Abstract
Background: Post-stroke depression (PSD) is a common and serious complication after stroke. In this systematic review and meta-analysis, we evaluated the association between early PSD and mortality, considering depressive symptoms occurring within the first 3 months after the neurological event.
Methods: This meta-analysis was conducted following Meta-analysis Of Observational Studies in Epidemiology (MOOSE) guidelines and based on studies indexed till May 2018 in PubMed and Web of Science databases. The relative risk (RR) for mortality in individuals with PSD, as compared with non-depressed ones, was estimated. Findings were pooled according to a random-effects model. Meta-regression and subgroup analyses were carried out.
Results: We included seven studies, accounting for 119,075 individuals, of whom 17,609 suffering from an early PSD. We found higher rates of mortality in subjects with PSD as compared with non-depressed ones (RR = 1.50; 95%CI: 1.28 to 1.75; p < 0.001). Heterogeneity across studies was moderate (I2 = 50.7%). Subgroup analysis showed a slightly higher effect of PSD on short-term mortality (RR = 1.70; p < 0.001), as compared with long-term one (RR = 1.35; p = 0.01). According to relevant meta-regression analyses, the estimate was influenced by sample proportion of men (p = 0.043).
Conclusions: Despite some limitations, our study shows the negative impact of early PSD on survival rates. Mechanisms underlying this association still need to be elucidated and several interpretations can be hypothesized. Future research should test if an early management of depression may increase life expectancy after stroke.
Keywords: stroke, depression, mortality, meta-analysis, meta-regression
Introduction
Stroke is the second leading cause of death worldwide, accounting for 6.3 million deaths in 2015 (1), and a major public health issue associated with an increasing global burden of disease (2) and different physical (3) and neuropsychological consequences (4). Post-stroke depression (PSD) is a common and serious complication after stroke (5), occurring in about one third of subjects, especially during the first stage after the acute event (6). The pathophysiology of PSD is probably multifactorial, deriving from a complex interaction between ischemia-induced neurobiological dysfunctions and psychological distress (7, 8). Although an early detection of PSD is a key issue in clinical practice, its identification remains challenging (9). This may be due to some neurological symptoms which could conceal primary mood abnormalities (5), as well as the lack of satisfactory diagnostic tools for case-finding (10). However, PSD represents one of the main factors limiting recovery and rehabilitation (9), predisposing to severe disability, functional and cognitive impairment, greater dependency in daily activities, and low treatment adherence (11, 12). In addition, several studies identified PSD as a potential risk factor for mortality (13–15). Consistently, a previous systematic review and meta-analysis (16), including 13 studies and 59,598 subjects, estimated a small, though significant, effect of PSD on mortality. However, findings were probably influenced by the methodological heterogeneity across studies. In particular, along with follow-up duration, high time variability for PSD assessment in different studies should be taken into account, considering that PSD frequency may change over time (5). Indeed, some studies evaluated early PSD (14), whereas other assessed depression up to several years after stroke (15). Since symptoms usually occur within the first 3 months after stroke (17), it seems important to evaluate the impact on survival of early PSD, including findings of recent studies published in this field (11, 18). We thus conducted a systematic review and meta-analysis, to investigate the association between early PSD and mortality, considering different factors that might potentially influence the estimated effect, including sample characteristics, assessment methods, follow-up duration, and quality.
Methods
This systematic review and meta-analysis was conducted following the Meta-analysis of Observational Studies in Epidemiology (MOOSE) guidelines (19).
Search strategy
We updated the strategy of our previous systematic review and meta-analysis (16), searching for articles indexed in PubMed and Web of Science electronic databases up to May 30th, 2018. No language restriction was set. We used the following terms for the PubMed search: “(depression [Mesh] or depression [all fields]) and (Stroke [Mesh] or post-stroke [title/abstract] or post stroke [title/abstract]) and (Mortality [Mesh] or mortal* [title/abstract] or death* [title/abstract]).” We used a similar search phrase for Web of Science database: “(depress* or mood* or affective*) and (stroke* or post-stroke or poststroke) and (mortal* or death*).”
Eligibility criteria
Studies which compared the survival rates in adults with and without PSD, respectively, were considered eligible. To be included, studies had to assess PSD within 3 months after an acute stroke. Both ischemic and hemorrhagic stroke were considered. We excluded articles providing continuous scores based on psychometric scales for depression, without dichotomization based on a cut-off value.
