Abstract
The recruitment of neutrophils to sites of inflammation, their battle against invading microorganisms through phagocytosis and the release of antimicrobial agents is a highly coordinated and tightly regulated process that involves the interplay of many different receptors, ion channels, and signaling pathways. Changes in intracellular calcium levels, caused by cytosolic Ca2+ store depletion and the influx of extracellular Ca2+ via ion channels play a critical role in synchronizing neutrophil activation and function. In this review, we provide an overview of how Ca2+ signaling is initiated in neutrophils and how changes in intracellular Ca2+ levels modulate neutrophil function.
Keywords: Neutrophil, leukocyte recruitment cascade, calcium signaling, SOCE, CRAC channels, Orai1, STIM1, Calcium channels, TRP channels, Potassium channels, P2X receptors
Neutrophil recruitment cascade
Neutrophils (polymorphonuclear leukocytes, PMNs) are a central component of the innate immune system and are among the first cells to be recruited to sites of infection and inflammation1. In doing so, the cells engage in a well-defined multistep cascade of adhesion and activation events. Initially, free flowing PMNs are recruited from the blood stream via selectin-glyco-ligand interactions, triggering rolling of the cells along inflamed postcapillary venules2. Engagement via selectins expressed on the inflamed endothelium with P-selectin glycoprotein ligand 1 (PSGL-1), L-selectin (in humans3) and other sialylated ligands initiates the activation of β2-integrins and subsequent deceleration of rolling neutrophils4,5. Additionally, PMNs ligate immobilized chemokines presented on inflamed endothelial cells, thereby inducing the high affinity conformation of β2-integrins lymphocyte function-associated antigen-1 (LFA-1) and macrophage-1 antigen (Mac-1), which leads to firm adhesion of neutrophils on the inflamed endothelium6. Post arrest modifications, including β2-integrin-clustering and anchoring to the cytoskeleton, results in neutrophil spreading, adhesion strengthening and intraluminal crawling7. Crawling of neutrophils along the inflamed vessel wall is a critical step for their localization at appropriate sites for extravasation (diapedesis). Similar to the intravascular adhesion steps, emigration of neutrophils into inflamed tissue also proceeds in a cascade like fashion including transmigration across the endothelial layer into the subendothelial space, followed by penetration of the vascular basement membrane8. Within tissue, neutrophils migrate along chemokine and cytokine gradients to the actual site of tissue insult, where they respond to invading pathogens by engaging in phagocytosis, degranulation and secretion of proteolytic enzymes and reactive oxygen species.
Ca2+ signaling/SOCE in neutrophils
The neutrophil recruitment cascade and its underlying molecular mechanisms have been the subject of intensive research over the last decade. Changes in intracellular Ca2+ levels are a hallmark in the activation process of neutrophils9. Hence, versatile regulation of Ca2+ fluxes during neutrophil recruitment and antimicrobial responses is a finely tuned process in terms of temporal and spatial organization9. Under resting conditions, levels of cytosolic Ca2+ in neutrophils (0.1 μM) are 10 000-fold lower than in the extracellular milieu 10. Upon stimulation, cytosolic Ca2+ levels rapidly increase by the release of Ca2+ from intracellular stores and/or by influx of extracellular Ca2+.
Entry of extracellular Ca2+ into immune cells, including neutrophils, is primarily mediated through Ca2+ release-activated Ca2+ (CRAC) channels, located in the plasma membrane (PM). The process of Ca2+ influx is termed store operated calcium entry (SOCE) (Figure 1A). In neutrophils, SOCE can be initiated by several ligand receptor interactions including the engagement of G-protein coupled receptors (GPCRs)11 and Fcγ-receptors (FcγRs)12, the activation of β2-integrins13,14 or the interaction of E-selectin with PSGL-1 and L-selectin4,15,16. Downstream signaling involves activation of phospholipase C (PLC). GPCRs mainly trigger the activation of the PLCβ subfamily members 2 and 3, while FcγRs, E- and P-selectin ligands, and β2-integrins predominantly activate PLCγ1 and PLCγ2, respectively4,17. PLCβ2/3 and PLCγ1/2 convert phosphatidylinositol 4,5 bisphosphate (PIP2) into diacylglycerol (DAG) and inositol-1,4,5 triphosphate (IP3 or InsP3). The IP3 receptor (IP3R) is localized in the membrane of the endoplasmic reticulum (ER) and the activation of this non-selective Ca2+ channel by IP3 triggers the release of ER-stored Ca2+ into the cytoplasm18. The drop in Ca2+ level in the ER is sensed by stromal interaction molecule 1 (STIM1), an ER transmembrane protein which then translocates to specific regions of the ER close to the PM (PM-ER junctions). At these sites, STIM1 binds to and activates the PM associated Ca2+ channel denoted CRAC modulator 1 (CRACM1), also termed Orai1, the predominant CRAC channel in neutrophils16,19. Stimulation of Orai1 leads to Ca2+ influx20 and concomitant increase of cytosolic Ca2+ concentration. FcγR activation is thought to exhibit an alternative mechanism to induce the release of Ca2+ from ER stores in a PLCγ (and therefore IP3) independent manner by activation of phospholipase D (PLD) via phosphatidylinositol 3-kinase (PI3K) and subsequent generation of sphingosine 1 phosphate (S1P) by sphingosine kinase (SK). Blockade of either SK or PI3K, was shown to reduce Ca2+ flux in neutrophils upon FcγRIIA and FcγRIIIB activation21. A role of S1P in calcium signaling was also demonstrated by Itagaki and Hauser22 who showed that S1P formation is dependent on ER store depletion. Furthermore, the authors found that inhibition of SK results in a reduction of Ca2+ entry. Recently, it was shown for glioblastoma cells that a member of the transient receptor potential (TRP) channel family (TRPC1; see also below) might be involved in S1P mediated calcium signaling23. However, the exact mechanism by which S1P mediates changes in intracellular Ca2+ and whether this signaling cascade cooperates with other activation pathways is currently unknown and requires further investigation.
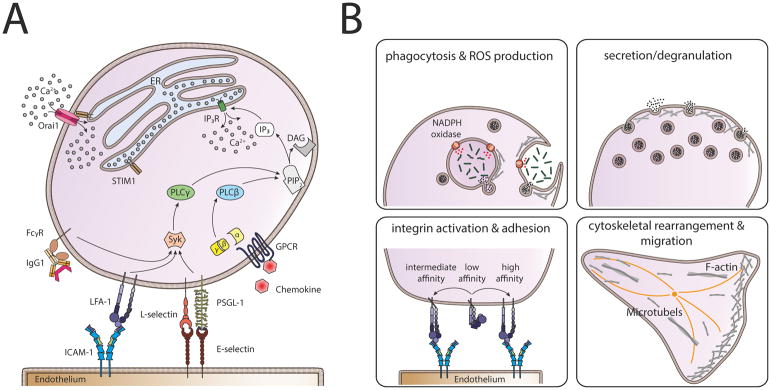
Figure 1. Calcium signaling cascade in neutrophils.
