Abstract
Altered expression of α-synuclein is linked to Parkinson’s disease (PD). A major challenge to explore how the increased α-synuclein affect neurotoxicity is the lack of a suitable human neuronal cell model that mimics this scenario. Its expression in neural precursors affects their differentiation process, in addition to the neuronal adaptability and variability in maintaining a constant level of expression across passages. Here, we describe an SH-SY5Y line harboring Tet-ON SNCA cDNA cassette that allows for induction of controlled α-synuclein expression after neuronal differentiation, which can be an important tool for PD research.
Keywords: α-synuclein, dopaminergic neurons, inducible α-synuclein expressing neuronal model for PD, Parkinson’s disease
INTRODUCTION
Parkinson’s disease (PD), the most common neurodegenerative disorder affecting movement, is characterized by the aggregation/misfolding of α-synuclein in dopaminergic neurons. α-synuclein fibrillary inclusions in Lewy bodies and Lewy neurites are the hallmark feature of PD and other synucleinopathies (reviewed in [1, 2]). In familial PD, in addition to various SNCA gene mutations [3–5], increased α-synuclein protein levels due to duplication or triplication of the SNCA gene [6, 7] has been directly linked to disease progression and severity. Subsequent studies in rodent models that overex-press wildtype or mutant α-synuclein provide strong in vivo evidence linking α-synuclein overexpression and PD-like phenotype as well as typical α-synuclein pathology [8, 9]. Similarly, intracerebral administration of preformed α-synuclein fibrils in rodents induces PD-like pathology and movement defects [10–13]. While these cumulative in vivo evidence directly links induction of α-synuclein expression to PD pathology, reliable modeling of this linkage in cultured human mature neurons has been challenging.
The majority of in situ studies utilize transient or constitutive (stable) overexpression of α-synuclein in neuroblastoma cells followed by their differentiation into dopamine neurons, and recent studies have highlighted the impact of overexpressed α-synuclein on neuronal differentiation [14–17]. In addition, controlling the level of ectopic expression to avoid non-physiologically high levels of expression has been a challenge. Furthermore, induced pluripotent cells (iPSC) derived from autosomal dominant PD patients with SNCA triplication (SNCA-tri), although showing high α-synuclein levels, their differentiation to neurons is affected by constitutively activated α-synuclein [14, 18]. The study tested prior knockdown of α-synuclein in SNCA-tri iPSC cells followed by neuronal differentiation; however, this approach also has limitations of knockdown efficiency, reproducibility as well as timing/controlling α-synuclein expression post differentiation.
Here, we generated and characterized a doxycycline (Dox)-inducible α-synuclein SH-SY5Y cell line, which not only evaded the adverse effects of high α-synuclein levels on differentiation but allowed for controlled, conditional, and reproducible overexpression of α-synuclein before/after neuronal differentiation. We propose that such an inducible neural cell line presents a reliable and more relevant cellular model for PD and other PD-like synucleinopathies, compared to other routinely used cell models.
MATERIALS AND METHODS
Construction of inducible mammalian FLAG-α-Syn expression vector
An inducible mammalian FLAG-α-Syn expression vector was generated by cloning blunt-ended full-length α-Syn amplified from pcDNA-WT-α Syn plasmid using Deep Vent DNA polymerase (M0258, NEB) into a pCW-Cas9 vector (gift from Eric Lander and David Sabatini, Addgene plasmid 50661) [19] and digested with restriction enzymes NheI (R0131, NEB) and BamHI (R0136, NEB) followed by treatment with DNA polymerase I, large (Klenow; M0210, NEB) to generate blunt DNA ends. The primers used to amplify C-terminal FLAG-tagged α-Syn were as follows: forward primer, 5′-ATG GAT GTA TTC ATG AAA GGA CT-3′; reverse primer, 5’–CAC TGT CGA CTT ACT TAT CGT CAT CGT CTT TGT AAT CGG CTT CAG GTT CGT AGT CTT GAT ACC-3′.
Culture and neuronal differentiation of the SH-SY5Y line
The human neuroblastoma SH-SY5Y line was routinely maintained in regular Dulbecco’s Modified Eagle’s Medium (DMEM) supplemented with 10% fetal bovine serum (FBS; Gibco) and 1% penicillin/streptomycin (Corning). Cells were cultured in Neurobasal medium (Gibco) supplemented with B-27, 1X Glutamax (Gibco), and 1% penicillin/streptomycin (Corning) containing 10 μM retinoic acid (RA) for seven days to induce neuronal differentiation.