Data extraction and quality assessment
Two authors (FB and CDB) independently completed the preliminary screening, based on titles and abstracts, in order to identify potentially eligible articles. The final inclusion decision was based on full text evaluation. We built a data extraction template, including key items for all eligible studies, i.e., year of publication, country, sample size, PSD and mortality assessment methods, follow up duration, rates of mortality in individuals with and without PSD, respectively.
In addition, we performed a quality evaluation of included studies, based on items derived from the Newcastle Ottawa Scale for non-randomized studies (20). We evaluated sample representativeness, recruitment source comparability between PSD and non-PSD groups, quality of methods to assess PSD and to retrieve information on cases of death, as well as the investigation of potential confounders.
Two authors (FB and CDB) independently extracted data and assessed quality for blind check of accuracy. Discordances were resolved by consensus with other co-authors.
Data analysis
We conducted a meta-analysis estimating the relative risk (RR) for mortality in individuals with PSD as compared with those without. We carried out a post-hoc stratification of data, according to study follow-up duration. Findings were pooled according to the random-effects model and were summarized using a conventional forest plot. In addition, we carried out random-effects meta-regression analyses with Hartung-Knapp modification (21), exploring whether the estimated association between PSD and mortality might vary according to different study characteristics. These included continuous variables such as sample size, mean age, male gender proportion, and follow-up duration, as well as a categorical variable distinguishing methods to assess PSD (i.e., standardized clinical interviews, psychometric scales, or clinical records). We also carried out sensitivity analyses excluding studies with potential methodological issues based on considered quality items.
Statistical significance was set at p < 0.05. Consistency across studies was measured using the I2 index, with standard values of 25, 50, and 75% taken to indicate low, moderate and high levels of heterogeneity, respectively (22). Analyses were performed using the Stata statistical software package (release 14).
Results
Study selection
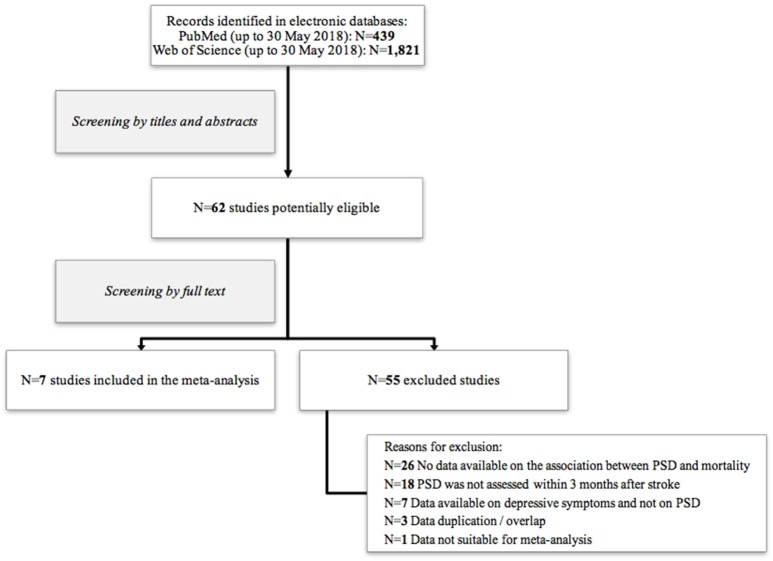
Our search generated 439 and 1,821 records from PubMed and Web of Science databases, respectively. The preliminary evaluation based on titles and abstracts, identified 62 potentially eligible studies. Further screening of full texts allowed to exclude 55 articles. Seven studies met eligibility criteria and were included in our meta-analysis (11, 14, 18, 23–26). Details on study selection process are shown in Figure 1.
Figure 1.
Flow chart of included/excluded studies.
Study characteristics and quality assessment
All included studies, published between 1993 (24, 25) and 2016 (18, 23), were written in English. Full characteristics are shown in Table 1. Most of studies could be considered sufficiently representative since they recruited subjects selected from specific catchment areas. However, one study (14) selected participants from a randomized-controlled trial on psychological treatments after stroke, who are likely to be different from the standard clinical population suffering from stroke. Moreover, all studies recruited both PSD and non-PSD individuals from the same setting, thus guaranteeing recruitment source comparability between cases and controls. In addition, all studies but one (23), deriving diagnosis from register-based information, tested PSD using clinical interviews or psychometric scales. One study (26) used only the first question of the Hamilton Depression Rating Scale, thus limiting its psychometric validity. In terms of mortality, adequate sources, such as death registries, were used in most of studies. In two studies (24, 25), information on mortality was unclear and probably not sufficiently valid, since the vital status of patients at follow-up could not be checked in a high proportion of cases (>10%). Finally, all studies but two (24, 25), which provided only unadjusted data, estimated the association between PSD and mortality after controlling for possible demographic and clinical confounders. Quality evaluation is summarized in Table 2.
