atrial fibrillation: subanalysis of the J-RHYTHM registry. Circ J. 2015;79:1719–1726. doi: 10.1253/circj.CJ-15-0095. [DOI] [PubMed] [Google Scholar]
- 138.Chao TF, Wang KL, Liu CJ, et al. Age threshold for increased stroke risk among patients with atrial fibrillation: a nationwide cohort study from Taiwan. J Am Coll Cardiol. 2015;66:1339–1347. doi: 10.1016/j.jacc.2015.07.026. [DOI] [PubMed] [Google Scholar]
- 139.Kim TH, Yang PS, Yu HT, et al. Age threshold for ischemic stroke risk in atrial fibrillation. Stroke. 2018;49:1872–1879. doi: 10.1161/STROKEAHA.118.021047. [DOI] [PubMed] [Google Scholar]
- 140.Zulkifly H, Lip GY, Lane DA. Use of the SAMe-TT2R2 score to predict anticoagulation control in atrial fibrillation and venous thromboembolism patients receiving vitamin K antagonists: a review. Heart Rhythm. 2018;15:615–623. doi: 10.1016/j.hrthm.2017.11.026. [DOI] [PubMed] [Google Scholar]
- 141.Yoon M, Yang PS, Jang E, et al. Dynamic changes of CHA2DS2-VASc score and the risk of ischaemic stroke in Asian patients with atrial fibrillation: a nationwide cohort study. Thromb Haemost. 2018;118:1296–1304. doi: 10.1055/s-0038-1651482. [DOI] [PubMed] [Google Scholar]
- 142.Chao TF, Lip GY, Liu CJ, et al. Relationship of aging and incident comorbidities to stroke risk in patients with atrial fibrillation. J Am Coll Cardiol. 2018;71:122–132. doi: 10.1016/j.jacc.2017.10.085. [DOI] [PubMed] [Google Scholar]
- 143.Chao TF, Lip GY, Lin YJ, et al. Incident risk factors and major bleeding in patients with atrial fibrillation treated with oral anticoagulants: a comparison of baseline, follow-up and delta HAS-BLED scores with an approach focused on modifiable bleeding risk factors. Thromb Haemost. 2018;118:768–777. doi: 10.1055/s-0038-1636534. [DOI] [PubMed] [Google Scholar]
- 144.Lip GY, Wang KL, Chiang CE. Non-vitamin K antagonist oral anticoagulants (NOACs) for stroke prevention in Asian patients with atrial fibrillation: time for a reappraisal. Int J Cardiol. 2015;180:246–254. doi: 10.1016/j.ijcard.2014.11.182. [DOI] [PubMed] [Google Scholar]
- 145.Cha MJ, Choi EK, Han KD, et al. Effectiveness and safety of non-vitamin K antagonist oral anticoagulants in Asian patients with atrial fibrillation. Stroke. 2017;48:3040–3048. doi: 10.1161/STROKEAHA.117.018773. [DOI] [PubMed] [Google Scholar]
- 146.Cho MY, Park J, Kim Y, et al. Clinical effectiveness and safety of standard-dose and low-dose non-vitamin K antagonist oral anticoagulants in patients with nonvalvular atrial fibrillation: a nationwide population-based cohort study. Heart Rhythm. 2017 [Google Scholar]
- 147.Lip GY, Clemens A, Noack H, Ferreira J, Connolly SJ, Yusuf S. Patient outcomes using the European label for dabigatran. A post-hoc analysis from the RE-LY database. Thromb Haemost. 2014;111:933–942. doi: 10.1160/TH13-09-0734. [DOI] [PubMed] [Google Scholar]
- 148.Chan NC, Coppens M, Hirsh J, et al. Real-world variability in dabigatran levels in patients with atrial fibrillation. J Thromb Haemost. 2015;13:353–359. doi: 10.1111/jth.12823. [DOI] [PubMed] [Google Scholar]
- 149.Li CH, Liu CJ, Chou AY, et al. European Society of Cardiology guideline-adherent antithrombotic treatment and risk of mortality in Asian patients with atrial fibrillation. Sci Rep. 2016;6:30734. doi: 10.1038/srep30734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Kim H, Kim TH, Cha MJ, et al. A prospective survey of atrial fibrillation management for real-world guideline adherence: COmparison study of Drugs for symptom control and complication prEvention of Atrial Fibrillation (CODE-AF) Registry. Korean Circ J. 2017;47:877–887. doi: 10.4070/kcj.2017.0146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Lee SR, Choi EK, Han KD, Cha MJ, Oh S, Lip GY. Temporal trends of antithrombotic therapy for stroke prevention in Korean patients with non-valvular atrial fibrillation in the era of non-vitamin K antagonist oral anticoagulants: a nationwide population-based study. PLoS One. 2017;12:e0189495. doi: 10.1371/journal.pone.0189495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Lip GY, Lane DA. Bleeding risk assessment in atrial fibrillation: observations on the use and misuse of bleeding risk scores. J Thromb Haemost. 2016;14:1711–1714. doi: 10.1111/jth.13386. [DOI] [PubMed] [Google Scholar]
- 153.Douketis JD, Spyropoulos AC, Kaatz S, et al. Perioperative bridging anticoagulation in patients with atrial fibrillation. N Engl J Med. 2015;373:823–833. doi: 10.1056/NEJMoa1501035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Douketis JD, Hasselblad V, Ortel TL. Bridging anticoagulation in patients with atrial fibrillation. N Engl J Med. 2016;374:93–94. doi: 10.1056/NEJMc1513255. [DOI] [PubMed] [Google Scholar]
- 155.Man-Son-Hing M, Nichol G, Lau A, Laupacis A. Choosing antithrombotic therapy for elderly patients with atrial fibrillation who are at risk for falls. Arch Intern Med. 1999;159:677–685. doi: 10.1001/archinte.159.7.677. [DOI] [PubMed] [Google Scholar]
- 156.Guo Y, Zhu H, Chen Y, Lip GY. Comparing bleeding risk assessment focused on modifiable risk factors only versus validated bleeding risk scores in atrial fibrillation. Am J Med. 2018;131:185–192. doi: 10.1016/j.amjmed.2017.09.009. [DOI] [PubMed] [Google Scholar]
- 157.Chao TF, Lip GY, Lin YJ, et al. Major bleeding and intracranial hemorrhage risk prediction in patients with atrial fibrillation: attention to modifiable bleeding risk factors or use of a bleeding risk stratification score? A nationwide cohort study. Int J Cardiol. 2018;254:157–161. doi: 10.1016/j.ijcard.2017.11.025. [DOI] [PubMed] [Google Scholar]
- 158.Esteve-Pastor MA, Rivera-Caravaca JM, Shantsila A, Roldán V, Lip GY, Marín F. Assessing bleeding risk in atrial fibrillation patients: comparing a bleeding risk score based only on modifiable bleeding risk factors against the HAS-BLED score. The AMADEUS trial. Thromb Haemost. 2017;117:2261–2266. doi: 10.1160/TH17-10-0710. [DOI] [PubMed] [Google Scholar]
