Abstract
Background:
Studies of air pollution exposure and arterial stiffness have reported inconsistent results and large studies employing the reference standard of arterial stiffness, carotid-femoral pulse-wave velocity (CFPWV), have not been conducted.
Aim:
To study long-term exposure to ambient fine particles (PM2.5), proximity to roadway, and short-term air pollution exposures in relation to multiple measures of arterial stiffness in the Framingham Heart Study.
Methods:
We assessed central arterial stiffness using CFPWV, forward pressure wave amplitude, mean arterial pressure and augmentation index. We investigated long-and short-term air pollution exposure associations with arterial stiffness with linear regressions using long-term residential PM2.5 (2003 average from a spatiotemporal model using satellite data) and proximity to roadway in addition to short-term averages of PM2.5, black carbon, particle number, sulfate, nitrogen oxides, and ozone from stationary monitors.
Results:
We examined 5,842 participants (mean age 51 ± 16, 54 % women). Living closer to a major roadway was associated with higher arterial stiffness (0.11 m/s higher CFPWV [95 % CI: 0.01, 0.22] living < 50 m vs 400 ≤ 1000 m). We did not observe association between arterial stiffness measures and long-term PM2.5 or short-term levels of PM2.5, particle number, sulfate or ozone. Higher levels of black carbon and nitrogen oxides in the previous days were unexpectedly associated with lower arterial stiffness.
Conclusions:
Long-term exposure to PM2.5 was not associated with arterial stiffness but positive associations with living close to a major road may suggest that pollutant mixtures very nearby major roads, rather than PM2.5, may affect arterial stiffness. Furthermore, short-term air pollution exposures were not associated with higher arterial stiffness.
Keywords: Arterial stiffness, air pollution, applanation tonometry, epidemiology
Introduction
Exposure to air pollution has been associated with cardiovascular morbidity and mortality in many studies (1). Atherosclerosis and vascular dysfunction contribute to the development of cardiovascular events and may represent a pathway by which air pollution may exert effects both as a result of short- and long-term exposure (2). Long-term exposure to particulate matter pollution over months to years has been associated with vascular risk factors and subclinical disease including high blood pressure (3), carotid intima media thickness (4, 5) and increased calcification of arteries (6), although associations were not consistent for these measures in all studies (7, 8).
Increased central arterial stiffness, another measure of vascular dysfunction, is a risk factor for the development of cardiovascular events and may be a precursor to hypertension (9, 10). To date, studies of long-term air pollution exposure and arterial stiffness have reported inconsistent results (11–17) but only one study measured carotid femoral pulse wave velocity (CFPWV), considered the reference standard measure of arterial stiffness (15). Instead most studies used measures such as augmentation index, that with age becomes a less sensitive marker for arterial stiffness and future CVD risk (10).
Short-term exposures to air pollution on the order of days, including both gaseous and particulate components, also have been associated with measures of vascular function including changes in blood pressure (18), peripheral vascular function (19–22), and coronary ischemia (23). Changes in arterial stiffness also have been studied in relation to short-term air pollution exposure in previous studies but with varying results and a lack of large-scale studies using CFPWV (24–28).
Whereas it is generally perceived that air pollution has an adverse effect on vascular function, resulting in increased risk of cardiovascular events in the short- and long-term, previous studies have yielded inconsistent results, possibly because the approach to measuring arterial stiffness has been problematic. Variation in study populations and constituents of air pollution mixtures may also explain inconsistencies across studies. In addition, none of the previous studies have assessed both long- and short-term air pollution exposure. We hypothesized that living close to a major road and at addresses with higher long-term averages of particulate matter is associated with higher arterial stiffness. We also hypothesized that higher short-term averages of particulate air pollution are associated with higher arterial stiffness. Therefore, we evaluated both long- and short-term exposure to air pollutants and traffic to the reference standard measure of arterial stiffness (and also several complementary measures) in a well-characterized large community-based cohort.
Methods
Study participants
Participants in the Framingham Heart Study Offspring and Third Generation cohorts were eligible based on having performed valid examinations of arterial stiffness in examination rounds 8 and 1 respectively and having a primary address in the Northeastern US corresponding to the catchment area of the spatially resolved fine particulate matter (PM2.5) satellite model, resulting in 5,842 participants. These cohorts have been described in detail previously (29, 30). Ethical approval was obtained from the Institutional Review Boards of the Beth Israel Deaconess Medical Center and Boston University Medical Center. Written informed consent was obtained from all participants.
Individual-level covariates were collected through physician interview, blood draws, and physical examination. Neighborhood-level data concerning socio-economic characteristics were collected through the U.S. Census 2000 based on the primary residential address of each participant. These included median household income and median value of owner-occupied housing units, at the census tract level.
Measures of arterial stiffness
Offspring cohort examination round 8 was conducted between 2005 and 2008 and Third Generation cohort examination round 1 was conducted between 2002 and 2005. The methodology of arterial stiffness measures in the Framingham cohorts has been described in detail elsewhere (31, 32). Briefly, using a custom transducer, electrocardiogram, and body-surface measurements, tonometric measurements were obtained from the carotid and femoral sites to calculate CFPWV. Left ventricular outflow tract diameter was measured with 2-dimensional echocardiography and Doppler flow velocity was multiplied by outflow tract area to obtain aortic volume flow. Wave separation analysis was used to determine the forward pressure wave amplitude (FWA) and carotid pressure waveform analysis was used to assesses augmentation index. Mean arterial pressure was calculated from the brachial pressure wave form based on tonometry or oscillometric cuff waveform.
Of the several methods used to quantify arterial stiffness CFPWV is considered the reference standard. CFPWV is primarily a measure of aortic wall stiffness. FWA is a function of the peak systolic blood flow and aortic impedance and increases with greater aortic wall stiffness or in the presence of mismatch between aortic diameter and flow. Mean arterial pressure provides a measure of steady-flow load and is influenced by cardiac output and peripheral vascular resistance. Augmentation index is based on pulse wave analysis in the carotid artery and is used to measure relative wave reflection.