Generation of stably transfected SH-SY5Y line with inducible α-synuclein vector and induction of α-synuclein expression
Transfection of SH-SY5Y cells with pCW-FLAG-(inducible)-α-Syn plasmids was carried out using Lipofectamine 2000 (Invitrogen) according to the manufacturer’s instructions. Dox-inducible α-Syn-expressing cells were selected against the antibiotic puromycin (InvivoGen) with a dose of 10 μg/mL. Dox (3 μg/mL for 0, 24, 48, or 72 h)-inducible (i) FLAG-α-Syn levels were optimized for 3–7-fold overexpression.
Cell viability assay
SHSY-5Y cells (4.0 × 106 cells/well) were plated in 96- well plates, and either treated with Dox at different time points or differentiated with RA for seven days followed by Dox treatment. Cell viability was evaluated at specific time points using the CCK-8 kit (CCK- 8; CK04–500, Dojindo Laboratories; Kumamoto, Japan) according to the manufacturer’s instructions.
Immunofluorescence microscopy
Cells grown in an 8-well chamber slide were fixed with 4% PFA, permeabilized with 0.2% Triton 100X in 1X PBS and blocked with 5% BSA-PBS-T (1× PBS with 0.1% Tween-20), before incubation with primary and secondary antibodies. Oligomer A11 polyclonal antibody (Thermo Scientific, Cat# AHB0052), and α-synuclein antibody LB 509 (Santa Cruz, Cat#sc-58480), ChAT antibody (Cat# AB144P, Millipore) and MAP2 antibody (Cat# GTX11267, Gene Tex) were used. After incubation with Alexa Fluor 488 (green) - and 558 (red)-conjugated secondary antibodies, coverslips were mounted in DAPI (Sigma). Images were taken using an Olympus BX61-Regular Upright BF & Fluorescent/Reflect Microscope using a 60 × objective. Images were analyzed with Image-J ROI Manager using the free hand selection tool. The corrected total cell fluorescence (CTCF) formula was used to obtain the final fluorescence values: CTCF = Integrated Density – (Area of selected cell X Mean fluorescence of background readings).
Real time-PCR analysis for mRNA quantitation
Total RNA was isolated using RNeasy Mini Kit (Qiagen #74104). Two microgram of RNA were used for cDNA synthesis in a 20 μL reaction using SuperScript III reverse transcriptase kit (Invitrogen, #18080–044). α-synuclein expression in the samples was analyzed by SYBR GREEN-based Real Time PCR (7000 Real-Time PCR System; Applied Biosystems) using SYBR Premix Ex Taq (TaKaRa) and primers for targeted mRNA HaSynTfw 5′-AGG GTGTTC TCT ATG TAG G-3′ and HaSynTrv 5′-ACT GTC TTC TGG GCT ACT GC-3′ [20]. HPRT expression (internal control) primer sequences used were as follows: forward primer, HPRT-F 5′-TGA CCTTGA TTT ATT TTG CAT ACC-3′ reverse primer, HPRT-R 5′-CGA GCA AGA CGT TCA GTC CT-3′. Data were presented as fold change mRNA expression with respect to the reference samples set at 1 based on 2–ΔΔCT method.
Immunoblotting of cell extracts
Preparation of whole cell extracts and protein quantitation was performed as previously described [21]. Following electrophoresis separation in a 4–16% gradient gel, proteins were transferred onto a PVDF membrane fixed with 0.4% paraformaldehyde (PFA) in 1X PBS for 30 min at room temperature (RT), and blocked with 5% skim milk in 1X TBS-T. The membranes were incubated with primary antibody diluted in 1% skim milk in TBS-T for 1 h at RT or overnight at 4°C, followed by washing in 1 × TBS-T and then incubation with secondary antibody for 1 h at RT and development with SuperSignal West Pico Chemiluminescent Substrate (Thermo Fisher).
Statistical analysis
Graphpad prism 6 software was used for data analysis. Comparisons of groups were generated with two-way ANOVA followed by Sidak’s or Tukey’s multiple comparison test to compare selected pair of means. p-values are indicated in the associated figure legends.