Table 1.
Characteristics of included studies.
| Study (reference) | City, Country | Participants | PSD assessment | Follow-up (yrs.) | ||
|---|---|---|---|---|---|---|
| N | Mean age (yrs.) | Men (%) | ||||
| Ayerbe et al. (11) | London, UK | 1,354 | 68.1 | 54.0 | HADS | 5 |
| de Mello et al. (18) | São Paulo, Brazil | 191 | 63 | 60.2 | PHQ-9 | 1 |
| House et al. (14) | Leeds and Bradford, UK | 448* | 70.7 | 53.8 | PSE | 2 |
| Jørgensen et al. (23) | Denmark | 116,569** | 68.7** | 53.8** | Clinical registries | 2 |
| Morris et al. (24) | Baltimore, USA | 91 | 60.4 | 59.3 | PSE | 10 |
| Morris et al. (25) | New South Wales, Australia | 84 | 71.1 | 53.6 | CIDI | 1.25 |
| Willey et al. (26) | New York, USA | 340 | 68.8 | 43.5 | Single item from HRSD | 5 |
BDI-II, Beck Depression Inventory-Second Edition; CIDI, Composite International Diagnostic Interview; HADS, Hospital Anxiety and Depression Scale; HRSD, Hamilton Rating Scale for Depression; PHQ-9, Patient Health Questionnaire; PSE, Present State Examination.
Data from 446 individuals were included in the meta-analysis, since data from two subjects were missing.
Data from the subsample of subjects survived and evaluated for depression within 3 months after stroke.
Table 2.
Quality assessment and sensitivity analyses.
| Study (reference) | Sample representativeness | Recruitment comparability | Depression assessment | Information on mortality | Control for confounders |
|---|---|---|---|---|---|
| Ayerbe et al. (11) | (+) | (+) | (+) | (+) | (+) |
| de Mello et al. (18) | (+) | (+) | (+) | (+) | (+) |
| House et al. (14) | (–) | (+) | (+) | (+) | (+) |
| Jørgensen et al. (23) | (+) | (+) | (–) | (+) | (+) |
| Morris et al. (24) | (+) | (+) | (+) | (–) | (–) |
| Morris et al. (25) | (+) | (+) | (+) | (–) | (–) |
| Willey et al. (26) | (+) | (+) | (–) | (+) | (+) |
| RR (95%CI) Sensitivity analyses | 1.49 (1.25 to 1.78) | – | 1.62 (1.27 to 2.05) | 1.45 (1.21 to 1.74) | 1.45 (1.21 to 1.74) |
Synthesis of results
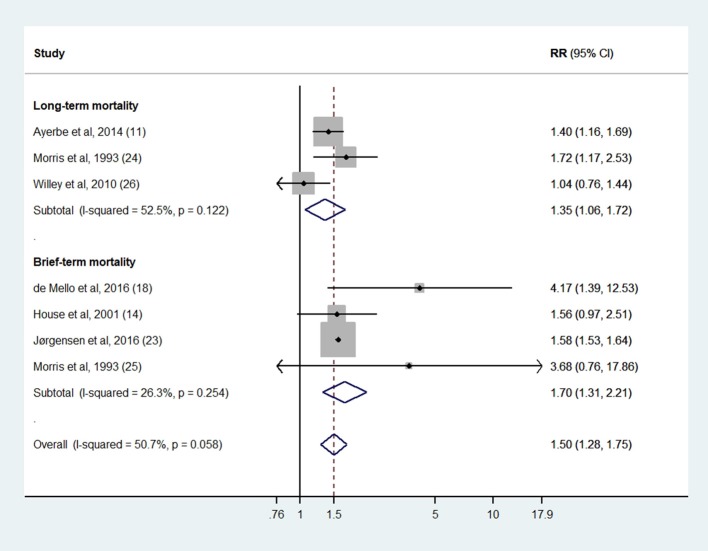
Seven studies (11, 14, 18, 23–26) based on 119,075 individuals (17,609 suffering from PSD and 101,466 non-depressed individuals) had data suitable to estimate the random-effect RR. We used unpublished information provided by the authors from four studies (11, 14, 23, 26). As a whole, 3,588 cases of death at follow-up were detected from PSD samples and 12,906 from comparison groups. Random-effects meta-analysis estimated higher rates of mortality in subjects suffering from PSD, as compared with non-depressed ones (RR = 1.50; 95%CI: 1.28 to 1.75; p < 0.001), with moderate heterogeneity across studies (I2 = 50.7%) (Figure 2). The subgroup analysis showed a slightly higher effect size in studies testing mortality within 2 years after stroke (RR = 1.70; p < 0.001), as compared with those based on a longer period of observation (RR = 1.35; p = 0.01) (Figure 2). However, a relevant meta-regression analysis showed no influence of follow-up duration on the estimated effect size (ß = −0.016; p = 0.711). Other meta-regression analyses, considering sample size, mean age, and PSD assessment methods, did not show any significant influence on effect size, apart from the proportion of recruited men (ß = 0.039; p = 0.043). In addition, sensitivity analyses based on quality issues confirmed the significant relationship between early PSD and lower life expectancy, with relative risk of mortality varying between 1.45 and 1.62 (Table 2).