Exposure assessment
Long-term PM2.5
We assessed annual averages of PM2.5 exposure at the primary residential address of each participant recorded at the examination round using modeled estimates of daily PM2.5 with a 1×1 km resolution. The basis of the model used satellite-derived aerosol optical depth (AOD), a quantitative measure of particle abundance in a column of air, to predict a PM2.5 concentration for a 1 × 1 km grid (33). These estimates were calibrated using 161 ground monitoring stations within 1 km of an AOD value and adjusted for meteorological parameters (temperature, daily visibility, sea land pressure, relative humidity, and wind speed) and local land-use regression parameters (distance to point source emissions, population density, percentage of land use, total area-source emissions, elevation, and traffic density). We then predicted daily PM2.5 concentrations in grid cells without monitors but with available AOD using the model fit from the preceding model. Finally, for grid cells without AOD measurements on a given day, we used region-specific associations between grid-cell AOD and PM2.5 levels using neighboring cells to calculate daily levels. The difference between ground-level PM2.5 data and model predictions were regressed against temporal and spatial predictors of monitored PM2.5 at a 200 × 200 m resolution including the following factors: distance to major roads, traffic density, percent urban, elevation, distance to point source emissions, population density, height of planetary boundary layer, and visibility. Our model predictions demonstrated an out-of-sample 10-fold cross-validated R2 of 0.88 (0.82–0.90 between 2003 and 2011). We summed daily grid predictions and localized residual PM2.5 predictions for each address and averaged them over a year.
We expected temporal trends for PM2.5 levels over the years in which arterial measurements were collected (2002–2008) as well as lower arterial stiffness in the generally healthier and younger Third Generation cohort examined between 2002–2005 compared to higher arterial stiffness in the older Offspring cohort examined later between 2005–2008. In an effort to avoid introducing the bias of these temporal trends we used a similar strategy employed in previous studies (34, 35), of assigning the annual PM2.5 concentration of a fixed year (2003) as a measure of recent, longer-term PM2.5 exposure. We used the address recorded at the time of their Framingham exam (Third Generation cohort Examination Round 1, 2002–2005 and Offspring cohort Examination Round 8, 2005–2008) and assigned the PM2.5 2003 annual average for these addresses for all participants. In this way we aimed to contrast the geographical differences in levels of exposure in our study sample while controlling for temporal trends. We picked 2003, a year preceding most exams, assuming that the rank-ordering of exposure would be reasonably well-preserved between 2002–2008.
Distance to a major roadway
We calculated the distance between geocoded primary residential addresses and the nearest major roadway classified as A1, A2 or A3 according to the U.S. Census Features Class. We examined proximity to roadways both as a natural logarithm as well as in categories of proximity <50 m, 50 to <100 m, 100 to <200 m, 200 to <400 m, and 400 to ≤1,000 m because previous studies have demonstrated log-linear associations between proximity to roadway and health outcomes (36) and a return to background ultrafine and PM2.5 levels within 100–300 m and 100–400 m respectively from major roadways (37). Residential addresses further than 1,000 m from a major roadway are likely to reflect different exposures compared to those addresses in urban or suburban areas. We, therefore, excluded these addresses (> 1,000 m, 635 observations or 11%) in analyses of proximity to roadway and arterial stiffness as distance to roadway for these addresses is not likely an indicator of traffic-related exposure resulting in 5,207 participants for these analyses.
Short-term averages of air pollutant exposure
In line with other studies of short-term air pollution exposure we made use of continuous measurements from fixed monitoring stations strategically situated to capture temporal variations in levels from day to day. Hourly levels of PM2.5, black carbon, particle number, sulfate, nitrogen oxides, and ozone retrieved from fixed site monitors were used to construct daily 9 AM to 9 AM means. We used PM2.5, black carbon, particle number and sulfate measurements from the Harvard Air Pollution Monitoring Supersite situated on the rooftop of the Francis A. Countway Library of Medicine, 5 stories above ground level and 50 m from the nearest street. We used a tapered-element oscillating microbalance (Model 1400A, Rupprecht & Patashnick Co. Inc., Albany, New York) to measure PM2.5, an Aethalometer (Model AE-16, Magee Scientific Corp., Berkeley, California) to measure black carbon on the basis of optical transmittance at a single wavelength (λ = 880nm), and a condensation particle counter (Model 3011A, TSI, Inc., Shoreview, Minnesota) to measure number of particles per cm3. Sulfate concentration was calculated from an elemental sulfur measured by x-ray fluorescence analysis of PM2.5 filter samples and on days when sulfur measurements were not available, by using a sulfate analyzer (Model 5020, Thermo Electron Corp., Franklin, Massachusetts). Nitrogen oxides and ozone were measured by local state monitors within the Greater Boston area and averaged from the available sites. We modeled separate 1-, 3-, 7-, and 14-day averages for each single pollutant model and restricted analyses of short-term air pollution exposure to arterial stiffness measures in participants living within 50 km of the Harvard Supersite monitor.
Meteorology
We calculated daily averages from hourly measurements of temperature and relative humidity from the Logan International Airport (Boston, Massachusetts) weather station, 12 km from the air pollution monitoring site.
Statistical methods
We fit multivariable linear regression models for all analyses using the same covariate model for both long and short-term analyses. We selected covariates a priori based on biological plausibility and consistency with our previous publications of air pollution and vascular function in this cohort (38, 39). All models were adjusted for age at examination (age, age2), sex, cohort, body mass index (BMI), triglycerides, ratio of total cholesterol/high density lipoprotein, heart rate at examination, diabetes status, smoking status (never, former or current), pack-years of smoking, individual-level education (high school or less, some college, college graduate), median census-tract household income in 2000, median census-tract value of owner-occupied housing in 2000, day of week, season (as sine and cosine functions of the exam date), time-trend (as a linear term for date), temperature and relative humidity of the day prior to outcome measurement as well as a multiplicative interaction term of temperature and relative humidity. We calculated means with standard deviations and counts with percent for continuous and categorical variables respectively. Spearman correlations were calculated for daily averages of air pollutants measured at the central monitoring site.
We explored effect modification for proximity to roadway analyses by including multiplicative terms of log proximity and categories of BMI (categorized <30 and ≥30 kg/m2), median age (<64 and ≥64 years) and sex.
We also performed several sensitivity analyses. In an attempt to investigate associations between long-term exposure and arterial stiffness independent of short-term variations in particulate pollution we performed analyses adjusting for PM2.5 levels obtained from the central site monitor for the day preceding tonometry examinations. We also relaxed our exclusion of participants living >1,000m from a major roadway for proximity to roadway analyses. Similarly, we performed sensitivity analyses using the same population for all analyses by restricting to individuals living within 50 km to the central site monitors in long-term exposure analyses. Previous literature has highlighted the dependence of pulse wave velocity on blood pressure (40), however, we did not include hypertension as a covariate in our main model since it may be an intermediate on the pathway of the association. Instead, we performed sensitivity analyses by including hypertension therapy in all models and in another separate analyses we included mean arterial blood pressure in models of CFPWV. We further explored possible over-adjustment of regression models by excluding heart rate in CFPWV models and in all long-term exposure models by excluding covariates predominately targeting short-term confounders (such as day of week, time-trend, season, ambient temperature, relative humidity, and season) in all long-term exposure models. Finally, we conducted cohort-specific analyses to assess the consistency of results across cohorts.
