Abstract
Diet is a major route of phthalate exposure in humans due to use in food packaging materials. School lunches may be an important contributor to phthalate exposure in children and adolescents in the US because of the large amount of packaging necessary for mass-produced foods. We used 2003–2014 National Health and Nutrition Examination Survey data to study the association between school lunch consumption and urinary phthalate metabolite concentrations in children (ages 6–11 years, N=2,196) and adolescents (ages 12–19 years, N=2,314). After adjustment for other covariates, children who Always consumed school lunch had significantly elevated urinary concentrations of the following phthalate metabolites compared to levels in children who Never ate school lunch: sum of di(2-ethylhexyl) phthalate metabolites, (28% higher, 95% confidence interval, CI: 10, 49%); mono-(carboxy-octyl) phthalate (MCOP; 43% higher, 95% CI: 17, 76%) and mono-n-butyl phthalate (18% higher, 95% CI: 3.5, 34%). We did not find statistically significant associations in adolescents, but the trend for MCOP concentrations was similar to that of children. In sensitivity analyses, associations between 24-hour recall of cafeteria food and urinary phthalate metabolites were not statistically significant, which could indicate that associations observed with Always consuming school lunch are due to residual confounding. Our findings show that children who Always eat school lunch had higher levels of exposure to some phthalates, but the source of differences in exposure in that category of school lunch consumers need to be evaluated in additional studies.
Keywords: Phthalates, diet, cafeteria, exposure assessment, plasticizers, endocrine disruptors
1. INTRODUCTION
Phthalates are chemical compounds made from alcohols and phthalic anhydride, and are commonly used as plasticizers in a wide range of industrialized and consumer goods. Common applications of phthalates are personal care and household products, as well as food packaging materials.1 Evidence suggests that phthalates are endocrine disruptors, with effects on androgenic2 and thyroidal activity.3,4 Exposure during childhood has been associated with abnormal pubertal development,5–7 asthma and allergy,8,9 increased blood pressure,10,11 and perturbations in thyroid hormone levels.12 In addition, there is concern over phthalate exposure in childhood because of higher observed urinary metabolite concentrations in this age group compared to adults.13 The reasons for these differences are not fully understood.
Due to their common use in food processing and packaging materials,14 a major route of phthalate exposure to humans is ingestion.15–17 Phthalates typically found in food packaging materials are di-n-butyl phthalate (DnBP), di-isobutyl phthalate (DiBP), di-cyclohexyl phthalate (DCHP), di-2-ethylhexyl (DEHP), di-n-octyl phthalate (DnOP) and di-isononyl phthalate (DiNP).13,17 Phthalates can transfer from food packaging to food, which then presents an opportunity for their ingestion.14 Given that many US children and adolescents consume lunch prepared at school, this may be an important source of phthalate exposure. Previously, a study of school lunches in Italy found that a high proportion of school lunch foods tested contained DEHP and other phthalates.15 However, this study did not investigate the relationship between this exposure source and biomarkers of intake. Addressing this knowledge gap may inform exposure reduction strategies for these vulnerable age groups.
In this study, we explored the association between eating lunch prepared at schools and urinary phthalate metabolites in US children and adolescents. To address this question, we utilized data from the 2003–2014 sampling cycles of the National Health and Nutrition Examination Survey (NHANES) on children, ages 6–11 years, and adolescents, ages 12–19 years.
2. METHODS
2.1. Study Participants
NHANES is a nationally representative cross-sectional survey of the health and nutrition of the US population conducted by the National Center for Health Statistics (NCHS). The survey involves questionnaires and a physical examination performed in a Mobile Examination Center (MEC) where urine is collected for assessment of exposure to environmental agents on a subset of participants. For the present analysis we used data from the 2003–2014 sampling cycles. Participants age 6–19 years, who attended kindergarten through high school, with information on urinary phthalate metabolite concentrations, and who responded to the questionnaire on school lunch consumption were included in this analysis (total of 4,510 participants). Response rates for participation in the examination component within this age range were good (75–85%).18
2.2. School Lunch Consumption Assessment
School lunch consumption was identified from the questionnaire portion of the NHANES examination. Participants were asked, “During the school year, about how many times a week do you usually get a complete school lunch?” in the Diet Behavior and Nutrition section. This question was restricted to complete lunches provided by the school.19 Answers were provided as a range of values, 0–5 days per week.20 The question was answered directly by participants, or by proxies (e.g., parents or caregivers) for participants younger than 16 years who could not answer it themselves.21 For our analysis we categorized the lunch consumption based on the value reported as follows: 0 days was categorized as Never; 1–4 days was categorized as Sometimes; and 5 days was categorized as Always.