Figure 2.
Association between early post-stroke depression and mortality. Short-term mortality = 1 or 2 years after stroke; Long-term mortality = 5 or more years after stroke.
Discussion
Summary and interpretation of findings
The current systematic review and meta-analysis, based on seven studies (11, 14, 18, 23–26), highlights the impact of early depression after stroke on survival rates. To our knowledge, this is the first meta-analysis estimating a higher risk of mortality in people suffering from PSD, considering depressive symptoms occurring in the early stage after the acute event. Subjects with early PSD had a risk of death about 1.5 higher as compared with non-depressed individuals, considering both short- and long-term mortality. Nevertheless, a comprehensive interpretation of these findings should take into account quality issues of included studies and a partial inconsistency of findings. Considering both internal and external validity (27, 28), we found that some studies included in this meta-analysis might have been influenced by the lack of representativeness (14) and control for confounders (24, 25), as well as by the potential low accuracy of PSD diagnosis (23) and mortality assessment at follow-up (24, 25). Nevertheless, quality-based sensitivity analyses seem consistent with the overall estimation.
In addition, our results should be interpreted considering the statistical heterogeneity, likely to be due to systematic differences in important methodological characteristics, such as target population, PSD definition, follow-up duration. The estimated excess of mortality in early PSD could not be explained by heterogeneity attributable to most of study characteristics, apart from gender. It should be considered that the strength of the relationship between PSD and mortality might be higher among men, although available data did not allow stratifying findings according to gender.
Even considering quality issues and heterogeneity, evidence from this meta-analysis is in line with findings of a previous systematic review and meta-analysis, showing an association between depression, assessed at any time after stroke, and mortality (16). Moreover, similar findings were reported in individuals with heart failure, in which depression was associated with higher risk of mortality (29). Our results are consistent with epidemiological evidence, showing the impact of depression on poor outcomes after acute vascular events (26).
Pathophysiological mechanisms which can explain the association between PSD and increased risk of mortality, remain to be clarified although several possible interpretations could be postulated. A first hypothesis may be related to depression effects on cardiovascular risk, possibly leading to recurrent cerebrovascular events and higher mortality. Indeed, individuals suffering from depressive disorders are more prone to unhealthy lifestyle behaviors, including higher rates of smoking, alcohol intake, physical inactivity, and poor dietary patterns (30, 31). Consistently, it should be considered that individuals with depression are generally less likely to be adherent and compliant to prescribed treatments and medications, being thus more vulnerable to poor clinical consequences (11, 32). Second, early depressive symptoms may occur more frequently in subjects with severe stroke-related consequences, such as disability, neurocognitive deficits, and functional impairment (33, 34). Thus, depression might be significantly related to stroke severity, that, in turn, might increase the risk of death. Third, evidence of pharmacological treatment for PSD have produced mixed results (35, 36) and benefits of selective serotonin reuptake inhibitors (SSRIs) have been questioned, considering the possible association with an even increased mortality (37). Consistently, even if antidepressants have shown to improve depressive symptoms and functional outcomes (38, 39), some studies estimated a higher likelihood of overall major bleeding and an increased risk of death in subjects treated with SSRIs (40). Thus, the excess of mortality in individuals with PSD might be partially explained by possible negative effects of a SSRI treatment on survival. Finally, mortality could be attributable also to non-natural causes of death. Stroke survivors show higher risk of death by suicide, especially if they suffer from current or lifetime mood disorder, recurrent stroke, and cognitive impairment (41). A recent systematic review and meta-analysis (42) including 10,400 stroke survivors, estimated that a clinically meaningful suicidal ideation may occur in about one out of eight individuals after the neurological event. Consistently, it has been reported that individuals suffering from stroke have rates of suicide attempts twice as high as the general population (43, 44).