Long-term PM2.5 exposure at residential address was modeled for the index year 2003 scaled to the interquartile range (1.46 μg/m3). Proximity to roadway was modeled both as a continuous log-linear independent variable scaled to the difference between living at the 75th (405 m) vs the 25th (61 m) percentile rounded off to 400 m vs 50 m from a major road as well as a categorical variable (<50 m, 50 to <100 m, 100 to <200 m, 200 to <400 m, and 400 to ≤1,000 m). Short-term associations were scaled to 5 μg/m3 PM2.5, 0.4 μg/m3 black carbon, 15,000 particles/cm3, 2 μg/m3 sulfate, and 0.01ppm nitrogen oxides and ozone. Consistent with previous publications of CFPWV in the Framingham Heart Study we inverse-transformed CFPWV to reduce heteroscedasticity and multiplied by −1000 to restore effect direction and convert units to milliseconds per meter(9). Results were interpreted focusing on describing and highlighting consistent association patterns across air pollution exposures and measures of arterial stiffness.
Results
Participant characteristics and measures of arterial stiffness
Participant characteristics are summarized in Table 1. Our study cohorts were almost exclusively white (99%) and consisted of a middle to older aged group of individuals with similar numbers of men and women. Few were current smokers and almost half had never smoked. Nearly half of participants completed college, and on average participants lived in census tracts with median household incomes that were above the US median in 2003 ($43,318)(41). Distributions for measures of arterial stiffness as well as the transformed CFPWV are provided in Table 2.
Table 1.
Characteristics of Study Participants, N=5,842
| Mean or N | SD or (%) | ||
|---|---|---|---|
| Age at exam, years | 51 | ± | 16 |
| Women, % | 3,170 | 54 | |
| Offspring Cohort, % | 2,424 | 41 | |
| Body Mass Index, kg/m2 | 28 | ± | 6 |
| Triglycerides, mg/dl | 117 | 82 | |
| Total cholesterol/HDL ratio | 3.7 | ± | 1.3 |
| Heart rate, beats/min | 62 | ± | 10 |
| Diabetes Mellitus, % | 490 | 8 | |
| Current smokers, % | 830 | 14 | |
| Former Smokers, % | 2,164 | 37 | |
| Pack-years of smoking | |||
| Current Smokers | 28 | ± | 17 |
| Former Smokers | 18 | ± | 19 |
| Education | |||
| Some College, % | 1,883 | 32 | |
| College Graduate, % | 2,608 | 45 | |
| Census-tract Median Household income, USD | 64,534 | ± | 20,769 |
| Census-tract Median Value of Owner-Occupied Housing units, USD | 220,221 | ± | 101,841 |
Table 2.
Distributions of measures of arterial stiffness
| Measure of arterial stiffness | N | Mean | Median | Standard deviation | Interquartile Range |
|---|---|---|---|---|---|
| CFPWV*, m/s | 5,598 | 8.5 | 7.6 | 3.2 | 2.9 |
| −1000/CFPW, ms/m | 5,598 | −129.7 | −131.6 | 34.7 | 47.5 |
| Augmentation index, % | 5,842 | 10.7 | 11.7 | 14.3 | 16.2 |
| Forward Pulse Pressure | 5,761 | 50.0 | 46,9 | 15.1 | 18.0 |
| Amplitude, mmHg Mean Arterial Pressure, mmHg |
5,842 | 93.3 | 92.0 | 12.3 | 17.0 |
CFPWV, Carotid Femoral Pulse Wave Velocity
Distributions of exposure
Distributions of measures of long-term exposures as well as short-term averages of pollutants are summarized in Table 3. The median level of PM2.5 at residential address in 2003 was 10.7 μg/m3 and 23 % lived within 50 m of a major roadway. Mean levels of long-term and short-term air pollutants were generally low. PM2.5, black carbon, and sulfates were moderately to highly correlated with each other (Spearman correlation coefficients >0.7, Table 4). Black carbon and NOx were moderately positively correlated and both were negatively correlated to ozone.
Table 3.
Distributions of PM2.5 and Proximity to a Major Roadway
| Median (IQR) or n [%] | Range (min, max) | Range (5th to 95th) | |
|---|---|---|---|
| 2003 Annual average PM2.5, μg/m3 | 10.7 (1.5) | 2.9–26.7 | 8.3–12.7 |
| Proximity to Major Roadway, m | 195.4 (342.8) | 0.01–999.7 | 7.1–804.9 |
| Residential Proximity in Categories, m | |||
| <50 | 1186 [23] | ||
| 50 to <100 | 541 [10] | ||
| 100 to <200 | 912 [18] | ||
| 200 to <400 | 1252 [24] | ||
| 400 to ≤1000 | 1316 [25] | ||
| Preceding Day PM2.5, μg/m3*(n=3,921) | 8.3 (6.2) | 0.9–75.9 | 3.5–21.1 |
| Preceding Day Black Carbon, μg/m3* (n=3,923) | 0.6 (0.5) | 0.1–4.2 | 0.3–1.5 |
| Preceding Day Particle Number, particles/cm3* (n=3,499) | 19,234 (15,000) | 3,791–63,866 | 7,983–40,771 |
| Preceding Day Sulfate, μg/m3* (n=3,589) | 2.4 (2.5) | 0.002–27.9 | 0.9–8.6 |
| Preceding Day NOx, ppm* (n=3,927) | 0.03 (0.02) | 0.006–0.17 | 0.02–0.07 |
| Preceding Day Ozone, ppm* (n=3,927) | 0.02 (0.02) | 0.001–0.09 | 0.01–0.05 |
| Preceding Day Temperature, °C | 10.5 (15.2) | −18.3–31.0 | −5.1–25.0 |
| Preceding Day Relative Humidity, % | 66 (26) | 21–99 | 40–92 |
For subpopulation living within 50 km from the central site monitor with non-missing pollutant data for days preceding examination cycle.
Table 4.