(A) Store operated calcium entry (SOCE) can be initiated either via activation of Fcγ-receptors (FcγRs), β2-integrins, the engagement of P-selectin glycoprotein ligand 1 (PSGL-1) and L-selectin with E-selectin expressed on inflamed endothelium, or via G-protein coupled receptors (GPCRs). Activation of GPCRs leads to dissociation of the G-protein subunits α and βγ and subsequent activation of phospholipase (PLC) β. FcγRs, β2-integrins and PSGL-1/L-selectin signaling in turn, activate PLCγ involving spleen tyrosine kinase (Syk). Both, activated PLCβ and PLCγ convert phosphatidylinositol 4,5 bisphosphate (PIP2) into diacylglycerol (DAG) and inositol-1,4,5 triphosphate (IP3). IP3 binds to and opens the IP3 receptor (IP3R) in the membrane of the endoplasmic reticulum (ER) which results in Ca2+ flux out of the ER into the cytoplasm via IP3R. Upon store depletion, the Ca2+ sensor stromal interaction molecule 1 (STIM1) translocates to PM-ER-junctions and activates Orai1, the predominant Ca2+ release activated Ca2+ (CRAC) channel in neutrophils. This allows the entry of extracellular Ca2+ into the neutrophil, exerting a variety of functions, including (B) phagocytosis, ROS production, secretion and degranulation of vesicles, activation of β2-integrins and cytoskeletal rearrangement leading to polarization and migration.
Local regulation of intracellular Ca2+ concentrations is a prerequisite for eliciting the broad machinery of innate immune defense processes in neutrophils for instance, phagocytosis, production of reactive oxygen species (ROS) and degranulation (reviewed in24). Furthermore, β2-integrin activation15, cytoskeletal rearrangement25 and migration of neutrophils26 within the tissue, are all Ca2+ signaling dependent processes (Figure 1B). The importance of STIM1 and Orai1 for SOCE in neutrophils has been validated using mouse mutant models27–30 and knockdown in neutrophil-like cell lines (i.e. HL-60 cells)16,31 and has been reviewed in detail in32. Additional evidence comes from patients with mutations in either Orai1 or STIM1 who suffer from severe combined immunodeficiency-like diseases and autoimmune disorders, accompanied by an increased susceptibility to infections, sepsis and neutropenia33,34. Surprisingly, neutrophils from patients with loss of function mutations in either STIM1 or Orai1 displayed no differences in ROS production, static adhesion, chemotaxis and IL-8 production and only a modest reduction of SOCE, and less sustained Ca2+ flux, respectively35. This indicates that the severe autoimmune disorders in these patients are not primary neutrophil dependent functions. One possible explanation might be the expression of Orai1 and STIM1 isoforms in neutrophils which contribute to SOCE and may compensate for a loss of function in Orai1 and STIM1, respectively. One potential candidate would be STIM2 which is an additional Ca2+ sensor expressed in neutrophils. Of note, STIM2 exerts additional functions in neutrophil activation, distinct from classical Ca2+ sensing. This was shown in STIM2 deficient neutrophils, which exhibit reduced cytokine production during inflammatory responses36. However, production of reactive oxygen species (ROS), degranulation and phagocytosis, which are impaired in STIM1 deficient PMNs, were normal in the absence of STIM228,29. Interestingly, both, STIM1 and STIM2 seem to be dispensable for neutrophil migration in the mouse27,36. The distinct and redundant functions of the two isoforms STIM1 and STIM2 have been summarized recently37. Evidence for the expression of the CRAC channel homologs Orai2 and Orai3 exists for HL-60 cells31,38 and most likely for human neutrophils35. Whereas Steinckwich and colleagues31 reported no significant differences in global cytosolic Ca2+ levels during phagocytosis in Orai2 and Orai3 knock down HL-60 cells, Diez-Bello et al.38 observed altered intracellular Ca2+ levels in HL-60 cells lacking ORAI2. Further functional data are necessary to better understand the functions of the ORAI1 and STIM1 isoforms and to decipher the differing compensatory properties in mouse and human neutrophils.
Temporal and spatial resolution
A fine balance exists between the protective antimicrobial functions of neutrophils and potential tissue damage associated with their unchecked recruitment during exuberant inflammation. Neutrophils have evolved mechano-regulatory mechanisms by which tensile forces exerted on cells in blood flow that act on selectin and integrin bonds, are converted to biochemical signals during the transition from rolling to cell arrest and transmigration (Figure 2). Recently, it was reported that an allosteric shift from low or intermediate LFA-1 to a high affinity state enables bond formation with intracellular adhesion molecules (ICAMs) that effectively functions like a clutch in transmission of sufficient tension to the transient LFA-1/Kindlin-3 linkage to elicit Orai1 mediated Ca2+ flux.14,15 Engagement of β2-integrins to their target ligands on inflamed endothelium is essential in activation of calcium dependent kinases and to achieve maximum cytosolic calcium influx via SOCE that activates the opening of intracellular Ca2+ release channels 39. As introduced above, the canonical pathway downstream from ligation of GPCR involves activation of PLC-β following release of the Gβγ subunit40. PLC cleaves membrane lipid PiP2 into DAG and IP3, resulting in Ca2+ flux and activation of protein kinase C (PKC)41. In conjunction with PKC, calcium itself activates adhesion receptors, whose ligation in turn amplifies downstream immune responses42. Thus, inside-out and outside-in signals cooperate in a synchronous manner in the emptying of cytosolic calcium stores, which in turn drives CRAC influx from the extracellular milieu. Regulation of the CRAC current has been carefully documented in T-cell immune synapse formation, where STIM1 and STIM2 act as the sensors of calcium store depletion and together with Orai1 play a central role in regulating local calcium transients that orient the polymerization of cellular actin during migration43,44. A key reason why CRAC current has not been widely studied in human neutrophils (hPMNs) is that they are much shorter-lived than T-cells and are not readily amenable to genetic manipulation. HL-60 cells are a myeloid cell line that are differentiated to neutrophils following culture with DMSO, and retain most features of primary hPMNs including inflammatory recruitment. Employing siRNA on differentiated HL-60 it has been shown that Orai1 is a primary mediator of membrane Ca2+ flux during neutrophil recruitment on inflamed endothelium16. More recently, it was reported that knockdown of Kindlin-3 elicited a similar phenotype of impaired neutrophil arrest in shear flow due in part to lack of Ca2+ flux and high affinity integrin clustering45. The picture that has emerged is that during arrest, neutrophils engage and exert force on a complex of LFA-1/ICAM-1/Kindlin-3 allowing the cell to ‘sense’ mechanical cues at the site of adhesive contact with inflamed endothelium. In this manner, the magnitude of shear stress directly regulates calcium flux locally through application of tensile force on LFA-1/ICAM-1 bonds, which strategically are positioned at the base of the uropod and pseudopod on polarized migrating neutrophils15,46 This provides intrinsic regulation on the number of PMNs that arrest and transmigrate under conditions of low flow such as during tissue ischemia.
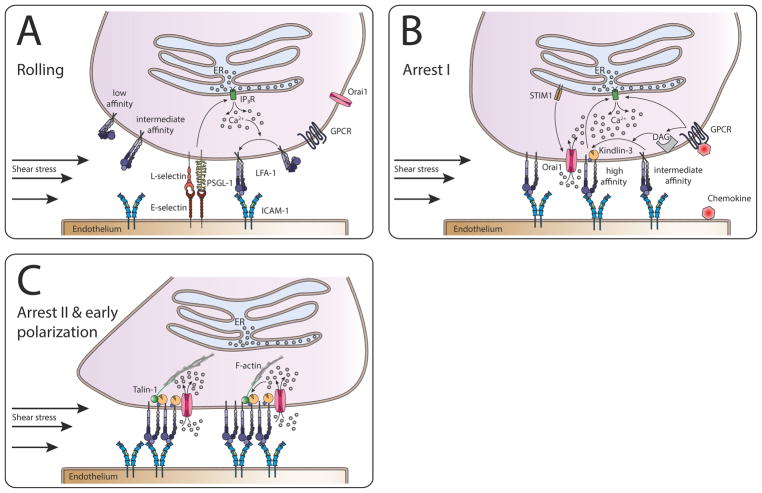
Figure 2. Calcium signaling functions to synchronize events in neutrophil recruitment.