Limitations
Although the current meta-analysis provided preliminary evidence on PSD effect on mortality, caution should be used in interpreting our findings, due to some limitations.
First, we could not systematically control for treatment-related factors, such as antidepressant medication as well as other interventions for depression prevention and management (5), which might influence the association between PSD and survival rates. This seems a key issue since previous studies highlighted a possible effect of antidepressants on mortality after stroke (40).
Second, it should be considered that there is not a gold standard for PSD diagnosis and that standardized scales, combining depressive and somatic items, are not currently available (10). Consistently, we found variability across studies in terms of assessment tools for depression, varying from clinical interviews such as PSE (14, 24) and CIDI (25), to psychometric scales (11, 18, 26). Diagnostic issues arise from specific features of stroke survivors, in which neurological symptoms and neurocognitive deficits could hide symptoms of independent mood disorders (5).
Finally, even if the number of subjects and cases included in this meta-analysis is adequate, only seven studies met inclusion criteria. Since < 10 studies were included in our meta-analysis (45), we could not assess risk of publication bias by using appropriate statistical approaches (46).
Clinical implications
Findings of this meta-analysis indirectly support the need of a regular assessment of depressive symptoms in subjects with stroke. Early depression after stroke often remains underdiagnosed and untreated, due to the lack of a gold standard for PSD assessment as well as of diagnostic issues related to clinical characteristics of this special population (10, 17). For example, stroke-related neurological symptoms such as aprosodic speech, abulia, or flat affect may hinder the clinical evaluation of depressive symptoms, whereas aphasia may lead to an underestimation of PSD (5). Consistently, evidence on pharmacological treatment of depression after stroke is limited and little is known about possible long-term adverse effects (47). In addition, further research is needed to explore efficacy of non-pharmacological interventions and prevention strategies for PSD. Various psychosocial, behavioral, and rehabilitative interventions (48–50) have been tested and may be possibly effective in reducing depressive symptoms. Although important gaps in terms of the most effective treatment for PSD remain, a greater awareness on depression after stroke and its impact on survival among patients, families, and health professionals, could facilitate recognition, earlier treatment, and improved outcomes (51).
Conclusions
Despite some limitations, our systematic review and meta-analysis suggests the potential impact of early PSD in influencing mortality of stroke survivors. Although the underlying mechanisms of this relationship remain unknown, PSD seems to negatively influence both short- and long-term life expectancy. Further research is required to evaluate whether an early management of mood disorders in individuals with stroke may improve their outcomes and related mortality gap.
Author contributions
All authors made substantial contributions to the intellectual content of the paper. FB and GC: conception and design; FB and CD: search and screening, data extraction, dataset management, and drafting of the manuscript; FB and CC: statistical analysis and interpretation of data; GC, MC, and CC: critical revision of the manuscript; GC and MC: supervision. All authors gave approval of the final version of the manuscript.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
We thank all authors of studies included in our meta-analysis, especially those providing additional data and information.