In addition, we performed a secondary analysis using 24-hour dietary recall data to assess associations between energy intake from the school cafeteria and urinary phthalate metabolites. The purpose of this secondary analysis was to use different questionnaire information to ask the same research question, in order assess the validity of our findings. Dietary recall data is collected at MECs by questionnaire, and children ages 6–11 are aided in their responses by adults.22 We used variables reflecting total energy intake (kcal) over the past 24 hours as well as total fat intake (% of total energy intake) over the past 24 hours, and also used the food source variable to identify percentages of those values that came from school cafeteria food.23 We calculated associations between urinary phthalate metabolites and total energy intake from cafeteria food (measured in % kcal from cafeteria food) as well as total energy intake from fat that was consumed in cafeteria food (measured in % kcal from fat in cafeteria food). Using the same approach as Zota et al. for examining associations between urinary phthalate metabolites and fast food consumption, we categorized these variables as Low (0% kcal from cafeteria food or from fat in cafeteria food), Medium (<median% kcal from cafeteria food or from fat in cafeteria food), and High ( median% kcal from cafeteria food or from fat in cafeteria food).23
2.3. Urinary Phthalate Metabolite Measurements
Spot urine samples were collected from the subjects in MECs at the same time as the questionnaire information was collected. Samples were stored at temperatures below −20 °C prior to analysis by high performance liquid chromatography-electrospray ionization-tandem mass spectrometry.24 The phthalate metabolites measured included: mono-(2-ethylhexyl) phthalate (MEHP), mono-(2-ethyl5-oxohexyl) phthalate (MEOHP), mono-(2-ethyl-5-hydroxyhexyl) phthalate (MEHHP), mono-(2-ethyl-5-carboxypentyl) phthalate (MECPP), mono-(carboxynonyl) phthalate (MCNP), mono-(carboxy-octyl) phthalate (MCOP), mono-benzyl phthalate (MBzP), mono-n-butyl phthalate (MnBP), mono-isobutyl phthalate (MiBP), and mono-ethyl phthalate (MEP). Results were expressed in ng/mL. Most of the phthalate metabolites of interest were measured in the sampling cycles (2003–2014); however, MCNP and MCOP were measured beginning in the 2005–2006 sampling cycle. The description of the phthalate metabolite analysis conducted by the laboratory is described in detail elsewhere.24 Phthalate metabolite concentrations below the limit of detection (LOD) were replaced with the LOD divided by the square root of 2.24
In addition to examining individual phthalate metabolites, we calculated the molar sum of DEHP metabolites (∑DEHP), expressed in nmol/mL, by the following formula in which each DEHP metabolite (ng/mL) is divided by the molarity of the chemical compound (mol/L) and then they are summed:
∑DEHP= (MEHP/278.34) + (MEOHP/292.33) + (MEHHP/294.34) + (MECPP/308.33)
This measure is used to obtain an estimate of total exposure to the parent phthalate, DEHP.25 To examine distributions of phthalate metabolites, we corrected for urinary dilution by dividing the phthalate concentration by the urinary creatinine concentration (µg/L) to obtain final units of µg/g creatinine (µmol/g creatinine for ∑DEHP).
2.4. Statistical Analysis
Analyses were conducted using R statistical software, version 3.4.0.26 Because NHANES uses a complex sampling design to create nationally representative data, we used the package “survey” (https://cran.r-project.org/web/packages/survey/survey.pdf) to correct for oversampling of certain populations and to make the results generalizable to the US population. We constructed a new sampling weight for each participant per the NHANES Analytic Guidelines.24
First, we assessed characteristics of the study population, including age, body mass index (BMI) z-score, gender, race/ethnicity, family poverty income ratio (PIR), and sampling cycle, by school lunch consumption categories. Age was categorized into two groups including children (ages 6–11 years) and adolescents (ages 12–19 years). Age cutoffs were based on what is typically used to analyze NHANES data.17 BMI z-score was calculated using World Health Organization (WHO) reference curves which incorporate information on BMI (kg/m2) as well as age and gender.27 BMI z-scores were then categorized as follows: <1, “normal and underweight”; 1–2, “overweight”; and >2, “obese”.27 Family PIR was categorized as follows: <1.3, below 130% of the federal poverty guidelines; 1.3–1.85, 130–185% of the federal poverty guidelines; and above 1.85, above 185% of the federal poverty guidelines. We categorized family PIR to reflect the eligibility of financial assistance based on the National School Lunch Program.28 Those below 130% federal poverty guidelines were eligible for free lunch; those within 130–185% were eligible for reduced priced lunch; and those above 185% received no assistance.28 The association between groups of population characteristics and school lunch consumption were tested using Pearson’s Χ2 test.