RESULTS AND DISCUSSION
Transient or constitutive expression of α-synuclein in cultured neural precursor cells and differentiated neurons have been modeled previously to study the biological implications of a high level of α-synuclein expression in neurons. These studies utilized conventional approaches to transfect α-synuclein expression vectors into neural precursor cells or differentiated neurons, using conventional transfection approaches. However, there are inherent limitations and challenges to this approach. First, expression of α-synuclein in neural precursor cells prior to differentiation significantly affects differentiation efficiency [14]. Moreover, such constitutive expression leads to strong cellular adaptability and a progressive decrease in sensitivity to PD-linked etiological factors, contrary to the progressive increase in neuronal dysfunction that is observed in PD. As shown in Supplementary Figure 1A–C, a constitutively overexpressing SH-SY5Y line failed to maintain similar levels of α-synuclein across passages and over time showed loss of overexpression. This was likely due to the mortality of cell population with higher levels of α-synuclein and survival/propagation of cells with moderate/reduced α-synuclein expression. Critically, cellular adaptability to a persistently increased amount of α-synuclein maybe a contributing factor to the loss of toxic effects. Likewise, transfection of differentiated neurons using viral vectors or electroporation is limited by variable efficiency as well as cell toxicity and thus its routine utilization is a challenge [22].
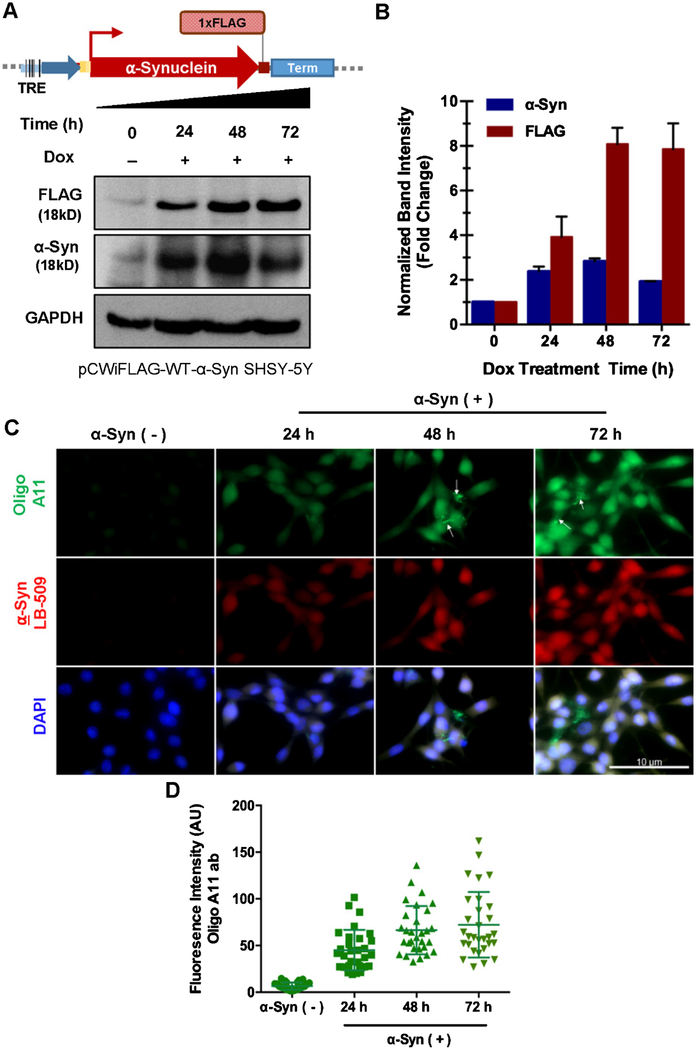
To overcome these limitations, we generated an inducible cell line by stably transfecting SH-SY5Y cells with an α-synuclein cDNA cassette containing a CMV promoter under the control of Tet-on response element (Fig. 1A and Supplementary Figure 1D). The vector also possesses a distal Tet-repressor sequence, which prevented any leaky expression of α-synuclein in uninduced cells. The stable clone selection using puromycin ensured survival of cells harboring the ectopic α-synuclein cassette. Treatment of pCW-iFLAG-α-Syn SH-SY5Y cells with 3 μg/mL Dox induced an ~3, 7, and 6-fold increase in FLAG α-synuclein at 24, 48, and 72 h, post induction, respectively (Fig. 1B). We then differentiated the pCW-iFLAG-α-Syn SH-SY5Y cells using retinoic acid [23, 24] for 7 days before inducing with Dox, which again yielded a similar expression pattern (Supplementary Figure 1E). This is consistent with α-synuclein mRNA levels at the same time points (Supplementary Figure 1F). The presence of a Tetrepressor element likely ensures a tight and highly reproducible pattern of protein induction. Notably, comparison of α-synuclein mRNA levels upon Dox-induction, before and after neuronal differentiation revealed that the increase in mRNA levels correlated with α-synuclein protein levels in differentiated neurons, but not in undifferentiated cells. This suggests a cross-talk between α-synuclein levels and neuronal differentiation that coincides with the critical role of α-synuclein in neurons.