- 159.Zulkifly H, Lip GY, Lane DA. Bleeding risk scores in atrial fibrillation and venous thromboembolism. Am J Cardiol. 2017;120:1139–1145. doi: 10.1016/j.amjcard.2017.06.058. [DOI] [PubMed] [Google Scholar]
- 160.Holmes DR, Jr, Kar S, Price MJ, et al. Prospective randomized evaluation of the Watchman Left Atrial Appendage Closure device in patients with atrial fibrillation versus long-term warfarin therapy: the PREVAIL trial. J Am Coll Cardiol. 2014;64:1–12. doi: 10.1016/j.jacc.2014.04.029. [DOI] [PubMed] [Google Scholar]
- 161.Holmes DR, Reddy VY, Turi ZG, et al. Percutaneous closure of the left atrial appendage versus warfarin therapy for prevention of stroke in patients with atrial fibrillation: a randomised non-inferiority trial. Lancet. 2009;374:534–542. doi: 10.1016/S0140-6736(09)61343-X. [DOI] [PubMed] [Google Scholar]
- 162.Reddy VY, Doshi SK, Sievert H, et al. Percutaneous left atrial appendage closure for stroke prophylaxis in patients with atrial fibrillation: 2.3-year follow-up of the PROTECT AF (Watchman Left Atrial Appendage System for Embolic Protection in Patients with Atrial Fibrillation) trial. Circulation. 2013;127:720–729. doi: 10.1161/CIRCULATIONAHA.112.114389. [DOI] [PubMed] [Google Scholar]
- 163.Reddy VY, Sievert H, Halperin J, et al. Percutaneous left atrial appendage closure vs warfarin for atrial fibrillation: a randomized clinical trial. JAMA. 2014;312:1988–1998. doi: 10.1001/jama.2014.15192. [DOI] [PubMed] [Google Scholar]
- 164.Holmes DR, Jr, Doshi SK, Kar S, et al. Left atrial appendage closure as an alternative to warfarin for stroke prevention in atrial fibrillation: a patient-level meta-analysis. J Am Coll Cardiol. 2015;65:2614–2623. doi: 10.1016/j.jacc.2015.04.025. [DOI] [PubMed] [Google Scholar]
- 165.Boersma LV, Schmidt B, Betts TR, et al. Implant success and safety of left atrial appendage closure with the WATCHMAN device: peri-procedural outcomes from the EWOLUTION registry. Eur Heart J. 2016;37:2465–2474. doi: 10.1093/eurheartj/ehv730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Badheka AO, Chothani A, Mehta K, et al. Utilization and adverse outcomes of percutaneous left atrial appendage closure for stroke prevention in atrial fibrillation in the United States: influence of hospital volume. Circ Arrhythm Electrophysiol. 2015;8:42–48. doi: 10.1161/CIRCEP.114.001413. [DOI] [PubMed] [Google Scholar]
- 167.Casu G, Gulizia MM, Molon G, et al. ANMCO/AIAC/SICI-GISE/SIC/SICCH consensus document: percutaneous occlusion of the left atrial appendage in non-valvular atrial fibrillation patients: indications, patient selection, staff skills, organisation, and training. Eur Heart J Suppl. 2017;19:D333–D353. doi: 10.1093/eurheartj/sux008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Whitlock R, Healey J, Vincent J, et al. Rationale and design of the Left Atrial Appendage Occlusion Study (LAAOS) III. Ann Cardiothorac Surg. 2014;3:45–54. doi: 10.3978/j.issn.2225-319X.2013.12.06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Schreck DM, Rivera AR, Tricarico VJ. Emergency management of atrial fibrillation and flutter: intravenous diltiazem versus intravenous digoxin. Ann Emerg Med. 1997;29:135–140. doi: 10.1016/s0196-0644(97)70319-6. [DOI] [PubMed] [Google Scholar]
- 170.Siu CW, Lau CP, Lee WL, Lam KF, Tse HF. Intravenous diltiazem is superior to intravenous amiodarone or digoxin for achieving ventricular rate control in patients with acute uncomplicated atrial fibrillation. Crit Care Med. 2009;37:2174–2179. doi: 10.1097/CCM.0b013e3181a02f56. [DOI] [PubMed] [Google Scholar]
- 171.Tisdale JE, Padhi ID, Goldberg AD, et al. A randomized, double-blind comparison of intravenous diltiazem and digoxin for atrial fibrillation after coronary artery bypass surgery. Am Heart J. 1998;135:739–747. doi: 10.1016/s0002-8703(98)70031-6. [DOI] [PubMed] [Google Scholar]
- 172.Scheuermeyer FX, Grafstein E, Stenstrom R, et al. Safety and efficiency of calcium channel blockers versus beta-blockers for rate control in patients with atrial fibrillation and no acute underlying medical illness. Acad Emerg Med. 2013;20:222–230. doi: 10.1111/acem.12091. [DOI] [PubMed] [Google Scholar]
- 173.Platia EV, Michelson EL, Porterfield JK, Das G. Esmolol versus verapamil in the acute treatment of atrial fibrillation or atrial flutter. Am J Cardiol. 1989;63:925–929. doi: 10.1016/0002-9149(89)90141-0. [DOI] [PubMed] [Google Scholar]
- 174.Ellenbogen KA, Dias VC, Plumb VJ, Heywood JT, Mirvis DM. A placebo-controlled trial of continuous intravenous diltiazem infusion for 24-hour heart rate control during atrial fibrillation and atrial flutter: a multicenter study. J Am Coll Cardiol. 1991;18:891–897. doi: 10.1016/0735-1097(91)90743-s. [DOI] [PubMed] [Google Scholar]
- 175.Steinberg JS, Katz RJ, Bren GB, Buff LA, Varghese PJ. Efficacy of oral diltiazem to control ventricular response in chronic atrial fibrillation at rest and during exercise. J Am Coll Cardiol. 1987;9:405–411. doi: 10.1016/s0735-1097(87)80396-0. [DOI] [PubMed] [Google Scholar]
- 176.Goldstein RE, Boccuzzi SJ, Cruess D, Nattel S. Diltiazem increases late-onset congestive heart failure in postinfarction patients with early reduction in ejection fraction. The Adverse Experience Committee; and the Multicenter Diltiazem Postinfarction Research Group. Circulation. 1991;83:52–60. doi: 10.1161/01.cir.83.1.52. [DOI] [PubMed] [Google Scholar]
- 177.Darby AE, Dimarco JP. Management of atrial fibrillation in patients with structural heart disease. Circulation. 2012;125:945–957. doi: 10.1161/CIRCULATIONAHA.111.019935. [DOI] [PubMed] [Google Scholar]
- 178.Clemo HF, Wood MA, Gilligan DM, Ellenbogen KA. Intravenous amiodarone for acute heart rate control in the critically ill patient with atrial tachyarrhythmias. Am J Cardiol. 1998;81:594–598. doi: 10.1016/s0002-9149(97)00962-4. [DOI] [PubMed] [Google Scholar]
- 179.Delle Karth G, Geppert A, Neunteufl T, et al. Amiodarone versus diltiazem for rate control in critically ill patients with atrial tachyarrhythmias. Crit Care Med. 2001;29:1149–1153. doi: 10.1097/00003246-200106000-00011. [DOI] [PubMed] [Google Scholar]