Daily Spearman’s Rank Correlation Coefficients for Pollutants
| Pollutant | PM2.5 | Black Carbon | Particle Number | Sulfate | Nitrogen oxides | Ozone |
|---|---|---|---|---|---|---|
| PM2.5 | 1.00 | |||||
| Black Carbon | 0.71 | 1.00 | ||||
| Particle Number | −0.19 | 0.18 | 1.00 | |||
| Sulfate | 0.85 | 0.59 | −0.17 | 1.00 | ||
| Nitrogen Oxides | 0.37 | 0.48 | 0.41 | 0.27 | 1.00 | |
| Ozone | 0.06 | −0.19 | −0.29 | 0.16 | −0.57 | 1.00 |
Long-term air pollution, proximity to major roadway and measures of arterial stiffness
Associations between PM2.5 and measures of arterial stiffness.
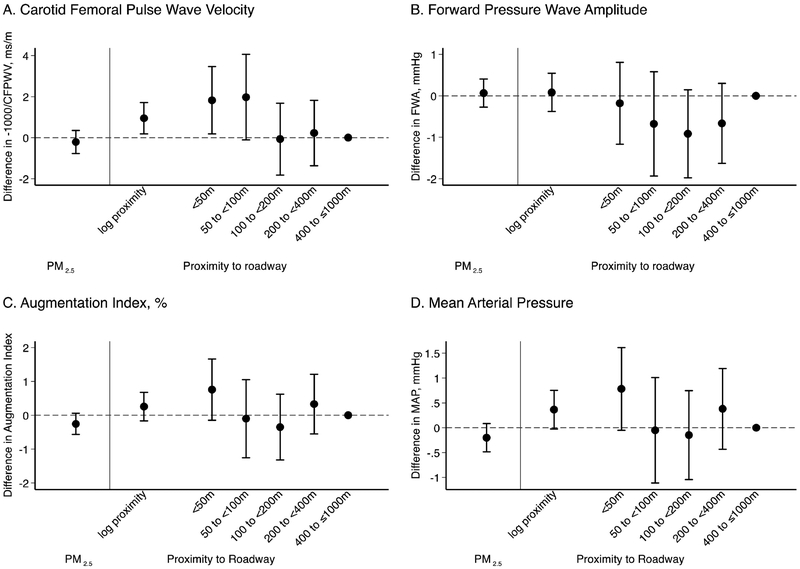
We did not observe associations among the 5,842 participants between higher residential PM2.5 levels and CFPWV, FWA or mean arterial pressure (Figure 1). The estimate for augmentation index gave some indication of lower augmentation index in association with higher PM2.5; however, the confidence intervals included the null.
Figure 1.
Long-term PM2.5 exposure at residential address in 2003 and residential proximity to major roadway and measures of arterial stiffness. Associations with arterial stiffness measures were scaled to the interquartile range in 2003 (1.46 μg/m3) of PM2.5, 75th (400 m) to the 25th (50 m) centile of the log-transformed residential proximity to a major roadway and as categorical variable for residential proximity to major roadway using the residences between 400 m and less than or equal to 1000 m as the reference category.
Associations between proximity to major roadway and measures of arterial stiffness.
In 5,207 participants with home addresses ≤ 1,000 m of a major road, living closer to major roadways was associated with higher CFPWV both when proximity was modeled as a continuous or categorical variable (Figure 1). Similar results were observed for mean arterial pressure and augmentation index although confidence interval estimates included the null (Figure 1). No association was observed between proximity to roadway and FWA.
Effect modification
There was no apparent difference in associations between proximity to roadway (continuous) and CFPWV by BMI (categorized <30 and ≥30 kg/m2), age (categorized <64 and ≥64 years), or sex (all p-values for interaction terms >0.05; Online Supplemental Figure E1).
Sensitivity analyses
Adjusting for PM2.5 levels obtained from the central site monitor for the day preceding tonometry examinations did not affect estimates (Online Supplemental Figure E2). Proximity to roadway associations were similar in models not excluding observations from addresses >1,000m from a major roadway (Online Supplemental Figure E3). Likewise, results from long-term analyses conducted using only the same individuals as in the short-term analyses (living within 50km from the central site monitor), including hypertension therapy in the covariate model, or excluding day of week, season, temperature, relative humidity and time-trend were very similar (Online Supplemental Figures E4, E5, and E6,). We observed consistent results in regression models assessing long-term exposure and CFPWV excluding adjustment for heart rate. Adjusting for mean arterial pressure in models of long-term exposure and CFPWV attenuated results, possibly reflecting an intermediate pathway of effect through blood pressure (Online Supplemental Figures E7).
Conducting separate exploratory analyses by cohort demonstrated consistent results for CFPWV across cohorts. Results for FWA did not show a clear pattern of association with PM2.5 or proximity to roadway in either cohort and we observed positive associations of proximity to roadway with augmentation index and mean arterial pressure only in the older Offspring cohort in comparison to the Third Generation cohort (Online Supplemental Figure E8).
Short-term air pollution and measures of arterial stiffness
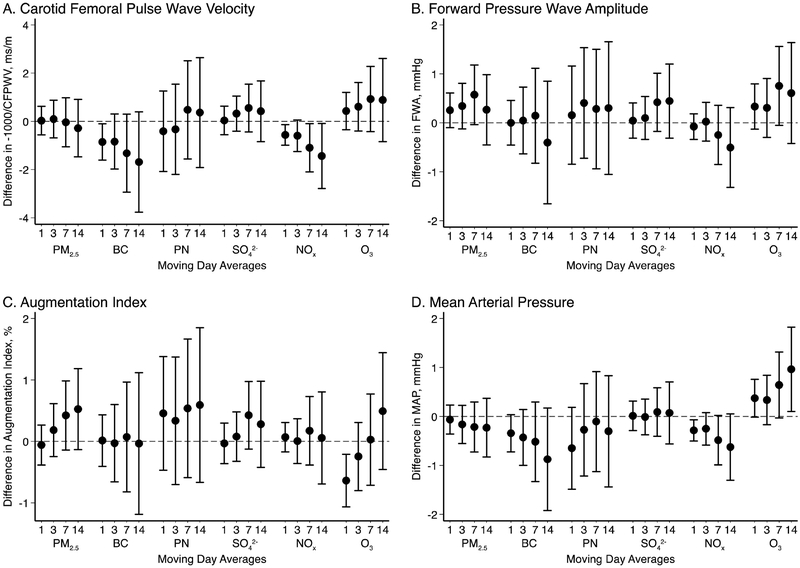
We examined short-term exposures to air pollution in 3,927 participants living within 50 km of the Harvard Supersite monitor. We did not observe consistent evidence to support associations between higher particulate air pollutants such as PM2.5 mass, particle number, and sulfate and measures of arterial stiffness (Figure 2). Higher short-term ozone levels were associated with higher estimates for mean arterial pressure and to a lesser degree CFPWV. Higher short-term levels of black carbon and NOx demonstrated a tendency of lower CFPWV and mean arterial pressure (Figure 2).