Fig. 1.
Generation and characterization of inducible α-synuclein (α-Syn) expressing neuronal line. A) Schematic representation of doxycycline-inducible pCW-iFLAG-α-Syn vector used to generate SHSY-5Y stable cell line and immunoblot showing time-dependent induction of FLAG α-synuclein. B) Densitometry analysis of FLAG and endogenous α-synuclein immunocontent normalized to GAPDH. C) Immunofluorescence of oligomer conformations upon α-synuclein expression. Anti-oligomer A11 antibody recognizes all types of oligomers, but not monomers and fibrils in the case of α-synuclein. D) Oligo A11 antibody fluorescence intensity per cells. Measurement from 30 cells from three different fields.
Formation of α-synuclein aggregates and inclusion bodies is a key feature of α-synuclein overexpression-mediated neurotoxicity [25, 26]. We observed a progressive increase in α-synuclein oligomers after α-synuclein induction as analyzed by microscopy using an oligomer-specific antibody A11 (Fig. 1C, D). Cell viability was moderately higher in differentiated cells compared to undifferentiated cells overexpressing α-synuclein (Supplementary Figure 1G), possibly due to the replication stress activated upon accumulation of DNA strand breaks induced by the presence of α-synuclein in the chromatin [21].
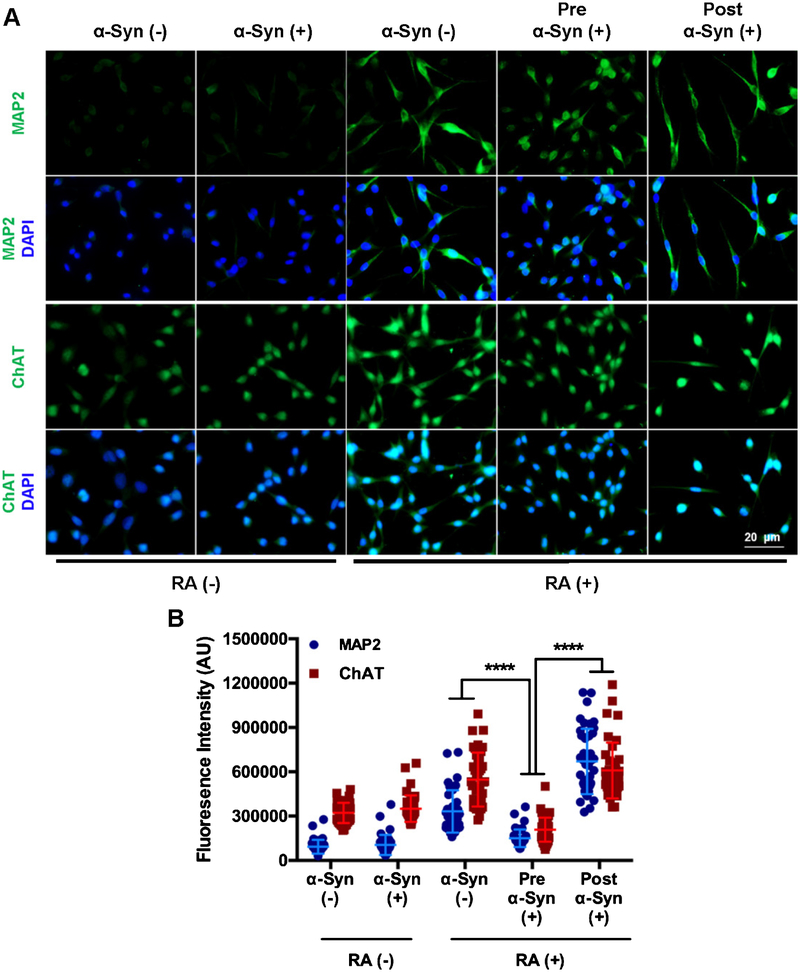
We next compared the differentiation efficiency before and after α-synuclein induction. Immunofluorescence microscopy of cells for markers of differentiated neurons microtubule-associated protein 2 (MAP2) and choline acetyltransferase (ChAT), clearly showed that α-synuclein induction significantly affected neuronal differentiation (Fig. 2A, B). α-synuclein induction after differentiation did not affect the MAP2 and ChAT levels. Moreover, higher α-synuclein levels during differentiation led to poor neuronal morphology and shorter neurites.