References
- 1.GBD 2015 Mortality and Causes of Death Collaborators Global, regional, and national life expectancy, all-cause mortality, and cause-specific mortality for 249 causes of death, 1980-2015: a systematic analysis for the Global Burden of Disease Study 2015. Lancet (2016) 388:1459–544. 10.1016/S0140-6736(16)31012-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Feigin VL, Forouzanfar MH, Krishnamurthi R, Mensah GA, Connor M, Bennett DA, et al. Global and regional burden of stroke during 1990-2010: findings from the Global Burden of Disease Study 2010. Lancet (2014) 383:245–54. 10.1016/S0140-6736(13)61953-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kumar S, Selim MH, Caplan LR. Medical complications after stroke. Lancet Neurol. (2010) 9:105–18. 10.1016/S1474-4422(09)70266-2 [DOI] [PubMed] [Google Scholar]
- 4.Hackett ML, Köhler S, O'Brien JT, Mead GE. Neuropsychiatric outcomes of stroke. Lancet Neurol. (2014) 13:525–34. 10.1016/S1474-4422(14)70016-X [DOI] [PubMed] [Google Scholar]
- 5.Towfighi A, Ovbiagele B, El Husseini N, Hackett ML, Jorge RE, Kissela BM, et al. Poststroke depression: a scientific statement for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. (2017) 48:e30–43. 10.1161/STR.0000000000000113 [DOI] [PubMed] [Google Scholar]
- 6.Hackett ML, Pickles K. Part I: frequency of depression after stroke: an updated systematic review and meta-analysis of observational studies. Int J Stroke. (2014) 9:1017–25. 10.1111/ijs.12357 [DOI] [PubMed] [Google Scholar]
- 7.Robinson RG, Jorge RE. Post-stroke depression: a review. Am J Psychiatry (2016) 173:221–31. 10.1176/appi.ajp.2015.15030363 [DOI] [PubMed] [Google Scholar]
- 8.Villa RF, Ferrari F, Moretti A. Post-stroke depression: mechanisms and pharmacological treatment. Pharmacol Ther. (2018) 184:131–44. 10.1016/j.pharmthera.2017.11.005 [DOI] [PubMed] [Google Scholar]
- 9.Das J, Rajanikant GK. Post stroke depression: the sequelae of cerebral stroke. Neurosci Biobehav Rev. (2018) 90:104–14. 10.1016/j.neubiorev.2018.04.005 [DOI] [PubMed] [Google Scholar]
- 10.Meader N, Moe-Byrne T, Llewellyn A, Mitchell AJ. Screening for post stroke major depression: a meta-analysis of diagnostic validity studies. J Neurol Neurosurg Psychiatry (2014) 85:198–206. 10.1136/jnnp-2012-304194 [DOI] [PubMed] [Google Scholar]
- 11.Ayerbe L, Ayis S, Crichton SL, Rudd AG, Wolfe CD. Explanatory factors for the increased mortality of stroke patients with depression. Neurology (2014) 83:2007–12. 10.1212/WNL.0000000000001029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.De Ryck A, Brouns R, Fransen E, Geurden M, Van Gestel G, Wilssens I, et al. A prospective study on the prevalence and risk factors of post-stroke depression. Cerebrovasc Dis Extra (2013) 3:1–13. 10.1159/000345557 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ellis C, Zhao Y, Egede LE. Depression and increased risk of death in adults with stroke. J Psychosom Res. (2010) 68:545–51. 10.1016/j.jpsychores.2009.11.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.House A, Knapp P, Bamford J, Vail A. Mortality at 12 and 24 months after stroke may be associated with depressive symptoms at 1 month. Stroke (2001) 32:696–701. 10.1161/01.STR.32.3.696 [DOI] [PubMed] [Google Scholar]
- 15.Williams LS, Ghose SS, Swindle RW. Depression and other mental health diagnoses increase mortality risk after ischemic stroke. Am J Psychiatry (2004) 161:1090–5. 10.1176/appi.ajp.161.6.1090 [DOI] [PubMed] [Google Scholar]
- 16.Bartoli F, Lillia N, Lax A, Crocamo C, Mantero V, Carrà G, et al. Depression after stroke and risk of mortality: a systematic review and meta-analysis. Stroke Res Treat. (2013) 2013:862978. 10.1155/2013/862978 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Espárrago Llorca G, Castilla-Guerra L, Fernández Moreno MC, Ruiz Doblado S, Jiménez Hernández MD. Post-stroke depression: an update. Neurologia (2015) 30:23–31. 10.1016/j.nrl.2012.06.008 [DOI] [PubMed] [Google Scholar]
- 18.de Mello RF, Santos Ide S, Alencar AP, Benseñor IM, Lotufo PA, Goulart AC. Major depression as a predictor of poor long-term survival in a Brazilian stroke cohort (Study of Stroke Mortality and Morbidity in Adults) EMMA study. J Stroke Cerebrovasc Dis. (2016) 25:618–25. 10.1016/j.jstrokecerebrovasdis.2015.11.021 [DOI] [PubMed] [Google Scholar]
- 19.Stroup DF, Berlin JA, Morton SC, Olkin I, Williamson GD, Rennie D, et al. Meta-analysis of observational studies in epidemiology: a proposal for reporting. Meta-analysis Of Observational Studies in Epidemiology (MOOSE) group. JAMA (2000) 283:2008–12. 10.1001/jama.283.15.2008 [DOI] [PubMed] [Google Scholar]
- 20.Wells GA, Shea B, O'Connell D, Peterson J, Welch V, Losos M, et al. The Newcastle-Ottawa Scale (NOS) for Assessing the Quality of Nonrandomized Studies in Meta-Analyses. Available online at: http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp (Accessed September 07, 2018).