Neutrophil rolling via PSGL-1, L-selectin, and E-selectin causes downstream activation of IP3R and subsequent release of ER stored Ca2+, which in turn shifts LFA-1 from a low to an intermediate and high affinity state. This shift leads to deceleration of the rolling neutrophil via LFA-1-ICAM-1 interaction. The activation of GPCRs by chemokines further increases intracellular Ca2+ levels via IP3R. Additionally, GPCR signaling via DAG activates additional LFA-1 which increases the number of LFA-1/ICAM-1 bonds, resulting in total arrest of the neutrophil (inside-out signaling). High affinity LFA-1 binding to ICAM-1 further enhances rise in intracellular Ca2+ concentrations (outside-in signaling), amplified by shear forces. Upon force transduction, Kindlin-3 binds to β2-integrin tails, recruits Orai1 and thus forms a complex which ensures increase in Ca2+ levels directly at focal adhesion spots, a process again highly dependent on shear forces to orient cell polarization and migration. This is followed by clustering of LFA-1 and the recruitment of Talin1 which links the focal adhesion spots to the cytoskeleton, enabling intraluminal crawling of the neutrophil.
Role of adaptor molecules in local regulation of Ca2+ flux
Absence of Kindlin-3 severely impairs β2-integrin function on leukocytes, resulting in leukocyte adhesion deficiency (LAD)-III in mouse and human47. As a direct consequence, Kindlin-3 deficiency in LAD-III patients results in severe bleeding and resistance to arterial thrombosis, as well as immunodeficiency48. Mechanistically, Kindlin-3 binds by its FERM F3 subdomain directly to regions of β-integrin tails distinct from Talin1, which in turn triggers integrin activation49. Kindlin-3 is required for the formation of micro-clusters (e.g. ~10–100 receptors) of high affinity LFA-1. Neutrophils engage and exert force on these receptors during bond formation with ICAM-1 that in turn enables the cell to ‘sense’ mechanical cues and transform them into localized sites of mechanotransduction at appropriate inflammatory sites on endothelial cells. It is currently unknown how force transmitted from the outside-in induces binding between the β-integrin tail and membrane bound Kindlin-3. This interaction precedes and is necessary to engage the CRAC machinery (e.g. STIM1 and STIM2) and catalyze the subsequent association with Orai1 adjacent to the endoplasmic reticulum for SOCE50. A clasp formed by a salt-bridge between the integrin α and β tails is crucial for maintaining integrins in the bent, inactive conformation. Intracellular proteins that interact with integrin tails, such as Talin1 and Kindlin-3 can induce conformational activation of the integrin by disrupting the integrin clasp51. Following inside-out signaling via GPCRs a separation of the clasp results in triggering extension of ectodomains and a conformational change to the activated and extended high affinity ligand binding site of LFA-1. In this manner, we propose that kindlins serve as coactivators, as they cooperate with Talin1 and Orai1 to activate integrins52. Further, we envision a mechanism by which engagement of high-affinity LFA-1 acts synergistically with chemokine receptor signaling to effectively amplify release of intracellular Ca2+ 45. Kindlin-3 association with high-affinity LFA-1 is necessary for this amplification in Ca2+ release in the presence of shear flow. We speculate that Kindlin-3 functions as a mechanosensor, since it binds constitutively with LFA-1 but its interaction increases by several-fold with an upshift to high-affinity as tensile force is applied to high-affinity bond clusters9. We and others have reported that silencing expression of Kindlin-3 or Orai1 impairs LFA-1 clustering at adhesive contacts, which correlates with a significant decrease in adhesion strengthening45. In contrast, silencing expression of Talin-1 does not alter high-affinity LFA-1 clustering and only slightly diminishes stable adhesion at high shear stress. This suggests that Talin-1 does not play an eminent role in clustering of LFA-1. What remains unknown is how force acting on LFA-1 bond clusters results in tight engagement between LFA-1 and Kindlin-3 and how it engages locally with CRAC channels in modulation of local Ca2+ release. It has been reported that as little as 5% of physiologic Kindlin-3 levels in hematopoietic cells are essential for the retention in bone marrow and sufficient for embryonic and postnatal development, but exposure to stress such as infection at this low level of Kindlin-3 significantly impaired the capacity for PMN adhesion and host response47,53. Thus, a ratio of two LFA-1 to each Kindlin-3 appears to be the optimal configuration for homeostatic regulation of adhesive function and to prevent spontaneous bleeding and microbial infections. We propose that the local engagement of adhesion receptors and regulation of cytosolic Ca2+ are rate limiting steps in mediating where and when neutrophils are activated to arrest and initiate a migratory phenotype, events that serve as a gatekeeper in neutrophil recruitment to sites of inflammation.
Other ion channels involved in Ca2+ signaling in neutrophils
Although the primary mechanism of calcium flux into the cytosol of neutrophils is predominantly mediated via IP3 dependent Ca2+ release out of ER stores and subsequent SOCE upon activation of the CRAC channel Orai1, there are additional ion channels involved in calcium signaling in neutrophils (Table 1). The literature within this broad field of Ca2+ signaling is rather controversial, as human and murine (mPMNs) neutrophils and even primary human neutrophils and HL-60 cells do not always display the same expression pattern of ion channels on their surface19,54,55. This makes it difficult to directly translate the different findings between species, cell lines and primary cells.
Table 1.
Known ion channels and sensors being expressed and involved in calcium signaling in neutrophils, their permeability and directionality. ↓: influx ↑: efflux; h=human; m= mouse (h̶/m̶= not expressed)
| Channels | Permeability | Directionality | Expression in neutrophils (Human/Mouse) | Reference |
|---|---|---|---|---|
| Orai1 | Ca2+ | ↓ | h,m | 16,19 |
| Orai2 | Ca2+ | ↓ | h | 35 |
| Orai3 | Ca2+ | ↓ | h | 35 |
| STIM1 | Ca2+-sensor | - | h,m | 28,90 |
| STIM2 | Ca2+-sensor | - | h,m | 36,90 |
| TRP Channels | ||||
| TRPC1 | non-selective; Ca2+, Na+ | ↓ | h, m | 57,61,91 |
| TRPC3 | non-selective; Ca2+, Na+ | ↓ | h, m̶ | 55,57,91 |
| TRPC4 | non-selective; Ca2+, Na+ | ↓ | h | 57,91 |
| TRPC6 | non-selective; Ca2+, Na+ | ↓ | h, m | 54,55,91 |
| TRPM2 | non-selective; Na+, K+, Ca2+ | ↓ | h, m | 54,65,91,92 |
| TRPM4 | non-selective; Na+, K+, Cs+ | ↓ | m | 55,91,92 |
| TRPV1 | non-selective; Ca2+ | ↓ | h, | 91,93 |
| TRPV2 | non-selective; Ca2+, Na+ | ↓ | h, | 91,93 |
| TRPV4 | non-selective; Ca2+ | ↓ | h, m | 55,68,91 |
| TRPV5 | non-selective; mainly Ca2+ | ↓ | h, | 91,93 |
| TRPV6 | non-selective; mainly Ca2+ | ↓ | h, | 91,93 |
| Potassium channels | ||||
| KV1.3 | K+ | ↑ | h | 73 |
| KCa3.1 | K+ | ↑ | h, m | 71 |
| P2X receptors | ||||
| P2X1R | non-selective; Ca2+, Na+ | ↓ | h, m | 74,76,77 |
| P2X3R | non-selective; Ca2+, Na+ | ↓ | h̶, m | 76,77 |
| P2X4R | non-selective; Ca2+, Na+ | ↓ | h | 74,75,77 |
| P2X5R | non-selective; Ca2+, Na+ | ↓ | h | 74,77 |
| P2X7R | non-selective; Ca2+, Na+, K+ | Ca2+, Na+: ↓ K+: ↑ | h, m | 77,81,82 |
| Proton channel | ||||
| VSOP/Hv1 | H+ | ↑ | h, m | 87 |
TRP-channels
The superfamily of transient receptor potential (TRP) channels is a group of non-selective ion channels consisting of six different subfamilies, namely the anykyrin (TRPA), the canonical (TRPC), the melastatin (TRPM), the mucolipin (TRPML), the polycystin (TRPP) and the vanilloid (TRPV) families. Human and murine PMNs only express members of the TRPC, TRPM and TRPV channels, which we will introduce within this section.