Figure 2.
Short-term exposures to air pollution and measures of arterial stiffness. Associations expressed as a change in unit of outcome per 5 μg/m3 PM2.5, 0.4 μg/m3 black carbon (BC), 15000 particles/cm3 (PN), 2 μg/m3 sulfate (SO42-) 0.01 parts per million nitrogen oxides (NOx), and 0.01 parts per million ozone (O3).
Sensitivity analyses
Models including mean arterial pressure as a covariate for estimating the association of short-term air pollution on CFPWV demonstrated very similar results, however, the negative associations (indicating protective effects) of black carbon and NOx became weaker and confidence intervals spanned the null. A similar but less pronounced pattern was observed for NOx in models excluding heart rate as a covariate (Online Supplemental Figure E9). Including hypertension therapy as a covariate for all models of arterial stiffness did not change results (Online Supplemental Figure E10). Similar to analyses of the overall participants, the cohort-specific analyses of short-term air pollution exposure and measures of arterial stiffness demonstrated large heterogeneity with wider confidence intervals and without any clear pattern of association (Online Supplemental Figure E11). In the Offspring cohort the negative associations between black carbon and CFPWV were slightly more pronounced and positive associations with wide confidence intervals were observed between several moving averages of particle number and augmentation index. In the Third Generation cohort we observed some associations between moving averages of ozone with higher FWA and mean arterial pressure, the latter largely concordant with the Offspring cohort.
Discussion
In our community-based sample in the Northeastern US, we observed higher levels of arterial stiffness among participants living closer to major roadways, but not among those exposed to higher levels of PM2.5. Short-term exposure to pollutants in the preceding days was not associated with arterial stiffness, and for some pollutants, there was unexpectedly lower stiffness measures following exposure. Taken together, these results add some evidence to suggest that long-term exposure to local near-roadway traffic-related exposures may be associated with higher arterial stiffness, but did not find evidence to support associations between short-term air pollution exposure and higher arterial stiffness.
Arterial stiffness increases with advancing age and is associated with the risk of hypertension (9) and cardiovascular events (10, 42). Increased inflammatory markers (43) and conditions of inflammation such as rheumatoid arthritis (44) have been associated with increased arterial stiffness and concordantly a reduction of inflammation has been associated with reduced central arterial stiffness (45). This implies both chronic structural changes, amenable for studies exploring effects of long-term air pollution exposure, and dynamic functional components, amenable to studies exploring effects of short-term air pollution exposure.
Long-term exposures and arterial stiffness
In our study, the long-term estimate of PM2.5 exposure was not associated with the reference standard measure of arterial stiffness CFPWV, nor any of our other measures including FWA, augmentation index, or mean arterial pressure. These results are in line with findings from the Multi-Ethnic Study of Atherosclerosis (MESA), perhaps the most comparable to our study sample, including almost 4,000 participants from 6 US cities in which no associations were observed between estimated 20-year exposure to PM10 or PM2.5 and carotid stiffness, assessed by Young’s modulus measure of elasticity, or stiffness assessed using radial artery applanation tonometry (16). Similarly, long-term PM2.5 was not associated with radial artery derived pulse wave velocity (PWV) or augmentation index in a study of 745 young adults in a Dutch study (15). In contrast, other studies have demonstrated associations between higher long-term PM2.5 or PM10 and measures of arterial stiffness such as brachial-ankle PWV or carotid stiffness using Young’s modulus, distensibility and stiffness index but in relation to prenatal exposure (11), or in specific subpopulations such as physically inactive older adults (12) or patient undergoing hemodialysis (17).
In contrast to results for PM2.5 exposure, we observed positive associations between living close to a major roadway and higher CFPWV and a similar pattern of association for mean arterial pressure and augmentation index, results that were more pronounced in the older Offspring cohort. These findings are largely consistent with several other studies of arterial stiffness using distance to major roadway as a proxy for long-term air pollution exposure. In 371 adults from an urban community in Shanghai, living within 50 m of a major road was associated with higher augmentation index (14) and in 52 children in southern Italy, living within 300 m from a major road was associated with higher carotid stiffness compared to living 330–730 m and 780–1,450 m (13).
The difference between associations observed for long-term PM2.5 and proximity to roadway in relation to arterial stiffness may reflect an estimation of different exposures. Proximity to roadway may better capture exposure to particle number than fine particle mass and include gaseous mixtures. Indeed, two large studies in the Netherlands and Switzerland observed positive associations between long-term exposure to NO2 and PWV (12, 15) and previous studies have demonstrated that particle number is highest near roadways and rapidly dissipates with increasing distance (36). In addition to being a proxy for air pollution exposure, proximity to roadway may also reflect noise exposure, a proposed risk factor for cardiovascular outcomes (46, 47), or socioeconomic status. Indeed, the apparent absence of association between long-term exposure to PM2.5 and arterial stiffness in our study coupled with the associations observed for proximity to roadway strengthen the argument of proximity to roadway as a proxy for noise pollution in this study. Unfortunately, we did not have noise pollution data to test this hypothesis.
Short-term exposures and arterial stiffness
In order to study short-term exposures and arterial stiffness, we limited our analyses to participants living within a 50 km radius from the monitoring facility. While reducing our sample size from 5,842 to 3,927 individuals this was an effort to maintain a reasonable assessment of day-to-day variation in pollution exposure using the central monitor. We found no strong evidence in our results to support an association between exposures to elevated short-term levels from 1 to 14 days and higher arterial stiffness. Results for black carbon and NOx suggested lower CFPWV and mean arterial pressure in association with higher pollutant levels and we observed largely reciprocal associations between ozone and these measures possibly reflecting to some extent the degree of negative correlations between these pollutants. In sensitivity analyses, the negative associations for black carbon and NOx were, however, attenuated in models including mean arterial pressure or excluding heart rate suggesting some sensitivity to model specification.
Our results are based on the largest cohort yet to consider short-term air pollution exposure and arterial stiffness and are in line with some, but not all previous studies. A study from Athens including 1,222 hypertension patients did not observe associations between 5-day or 24-hour PM10 levels and augmentation pressure or index in the full sample, consistent with our results, but reported higher augmentation pressure in men following higher 5-day PM10 levels (24). Other small studies using personal samplers for black carbon, NO2 or PM2.5 did not observe any associations between daily levels of these pollutants and higher PWV or augmentation index in patients with metabolic syndrome, in rural mothers, or healthy workers in China (48–50).