- 21.Knapp G, Hartung J. Improved tests for a random effects meta-regression with a single covariate. Stat Med. (2003) 22:2693–710. 10.1002/sim.1482 [DOI] [PubMed] [Google Scholar]
- 22.Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ (2003) 327:557–60. 10.1136/bmj.327.7414.557 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jørgensen TS, Wium-Andersen IK, Wium-Andersen MK, Jørgensen MB, Prescott E, Maartensson S, et al. Incidence of depression after stroke, and associated risk factors and mortality outcomes, in a large cohort of Danish patients. JAMA Psychiatry (2016) 73:1032–40. 10.1001/jamapsychiatry.2016.1932 [DOI] [PubMed] [Google Scholar]
- 24.Morris PL, Robinson RG, Andrzejewski P, Samuels J, Price TR. Association of depression with 10-year poststroke mortality. Am J Psychiatry (1993) 150:124–9. 10.1176/ajp.150.1.124 [DOI] [PubMed] [Google Scholar]
- 25.Morris PL, Robinson RG, Samuels J. Depression, introversion and mortality following stroke. Aust N Z J Psychiatry (1993) 27:443–9. 10.3109/00048679309075801 [DOI] [PubMed] [Google Scholar]
- 26.Willey JZ, Disla N, Moon YP, Paik MC, Sacco RL, Boden-Albala B, et al. Early depressed mood after stroke predicts long-term disability: the Northern Manhattan Stroke Study (NOMASS). Stroke (2010) 41:1896–900. 10.1161/STROKEAHA.110.583997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Egger M, Schneider M, Davey Smith G. Spurious precision? Meta-analysis of observational studies. BMJ (1998) 316:140–4. 10.1136/bmj.316.7125.140 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Grimes DA, Schulz KF. Bias and causal associations in observational research. Lancet (2002) 359:248–52. 10.1016/S0140-6736(02)07451-2 [DOI] [PubMed] [Google Scholar]
- 29.Gathright EC, Goldstein CM, Josephson RA, Hughes JW. Depression increases the risk of mortality in patients with heart failure: a meta-analysis. J Psychosom Res. (2017) 94:82–9. 10.1016/j.jpsychores.2017.01.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Barth J, Schumacher M, Herrmann-Lingen C. Depression as a risk factor for mortality in patients with coronary heart disease: a meta-analysis. Psychosom Med. (2004) 66:802–13. 10.1097/01.psy.0000146332.53619.b2 [DOI] [PubMed] [Google Scholar]
- 31.Hare DL, Toukhsati SR, Johansson P, Jaarsma T. Depression and cardiovascular disease: a clinical review. Eur Heart J. (2014) 35:1365–72. 10.1093/eurheartj/eht462 [DOI] [PubMed] [Google Scholar]
- 32.Grenard JL, Munjas BA, Adams JL, Suttorp M, Maglione M, McGlynn EA, et al. Depression and medication adherence in the treatment of chronic diseases in the United States: a meta-analysis. J General Inter Med. (2011) 26:1175–82. 10.1007/s11606-011-1704-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hackett ML, Anderson C. Predictors of depression after stroke: a systematic review of observational studies. Stroke (2005) 36:2296–301. 10.1161/01.STR.0000183622.75135.a4 [DOI] [PubMed] [Google Scholar]
- 34.Nys GM, van Zandvoort MJ, van der Worp HB, de Haan EH, de Kort PL, Kappelle LJ. Early depressive symptoms after stroke: neuropsychological correlates and lesion characteristics. J Neurol Sci. (2005) 228:27–33. 10.1016/j.jns.2004.09.031 [DOI] [PubMed] [Google Scholar]
- 35.Hackett ML, Anderson CS, House AO. Management of depression after stroke: a systematic review of pharmacological therapies. Stroke (2005) 36:1098–103. 10.1161/01.STR.0000162391.27991.9d [DOI] [PubMed] [Google Scholar]
- 36.Paolucci S. Advances in antidepressants for treating post-stroke depression. Expert Opin Pharmacother. (2017) 18:1011–7. 10.1080/14656566.2017.1334765 [DOI] [PubMed] [Google Scholar]