TRPC channels are non-selective cation channels for Ca2+ and Na+, displaying a strong tendency to form heterodimers56. Four members of TRPC channels are described in neutrophils, namely TRPC1, TRPC3, TRPC4, and TRPC6 which is the best studied TRPC channel in this cell type19,54,55,57–59. Knockdown of TRPC6 in HL-60 cells reduced NADPH oxidase activity and therefore ROS production, demonstrating its contribution to changes in intracellular Ca2+ levels19. Subsequently, the use of TRPC6 deficient mPMNs demonstrated that TRPC6 plays a role in CXCL2 mediated Ca2+ influx55. However, TRPC6 dependent changes in cytosolic Ca2+ levels are stimulus dependent, as formyl-methionyl-leucyl-phenylalanine (fMLF) was unable to initiate this process of Ca2+ entry55. Further, knockout of TRPC6 resulted in delayed Ca2+ responses and altered cytoskeletal dynamics in CXCL2 stimulated neutrophils. In 2013, Lindemann et al.59 confirmed the relevance of TRPC6 in chemokine receptor mediated actin remodeling and subsequent chemotaxis in neutrophils. TRPC6−/− mPMNs were unable to efficiently transmigrate into the inflamed peritoneal cavity in a mouse peritonitis model and additionally, knockout mPMNs displayed a reduction in intracellular Ca2+peaks following CXCL1 stimulation compared to wildtype control cells. It is known that TRPC6 (together with TRPC3) is activated independently of ER Ca2+ store depletion directly via DAG60, the second cleavage product of PIP2 conversion into IP3. This mechanism has been termed receptor operated calcium entry (ROCE)59. Hence, the findings in the context of TRPC6 function in neutrophils reveals an alternative process of Ca2+ influx through the PM, which functions independent of the classical IP3/IP3R/STIM1-axis.
TRPC3 is another member of the TRPC family expressed on human, but not on mPMNs55,57. Similar to TRPC6, it was reported to be involved in the regulation of ROS production in neutrophils19.
TRPC1 may primarily function as a regulator of neutrophil migration and chemotaxis. TRPC1−/− mPMNs were described to display reduced migratory and chemotactic properties following fMLF stimulation61. Surprisingly, Ca2+ influx was not diminished in TRPC1−/− mPMNs, but rather increased. However, absence of TPRC1 resulted in abnormal subcellular Ca2+ distribution with higher Ca2+ levels at the uropod, leading to reduced migratory capacity of the neutrophils. The authors suggested that TRPC1 may regulate the activity of other members of the signaling cascade, rather than being directly involved in SOCE or ROCE. These findings are in line with a study from Storch et al.62, who showed that loss of TRPC1 resulted in an increase in Ca2+ influx in neuronal cells. Only one publication describes a putative role of TPRC1 in SOCE19, where silencing of TRPC1 in HL-60 cells led to reduced intracellular Ca2+ levels and NADPH oxidase activation upon fMLF stimulation.
Members of the TRPM family are monovalent-cation channels, characterized by a variable selectivity for divalent ions56. TRPM2, for instance is permeable for Ca2+ 56 and is the only member of the TRPM family known to be expressed on human and murine neutrophils54,63. TRPM2 may play an important role as a stress sensor, translating metabolic and oxidative signals into Ca2+ flux64. This channel is activated by ADP ribose (ADPR), cyclic ADPR (cADPR), adenosine monophosphate (AMP), nicotinic acid adenine dinucleotide phosphate (NAADP) and by hydrogen peroxide (H2O2)64. Additionally, TRMP2 may be involved in the Ca2+ signaling downstream of formyl-peptide receptors (FPRs), but not downstream of chemokine receptors, since impaired Ca2+ flux was shown in TRPM2−/− mPMNs following fMLF, but not CXCL2 stimulation65. Further evidence for a specific role of TRPM2 in fMLF induced signaling comes from the observation that in hPMNs, TRPM2 acts exclusively downstream of FPRs66. Moreover, TRPM2 may restrain neutrophil migration, as the channel is internalized together with FPR1 at the site of infection, hence permitting and initiating neutrophil mediated host defense, namely phagocytosis and secretion of antimicrobial agents63.
Expression of TRPM4 on mRNA level has been shown via qPCR55,67. However, no functional role in the context of Ca2+ signaling could be attributed to TRPM4 in neutrophils. Serafini and colleagues67 did not detect any differences in mobilization and function of TRPM4−/− neutrophils in a mouse model for sepsis and in intracellular Ca2+ levels in these cells activated with E.coli.
Finally, neutrophils have been reported to express TRPV channels, non-selective cation channels, which exhibit only a moderate permeability to Ca2+ (with the exception of TRPV5 and TRPV6) and which sense and react to chemical compounds like capsaicin and physical environmental cues like temperature or changes in osmotic pressure56. Whereas TRPV4 is expressed on both, human and murine PMNs55,68, TRPV1, TRPV2, TRPV5 and TRPV6 were up to now only found on hPMNs54. TRPV4−/− mPMNs, as well as hPMNs pretreated with a TRPV4 inhibitor (HC-067047), exhibited a reduction in Ca2+ flux upon stimulation with platelet activating factor (PAF), resulting in reduced ROS production68. In a mouse model for acute lung injury (ALI), TRPV4 deficient cells were not recruited efficiently to inflamed lung tissue, pointing out a critical role of this Ca2+ channel in leukocyte migration and activation68.
Very little is known about the role of TRPV1 in neutrophil function. This receptor (also known as capsaicin receptor) is permeable to Ca2+ and can be activated by capsaicin or high temperatures in the noxious range69. Nevertheless, no Ca2+ flux was detectable in hPMNs treated with capsaicin54. Besides the presence of TRPV2, TRPV5 TPRV6 on hPMNs, their respective roles in neutrophil function remains unknown. Further studies are needed to elucidate whether TRPV1, TRPV2, TRPV5 and/or TPRV6 are involved in Ca2+ dependent neutrophil activation.
Potassium channels
K+ efflux has been shown to be important for sustained Ca2+ influx in immune cells via Ca2+ permeable channels. It preserves the depolarization of the PM by maintaining the electrochemical gradient to drive further Ca2+ influx18. Using patch clamp technology, Krause and Welsh70 observed two distinct K+ currents in hPMNs: one of them a voltage dependent K+ current and a second class that relies on elevation of cytoplasmic Ca2+ concentration. Recently, the latter K+ current was related to the presence of KCa3.1 (also known as IKCa1)71. Interestingly, inhibition of KCa3.1 via the specific inhibitor TRAM-34 did not alter increased intracellular Ca2+ levels in response to fMLF. This observation was surprising, as an influence of K+ efflux via KCa3.1 on intracellular Ca2+ levels had been shown for other cell types (eg. Jurkat cells)72. However, pharmacological blockade of this channel resulted in functional deficiencies of hPMNs. Likewise, KCa3.1−/− neutrophils displayed a reduction of their migratory behavior in a transwell migration assay compared to control cells71. In line with these observations, recruitment of KCa3.1−/− mPMNs was reduced in an acute lung injury model71. One candidate for the voltage dependent K+ current in neutrophils might be KV1.3, which was shown to be expressed on hPMNs73. However, functional data on the role of KV1.3 in Ca2+ signaling in PMNs are still missing.