In contrast, several studies have reported positive associations between short-term air pollution levels and arterial stiffness in various populations. Particle number levels and to a lesser extent PM2.5 was associated with higher augmentation index and pressure for exposure periods ranging from 1-day to 14-day averages among 370 older men in the New England area, where our study was also conducted (28). Same day black carbon, PM2.5 and NO2 levels were associated with higher PWV and measures of carotid artery stiffness in small studies including 54 healthy Belgian nurses (51) or 89 healthy Taiwanese bankers (52). In addition, welding and exposure to high levels of PM2.5 was associated with higher augmentation index on the day of exposure but a decrease subsequent day (53) suggesting that this rapid change in pulse-wave reflection may indicate a change in ventricular preload or peripheral vascular tone rather than structural changes in arterial stiffness.
In addition to panel studies, a few studies of controlled short-term exposure to diesel exhaust and wood-smoke with a randomized double-blind crossover approach have been conducted, with inconsistent results. In two of the diesel chamber studies, CFPWV was examined with null associations in one study (27) and higher PWV in another when participants where infused with nitric oxide synthase inhibition, possibly suggesting pathways dependent on NO bioavailability (26). Similarly, diesel exposure demonstrated conflicting associations with augmentation index and pressure with positive associations reported immediately following diesel exposure in one study but lower augmentation index (indicating lower arterial stiffness) in another study. Conflicting results are also reported in chamber studies of wood-smoke exposure for both PWV and augmentation index measures of arterial stiffness (25, 54). Thus, studies of short-term studies of air pollution, including controlled exposure studies, have not consistently demonstrated associations with arterial stiffness.
Limitations
We only assessed arterial stiffness at a single visit for each participant and therefore we were not able to analyze air pollution exposure in relation to a longitudinal change in arterial stiffness. We based assessment of long-term exposure on residential address as a proxy for average exposure and could not account for time spent away from home. In order to preserve the rank-ordering of long-term pollution exposure regardless of the downward trends of air pollution exposure over time, we assigned exposure at the home address recorded when tonometry measures were performed according to that address’ annual level in 2003. These approximations of long-term exposure will contribute to some exposure misclassification but minimize the potential bias based on cohort and temporal air pollution trends and are unlikely to introduce major bias in relation to stiffness measures. For short-term exposure, we used a central monitoring site to capture day-to-day variations in pollution levels in a region within 50 km. This disregards the spatial variation in exposure. However, within this 50 km radius, this variation is likely lower than the temporal variability within short-term exposure periods of up to 14 days.
Strengths
We applied a multitude of sensitivity analyses with consistent results. We benefitted from a very large community sample of a well-characterized middle aged to older adults living in a region of the US where air pollution levels are mostly below air quality standards. Arterial stiffness was measured using multiple methods following a rigorous protocol. In addition, we studied the associations of both short-term and long-term air pollution exposures in the same sample, allowing us to study both functional and structural changes in arterial stiffness in the same population.
Conclusions
In conclusion, we observed that living closer to a major roadway was associated with higher arterial stiffness, indicating possible harmful effects of traffic-related exposure on vascular function in this generally healthy cohort of adults. However, the lack of associations between long-term exposure to PM2.5 and arterial stiffness suggest the associations may be driven by the mixture of pollutants in close proximity to major roads, rather than PM2.5, or possibly by unmeasured effects from traffic-noise. Furthermore, in contrast to some previous studies, we did not observe higher arterial stiffness in association with short-term exposures to air pollution.
Supplementary Material
Highlights.
Living nearby a major road was associated with higher arterial stiffness
Particle matter measured by satellite was not associated with arterial stiffness
No associations were observed for short-term exposure to air pollution
Acknowledgments
This publication was made possible by USEPA grant RD-835872–01. Its contents are solely the responsibility of the grantee and do not necessarily represent the official views of the USEPA. Further, USEPA does not endorse the purchase of any commercial products or services mentioned in the publication. PLSL received funding from the Swedish Heart-Lung Foundation. Funding of the Framingham Heart Study was via HHSN268201500001I; N01-HC 25195. Funding sources had no involvement in study design, analysis, data collection, interpretation, writing the report, or decision of submission.
GFM is owner and of Cardiovascular Engineering Inc., a company that develops and manufactures devices to measure vascular stiffness, and serves as a consultant to and receives honoraria from Merck and Novartis.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Declarations of interest:
PLSL, WL, MBR, EHW, JS, DRG, PK, EJB, RSV, NMH, and MAM declare no conflicts of interest.