- 180.Van Gelder IC, Groenveld HF, Crijns HJ, et al. Lenient versus strict rate control in patients with atrial fibrillation. N Engl J Med. 2010;362:1363–1373. doi: 10.1056/NEJMoa1001337. [DOI] [PubMed] [Google Scholar]
- 181.Al-Khatib SM, Allen LaPointe NM, Chatterjee R, et al. Rate- and rhythm-control therapies in patients with atrial fibrillation: a systematic review. Ann Intern Med. 2014;160:760–773. doi: 10.7326/M13-1467. [DOI] [PubMed] [Google Scholar]
- 182.Roy D, Talajic M, Nattel S, et al. Rhythm control versus rate control for atrial fibrillation and heart failure. N Engl J Med. 2008;358:2667–2677. doi: 10.1056/NEJMoa0708789. [DOI] [PubMed] [Google Scholar]
- 183.Kirchhof P, Andresen D, Bosch R, et al. Short-term versus long-term antiarrhythmic drug treatment after cardioversion of atrial fibrillation (Flec-SL): a prospective, randomised, open-label, blinded endpoint assessment trial. Lancet. 2012;380:238–246. doi: 10.1016/S0140-6736(12)60570-4. [DOI] [PubMed] [Google Scholar]
- 184.Lafuente-Lafuente C, Valembois L, Bergmann JF, Belmin J. Antiarrhythmics for maintaining sinus rhythm after cardioversion of atrial fibrillation. Cochrane Database Syst Rev. 2015;(3):CD005049. doi: 10.1002/14651858.CD005049.pub4. [DOI] [PubMed] [Google Scholar]
- 185.Wyse DG, Waldo AL, DiMarco JP, et al. A comparison of rate control and rhythm control in patients with atrial fibrillation. N Engl J Med. 2002;347:1825–1833. doi: 10.1056/NEJMoa021328. [DOI] [PubMed] [Google Scholar]
- 186.Van Gelder IC, Hagens VE, Bosker HA, et al. A comparison of rate control and rhythm control in patients with recurrent persistent atrial fibrillation. N Engl J Med. 2002;347:1834–1840. doi: 10.1056/NEJMoa021375. [DOI] [PubMed] [Google Scholar]
- 187.Opolski G, Torbicki A, Kosior DA, et al. Rate control vs rhythm control in patients with nonvalvular persistent atrial fibrillation: the results of the Polish How to Treat Chronic Atrial Fibrillation (HOT CAFE) Study. Chest. 2004;126:476–486. doi: 10.1378/chest.126.2.476. [DOI] [PubMed] [Google Scholar]
- 188.Chatterjee S, Sardar P, Lichstein E, Mukherjee D, Aikat S. Pharmacologic rate versus rhythm-control strategies in atrial fibrillation: an updated comprehensive review and meta-analysis. Pacing Clin Electrophysiol. 2013;36:122–133. doi: 10.1111/j.1540-8159.2012.03513.x. [DOI] [PubMed] [Google Scholar]
- 189.de Denus S, Sanoski CA, Carlsson J, Opolski G, Spinler SA. Rate vs rhythm control in patients with atrial fibrillation: a meta-analysis. Arch Intern Med. 2005;165:258–262. doi: 10.1001/archinte.165.3.258. [DOI] [PubMed] [Google Scholar]
- 190.Kotecha D, Kirchhof P. Rate and rhythm control have comparable effects on mortality and stroke in atrial fibrillation but better data are needed. Evid Based Med. 2014;19:222–223. doi: 10.1136/ebmed-2014-110062. [DOI] [PubMed] [Google Scholar]
- 191.Wilber DJ, Pappone C, Neuzil P, et al. Comparison of antiarrhythmic drug therapy and radiofrequency catheter ablation in patients with paroxysmal atrial fibrillation: a randomized controlled trial. JAMA. 2010;303:333–340. doi: 10.1001/jama.2009.2029. [DOI] [PubMed] [Google Scholar]
- 192.Arbelo E, Brugada J, Hindricks G, et al. The atrial fibrillation ablation pilot study: a European survey on methodology and results of catheter ablation for atrial fibrillation conducted by the European Heart Rhythm Association. Eur Heart J. 2014;35:1466–1478. doi: 10.1093/eurheartj/ehu001. [DOI] [PubMed] [Google Scholar]
- 193.Anselmino M, Matta M, D'Ascenzo F, et al. Catheter ablation of atrial fibrillation in patients with left ventricular systolic dysfunction: a systematic review and meta-analysis. Circ Arrhythm Electrophysiol. 2014;7:1011–1018. doi: 10.1161/CIRCEP.114.001938. [DOI] [PubMed] [Google Scholar]
- 194.Kirchhof P, Breithardt G, Camm AJ, et al. Improving outcomes in patients with atrial fibrillation: rationale and design of the Early treatment of Atrial fibrillation for Stroke prevention Trial. Am Heart J. 2013;166:442–448. doi: 10.1016/j.ahj.2013.05.015. [DOI] [PubMed] [Google Scholar]
- 195.Shi LZ, Heng R, Liu SM, Leng FY. Effect of catheter ablation versus antiarrhythmic drugs on atrial fibrillation: a meta-analysis of randomized controlled trials. Exp Ther Med. 2015;10:816–822. doi: 10.3892/etm.2015.2545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Choi YJ, Kang KW, Kim TH, et al. Comparison of rhythm and rate control strategies for stroke occurrence in a prospective cohort of atrial fibrillation patients. Yonsei Med J. 2018;59:258–264. doi: 10.3349/ymj.2018.59.2.258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Kirchhof P, Mönnig G, Wasmer K, et al. A trial of self-adhesive patch electrodes and hand-held paddle electrodes for external cardioversion of atrial fibrillation (MOBIPAPA) Eur Heart J. 2005;26:1292–1297. doi: 10.1093/eurheartj/ehi160. [DOI] [PubMed] [Google Scholar]
- 198.Mittal S, Ayati S, Stein KM, et al. Transthoracic cardioversion of atrial fibrillation: comparison of rectilinear biphasic versus damped sine wave monophasic shocks. Circulation. 2000;101:1282–1287. doi: 10.1161/01.cir.101.11.1282. [DOI] [PubMed] [Google Scholar]
- 199.Kirchhof P, Eckardt L, Loh P, et al. Anterior-posterior versus anterior-lateral electrode positions for external cardioversion of atrial fibrillation: a randomised trial. Lancet. 2002;360:1275–1279. doi: 10.1016/s0140-6736(02)11315-8. [DOI] [PubMed] [Google Scholar]
- 200.Furniss SS, Sneyd JR. Safe sedation in modern cardiological practice. Heart. 2015;101:1526–1530. doi: 10.1136/heartjnl-2015-307656. [DOI] [PubMed] [Google Scholar]
- 201.Bianconi L, Mennuni M, Lukic V, Castro A, Chieffi M, Santini M. Effects of oral propafenone administration before electrical cardioversion of chronic atrial fibrillation: a placebo-controlled study. J Am Coll Cardiol. 1996;28:700–706. doi: 10.1016/0735-1097(96)00230-6. [DOI] [PubMed] [Google Scholar]