P2X receptors
P2X receptors are ATP-gated non-selective cation channels. Two members, P2X1R and P2X7R are expressed on human and mouse neutrophils, whereas P2X4R and P2X5R may be expressed on hPMNs74,75 and P2X3R on mPMNs76. However, functional data is not available. Extracellular ATP levels are elevated at sites of inflammation and influence intracellular Ca2+ levels via two different pathways. ATP can either bind and activate GPCRs (P2Y receptors) and affect Ca2+ signaling via SOCE or directly via P2X receptors77. P2X1R is expressed on human and murine neutrophils74,76 and its activation allows Na+ and Ca2+ to enter the cell77. Lecut et al. showed that P2X1R engagement leads to downstream activation of RhoA and promotes neutrophil migration76. In vivo, loss of P2X1R resulted in impaired migratory behavior and reduced extravasation of mPMNs into the inflamed peritoneal cavity. This observation was confirmed by Maître and colleagues, where P2X1−/− mice showed a defect in LPS induced neutrophil emigration from cremaster venules into inflamed tissue78. Furthermore, these mice displayed diminished responses in a model of acute systemic inflammation provoked by LPS78. In contrast to these reports, more recently Lecut et al.79 found an increased susceptibility to LPS induced endotoxemia and an increase in ROS production in P2X1−/− mice. They concluded that P2X1R may have a protective role in endotoxemia by negatively regulating systemic neutrophil activation. Wang et al.80 assumed that P2X1R may be involved in stopping chemotactic migration of neutrophils during LPS induced autocrine ATP signaling, thereby providing a signal to neutrophils at the inflammatory site to exert their antimicrobial functions.
Data on the presence of P2X7R on neutrophils is still conflicting, but emerging evidence suggests that hPMNs81 and mPMNs82 both express P2X7R. Activation of P2X7R via binding of extracellular ATP leads to rapid influx of Na+ and Ca2+, as well as K+ efflux via this channel77. This in turn was shown to activate the NLRP3 inflammasome and to initiate neutrophil recruitment83 and IL-1β secretion82. It is broadly accepted that activation of the NLRP3 inflammasome is associated with K+ efflux in myeloid cells84,85.
Proton channel VSOP/Hv1
The proton channel murine voltage-sensing domain only protein (VSOP) and its human homologue Hv1 have been shown to inhibit azurophilic granule release in neutrophils. VSOP/Hv1−/− deficient neutrophils displayed an enhanced release of MPO and neutrophil elastase compared to WT cells when activated with PMA86. Furthermore, VSOP/Hv1 was described to play an important role in NADPH oxidase mediated phagosomal ROS production in neutrophils by counterbalancing the oxidase induced cytosolic acidification and cell depolarization87,88. This compensatory effect has been shown to influence Ca2+ influx as well, since VSOP/Hv1−/− mPMNs exhibited a reduction in intracellular Ca2+ levels upon activation with PMA/fMIVIL (a pentapeptide from Listeria monocytogenes), leading to migration defects in these mice89.
Conclusion
Neutrophil recruitment to vascular sites of inflammation is a cascade-like molecular event that is initiated by selectin and chemokine bond formation superposed to activate the transition from cell rolling to integrin dependent arrest and transmigration into the parenchyma. Uninterrupted PMN recruitment is a robust and synchronous process that by nature’s design is faithfully reproduced a million-fold in healthy individuals during the innate immune response to inflammatory stimuli. Deletion or disruption of any single receptor-ligand interaction in this multistep process results in defective emigration and diminished accumulation of neutrophils to the site of injury or infection. Calcium signaling is an integral part of the integrin and chemokine signaling pathways that cooperate to synchronize the recruitment process. Although Orai1 expressed on circulating neutrophils is the predominant SOCE channel, it is clear that it cooperates in a dynamic sense with additional ion channels such as TRP, P2X, Potassium, and proton channels to maintain a balance in the electrochemical potential across the plasma membrane. As discussed, genetic deletion or pharmacological inhibition of individual channels disrupts a diverse set of functions from degranulation and ROS production to activation of β2-integrin mediated cell adhesion and migratory functions. Calcium influx via CRAC channels provide a means by which neutrophils initiate cell activation within the plane of adhesive contact, where focal clusters of integrin bonds form and trigger IP3-gated channels downstream of PLC to activate SOCE and synchronize the transition from cell rolling to arrest and shape polarization. Individuals with a point mutation in the Orai1 or STIM1 gene suffer from severe immune deficiencies with frequent infections due to insufficient immune cell recruitment to sites of inflammation as well as defects in hair, skin and sweat gland morphogenesis. Although, these observations point to an involvement of neutrophils in the development of diseases, PMNs of these patients show only moderate changes in intracellular Ca2+ levels. However, physiological shear stress is necessary to observe significant Ca2+ flux and with this integrin mediated outside-in signaling in neutrophils. Since the studies on neutrophil function in patients with Orai1 or STIM1 mutation were performed in the absence of shear, this, together with the assumed compensatory capacities of Orai and STIM isoforms might explain the discrepancy between preserved neutrophil and impaired immune function. Nevertheless, over the past 80 million years, human neutrophils have acquired even more ion channels than mouse neutrophils to regulate their activity. Key questions that remain are why neutrophils require so many ion channels and what are the detailed mechanisms by which neutrophils use their channels to carry out their relatively short-lived innate immune functions.
Acknowledgments
This work was supported by the Deutsche Forschungsgemeinschaft DFG – SFB914, project B1 and Z3 to MS. SIS is funded by the United States National Institute of Health under two NIAID grants; AI047924 and AI129302.