- 37.Bartoli F, Paolucci S. Association of depression and SSRIs with mortality after stroke. Neurology (2014) 83:1998–9. 10.1212/WNL.0000000000001040 [DOI] [PubMed] [Google Scholar]
- 38.Jorge RE, Robinson RG, Arndt S, Starkstein S. Mortality and poststroke depression: a placebo-controlled trial of antidepressants. Am J Psychiatry (2003) 160:1823–9. 10.1176/appi.ajp.160.10.1823 [DOI] [PubMed] [Google Scholar]
- 39.Mortensen JK, Johnsen SP, Larsson H, Andersen G. Early antidepressant treatment and all-cause 30-day mortality in patients with ischemic stroke. Cerebrovasc Dis. (2015) 40:81–90. 10.1159/000435819 [DOI] [PubMed] [Google Scholar]
- 40.Mortensen JK, Larsson H, Johnsen SP, Andersen G. Post stroke use of selective serotonin reuptake inhibitors and clinical outcome among patients with ischemic stroke: a nationwide propensity score-matched follow-up study. Stroke (2013) 44:420–6. 10.1161/STROKEAHA.112.674242 [DOI] [PubMed] [Google Scholar]
- 41.Pompili M, Venturini P, Campi S, Seretti ME, Montebovi F, Lamis DA, et al. Do stroke patients have an increased risk of developing suicidal ideation or dying by suicide? An overview of the current literature. CNS Neurosci Ther. (2012) 18:711–21. 10.1111/j.1755-5949.2012.00364.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Bartoli F, Pompili M, Lillia N, Crocamo C, Salemi G, Clerici M, et al. Rates and correlates of suicidal ideation among stroke survivors: a meta-analysis. J Neurol Neurosurg Psychiatry (2017) 88:498–504. 10.1136/jnnp-2017-315660 [DOI] [PubMed] [Google Scholar]
- 43.Eriksson M, Glader EL, Norrving B, Asplund K. Poststroke suicide attempts and completed suicides: a socioeconomic and nationwide perspective. Neurology (2015) 84:1732–8. 10.1212/WNL.0000000000001514 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Harnod T, Lin CL, Kao CH. Risk of suicide attempt in poststroke patients: a population-based cohort study. J Am Heart Assoc. (2018) 7:e007830. 10.1161/JAHA.117.007830 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Sterne JAC, Egger M, Moher D. Addressing reporting biases. In: Higgins JP, Green S. editors. The Cochrane Handbook for Systematic Reviews of Interventions. Chichester: John Wiley & Sons, Ltd; (2008). 10.1002/9780470712184.ch10 [DOI] [Google Scholar]
- 46.Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ (1997) 315:629–34. 10.1136/bmj.315.7109.629 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hackett ML, Anderson CS, House A, Xia J. Interventions for treating depression after stroke. Cochrane Database Syst Rev. (2008) 4:CD003437 10.1002/14651858.CD003437.pub3 [DOI] [PubMed] [Google Scholar]
- 48.Baker C, Worrall L, Rose M, Hudson K, Ryan B, O'Byrne L. A systematic review of rehabilitation interventions to prevent and treat depression in post-stroke aphasia. Disabil Rehabil. (2018) 40:1870–92. 10.1080/09638288.2017.1315181 [DOI] [PubMed] [Google Scholar]
- 49.Mitchell PH, Veith RC, Becker KJ, Buzaitis A, Cain KC, Fruin M, et al. Brief psychosocial-behavioral intervention with antidepressant reduces poststroke depression significantly more than usual care with antidepressant: living well with stroke: randomized, controlled trial. Stroke (2009) 40:3073–8. 10.1161/STROKEAHA.109.549808 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Wang SB, Wang YY, Zhang QE, Wu SL, Ng CH, Ungvari GS, et al. Cognitive behavioral therapy for post-stroke depression: a meta-analysis. J Affect Disord. (2018) 235:589–96. 10.1016/j.jad.2018.04.011 [DOI] [PubMed] [Google Scholar]
- 51.Anderson CS. Depression After Stroke-Frequency, Risk Factors, and Mortality Outcomes. JAMA Psychiatry (2016) 73:1013–4. 10.1001/jamapsychiatry.2016.1868 [DOI] [PubMed] [Google Scholar]