- 202.Singh SN, Tang XC, Reda D, Singh BN. Systematic electrocardioversion for atrial fibrillation and role of antiarrhythmic drugs: a substudy of the SAFE-T trial. Heart Rhythm. 2009;6:152–155. doi: 10.1016/j.hrthm.2008.10.036. [DOI] [PubMed] [Google Scholar]
- 203.Channer KS, Birchall A, Steeds RP, et al. A randomized placebo-controlled trial of pre-treatment and short- or long-term maintenance therapy with amiodarone supporting DC cardioversion for persistent atrial fibrillation. Eur Heart J. 2004;25:144–150. doi: 10.1016/j.ehj.2003.10.020. [DOI] [PubMed] [Google Scholar]
- 204.Hemels ME, Van Noord T, Crijns HJ, et al. Verapamil versus digoxin and acute versus routine serial cardioversion for the improvement of rhythm control for persistent atrial fibrillation. J Am Coll Cardiol. 2006;48:1001–1009. doi: 10.1016/j.jacc.2006.05.043. [DOI] [PubMed] [Google Scholar]
- 205.Villani GQ, Piepoli MF, Terracciano C, Capucci A. Effects of diltiazem pretreatment on direct-current cardioversion in patients with persistent atrial fibrillation: a single-blind, randomized, controlled study. Am Heart J. 2000;140:e12. doi: 10.1067/mhj.2000.107179. [DOI] [PubMed] [Google Scholar]
- 206.De Simone A, Stabile G, Vitale DF, et al. Pretreatment with verapamil in patients with persistent or chronic atrial fibrillation who underwent electrical cardioversion. J Am Coll Cardiol. 1999;34:810–814. doi: 10.1016/s0735-1097(99)00256-9. [DOI] [PubMed] [Google Scholar]
- 207.The Digitalis in Acute Atrial Fibrillation (DAAF) Trial Group. Intravenous digoxin in acute atrial fibrillation. Results of a randomized, placebo-controlled multicentre trial in 239 patients. Eur Heart J. 1997;18:649–654. doi: 10.1093/oxfordjournals.eurheartj.a015311. [DOI] [PubMed] [Google Scholar]
- 208.Atarashi H, Inoue H, Fukunami M, et al. Double-blind placebo-controlled trial of aprindine and digoxin for the prevention of symptomatic atrial fibrillation. Circ J. 2002;66:553–556. doi: 10.1253/circj.66.553. [DOI] [PubMed] [Google Scholar]
- 209.Schädlich PK, Schmidt-Lucke C, Huppertz E, et al. Economic evaluation of enoxaparin for anticoagulation in early cardioversion of persisting nonvalvular atrial fibrillation: a statutory health insurance perspective from Germany. Am J Cardiovasc Drugs. 2007;7:199–217. doi: 10.2165/00129784-200707030-00006. [DOI] [PubMed] [Google Scholar]
- 210.Schmidt-Lucke C, Paar WD, Stellbrink C, et al. Quality of anticoagulation with unfractionated heparin plus phenprocoumon for the prevention of thromboembolic complications in cardioversion for non-valvular atrial fibrillation. Sub-analysis from the Anticoagulation in Cardioversion using Enoxaparin (ACE) trial. Thromb Res. 2007;119:27–34. doi: 10.1016/j.thromres.2005.11.016. [DOI] [PubMed] [Google Scholar]
- 211.Stellbrink C, Nixdorff U, Hofmann T, et al. Safety and efficacy of enoxaparin compared with unfractionated heparin and oral anticoagulants for prevention of thromboembolic complications in cardioversion of nonvalvular atrial fibrillation: the Anticoagulation in Cardioversion using Enoxaparin (ACE) trial. Circulation. 2004;109:997–1003. doi: 10.1161/01.CIR.0000120509.64740.DC. [DOI] [PubMed] [Google Scholar]
- 212.Shin DG, Cho I, Hartaigh B, et al. Cardiovascular events of electrical cardioversion under optimal anticoagulation in atrial fibrillation: the multicenter analysis. Yonsei Med J. 2015;56:1552–1558. doi: 10.3349/ymj.2015.56.6.1552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Hansen ML, Jepsen RM, Olesen JB, et al. Thromboembolic risk in 16 274 atrial fibrillation patients undergoing direct current cardioversion with and without oral anticoagulant therapy. Europace. 2015;17:18–23. doi: 10.1093/europace/euu189. [DOI] [PubMed] [Google Scholar]
- 214.Gwag HB, Chun KJ, Hwang JK, et al. Which antiarrhythmic drug to choose after electrical cardioversion: a study on non-valvular atrial fibrillation patients. PLoS One. 2018;13:e0197352. doi: 10.1371/journal.pone.0197352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Khan IA. Oral loading single dose flecainide for pharmacological cardioversion of recent-onset atrial fibrillation. Int J Cardiol. 2003;87:121–128. doi: 10.1016/s0167-5273(02)00467-9. [DOI] [PubMed] [Google Scholar]
- 216.Chevalier P, Durand-Dubief A, Burri H, Cucherat M, Kirkorian G, Touboul P. Amiodarone versus placebo and class Ic drugs for cardioversion of recent-onset atrial fibrillation: a meta-analysis. J Am Coll Cardiol. 2003;41:255–262. doi: 10.1016/s0735-1097(02)02705-5. [DOI] [PubMed] [Google Scholar]
- 217.Letelier LM, Udol K, Ena J, Weaver B, Guyatt GH. Effectiveness of amiodarone for conversion of atrial fibrillation to sinus rhythm: a meta-analysis. Arch Intern Med. 2003;163:777–785. doi: 10.1001/archinte.163.7.777. [DOI] [PubMed] [Google Scholar]
- 218.Khan IA, Mehta NJ, Gowda RM. Amiodarone for pharmacological cardioversion of recent-onset atrial fibrillation. Int J Cardiol. 2003;89:239–248. doi: 10.1016/s0167-5273(02)00477-1. [DOI] [PubMed] [Google Scholar]
- 219.Thomas SP, Guy D, Wallace E, et al. Rapid loading of sotalol or amiodarone for management of recent onset symptomatic atrial fibrillation: a randomized, digoxin-controlled trial. Am Heart J. 2004;147:E3. doi: 10.1016/s0002-8703(03)00526-x. [DOI] [PubMed] [Google Scholar]
- 220.Vijayalakshmi K, Whittaker VJ, Sutton A, et al. A randomized trial of prophylactic antiarrhythmic agents (amiodarone and sotalol) in patients with atrial fibrillation for whom direct current cardioversion is planned. Am Heart J. 2006;151:863.e1–863.e6. doi: 10.1016/j.ahj.2005.09.009. [DOI] [PubMed] [Google Scholar]
- 221.Singh BN, Singh SN, Reda DJ, et al. Amiodarone versus sotalol for atrial fibrillation. N Engl J Med. 2005;352:1861–1872. doi: 10.1056/NEJMoa041705. [DOI] [PubMed] [Google Scholar]
- 222.Roy D, Pratt CM, Torp-Pedersen C, et al. Vernakalant hydrochloride for rapid conversion of atrial fibrillation: a phase 3, randomized, placebo-controlled trial. Circulation. 2008;117:1518–1525. doi: 10.1161/CIRCULATIONAHA.107.723866. [DOI] [PubMed] [Google Scholar]