References
- 1.Kolaczkowska E, Kubes P. Neutrophil recruitment and function in health and inflammation. Nat Rev Immunol. 2013;13:159–175. doi: 10.1038/nri3399. [DOI] [PubMed] [Google Scholar]
- 2.Sperandio M, Gleissner Ca, Ley K. Glycosylation in immune cell trafficking. Immunol Rev. 2009;230:97–113. doi: 10.1111/j.1600-065X.2009.00795.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Morikis VA, et al. Selectin catch-bonds mechanotransduce integrin activation and neutrophil arrest on inflamed endothelium under shear flow. Blood. 2017;130:2101–2110. doi: 10.1182/blood-2017-05-783027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mueller H, et al. Tyrosine kinase Btk regulates E-selectin-mediated integrin activation and neutrophil recruitment by controlling phospholipase C (PLC) γ2 and PI3Kγ pathways. Blood. 2010;115:3118–3127. doi: 10.1182/blood-2009-11-254185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pruenster M, et al. Extracellular MRP8/14 is a regulator of β2 integrin-dependent neutrophil slow rolling and adhesion. Nat Commun. 2015;6:6915. doi: 10.1038/ncomms7915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Schmidt S, Moser M, Sperandio M. The molecular basis of leukocyte recruitment and its deficiencies. Mol Immunol. 2013;55:49–58. doi: 10.1016/j.molimm.2012.11.006. [DOI] [PubMed] [Google Scholar]
- 7.Begandt D, Thome S, Sperandio M, Walzog B. How neutrophils resist shear stress at blood vessel walls: molecular mechanisms, subcellular structures, and cell–cell interactions. J Leukoc Biol. 2017;102:699–709. doi: 10.1189/jlb.3MR0117-026RR. [DOI] [PubMed] [Google Scholar]
- 8.Vestweber D. How leukocytes cross the vascular endothelium. Nat Rev Immunol. 2015;15:692–704. doi: 10.1038/nri3908. [DOI] [PubMed] [Google Scholar]
- 9.Dixit N, Simon SI. Chemokines, selectins and intracellular calcium flux: Temporal and spatial cues for leukocyte arrest. Front Immunol. 2012;3:1–9. doi: 10.3389/fimmu.2012.00188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bagur R, Hajnóczky G. Intracellular Ca2+Sensing: Its Role in Calcium Homeostasis and Signaling. Mol Cell. 2017;66:780–788. doi: 10.1016/j.molcel.2017.05.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jiang H, et al. Roles of phospholipase C beta2 in chemoattractant-elicited responses. Proc Natl Acad Sci U S A. 1997;94:7971–5. doi: 10.1073/pnas.94.15.7971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kimberly RP, Ahlstrom JW, Click ME, Edberg JC. The glycosyl phosphatidylinositol-linked FcgammaRIII PMN mediates transmembrane signaling events distinct from FcgammaRII. J Exp Med. 1990;171:1239–1255. doi: 10.1084/jem.171.4.1239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Graham DB, et al. Neutrophil-mediated oxidative burst and host defense are controlled by a Vav-PLCγ2 signaling axis in mice. J Clin Invest. 2007;117:3445–3452. doi: 10.1172/JCI32729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jakus Z, Simon E, Frommhold D, Sperandio M, Mócsai A. Critical role of phospholipase Cγ2 in integrin and Fc receptor-mediated neutrophil functions and the effector phase of autoimmune arthritis. J Exp Med. 2009;206:577–593. doi: 10.1084/jem.20081859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Schaff UY, et al. Calcium flux in neutrophils synchronizes β2 integrin adhesive and signaling events that guide inflammatory recruitment. Ann Biomed Eng. 2008;36:632–646. doi: 10.1007/s10439-008-9453-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Schaff UY, et al. Orai1 regulates intracellular calcium, arrest, and shape polarization during neutrophil recruitment in shear flow. Blood. 2010;115:657–666. doi: 10.1182/blood-2009-05-224659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Futosi K, Fodor S, Mócsai A. Neutrophil cell surface receptors and their intracellular signal transduction pathways. Int Immunopharmacol. 2013;17:638–650. doi: 10.1016/j.intimp.2013.06.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Feske S, Wulff H, Skolnik EY. Ion channels in innate and adaptive immunity. Annu Rev Immunol. 2015;33:291–353. doi: 10.1146/annurev-immunol-032414-112212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bréchard S, Melchior C, Plançon S, Schenten V, Tschirhart EJ. Store-operated Ca2+ channels formed by TRPC1, TRPC6 and Orai1 and non-store-operated channels formed by TRPC3 are involved in the regulation of NADPH oxidase in HL-60 granulocytes. Cell Calcium. 2008;44:492–506. doi: 10.1016/j.ceca.2008.03.002. [DOI] [PubMed] [Google Scholar]
- 20.Zhang SL, et al. STIM1 is a Ca2+ sensor that activates CRAC channels and migrates from the Ca2+ store to the plasma membrane. Nature. 2005;437:902–905. doi: 10.1038/nature04147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chuang FY, Sassaroli M, Unkeless JC. Convergence of Fc gamma receptor IIA and Fc gamma receptor IIIB signaling pathways in human neutrophils. J Immunol. 2000;164:350–360. doi: 10.4049/jimmunol.164.1.350. [DOI] [PubMed] [Google Scholar]
- 22.Itagaki K, Hauser CJ. Sphingosine 1-phosphate, a diffusible calcium influx factor mediating store-operated calcium entry. J Biol Chem. 2003;278:27540–27547. doi: 10.1074/jbc.M301763200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lepannetier S, et al. Sphingosine-1-phosphate-activated TRPC1 channel controls chemotaxis of glioblastoma cells. Cell Calcium. 2016;60:373–383. doi: 10.1016/j.ceca.2016.09.002. [DOI] [PubMed] [Google Scholar]
- 24.Nunes P, Demaurex N. The role of calcium signaling in phagocytosis. J Leukoc Biol. 2010;88:57–68. doi: 10.1189/jlb.0110028. [DOI] [PubMed] [Google Scholar]
- 25.Kruskal BA, Shak S, Maxfield FR. Spreading of human neutrophils is immediately preceded by a large increase in cytoplasmic free calcium. Proc Natl Acad Sci U S A. 1986;83:2919–2923. doi: 10.1073/pnas.83.9.2919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Schwab A, Fabian A, Hanley PJ, Stock C. Role of Ion Channels and Transporters in Cell Migration. Physiol Rev. 2012;92:1865–1913. doi: 10.1152/physrev.00018.2011. [DOI] [PubMed] [Google Scholar]
- 27.Braun A, et al. STIM1 is essential for Fcy receptor activation and autoimmune inflammation. Blood. 2009;113:1097–1105. doi: 10.1182/blood-2008-05-158477. [DOI] [PubMed] [Google Scholar]
- 28.Nunes P, et al. STIM1 juxtaposes ER to phagosomes, generating Ca2+ hotspots that boost phagocytosis. Curr Biol. 2012;22:1990–1997. doi: 10.1016/j.cub.2012.08.049. [DOI] [PubMed] [Google Scholar]
- 29.Zhang H, et al. STIM1 calcium sensor is required for activation of the phagocyte oxidase during in fl ammation and host defense. Blood. 2014;123:2238–2250. doi: 10.1182/blood-2012-08-450403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sogkas G, et al. Orai1 controls C5a-induced neutrophil recruitment in inflammation. Eur J Immunol. 2015;45:2143–2153. doi: 10.1002/eji.201445337. [DOI] [PubMed] [Google Scholar]
- 31.Steinckwich N, Schenten V, Melchior C, Brechard S, Tschirhart EJ. An Essential Role of STIM1, Orai1, and S100A8-A9 Proteins for Ca2+ Signaling and Fc R-Mediated Phagosomal Oxidative Activity. J Immunol. 2011;186:2182–2191. doi: 10.4049/jimmunol.1001338. [DOI] [PubMed] [Google Scholar]