Fig. 2.
Impact of α-synuclein expression prior to and post differentiation with retinoic acid (RA). A) Immunofluorescence analysis to determine α-synuclein effect on differentiation markers. Pre α-Syn (+) cells were first induced with Dox for 3 days and then differentiated for 7 days. Post α-Syn (+) cells were first differentiated for 7 days and then induced with dox for 3 days. B) MAP2 fluorescence intensity per cells. Measurement from 40 cells from four different fields. ****p ≤ 0.0001.
Together, these data underscore the importance of avoiding α-synuclein overexpression during neuronal differentiation. Efficient physiologically relevant differentiation can be achieved with an inducible α-synuclein expressing neural cell line as we show here. The pros and cons of an inducible α-synuclein cell line versus a constitutive and transient expression models is tabulated in Table 1. Our inducible α-synuclein cell line thus provides an ideal system for obtaining mechanistic insights of α-synuclein-mediated neuronal degeneration in PD pathogenesis.
Table 1.
Pros and Cons of inducible α-synuclein expression over transiently or constitutive α-synuclein expressing lines
| Inducible | Constitutive | Transient |
|---|---|---|
| Not limited by transfection efficiency | Not limited by transfection efficiency | Limited by transfection efficiency |
| Controlled overexpression that is unaffected across cell passages | Controlled overexpression for a limited number of passages due to mortality of overexpressing cells | Short time overexpression, whose efficiency/consistency varies from experiment to experiment |
| Allows for the comparison of α-Syn effect in the same cell lineage (i.e., treated vs. untreated with Dox) | A cell line with no α- Syn overexpression is needed as a control | Allows for the comparison of α-Syn effect in the same cell lineage (i.e., transfected with α-Syn vector vs. transfected with empty vector). |
| α-Syn overexpression can be induced after differentiation to avoid α-Syn effect on differentiation | Differentiation is affected by the presence of α-Syn | Viral transductions or electroporation are required for overexpressing α-Syn, which are limited by transfection efficiency and toxicity |
Green shades indicate pros and cells in orange shade indicate cons.
CONCLUSIONS
Modeling of α-synuclein pathology in cellular and animal models that closely represent key aspects of human PD has been a fundamental challenge in understanding the molecular pathways involved in α-synuclein induced neurotoxicity in PD and in exploring ways to ameliorate these pathways. The early promise of PD patient-derived iPSC cells with (SNCA-tri), although, consistently shows higher α-synuclein levels and many aspects of neurotoxicity, the constitutively increased α-synuclein limits its faithful neuronal differentiation [14]. The limitations of existing models in presenting key features of human synucleinopathies together with limited tight-reproducibility of the underlying pathology poses a significant challenge for translating the in-cellulo findings to in vivo models and human PD. From this perspective, the Dox-inducible pCW-iFLAG-α-Syn SH-SY5Y line reported here, allows ectopic expression of α-synuclein in a controlled and reproducible fashion. An important advantage of this stable cell line is its post-differentiation induction of expression, thereby preventing any non-physiological protein expressions during the differentiation process. Although, a neuroblastoma line was reported here as a proof-of-concept to demonstrate the advantages of an inducible α-synuclein expression system, a similar approach can be utilized to generate iPSC or neural progenitor cell line, which can be differentiated to various neuronal types before α-synuclein induction.