- 223.Kowey PR, Dorian P, Mitchell LB, et al. Vernakalant hydrochloride for the rapid conversion of atrial fibrillation after cardiac surgery: a randomized, double-blind, placebo-controlled trial. Circ Arrhythm Electrophysiol. 2009;2:652–659. doi: 10.1161/CIRCEP.109.870204. [DOI] [PubMed] [Google Scholar]
- 224.Camm AJ, Capucci A, Hohnloser SH, et al. A randomized active-controlled study comparing the efficacy and safety of vernakalant to amiodarone in recent-onset atrial fibrillation. J Am Coll Cardiol. 2011;57:313–321. doi: 10.1016/j.jacc.2010.07.046. [DOI] [PubMed] [Google Scholar]
- 225.Bash LD, Buono JL, Davies GM, et al. Systematic review and meta-analysis of the efficacy of cardioversion by vernakalant and comparators in patients with atrial fibrillation. Cardiovasc Drugs Ther. 2012;26:167–179. doi: 10.1007/s10557-012-6374-4. [DOI] [PubMed] [Google Scholar]
- 226.Reisinger J, Gatterer E, Lang W, et al. Flecainide versus ibutilide for immediate cardioversion of atrial fibrillation of recent onset. Eur Heart J. 2004;25:1318–1324. doi: 10.1016/j.ehj.2004.04.030. [DOI] [PubMed] [Google Scholar]
- 227.Stambler BS, Wood MA, Ellenbogen KA, Perry KT, Wakefield LK, VanderLugt JT. Efficacy and safety of repeated intravenous doses of ibutilide for rapid conversion of atrial flutter or fibrillation. Ibutilide Repeat Dose Study Investigators. Circulation. 1996;94:1613–1621. doi: 10.1161/01.cir.94.7.1613. [DOI] [PubMed] [Google Scholar]
- 228.Reisinger J, Gatterer E, Heinze G, et al. Prospective comparison of flecainide versus sotalol for immediate cardioversion of atrial fibrillation. Am J Cardiol. 1998;81:1450–1454. doi: 10.1016/s0002-9149(98)00223-9. [DOI] [PubMed] [Google Scholar]
- 229.Mun HS, Shen C, Pak HN, et al. Chronic amiodarone therapy impairs the function of the superior sinoatrial node in patients with atrial fibrillation. Circ J. 2013;77:2255–2263. doi: 10.1253/circj.cj-12-1615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230.Alboni P, Botto GL, Baldi N, et al. Outpatient treatment of recent-onset atrial fibrillation with the “pill-in-the-pocket” approach. N Engl J Med. 2004;351:2384–2391. doi: 10.1056/NEJMoa041233. [DOI] [PubMed] [Google Scholar]
- 231.Saborido CM, Hockenhull J, Bagust A, Boland A, Dickson R, Todd D. Systematic review and cost-effectiveness evaluation of ‘pill-in-the-pocket’ strategy for paroxysmal atrial fibrillation compared to episodic in-hospital treatment or continuous antiarrhythmic drug therapy. Health Technol Assess. 2010;14:iii–iv. doi: 10.3310/hta14310. [DOI] [PubMed] [Google Scholar]
- 232.Lau DH, Nattel S, Kalman JM, Sanders P. Modifiable risk factors and atrial fibrillation. Circulation. 2017;136:583–596. doi: 10.1161/CIRCULATIONAHA.116.023163. [DOI] [PubMed] [Google Scholar]
- 233.Van Gelder IC, Crijns HJ, Van Gilst WH, Van Wijk LM, Hamer HP, Lie KI. Efficacy and safety of flecainide acetate in the maintenance of sinus rhythm after electrical cardioversion of chronic atrial fibrillation or atrial flutter. Am J Cardiol. 1989;64:1317–1321. doi: 10.1016/0002-9149(89)90574-2. [DOI] [PubMed] [Google Scholar]
- 234.Echt DS, Liebson PR, Mitchell LB, et al. Mortality and morbidity in patients receiving encainide, flecainide, or placebo. The Cardiac Arrhythmia Suppression Trial. N Engl J Med. 1991;324:781–788. doi: 10.1056/NEJM199103213241201. [DOI] [PubMed] [Google Scholar]
- 235.Chimienti M, Cullen MT, Jr, Casadei G. Safety of flecainide versus propafenone for the long-term management of symptomatic paroxysmal supraventricular tachyarrhythmias. Report from the Flecainide and Propafenone Italian Study (FAPIS) Group. Eur Heart J. 1995;16:1943–1951. doi: 10.1093/oxfordjournals.eurheartj.a060852. [DOI] [PubMed] [Google Scholar]
- 236.Kim DS, Koh CW, Cho HK, et al. Comparision of the efficacy of propafenone and flecainide in patients with atrial fibrillation. Korean Circ J. 1997;27:860–866. [Google Scholar]
- 237.Aliot E, Capucci A, Crijns HJ, Goette A, Tamargo J. Twenty-five years in the making: flecainide is safe and effective for the management of atrial fibrillation. Europace. 2011;13:161–173. doi: 10.1093/europace/euq382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 238.Freemantle N, Lafuente-Lafuente C, Mitchell S, Eckert L, Reynolds M. Mixed treatment comparison of dronedarone, amiodarone, sotalol, flecainide, and propafenone, for the management of atrial fibrillation. Europace. 2011;13:329–345. doi: 10.1093/europace/euq450. [DOI] [PubMed] [Google Scholar]
- 239.Chun KJ, Byeon K, Im SI, et al. Efficacy of dronedarone versus propafenone in the maintenance of sinus rhythm in patients with atrial fibrillation after electrical cardioversion. Clin Ther. 2014;36:1169–1175. doi: 10.1016/j.clinthera.2014.07.013. [DOI] [PubMed] [Google Scholar]
- 240.Singh SN, Fletcher RD, Fisher SG, et al. Amiodarone in patients with congestive heart failure and asymptomatic ventricular arrhythmia. Survival Trial of Antiarrhythmic Therapy in Congestive Heart Failure. N Engl J Med. 1995;333:77–82. doi: 10.1056/NEJM199507133330201. [DOI] [PubMed] [Google Scholar]
- 241.Kirchhof P, Franz MR, Bardai A, Wilde AM. Giant T–U waves precede torsades de pointes in long QT syndrome: a systematic electrocardiographic analysis in patients with acquired and congenital QT prolongation. J Am Coll Cardiol. 2009;54:143–149. doi: 10.1016/j.jacc.2009.03.043. [DOI] [PubMed] [Google Scholar]
- 242.Goldschlager N, Epstein AE, Naccarelli GV, et al. A practical guide for clinicians who treat patients with amiodarone: 2007. Heart Rhythm. 2007;4:1250–1259. doi: 10.1016/j.hrthm.2007.07.020. [DOI] [PubMed] [Google Scholar]
- 243.Singh BN, Connolly SJ, Crijns HJ, et al. Dronedarone for maintenance of sinus rhythm in atrial fibrillation or flutter. N Engl J Med. 2007;357:987–999. doi: 10.1056/NEJMoa054686. [DOI] [PubMed] [Google Scholar]
- 244.Hohnloser SH, Crijns HJ, van Eickels M, et al. Effect of dronedarone on cardiovascular events in atrial fibrillation. N Engl J Med. 2009;360:668–678. doi: 10.1056/NEJMoa0803778. [DOI] [PubMed] [Google Scholar]