We then examined the distribution of the creatinine-corrected urinary phthalate metabolite concentrations by calculating the median for each age group, school lunch consumption category, and sampling cycle. One participant was excluded from the analysis because their creatinine concentration was missing, resulting in the final sample size of 4,509.
We used multivariable linear regression models to analyze the relationship between school lunch consumption and the uncorrected natural log transformed urinary phthalate metabolite concentrations. The multivariable linear regression models were created for children and adolescents separately because of a priori knowledge of differences in phthalate metabolite concentrations, metabolism, and excretion in these groups,29,30 and because of differences in frequency of school lunch consumption by age category in the present population. We modeled school lunch consumption categorically using Never as the reference group. Crude models included urinary creatinine (natural log-transformed, continuous) only as a covariate, and were restricted to individuals who had all information available on covariates included in adjustment models for better comparison.29
We selected a set of covariates for inclusion in our fully adjusted models based on known associations with phthalate exposure,13,29 associations with school lunch consumption in the current population, and adjustment factors from previous studies examining associations between phthalates and diet.23,31 These included: age (continuous), gender (categorical), race/ethnicity (categorical), family PIR (continuous), BMI z-score (continuous), and sampling cycle (categorical). Because some studies suggest that phthalate exposure could cause changes in BMI, this variable could act as a collider rather than a confounder in our analyses; thus, in a sensitivity analysis, we also examined models without adjusting for BMI.
For crude and adjusted results, effect estimates were presented as percent change in urinary phthalate metabolite concentration for Sometimes or Always school lunch consumption compared to Never. Finally, we ran the aforementioned models treating school lunch consumption as a continuous variable to test for trends in the associations between school lunch consumption and urinary phthalate metabolite concentrations. We considered a p value < 0.05 to be statistically significant.
Because examinations occurred over the calendar year and not the school year, we performed a sensitivity analysis in which we restricted models to subjects who participated in NHANES between November 1 and April 30. (The binary variable capturing 6 month period of participation was the closest approximation to the school year available in the NHANES dataset.32)
In our secondary analysis, we examined associations between energy intake categories and urinary phthalate metabolites using similar multivariable linear regression models as our school lunch analysis. We adjusted for the same covariates as before and similarly modeled the variables continuously in order to estimate p values for trends.
3. RESULTS
Weighted population characteristics by school lunch consumption categories are summarized in Table 1. Overall, more than half of participants ages 6–19 years Always ate school lunch (60%). Children were more likely to Always eat school lunch (67%) compared to adolescents (55%); obese participants were more likely to Always eat school lunch (66%) compared to those who were normal or underweight (58%); and non-White populations, including Mexican-Americans (74%) and non-Hispanic Blacks (71%), were more likely to Always eat school lunch compared to Whites (54%). Additionally, participants with a family PIR of <1.3 and 1.3–1.85 were more likely to Always eat school lunch (79% and 69%, respectively) compared to subjects with a higher family PIR (48%). Males were more likely to Always eat school lunch (65%) than females (56%). School lunch consumption was comparable across sampling cycles. The associations between population characteristics and school lunch consumption categories presented in Table 1 were all statistically significant. We additionally examined associations between race/ethnicity, family PIR, and BMI z-score category in a mutually adjusted model comparing Always and Never school lunch consumption. In this model Mexican-Americans, non-Hispanic Blacks, and those eligible for free or reduced lunch were still more likely to Always consume school lunch.
Table 1.