References
- 1.Brook RD, Franklin B, Cascio W, Hong Y, Howard G, Lipsett M, et al. Air pollution and cardiovascular disease: a statement for healthcare professionals from the Expert Panel on Population and Prevention Science of the American Heart Association. Circulation. 2004;109(21):2655–71. [DOI] [PubMed] [Google Scholar]
- 2.Newby DE, Mannucci PM, Tell GS, Baccarelli AA, Brook RD, Donaldson K, et al. Expert position paper on air pollution and cardiovascular disease. Eur Heart J. 2015;36(2):83–93b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zhang Z, Guo C, Lau AKH, Chan TC, Chuang YC, Lin C, et al. Long-Term Exposure to Fine Particulate Matter, Blood Pressure, and Incident Hypertension in Taiwanese Adults. Environmental health perspectives. 2018;126(1):017008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wilker EH, Mittleman MA, Coull BA, Gryparis A, Bots ML, Schwartz J, et al. Long-term exposure to black carbon and carotid intima-media thickness: the normative aging study. Environmental health perspectives. 2013;121(9):1061–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bauer M, Moebus S, Mohlenkamp S, Dragano N, Nonnemacher M, Fuchsluger M, et al. Urban particulate matter air pollution is associated with subclinical atherosclerosis: results from the HNR (Heinz Nixdorf Recall) study. J Am Coll Cardiol. 2010;56(22):1803–8. [DOI] [PubMed] [Google Scholar]
- 6.Hoffmann B, Moebus S, Mohlenkamp S, Stang A, Lehmann N, Dragano N, et al. Residential exposure to traffic is associated with coronary atherosclerosis. Circulation. 2007;116(5):489–96. [DOI] [PubMed] [Google Scholar]
- 7.Diez Roux AV, Auchincloss AH, Franklin TG, Raghunathan T, Barr RG, Kaufman J, et al. Long-term exposure to ambient particulate matter and prevalence of subclinical atherosclerosis in the Multi-Ethnic Study of Atherosclerosis. American journal of epidemiology. 2008;167(6):667–75. [DOI] [PubMed] [Google Scholar]
- 8.Dorans KS, Wilker EH, Li W, Rice MB, Ljungman PL, Schwartz J, et al. Residential proximity to major roads, exposure to fine particulate matter and aortic calcium: the Framingham Heart Study, a cohort study. BMJ Open. 2017;7(3):e013455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kaess BM, Rong J, Larson MG, Hamburg NM, Vita JA, Levy D, et al. Aortic stiffness, blood pressure progression, and incident hypertension. JAMA. 2012;308(9):875–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mitchell GF. Arterial Stiffness and Wave Reflection: Biomarkers of Cardiovascular Risk. Artery Res. 2009;3(2):56–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Breton CV, Mack WJ, Yao J, Berhane K, Amadeus M, Lurmann F, et al. Prenatal Air Pollution Exposure and Early Cardiovascular Phenotypes in Young Adults. PloS one. 2016;11(3):e0150825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Endes S, Schaffner E, Caviezel S, Dratva J, Stolz D, Schindler C, et al. Is physical activity a modifier of the association between air pollution and arterial stiffness in older adults: The SAPALDIA cohort study. International journal of hygiene and environmental health. 2017;220(6):1030–8. [DOI] [PubMed] [Google Scholar]
- 13.Iannuzzi A, Verga MC, Renis M, Schiavo A, Salvatore V, Santoriello C, et al. Air pollution and carotid arterial stiffness in children. Cardiol Young. 2010;20(2):186–90. [DOI] [PubMed] [Google Scholar]
- 14.Jiang S, Bo L, Gong C, Du X, Kan H, Xie Y, et al. Traffic-related air pollution is associated with cardio-metabolic biomarkers in general residents. Int Arch Occup Environ Health. 2016;89(6):911–21. [DOI] [PubMed] [Google Scholar]
- 15.Lenters V, Uiterwaal CS, Beelen R, Bots ML, Fischer P, Brunekreef B, et al. Long-term exposure to air pollution and vascular damage in young adults. Epidemiology. 2010;21(4):512–20. [DOI] [PubMed] [Google Scholar]
- 16.O’Neill MS, Diez-Roux AV, Auchincloss AH, Shen M, Lima JA, Polak JF, et al. Long-term exposure to airborne particles and arterial stiffness: the Multi-Ethnic Study of Atherosclerosis (MESA). Environmental health perspectives. 2011;119(6):844–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Weng CH, Hu CC, Yen TH, Huang WH. Association between environmental particulate matter and arterial stiffness in patients undergoing hemodialysis. BMC Cardiovasc Disord. 2015;15:115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yang BY, Qian Z, Howard SW, Vaughn MG, Fan SJ, Liu KK, et al. Global association between ambient air pollution and blood pressure: A systematic review and meta-analysis. Environmental pollution (Barking, Essex : 1987). 2018;235:576–88. [DOI] [PubMed] [Google Scholar]
- 19.Ljungman PL, Wilker EH, Rice MB, Austin E, Schwartz J, Gold DR, et al. The Impact of Multipollutant Clusters on the Association Between Fine Particulate Air Pollution and Microvascular Function. Epidemiology. 2016;27(2):194–201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zanobetti A, Luttmann-Gibson H, Horton ES, Cohen A, Coull BA, Hoffmann B, et al. Brachial artery responses to ambient pollution, temperature, and humidity in people with type 2 diabetes: a repeated-measures study. Environmental health perspectives. 2014;122(3):242–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sack CS, Jansen KL, Cosselman KE, Trenga CA, Stapleton PL, Allen J, et al. Pretreatment with Antioxidants Augments the Acute Arterial Vasoconstriction Caused by Diesel Exhaust Inhalation. Am J Respir Crit Care Med. 2016;193(9):1000–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Schneider A, Neas L, Herbst MC, Case M, Williams RW, Cascio W, et al. Endothelial dysfunction: associations with exposure to ambient fine particles in diabetic individuals. Environmental health perspectives. 2008;116(12):1666–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mills NL, Tornqvist H, Gonzalez MC, Vink E, Robinson SD, Soderberg S, et al. Ischemic and thrombotic effects of dilute diesel-exhaust inhalation in men with coronary heart disease. N Engl J Med. 2007;357(11):1075–82. [DOI] [PubMed] [Google Scholar]
- 24.Adamopoulos D, Vyssoulis G, Karpanou E, Kyvelou SM, Argacha JF, Cokkinos D, et al. Environmental determinants of blood pressure, arterial stiffness, and central hemodynamics. J Hypertens. 2010;28(5):903–9. [DOI] [PubMed] [Google Scholar]
- 25.Hunter AL, Unosson J, Bosson JA, Langrish JP, Pourazar J, Raftis JB, et al. Effect of wood smoke exposure on vascular function and thrombus formation in healthy fire fighters. Part Fibre Toxicol. 2014;11:62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Langrish JP, Unosson J, Bosson J, Barath S, Muala A, Blackwell S, et al. Altered nitric oxide bioavailability contributes to diesel exhaust inhalation-induced cardiovascular dysfunction in man. J Am Heart Assoc. 2013;2(1):e004309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lundback M, Mills NL, Lucking A, Barath S, Donaldson K, Newby DE, et al. Experimental exposure to diesel exhaust increases arterial stiffness in man. Part Fibre Toxicol. 2009;6:7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mehta AJ, Zanobetti A, Koutrakis P, Mittleman MA, Sparrow D, Vokonas P, et al. Associations between short-term changes in air pollution and correlates of arterial stiffness: The Veterans Affairs Normative Aging Study, 2007–2011. American journal of epidemiology. 2014;179(2):192–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kannel WB, Feinleib M, McNamara PM, Garrison RJ, Castelli WP. An investigation of coronary heart disease in families. The Framingham offspring study. American journal of epidemiology. 1979;110(3):281–90. [DOI] [PubMed] [Google Scholar]