- 245.Køber L, Torp-Pedersen C, McMurray JJ, et al. Increased mortality after dronedarone therapy for severe heart failure. N Engl J Med. 2008;358:2678–2687. doi: 10.1056/NEJMoa0800456. [DOI] [PubMed] [Google Scholar]
- 246.Connolly SJ, Camm AJ, Halperin JL, et al. Dronedarone in high-risk permanent atrial fibrillation. N Engl J Med. 2011;365:2268–2276. doi: 10.1056/NEJMoa1109867. [DOI] [PubMed] [Google Scholar]
- 247.Roy D, Talajic M, Dorian P, et al. Amiodarone to prevent recurrence of atrial fibrillation. N Engl J Med. 2000;342:913–920. doi: 10.1056/NEJM200003303421302. [DOI] [PubMed] [Google Scholar]
- 248.Klein AL, Grimm RA, Murray RD, et al. Use of transesophageal echocardiography to guide cardioversion in patients with atrial fibrillation. N Engl J Med. 2001;344:1411–1420. doi: 10.1056/NEJM200105103441901. [DOI] [PubMed] [Google Scholar]
- 249.Nagarakanti R, Ezekowitz MD, Oldgren J, et al. Dabigatran versus warfarin in patients with atrial fibrillation: an analysis of patients undergoing cardioversion. Circulation. 2011;123:131–136. doi: 10.1161/CIRCULATIONAHA.110.977546. [DOI] [PubMed] [Google Scholar]
- 250.Piccini JP, Stevens SR, Lokhnygina Y, et al. Outcomes after cardioversion and atrial fibrillation ablation in patients treated with rivaroxaban and warfarin in the ROCKET AF trial. J Am Coll Cardiol. 2013;61:1998–2006. doi: 10.1016/j.jacc.2013.02.025. [DOI] [PubMed] [Google Scholar]
- 251.Flaker G, Lopes RD, Al-Khatib SM, et al. Efficacy and safety of apixaban in patients after cardioversion for atrial fibrillation: insights from the ARISTOTLE trial (Apixaban for Reduction in Stroke and Other Thromboembolic Events in Atrial Fibrillation) J Am Coll Cardiol. 2014;63:1082–1087. doi: 10.1016/j.jacc.2013.09.062. [DOI] [PubMed] [Google Scholar]
- 252.Cappato R, Ezekowitz MD, Klein AL, et al. Rivaroxaban vs. vitamin K antagonists for cardioversion in atrial fibrillation. Eur Heart J. 2014;35:3346–3355. doi: 10.1093/eurheartj/ehu367. [DOI] [PubMed] [Google Scholar]
- 253.Goette A, Merino JL, Ezekowitz MD, et al. Edoxaban versus enoxaparin-warfarin in patients undergoing cardioversion of atrial fibrillation (ENSURE-AF): a randomised, open-label, phase 3b trial. Lancet. 2016;388:1995–2003. doi: 10.1016/S0140-6736(16)31474-X. [DOI] [PubMed] [Google Scholar]
- 254.Ezekowitz MD, Pollack CV, Jr, Halperin JL, et al. Apixaban compared to heparin/vitamin K antagonist in patients with atrial fibrillation scheduled for cardioversion: the EMANATE trial. Eur Heart J. 2018;39:2959–2971. doi: 10.1093/eurheartj/ehy148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 255.Steffel J, Verhamme P, Potpara TS, et al. The 2018 European Heart Rhythm Association Practical Guide on the use of non-vitamin K antagonist oral anticoagulants in patients with atrial fibrillation. Eur Heart J. 2018;39:1330–1393. doi: 10.1093/eurheartj/ehy136. [DOI] [PubMed] [Google Scholar]
- 256.Lip GYH, Collet JP, Haude M, et al. 2018 Joint European consensus document on the management of antithrombotic therapy in atrial fibrillation patients presenting with acute coronary syndrome and/or undergoing percutaneous cardiovascular interventions: a joint consensus document of the European Heart Rhythm Association (EHRA), European Society of Cardiology Working Group on Thrombosis, European Association of Percutaneous Cardiovascular Interventions (EAPCI), and European Association of Acute Cardiac Care (ACCA) endorsed by the Heart Rhythm Society (HRS), Asia-Pacific Heart Rhythm Society (APHRS), Latin America Heart Rhythm Society (LAHRS), and Cardiac Arrhythmia Society of Southern Africa (CASSA) Europace. 2018:[Epub ahead of print]. [Google Scholar]
- 257.Leon MB, Baim DS, Popma JJ, et al. A clinical trial comparing three antithrombotic-drug regimens after coronary-artery stenting. N Engl J Med. 1998;339:1665–1671. doi: 10.1056/NEJM199812033392303. [DOI] [PubMed] [Google Scholar]
- 258.ACTIVE Writing Group of the ACTIVE Investigators. Connolly S, Pogue J, et al. Clopidogrel plus aspirin versus oral anticoagulation for atrial fibrillation in the Atrial fibrillation Clopidogrel Trial with Irbesartan for prevention of Vascular Events (ACTIVE W): a randomised controlled trial. Lancet. 2006;367:1903–1912. doi: 10.1016/S0140-6736(06)68845-4. [DOI] [PubMed] [Google Scholar]
- 259.Dewilde WJ, Oirbans T, Verheugt FW, et al. Use of clopidogrel with or without aspirin in patients taking oral anticoagulant therapy and undergoing percutaneous coronary intervention: an open-label, randomised, controlled trial. Lancet. 2013;381:1107–1115. doi: 10.1016/S0140-6736(12)62177-1. [DOI] [PubMed] [Google Scholar]
- 260.Rubboli A, Faxon DP, Juhani Airaksinen KE, et al. The optimal management of patients on oral anticoagulation undergoing coronary artery stenting. The 10th anniversary overview. Thromb Haemost. 2014;112:1080–1087. doi: 10.1160/TH14-08-0681. [DOI] [PubMed] [Google Scholar]
- 261.Choi HI, Ahn JM, Kang SH, et al. Prevalence, management, and long-term (6-year) outcomes of atrial fibrillation among patients receiving drug-eluting coronary stents. JACC Cardiovasc Interv. 2017;10:1075–1085. doi: 10.1016/j.jcin.2017.02.028. [DOI] [PubMed] [Google Scholar]
- 262.Cannon CP, Bhatt DL, Oldgren J, et al. Dual antithrombotic therapy with dabigatran after PCI in atrial fibrillation. N Engl J Med. 2017;377:1513–1524. doi: 10.1056/NEJMoa1708454. [DOI] [PubMed] [Google Scholar]
- 263.Gibson CM, Mehran R, Bode C, et al. Prevention of bleeding in patients with atrial fibrillation undergoing PCI. N Engl J Med. 2016;375:2423–2434. doi: 10.1056/NEJMoa1611594. [DOI] [PubMed] [Google Scholar]
- 264.Golwala HB, Cannon CP, Steg PG, et al. Safety and efficacy of dual vs. triple antithrombotic therapy in patients with atrial fibrillation following percutaneous coronary intervention: a systematic review and meta-analysis of randomized clinical trials. Eur Heart J. 2018;39:1726–1735a. doi: 10.1093/eurheartj/ehy162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 265.Connolly SJ, Ezekowitz MD, Yusuf S, et al. Dabigatran versus warfarin in patients with atrial fibrillation. N Engl J Med. 2009;361:1139–1151. doi: 10.1056/NEJMoa0905561. [DOI] [PubMed] [Google Scholar]