Supplementary Material
ACKNOWLEDGMENTS
This research was supported by grants from the National Institutes of Health (USPHS R01 NS088645) and Houston Methodist Research Institute to M.L.H. and Melo Brain Funds to M.L.H. and K.S.R. V.V. is supported by a doctoral scholarship from the Institute for Training and Development of Human Resources of Panama (IFARHU) and the National Secretariat for Science, Technology, and Innovation of Panama (SENACYT). K.S.R. is thankful for SENACYT for financial support through SNI system.
Authors’ disclosures available online (https://www.j-alz.com/manuscript-disclosures/18-0610r1).
Footnotes
The pCW-FLAG-(inducible)-α-Syn plasmid is being made available in Addgene and the pCW-iFLAG-α-Syn SH-SY5Y cell line is available upon direct request to MLH.
SUPPLEMENTARY MATERIAL
The supplementary material is available in the electronic version of this article: http://dx.doi.org/10.3233/JAD-180610.
REFERENCES
- [1].Kim WS, Kagedal K, Halliday GM (2014) Alpha-synuclein biology in Lewy body diseases. Alzheimers Res Ther 6, 73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Shults CW (2006) Lewy bodies. Proc Natl Acad Sci U S A 103, 1661–1668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Kruger R, Kuhn W, Muller T, Woitalla D, Graeber M, Kosel S, Przuntek H, Epplen JT, Schols L, Riess O (1998) Ala30Pro mutation in the gene encoding alpha-synuclein in Parkinson’s disease. Nat Genet 18, 106–108. [DOI] [PubMed] [Google Scholar]
- [4].Ostrerova-Golts N, Petrucelli L, Hardy J, Lee JM, Farer M, Wolozin B (2000) The A53T alpha-synuclein mutation increases iron-dependent aggregation and toxicity. J Neurosci 20, 6048–6054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Zarranz JJ, Alegre J, Gomez-Esteban JC, Lezcano E, Ros R, Ampuero I, Vidal L, Hoenicka J, Rodriguez O, Atares B, Llorens V, Gomez Tortosa E, del Ser T, Munoz DG, de Yebenes JG (2004) The new mutation, E46K, of alpha-synuclein causes Parkinson and Lewy body dementia. Ann Neurol 55, 164–173. [DOI] [PubMed] [Google Scholar]
- [6].Singleton AB, Farrer M, Johnson J, Singleton A, Hague S, Kachergus J, Hulihan M, Peuralinna T, Dutra A, Nussbaum R, Lincoln S, Crawley A, Hanson M, Maraganore D, Adler C, Cookson MR, Muenter M, Baptista M, Miller D, Blancato J, Hardy J, Gwinn-Hardy K (2003) alpha-Synuclein locus triplication causes Parkinson’s disease. Science 302, 841. [DOI] [PubMed] [Google Scholar]
- [7].Chartier-Harlin MC, Kachergus J, Roumier C, Mouroux V, Douay X, Lincoln S, Levecque C, Larvor L, Andrieux J, Hulihan M, Waucquier N, Defebvre L, Amouyel P, Farrer M, Destee A (2004) Alpha-synuclein locus duplication as a cause of familial Parkinson’s disease. Lancet 364, 1167–1169. [DOI] [PubMed] [Google Scholar]
- [8].Visanji NP, Brotchie JM, Kalia LV, Koprich JB, Tandon A, Watts JC, Lang AE (2016) Alpha-Synuclein-based animal models of Parkinson’s disease: Challenges and opportunities in a new era. Trends Neurosci 39, 750–762. [DOI] [PubMed] [Google Scholar]
- [9].Volpicelli-Daley LA, Kirik D, Stoyka LE, Standaert DG, Harms AS (2016) How can rAAV-alpha-synuclein and the fibril alpha-synuclein models advance our understanding of Parkinson’s disease? J Neurochem 139(Suppl 1), 131–155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Abdelmotilib H, Maltbie T, Delic V, Liu Z, Hu X, Fraser KB, Moehle MS, Stoyka L, Anabtawi N, Krendelchtchikova V, Volpicelli-Daley LA, West A (2017) Alpha-Synuclein fibril-induced inclusion spread in rats and mice correlates with dopaminergic Neurodegeneration. Neurobiol Dis 105, 84–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Luk KC, Kehm VM, Zhang B, O’Brien P, Trojanowski JQ, Lee VM (2012) Intracerebral inoculation of pathological alpha-synuclein initiates a rapidly progressive neurodegenerative alpha-synucleinopathy in mice. J Exp Med 209, 975–986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Osterberg VR, Spinelli