Distributions of participants by school lunch consumption categories and population characteristics in NHANES data 2003–2014 among 4510 participants 6–19 years of age: N (weighted %).
| School Lunch Consumption | ||||
|---|---|---|---|---|
| Overall | Never | Sometimes | Always | |
| Total population | 4510 | 744 (20) | 805 (19) | 2961 (60) |
| Age | ||||
| Children (6–11) | 2196 | 217 (13) | 334 (20) | 1645 (67) |
| Adolescents (12–19) | 2314 | 527 (27) | 471 (18) | 1316 (55) |
| Gender | ||||
| Male | 2268 | 330 (18) | 357 (17) | 1581 (65) |
| Female | 2242 | 414 (22) | 448 (22) | 1380 (56) |
| BMI z-scorea (nmiss=28) | ||||
| Normal & Underweight | 2410 | 414 (22) | 453 (21) | 1543 (58) |
| Overweight | 1032 | 177 (21) | 180 (16) | 675 (62) |
| Obese | 1040 | 147 (16) | 170 (18) | 723 (66) |
| Race/ethnicity | ||||
| Mexican American | 1236 | 151 (11) | 183 (15) | 902 (74) |
| Other Hispanic | 386 | 56 (18) | 65 (16) | 265 (66) |
| Non-Hispanic White | 1174 | 262 (25) | 245 (22) | 667 (54) |
| Non-Hispanic Black | 1338 | 186 (13) | 225 (16) | 927 (71) |
| Other/Multi-racial | 376 | 89 (23) | 87 (21) | 200 (56) |
| Family PIRb (nmiss=276) | ||||
| <1.3 | 1877 | 176 (10) | 211 (11) | 1490 (79) |
| 1.3–1.85 | 603 | 80 (14) | 104 (18) | 419 (69) |
| >1.85 | 1754 | 436 (27) | 448 (25) | 870 (48) |
| Sampling Cycle | ||||
| 2003–2004 | 860 | 168 (22) | 188 (21) | 504 (57) |
| 2005–2006 | 857 | 151 (22) | 157 (19) | 549 (59) |
| 2007–2008 | 676 | 98 (23) | 98 (17) | 480 (60) |
| 2009–2010 | 710 | 89 (16) | 112 (18) | 509 (66) |
| 2011–2012 | 674 | 100 (16) | 123 (22) | 451 (63) |
| 2013–2014 | 733 | 138 (23) | 127 (20) | 468 (57) |
BMI z-score categories: <1: Normal & Underweight; 1–2: Obese; >2: Obese.
Family PIR categories: <1.3: below 130%, 1.3–1.85: 130–185%, >1.85: above 185% federal poverty guidelines.
Abbreviations: NHANES, National Health and Nutrition Examination Survey; BMI, body mass index; PIR, poverty income ratio.
Abbreviations: BMI, body mass index; nmiss, n missing; PIR, poverty income ratio.
Median creatinine corrected urinary phthalate metabolite concentrations by age group (children vs. adolescents) for each NHANES sampling period are presented in Figure S1. Most urinary phthalate metabolite concentrations decreased over time for both children and adolescents, except for MCOP and MiBP concentrations, which increased over time.
Creatinine corrected urinary phthalate metabolite concentration distributions by age group and school lunch consumption categories are presented in Figure 1. Generally, urinary phthalate metabolite concentrations were higher in children compared to adolescents, except for MEP. Median concentrations of ∑DEHP, MCNP, MBzP, MnBP and MEP were higher among children who Always ate school lunch compared to Sometimes and Never. Median concentrations of MCOP, MBzP, MnBP and MiBP were higher among adolescents who Always ate school lunch compared to Sometimes and Never.
Figure 1.