- 266.Lopes RD, Vora AN, Liaw D, et al. An open-Label, 2 × 2 factorial, randomized controlled trial to evaluate the safety of apixaban vs. vitamin K antagonist and aspirin vs. placebo in patients with atrial fibrillation and acute coronary syndrome and/or percutaneous coronary intervention: rationale and design of the AUGUSTUS trial. Am Heart J. 2018;200:17–23. doi: 10.1016/j.ahj.2018.03.001. [DOI] [PubMed] [Google Scholar]
- 267.Vranckx P, Lewalter T, Valgimigli M, et al. Evaluation of the safety and efficacy of an edoxaban-based antithrombotic regimen in patients with atrial fibrillation following successful percutaneous coronary intervention (PCI) with stent placement: Rationale and design of the ENTRUST-AF PCI trial. Am Heart J. 2018;196:105–112. doi: 10.1016/j.ahj.2017.10.009. [DOI] [PubMed] [Google Scholar]
- 268.Lamberts M, Gislason GH, Lip GY, et al. Antiplatelet therapy for stable coronary artery disease in atrial fibrillation patients taking an oral anticoagulant: a nationwide cohort study. Circulation. 2014;129:1577–1585. doi: 10.1161/CIRCULATIONAHA.113.004834. [DOI] [PubMed] [Google Scholar]
- 269.Valgimigli M, Bueno H, Byrne RA, et al. 2017 ESC focused update on dual antiplatelet therapy in coronary artery disease developed in collaboration with EACTS: The Task Force for dual antiplatelet therapy in coronary artery disease of the European Society of Cardiology (ESC) and of the European Association for Cardio-Thoracic Surgery (EACTS) Eur Heart J. 2018;39:213–260. doi: 10.1093/eurheartj/ehx419. [DOI] [PubMed] [Google Scholar]
- 270.Calkins H, Hindricks G, Cappato R, et al. 2017 HRS/EHRA/ECAS/APHRS/SOLAECE expert consensus statement on catheter and surgical ablation of atrial fibrillation. Heart Rhythm. 2017;14:e275–444. doi: 10.1016/j.hrthm.2017.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 271.Hohnloser SH, Camm AJ. Safety and efficacy of dabigatran etexilate during catheter ablation of atrial fibrillation: a meta-analysis of the literature. Europace. 2013;15:1407–1411. doi: 10.1093/europace/eut241. [DOI] [PubMed] [Google Scholar]
- 272.Di Biase L, Lakkireddy D, Trivedi C, et al. Feasibility and safety of uninterrupted periprocedural apixaban administration in patients undergoing radiofrequency catheter ablation for atrial fibrillation: results from a multicenter study. Heart Rhythm. 2015;12:1162–1168. doi: 10.1016/j.hrthm.2015.02.028. [DOI] [PubMed] [Google Scholar]
- 273.Lakkireddy D, Reddy YM, Di Biase L, et al. Feasibility and safety of uninterrupted rivaroxaban for periprocedural anticoagulation in patients undergoing radiofrequency ablation for atrial fibrillation: results from a multicenter prospective registry. J Am Coll Cardiol. 2014;63:982–988. doi: 10.1016/j.jacc.2013.11.039. [DOI] [PubMed] [Google Scholar]
- 274.Kottmaier M, Bourier F, Pausch H, et al. Safety of uninterrupted periprocedural edoxaban versus phenprocoumon for patients who underwent left atrial catheter ablation procedures. Am J Cardiol. 2018;121:445–449. doi: 10.1016/j.amjcard.2017.11.015. [DOI] [PubMed] [Google Scholar]
- 275.Cappato R, Marchlinski FE, Hohnloser SH, et al. Uninterrupted rivaroxaban vs. uninterrupted vitamin K antagonists for catheter ablation in non-valvular atrial fibrillation. Eur Heart J. 2015;36:1805–1811. doi: 10.1093/eurheartj/ehv177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 276.Calkins H, Willems S, Gerstenfeld EP, et al. Uninterrupted dabigatran versus warfarin for ablation in atrial fibrillation. N Engl J Med. 2017;376:1627–1636. doi: 10.1056/NEJMoa1701005. [DOI] [PubMed] [Google Scholar]
- 277.Kirchhof P, Haeusler KG, Blank B, et al. Apixaban in patients at risk of stroke undergoing atrial fibrillation ablation. Eur Heart J. 2018;39:2942–2955. doi: 10.1093/eurheartj/ehy176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 278.Fox KA, Piccini JP, Wojdyla D, et al. Prevention of stroke and systemic embolism with rivaroxaban compared with warfarin in patients with non-valvular atrial fibrillation and moderate renal impairment. Eur Heart J. 2011;32:2387–2394. doi: 10.1093/eurheartj/ehr342. [DOI] [PubMed] [Google Scholar]
- 279.Eikelboom JW, Connolly SJ, Gao P, et al. Stroke risk and efficacy of apixaban in atrial fibrillation patients with moderate chronic kidney disease. J Stroke Cerebrovasc Dis. 2012;21:429–435. doi: 10.1016/j.jstrokecerebrovasdis.2012.05.007. [DOI] [PubMed] [Google Scholar]
- 280.Hohnloser SH, Hijazi Z, Thomas L, et al. Efficacy of apixaban when compared with warfarin in relation to renal function in patients with atrial fibrillation: insights from the ARISTOTLE trial. Eur Heart J. 2012;33:2821–2830. doi: 10.1093/eurheartj/ehs274. [DOI] [PubMed] [Google Scholar]
- 281.Hijazi Z, Hohnloser SH, Oldgren J, et al. Efficacy and safety of dabigatran compared with warfarin in relation to baseline renal function in patients with atrial fibrillation: a RE-LY (Randomized Evaluation of Long-term Anticoagulation Therapy) trial analysis. Circulation. 2014;129:961–970. doi: 10.1161/CIRCULATIONAHA.113.003628. [DOI] [PubMed] [Google Scholar]
- 282.Hart RG, Pearce LA, Asinger RW, Herzog CA. Warfarin in atrial fibrillation patients with moderate chronic kidney disease. Clin J Am Soc Nephrol. 2011;6:2599–2604. doi: 10.2215/CJN.02400311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 283.Ruff CT, Giugliano RP, Braunwald E, et al. Comparison of the efficacy and safety of new oral anticoagulants with warfarin in patients with atrial fibrillation: a meta-analysis of randomised trials. Lancet. 2014;383:955–962. doi: 10.1016/S0140-6736(13)62343-0. [DOI] [PubMed] [Google Scholar]