- 32.Demaurex N, Nunes P. The role of STIM and ORAI proteins in phagocytic immune cells. Am J Physiol - Cell Physiol. 2016;310:C496–C508. doi: 10.1152/ajpcell.00360.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Berna-Erro A, Woodard GE, Rosado JA. Orais and STIMs: Physiological mechanisms and disease. J Cell Mol Med. 2012;16:407–424. doi: 10.1111/j.1582-4934.2011.01395.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lacruz RS, Feske S. Diseases caused by mutations in ORAI1 and STIM1. Ann N Y Acad Sci. 2015;1356:45–79. doi: 10.1111/nyas.12938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Elling R, et al. Preserved effector functions of human ORAI1- and STIM1-deficient neutrophils. J Allergy Clin Immunol. 2016;137:1587–1591.e7. doi: 10.1016/j.jaci.2015.09.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Clemens RA, Chong J, Grimes D, Hu Y, Lowell CA. STIM1 and STIM2 cooperatively regulate mouse neutrophil store operated calcium entry and cytokine production. Blood. 2017;130:1565–1577. doi: 10.1182/blood-2016-11-751230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Demaurex N, Saul S. The role of STIM proteins in neutrophil functions. J Physiol. 2018;0:1–10. doi: 10.1113/JP275639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Diez-Bello R, Jardin I, Salido GM, Rosado JA. Orai1 and Orai2 mediate store-operated calcium entry that regulates HL60 cell migration and FAK phosphorylation. Biochim Biophys Acta - Mol Cell Res. 2017;1864:1064–1070. doi: 10.1016/j.bbamcr.2016.11.014. [DOI] [PubMed] [Google Scholar]
- 39.Rodrıguez-Ferandez JL, et al. LFA-1 integrin and the microtubular cytoskeleton are involved in the Ca 2+-mediated regulation of the activity of the tyrosine kinase PYK2 in T cells. J Leukoc Biol. 2002;71:520–530. [PubMed] [Google Scholar]
- 40.Schorr W, Swandulla D, Zeilhofer HU. Mechanisms of IL-8-induced Ca2+ signaling in human neutrophil granulocytes. Eur J Immunol. 1999;29:897–904. doi: 10.1002/(SICI)1521-4141(199903)29:03<897::AID-IMMU897>3.0.CO;2-5. [DOI] [PubMed] [Google Scholar]
- 41.Davies MP, Hallam TJ, Merritt JE. A role for calcium and protein kinase C in agonist-stimulated adhesion of human neutrophils. Biochem J. 1990;267:13–16. doi: 10.1042/bj2670013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Truneh A, Albert F, Golstein P, Schmitt-Verhulst AM. Calcium ionophore plus phorbol ester can substitute for antigen in the induction of cytolytic T lymphocytes from specifically primed precursors. J Immunol. 1985;135:2262–2267. [PubMed] [Google Scholar]
- 43.Lioudyno MI, et al. Orai1 and STIM1 move to the immunological synapse and are up-regulated during T cell activation. Proc Natl Acad Sci U S A. 2008;105:2011–2016. doi: 10.1073/pnas.0706122105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hartzell CA, Jankowska KI, Burkhardt JK, Lewis RS. Calcium influx through CRAC channels controls actin organization and dynamics at the immune synapse. Elife. 2016;5:1–28. doi: 10.7554/eLife.14850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Dixit N, et al. Leukocyte function antigen-1, Kindlin-3, and calcium flux orchestrate neutrophil recruitment during inflammation. J Immunol. 2012;189:5954–5964. doi: 10.4049/jimmunol.1201638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Green CE, et al. Dynamic shifts in LFA-1 affinity regulate neutrophil rolling, arrest, and transmigration on inflamed endothelium. Blood. 2006;107:2101–2111. doi: 10.1182/blood-2005-06-2303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Moser M, et al. Kindlin-3 is required for beta2 integrin-mediated leukocyte adhesion to endothelial cells. Nat Med. 2009;15:300–5. doi: 10.1038/nm.1921. [DOI] [PubMed] [Google Scholar]
- 48.Svensson L, et al. Leukocyte adhesion deficiency-III is caused by mutations in KINDLIN3 affecting integrin activation. Nat Med. 2009;15:306–312. doi: 10.1038/nm.1931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Moser M, Nieswandt B, Ussar S, Pozgajova M, Fässler R. Kindlin-3 is essential for integrin activation and platelet aggregation. Nat Med. 2008;14:325–330. doi: 10.1038/nm1722. [DOI] [PubMed] [Google Scholar]
- 50.Simon SI, Sarantos MR, Green CE, Schaff UY. Leucocyte recruitment under fluid shear: Mechanical and molecular regulation within the inflammatory synapse. Clin Exp Pharmacol Physiol. 2009;36:217–224. doi: 10.1111/j.1440-1681.2008.05083.x. [DOI] [PubMed] [Google Scholar]
- 51.Ye F, Kim C, Ginsberg MH. Molecular mechanism of inside-out integrin regulation. J Thromb Haemost. 2011;9:20–25. doi: 10.1111/j.1538-7836.2011.04355.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Rognoni E, Ruppert R, Fässler R. The kindlin family: functions, signaling properties and implications for human disease. J Cell Sci. 2016;129:17–27. doi: 10.1242/jcs.161190. [DOI] [PubMed] [Google Scholar]
- 53.Ruppert R, et al. Kindlin-3–mediated integrin adhesion is dispensable for quiescent but essential for activated hematopoietic stem cells. J Exp Med. 2015;212:1415–1432. doi: 10.1084/jem.20150269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Heiner I, et al. Expression profile of the transient receptor potential (TRP) family in neutrophil granulocytes: evidence for currents through long TRP channel 2 induced by ADP-ribose and NAD. Biochem J. 2003;371:1045–1053. doi: 10.1042/BJ20021975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Damann N, Owsianik G, Li S, Poll C, Nilius B. The calcium-conducting ion channel transient receptor potential canonical 6 is involved in macrophage inflammatory protein-2-induced migration of mouse neutrophils. Acta Physiol. 2009;195:3–11. doi: 10.1111/j.1748-1716.2008.01918.x. [DOI] [PubMed] [Google Scholar]
- 56.Parenti A, De Logu F, Geppetti P, Benemei S. What is the evidence for the role of TRP channels in inflammatory and immune cells? Br J Pharmacol. 2016;173:953–969. doi: 10.1111/bph.13392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Itagaki K, Kannan KB, Singh BB, Hauser CJ. Cytoskeletal reorganization internalizes multiple transient receptor potential channels and blocks calcium entry into human neutrophils. J Immunol. 2004;172:601–607. doi: 10.4049/jimmunol.172.1.601. [DOI] [PubMed] [Google Scholar]
- 58.McMeekin SR, Dransfield I, Rossi AG, Haslett C, Walker TR. E-selectin permits communication between PAF receptors and TRPC channels in human neutrophils. Blood. 2006;107:4938–4945. doi: 10.1182/blood-2005-09-3803. [DOI] [PubMed] [Google Scholar]
- 59.Lindemann O, et al. TRPC6 Regulates CXCR2-Mediated Chemotaxis of Murine Neutrophils. J Immunol. 2013;190:5496–5505. doi: 10.4049/jimmunol.1201502. [DOI] [PubMed] [Google Scholar]
- 60.Hofmann T, et al. Direct activation of human TRPC6 and TRPC3 channels by diacylglycerol. Nature. 1999;397:259–263. doi: 10.1038/16711. [DOI] [PubMed] [Google Scholar]
- 61.Lindemann O, et al. TRPC1 regulates fMLP-stimulated migration and chemotaxis of neutrophil granulocytes. Biochim Biophys Acta - Mol Cell Res. 2015;1853:2122–2130. doi: 10.1016/j.bbamcr.2014.12.037. [DOI] [PubMed] [Google Scholar]