KJ, Weston LJ, Luk KC, Woltjer RL, Unni VK (2015) Progressive aggregation of alpha-synuclein and selective degeneration of lewy inclusion-bearing neurons in a mouse model of parkinsonism. Cell Rep 10, 1252–1260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Thakur P, Breger LS, Lundblad M, Wan OW, Mattsson B, Luk KC, Lee VMY, Trojanowski JQ, Bjorklund A (2017) Modeling Parkinson’s disease pathology by combination of fibril seeds and alpha-synuclein overexpression in the rat brain. Proc Natl Acad Sci U S A 114, E8284–E8293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Oliveira LM, Falomir-Lockhart LJ, Botelho MG, Lin KH, Wales P, Koch JC, Gerhardt E, Taschenberger H, Outeiro TF, Lingor P, Schule B, Arndt-Jovin DJ, Jovin TM (2015) Elevated alpha-synuclein caused by SNCA gene triplication impairs neuronal differentiation and maturation in Parkinson’s patient-derived induced pluripotent stem cells. Cell Death Dis 6, e1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Schneider BL, Seehus CR, Capowski EE, Aebischer P, Zhang SC, Svendsen CN (2007) Over-expression of alpha-synuclein in human neural progenitors leads to specific changes in fate and differentiation. Hum Mol Genet 16, 651–666. [DOI] [PubMed] [Google Scholar]
- [16].Kumar B, Nahreini P, Hanson AJ, Andreatta C, Prasad JE, Prasad KN (2005) Selenomethionine prevents degeneration induced by overexpression of wild-type human alpha-synuclein during differentiation of neuroblastoma cells. J Am Coll Nutr 24, 516–523. [DOI] [PubMed] [Google Scholar]
- [17].Ettle B, Reiprich S, Deusser J, Schlachetzki JC, Xiang W, Prots I, Masliah E, Winner B, Wegner M, Winkler J (2014) Intracellular alpha-synuclein affects early maturation of primary oligodendrocyte progenitor cells. Mol Cell Neurosci 62, 68–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Desplats P, Spencer B, Crews L, Pathel P, Morvinski-Friedmann D, Kosberg K, Roberts S, Patrick C, Winner B, Winkler J, Masliah E (2012) alpha-Synuclein induces alterations in adult neurogenesis in Parkinson disease models via p53-mediated repression of Notch1. J Biol Chem 287, 31691–31702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Wang T, Wei JJ, Sabatini DM, Lander ES (2014) Genetic screens in human cells using the CRISPR-Cas9 system. Science 343, 80–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Rhinn H, Qiang L, Yamashita T, Rhee D, Zolin A, Vanti W, Abeliovich A (2012) Alternative alpha-synuclein transcript usage as a convergent mechanism in Parkinson’s disease pathology. Nat Commun 3, 1084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Vasquez V, Mitra J, Hegde PM, Pandey A, Sengupta S, Mitra S, Rao KS, Hegde ML (2017) Chromatin-bound oxidized alpha-synuclein causes strand breaks in neuronal genomes in in vitro models of Parkinson’s disease. J Alzheimers Dis 60(s1), S133–S150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Karra D, Dahm R (2010) Transfection techniques for neuronal cells. J Neurosci 30, 6171–6177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Shipley MM, Mangold CA, Szpara ML (2016) Differentiation of the SH-SY5Y human neuroblastoma cell line. J Vis Exp, 53193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Kovalevich J, Langford D (2013) Considerations for the use of SH-SY5Y neuroblastoma cells in neurobiology. Methods Mol Biol 1078, 9–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Spinelli KJ, Taylor JK, Osterberg VR, Churchill MJ, Pol-lock E, Moore C, Meshul CK, Unni VK (2014) Presynaptic alpha-synuclein aggregation in a mouse model of Parkinson’s disease. J Neurosci 34, 2037–2050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Lee HJ, Lee SJ (2002) Characterization of cytoplasmic alpha-synuclein aggregates. Fibril formation is tightly linked to the inclusion-forming process in cells. J Biol Chem 277, 48976–48983. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.