- 284.Del-Carpio Munoz F, Gharacholou SM, Munger TM, et al. Meta-analysis of renal function on the safety and efficacy of novel oral anticoagulants for atrial fibrillation. Am J Cardiol. 2016;117:69–75. doi: 10.1016/j.amjcard.2015.09.046. [DOI] [PubMed] [Google Scholar]
- 285.Heidbuchel H, Verhamme P, Alings M, et al. Updated European Heart Rhythm Association Practical Guide on the use of non-vitamin K antagonist anticoagulants in patients with non-valvular atrial fibrillation. Europace. 2015;17:1467–1507. doi: 10.1093/europace/euv309. [DOI] [PubMed] [Google Scholar]
- 286.Patel MR, Mahaffey KW, Garg J, et al. Rivaroxaban versus warfarin in nonvalvular atrial fibrillation. N Engl J Med. 2011;365:883–891. doi: 10.1056/NEJMoa1009638. [DOI] [PubMed] [Google Scholar]
- 287.Granger CB, Alexander JH, McMurray JJ, et al. Apixaban versus warfarin in patients with atrial fibrillation. N Engl J Med. 2011;365:981–992. doi: 10.1056/NEJMoa1107039. [DOI] [PubMed] [Google Scholar]
- 288.Giugliano RP, Ruff CT, Braunwald E, et al. Edoxaban versus warfarin in patients with atrial fibrillation. N Engl J Med. 2013;369:2093–2104. doi: 10.1056/NEJMoa1310907. [DOI] [PubMed] [Google Scholar]
- 289.Wizemann V, Tong L, Satayathum S, et al. Atrial fibrillation in hemodialysis patients: clinical features and associations with anticoagulant therapy. Kidney Int. 2010;77:1098–1106. doi: 10.1038/ki.2009.477. [DOI] [PubMed] [Google Scholar]
- 290.Chan KE, Lazarus JM, Thadhani R, Hakim RM. Warfarin use associates with increased risk for stroke in hemodialysis patients with atrial fibrillation. J Am Soc Nephrol. 2009;20:2223–2233. doi: 10.1681/ASN.2009030319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 291.Winkelmayer WC, Liu J, Setoguchi S, Choudhry NK. Effectiveness and safety of warfarin initiation in older hemodialysis patients with incident atrial fibrillation. Clin J Am Soc Nephrol. 2011;6:2662–2668. doi: 10.2215/CJN.04550511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 292.Schwartzenberg S, Lev EI, Sagie A, Korzets A, Kornowski R. The quandary of oral anticoagulation in patients with atrial fibrillation and chronic kidney disease. Am J Cardiol. 2016;117:477–482. doi: 10.1016/j.amjcard.2015.10.065. [DOI] [PubMed] [Google Scholar]
- 293.Potpara TS, Ferro CJ, Lip GY. Use of oral anticoagulants in patients with atrial fibrillation and renal dysfunction. Nat Rev Nephrol. 2018;14:337–351. doi: 10.1038/nrneph.2018.19. [DOI] [PubMed] [Google Scholar]
- 294.Siontis KC, Zhang X, Eckard A, et al. Outcomes associated with apixaban use in end-stage kidney disease patients with atrial fibrillation in the United States. Circulation. 2018;138:1519–1529. doi: 10.1161/CIRCULATIONAHA.118.035418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 295.Lee SR, Choi EK, Han KD, Jung JH, Oh S, Lip GY. Edoxaban in Asian patients with atrial fibrillation: effectiveness and safety. J Am Coll Cardiol. 2018;72:838–853. doi: 10.1016/j.jacc.2018.05.066. [DOI] [PubMed] [Google Scholar]
- 296.Yu HY, Kim P, Jang TH, et al. Impact of renal function on outcomes with edoxaban in real-world patients with atrial fibrillation: a nationwide cohort study. Stroke. 2018;49:2421–2429. doi: 10.1161/STROKEAHA.118.021387. [DOI] [PubMed] [Google Scholar]
- 297.Hylek EM, D'Antonio J, Evans-Molina C, Shea C, Henault LE, Regan S. Translating the results of randomized trials into clinical practice: the challenge of warfarin candidacy among hospitalized elderly patients with atrial fibrillation. Stroke. 2006;37:1075–1080. doi: 10.1161/01.STR.0000209239.71702.ce. [DOI] [PubMed] [Google Scholar]
- 298.Sharma M, Cornelius VR, Patel JP, Davies JG, Molokhia M. Efficacy and harms of direct oral anticoagulants in the elderly for stroke prevention in atrial fibrillation and secondary prevention of venous thromboembolism: systematic review and meta-analysis. Circulation. 2015;132:194–204. doi: 10.1161/CIRCULATIONAHA.114.013267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 299.Mant J, Hobbs FD, Fletcher K, et al. Warfarin versus aspirin for stroke prevention in an elderly community population with atrial fibrillation (the Birmingham Atrial Fibrillation Treatment of the Aged Study, BAFTA): a randomised controlled trial. Lancet. 2007;370:493–503. doi: 10.1016/S0140-6736(07)61233-1. [DOI] [PubMed] [Google Scholar]
- 300.Gage BF, Birman-Deych E, Kerzner R, Radford MJ, Nilasena DS, Rich MW. Incidence of intracranial hemorrhage in patients with atrial fibrillation who are prone to fall. Am J Med. 2005;118:612–617. doi: 10.1016/j.amjmed.2005.02.022. [DOI] [PubMed] [Google Scholar]
- 301.Chao TF, Liu CJ, Lin YJ, et al. Oral anticoagulation in very elderly patients with atrial fibrillation: a nationwide cohort study. Circulation. 2018;138:37–47. doi: 10.1161/CIRCULATIONAHA.117.031658. [DOI] [PubMed] [Google Scholar]
- 302.Yamashita Y, Hamatani Y, Esato M, et al. Clinical characteristics and outcomes in extreme elderly (age ≥ 85 years) Japanese patients with atrial fibrillation: the Fushimi AF registry. Chest. 2016;149:401–412. doi: 10.1378/chest.15-1095. [DOI] [PubMed] [Google Scholar]
- 303.Halvorsen S, Atar D, Yang H, et al. Efficacy and safety of apixaban compared with warfarin according to age for stroke prevention in atrial fibrillation: observations from the ARISTOTLE trial. Eur Heart J. 2014;35:1864–1872. doi: 10.1093/eurheartj/ehu046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 304.Hori M, Matsumoto M, Tanahashi N, et al. Rivaroxaban vs. warfarin in Japanese patients with non-valvular atrial fibrillation in relation to age. Circ J. 2014;78:1349–1356. doi: 10.1253/circj.cj-13-1324. [DOI] [PubMed] [Google Scholar]
- 305.Lin YC, Chien SC, Hsieh YC, et al. Effectiveness and safety of standard- and low-dose rivaroxaban in Asians with atrial fibrillation. J Am Coll Cardiol. 2018;72:477–485. doi: 10.1016/j.jacc.2018.04.084. [DOI] [PubMed] [Google Scholar]
- 306.Joung B. Real-world data and recommended dosage of non-vitamin K oral anticoagulants for Korean patients. Korean Circ J. 2017;47:833–841. doi: 10.4070/kcj.2017.0158. [DOI] [PMC free article] [PubMed] [Google Scholar]