- 62.Storch U, Forst AL, Philipp M, Gudermann T, Mederos Y Schnitzler M. Transient receptor potential channel 1 (TRPC1) reduces calcium permeability in heteromeric channel complexes. J Biol Chem. 2012;287:3530–3540. doi: 10.1074/jbc.M111.283218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Wang G, et al. Oxidant Sensing by TRPM2 Inhibits Neutrophil Migration and Mitigates Inflammation. Dev Cell. 2016;38:453–462. doi: 10.1016/j.devcel.2016.07.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Zierler S, Hampe S, Nadolni W. TRPM channels as potential therapeutic targets against pro-inflammatory diseases. Cell Calcium. 2017;67:105–115. doi: 10.1016/j.ceca.2017.05.002. [DOI] [PubMed] [Google Scholar]
- 65.Yamamoto S, et al. TRPM2-mediated Ca2+ influx induces chemokine production in monocytes that aggravates inflammatory neutrophil infiltration. Nat Med. 2008;14:738–747. doi: 10.1038/nm1758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Pantaler E, Lückhoff A. Inhibitors of TRP channels reveal stimulus-dependent differential activation of Ca2+ influx pathways in human neutrophil granulocytes. Naunyn Schmiedebergs Arch Pharmacol. 2009;380:497–507. doi: 10.1007/s00210-009-0464-2. [DOI] [PubMed] [Google Scholar]
- 67.Serafini N, et al. The TRPM4 Channel Controls Monocyte and Macrophage, but Not Neutrophil, Function for Survival in Sepsis. J Immunol. 2012;189:3689–3699. doi: 10.4049/jimmunol.1102969. [DOI] [PubMed] [Google Scholar]
- 68.Yin J, et al. Role of transient receptor potential vanilloid 4 in neutrophil activation and acute lung injury. Am J Respir Cell Mol Biol. 2016;54:370–383. doi: 10.1165/rcmb.2014-0225OC. [DOI] [PubMed] [Google Scholar]
- 69.Caterina MJ, et al. The capsaicin receptor: A heat-activated ion channel in the pain pathway. Nature. 1997;389:816–824. doi: 10.1038/39807. [DOI] [PubMed] [Google Scholar]
- 70.Krause KH, Welsh MJ. Voltage-dependent and Ca2+-activated ion channels in human neutrophils. J Clin Invest. 1990;85:491–498. doi: 10.1172/JCI114464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Henríquez C, et al. The calcium-activated potassium channel KCa3.1 plays a central role in the chemotactic response of mammalian neutrophils. Acta Physiol. 2016;216:132–145. doi: 10.1111/apha.12548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Fanger CM, et al. Calcium-activated potassium channels sustain calcium signaling in T lymphocytes. Selective blockers and manipulated channel expression levels. J Biol Chem. 2001;276:12249–12256. doi: 10.1074/jbc.M011342200. [DOI] [PubMed] [Google Scholar]
- 73.Kindzelskii AL, Petty HR. Ion channel clustering enhances weak electric field detection by neutrophils: Apparent roles of SKF96365-sensitive cation channels and myeloperoxidase trafficking in cellular responses. Eur Biophys J. 2005;35:1–26. doi: 10.1007/s00249-005-0001-2. [DOI] [PubMed] [Google Scholar]
- 74.Mohanty JG, Raible DG, McDermott LJ, Pelleg A, Schulman ES. Effects of purine and pyrimidine nucleotides on intracellular Ca2+in human eosinophils: Activation of purinergic P2Y receptors. J Allergy Clin Immunol. 2001;107:849–855. doi: 10.1067/mai.2001.114658. [DOI] [PubMed] [Google Scholar]
- 75.Chen Y, et al. ATP Release Chemotaxis A3 Receptors Neutrophil via P2Y2 and Guides. Science (80- ) 2006;314:1792–1795. doi: 10.1126/science.1132559. [DOI] [PubMed] [Google Scholar]
- 76.Lecut C, et al. P2X1 Ion Channels Promote Neutrophil Chemotaxis through Rho Kinase Activation. J Immunol. 2009;183:2801–2809. doi: 10.4049/jimmunol.0804007. [DOI] [PubMed] [Google Scholar]
- 77.Di Virgilio F, Dal Ben D, Sarti AC, Giuliani AL, Falzoni S. The P2X7 Receptor in Infection and Inflammation. Immunity. 2017;47:15–31. doi: 10.1016/j.immuni.2017.06.020. [DOI] [PubMed] [Google Scholar]
- 78.Maître B, et al. The P2X 1 Receptor Is Required for Neutrophil Extravasation during Lipopolysaccharide-Induced Lethal Endotoxemia in Mice. J Immunol. 2015;194:739–749. doi: 10.4049/jimmunol.1401786. [DOI] [PubMed] [Google Scholar]
- 79.Lecut C, et al. ATP-gated P2X 1 ion channels protect against endotoxemia by dampening neutrophil activation. J Thromb Haemost. 2012;10:453–465. doi: 10.1111/j.1538-7836.2011.04606.x. [DOI] [PubMed] [Google Scholar]
- 80.Wang X, et al. Endotoxin-induced autocrine ATP signaling inhibits neutrophil chemotaxis through enhancing myosin light chain phosphorylation. Proc Natl Acad Sci. 2017;114:4483–4488. doi: 10.1073/pnas.1616752114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Suh BC, Kim JS, Namgung U, Ha H, Kim KT. P2X7 Nucleotide Receptor Mediation of Membrane Pore Formation and Superoxide Generation in Human Promyelocytes and Neutrophils. J Immunol. 2001;166:6754–6763. doi: 10.4049/jimmunol.166.11.6754. [DOI] [PubMed] [Google Scholar]
- 82.Karmakar M, Katsnelson MA, Dubyak GR, Pearlman E. Neutrophil P2X7 receptors mediate NLRP3 inflammasome-dependent IL-1b secretion in response to ATP. Nat Commun. 2016;7:1–13. doi: 10.1038/ncomms10555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.March C, et al. Intravascular Danger Signals Guide Neutrophils to Sites of Sterile Inflammation. Science (80- ) 2011;330:362–367. doi: 10.1126/science.1195491. [DOI] [PubMed] [Google Scholar]
- 84.Karmakar M, et al. Neutrophil IL-1β Processing Induced by Pneumolysin Is Mediated by the NLRP3/ASC Inflammasome and Caspase-1 Activation and Is Dependent on K + Efflux. J Immunol. 2015;194:1763–1775. doi: 10.4049/jimmunol.1401624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Broz P, Dixit VM. Inflammasomes: mechanism of assembly, regulation and signalling. Nat Rev Immunol. 2016;16:407–420. doi: 10.1038/nri.2016.58. [DOI] [PubMed] [Google Scholar]
- 86.Okochi Y, et al. The voltage-gated proton channel Hv1/VSOP inhibits neutrophil granule release. J Leukoc Biol. 2016;99:7–19. doi: 10.1189/jlb.3HI0814-393R. [DOI] [PubMed] [Google Scholar]
- 87.Okochi Y, Sasaki M, Iwasaki H, Okamura Y. Voltage-gated proton channel is expressed on phagosomes. Biochem Biophys Res Commun. 2009;382:274–279. doi: 10.1016/j.bbrc.2009.03.036. [DOI] [PubMed] [Google Scholar]
- 88.Ramsey IS, Ruchti E, Kaczmarek JS, Clapham DE. Hv1 proton channels are required for high-level NADPH oxidase-dependent superoxide production during the phagocyte respiratory burst. Proc Natl Acad Sci. 2009;106:7642–7647. doi: 10.1073/pnas.0902761106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.El Chemaly A, et al. VSOP/Hv1 proton channels sustain calcium entry, neutrophil migration, and superoxide production by limiting cell depolarization and acidification. J Exp Med. 2010;207:129–139. doi: 10.1084/jem.20091837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Feske S. ORAI1 and STIM1 deficiency in human and mice: roles of store-operated Ca2+ entry in the immune system and beyond. Immunol Rev. 2009;231:189–209. doi: 10.1111/j.1600-065X.2009.00818.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Owsianik G, Talavera K, Voets T, Nilius B. Permeation and selectivity of TRP channels. Annu Rev Physiol. 2006;68:685–717. doi: 10.1146/annurev.physiol.68.040204.101406. [DOI] [PubMed] [Google Scholar]
- 92.Massullo P, Sumoza-Toledo A, Bhagat H, Partida-Sánchez S. TRPM channels, calcium and redox sensors during innate immune responses. Semin Cell Dev Biol. 2006;17:654–666. doi: 10.1016/j.semcdb.2006.11.006. [DOI] [PubMed] [Google Scholar]
- 93.Heiner I, Eisfeld J, Lückhoff A. Role and regulation of TRP channels in neutrophil granulocytes. Cell Calcium. 2003;33:533–540. doi: 10.1016/s0143-4160(03)00058-7. [DOI] [PubMed] [Google Scholar]