- 30.Splansky GL, Corey D, Yang Q, Atwood LD, Cupples LA, Benjamin EJ, et al. The Third Generation Cohort of the National Heart, Lung, and Blood Institute’s Framingham Heart Study: design, recruitment, and initial examination. American journal of epidemiology. 2007;165(11):1328–35. [DOI] [PubMed] [Google Scholar]
- 31.Mitchell GF, Parise H, Benjamin EJ, Larson MG, Keyes MJ, Vita JA, et al. Changes in arterial stiffness and wave reflection with advancing age in healthy men and women: the Framingham Heart Study. Hypertension. 2004;43(6):1239–45. [DOI] [PubMed] [Google Scholar]
- 32.Mitchell GF, Wang N, Palmisano JN, Larson MG, Hamburg NM, Vita JA, et al. Hemodynamic correlates of blood pressure across the adult age spectrum: noninvasive evaluation in the Framingham Heart Study. Circulation. 2010;122(14):1379–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kloog I, Chudnovsky AA, Just AC, Nordio F, Koutrakis P, Coull BA, et al. A new hybrid spatio-temporal model for estimating daily multi-year PM2.5 concentrations across northeastern USA using high resolution aerosol optical depth data. Atmos Environ. 2014;95:581–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Miller KA, Siscovick DS, Sheppard L, Shepherd K, Sullivan JH, Anderson GL, et al. Long-term exposure to air pollution and incidence of cardiovascular events in women. New Engl J Med. 2007;356(5):447–58. [DOI] [PubMed] [Google Scholar]
- 35.Rice MB, Ljungman PL, Wilker EH, Dorans KS, Gold DR, Schwartz J, et al. Long-term exposure to traffic emissions and fine particulate matter and lung function decline in the Framingham heart study. Am J Respir Crit Care Med. 2015;191(6):656–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zhu Y, Hinds WC, Kim S, Sioutas C. Concentration and size distribution of ultrafine particles near a major highway. J Air Waste Manag Assoc. 2002;52(9):1032–42. [DOI] [PubMed] [Google Scholar]
- 37.Zhou Y, Levy JI. Factors influencing the spatial extent of mobile source air pollution impacts: a meta-analysis. BMC Public Health. 2007;7:89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ljungman PL, Wilker EH, Rice MB, Schwartz J, Gold DR, Koutrakis P, et al. Short-term exposure to air pollution and digital vascular function. American Journal of Epidemiology. 2014;180(5):482–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wilker EH, Ljungman PL, Rice MB, Kloog I, Schwartz J, Gold DR, et al. Relation of long-term exposure to air pollution to brachial artery flow-mediated dilation and reactive hyperemia. Am J Cardiol. 2014;113(12):2057–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Reference Values for Arterial Stiffness C. Determinants of pulse wave velocity in healthy people and in the presence of cardiovascular risk factors: ‘establishing normal and reference values’. Eur Heart J. 2010;31(19):2338–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.DeNavas-Walt C, Proctor BD, Mills RJ. Income, Poverty, and Health Insurance Coverage in the United States: 2003. Washington DC: U.S. Census Bureau; 2004. p. p60–226. [Google Scholar]
- 42.Cavalcante JL, Lima JA, Redheuil A, Al-Mallah MH. Aortic stiffness: current understanding and future directions. J Am Coll Cardiol. 2011;57(14):1511–22. [DOI] [PubMed] [Google Scholar]
- 43.Muhammad IF, Borne Y, Ostling G, Kennback C, Gottsater M, Persson M, et al. Acute phase proteins as prospective risk markers for arterial stiffness: The Malmo Diet and Cancer cohort. PloS one. 2017;12(7):e0181718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Turesson C, Jacobsson L, Ryden Ahlgren A, Sturfelt G, Wollmer P, Lanne T. Increased stiffness of the abdominal aorta in women with rheumatoid arthritis. Rheumatology (Oxford). 2005;44(7):896–901. [DOI] [PubMed] [Google Scholar]
- 45.Maki-Petaja KM, Hall FC, Booth AD, Wallace SM, Yasmin, Bearcroft PW, et al. Rheumatoid arthritis is associated with increased aortic pulse-wave velocity, which is reduced by anti-tumor necrosis factor-alpha therapy. Circulation. 2006;114(11):1185–92. [DOI] [PubMed] [Google Scholar]
- 46.Fuks KB, Weinmayr G, Basagana X, Gruzieva O, Hampel R, Oftedal B, et al. Long-term exposure to ambient air pollution and traffic noise and incident hypertension in seven cohorts of the European study of cohorts for air pollution effects (ESCAPE). European heart journal. 2017;38(13):983–90. [DOI] [PubMed] [Google Scholar]
- 47.Selander J, Nilsson ME, Bluhm G, Rosenlund M, Lindqvist M, Nise G, et al. Long-term exposure to road traffic noise and myocardial infarction. Epidemiology. 2009;20(2):272–9. [DOI] [PubMed] [Google Scholar]
- 48.Shan M, Yang X, Ezzati M, Chaturvedi N, Coady E, Hughes A, et al. A feasibility study of the association of exposure to biomass smoke with vascular function, inflammation, and cellular aging. Environmental research. 2014;135:165–72. [DOI] [PubMed] [Google Scholar]
- 49.Zhao X, Sun Z, Ruan Y, Yan J, Mukherjee B, Yang F, et al. Personal Black Carbon Exposure Influences Ambulatory Blood Pressure: Air Pollution and Cardiometabolic Disease (AIRCMD-China) Study. Hypertension. 2014;63(4):871–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Day DB, Xiang J, Mo J, Li F, Chung M, Gong J, et al. Association of Ozone Exposure With Cardiorespiratory Pathophysiologic Mechanisms in Healthy Adults. JAMA Intern Med. 2017;177(9):1344–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Provost EB, Louwies T, Cox B, Op ‘t Roodt J, Solmi F, Dons E, et al. Short-term fluctuations in personal black carbon exposure are associated with rapid changes in carotid arterial stiffening. Environment international. 2016;88:228–34. [DOI] [PubMed] [Google Scholar]
- 52.Wu CF, Shen FH, Li YR, Tsao TM, Tsai MJ, Chen CC, et al. Association of short-term exposure to fine particulate matter and nitrogen dioxide with acute cardiovascular effects. Sci Total Environ. 2016;569–570:300–5. [DOI] [PubMed] [Google Scholar]
- 53.Fang SC, Eisen EA, Cavallari JM, Mittleman MA, Christiani DC. Acute changes in vascular function among welders exposed to metal-rich particulate matter. Epidemiology (Cambridge, Mass). 2008;19(2):217–25. [DOI] [PubMed] [Google Scholar]
- 54.Unosson J, Blomberg A, Sandstrom T, Muala A, Boman C, Nystrom R, et al. Exposure to wood smoke increases arterial stiffness and decreases heart rate variability in humans. Part Fibre Toxicol. 2013;10:20. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.