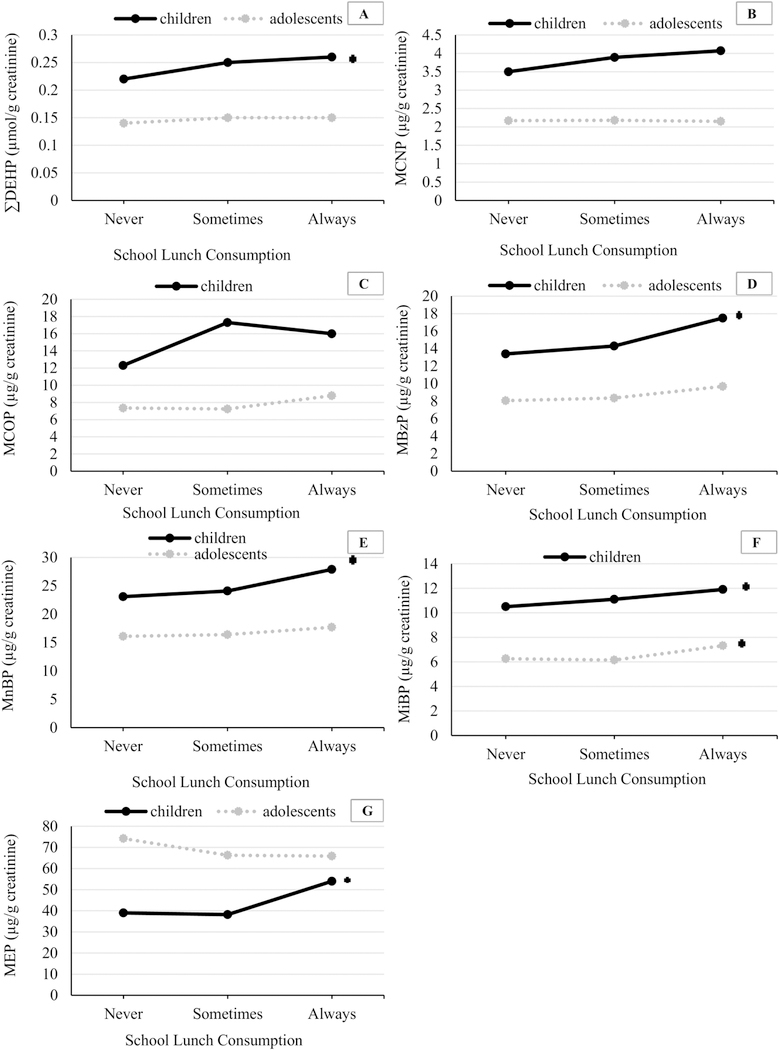
Median urinary phthalate metabolite concentrations (µg/g creatinine) in children (ages 6–11) and adolescents (ages 12–19) by school lunch consumption category.
Footnote: In children: n (Never) were: 184, for MCNP and MCOP, and 217 for the rest of the urinary metabolites; n (Sometimes) were: 278 for MCNP and MCOP, and 334 for the rest of the urinary metabolites; n (Always) were: 1407 for MCNP and MCOP, 1644 for the rest of the urinary metabolites. In adolescents: n (Never) were: 392 for MCNP and MCOP, and 527 for the rest of the urinary metabolites; n (Sometimes) were: 339 for MCNP and MCOP, and 471 for the rest of the urinary metabolites; n (Always) were: 1049 for MCNP and MCOP, and 1316 for the rest of the urinary metabolites. *significant p trend – Models adjusted for urinary creatinine (continuous, ln-transformed)
Urinary phthalate metabolite concentration associations with school lunch consumption categories in fully adjusted models are presented in Table 2. Among children, we found that for ∑DEHP, urinary phthalate metabolite concentrations were 28% higher (95% confidence interval [CI]: 10–49%) in children who Always ate school lunch compared to Never. Urinary concentrations of MCOP (43%, 95% CI: 17–76%) and MnBP (18%, 95% CI: 4–34%) were also significantly higher in the Always compared to Never group. When school lunch consumption categories were modeled continuously instead of categorically (i.e., to examine linear trend), we detected significant positive associations with each of these metabolites (p=0.01 for ∑DEHP; p<0.01 for MCOP; p=0.01 for MnBP). These associations were similar, but attenuated, compared to those observed in crude models where the population was restricted to participants with data on covariates (Table S1). Additionally, results from models without adjustment for BMI were similar to fully adjusted results (Table S2) and results were not sensitive to the method of creatinine adjustment (data not shown).
Table 2.
Adjusteda percent change in urinary phthalate metabolite concentrations for those who Sometimes or Always vs. Never eat school lunch in weighted NHANES data 2003–2014.
| School Lunch Consumption | ||||||
|---|---|---|---|---|---|---|
| Sometimes | Always | |||||
| Children (6–11) | n | %∆ | 95% CI | %∆ | 95% CI | p trend |
| ∑DEHP | 2055 | 11.1 | (−2.94, 27.2) | 28.2 | (10.0, 49.3) | 0.01 |
| MCNP | 1741 | 11.5 | (−10.0, 38.2) | 15.4 | (−4.16, 38.9) | 0.17 |
| MCOP | 1741 | 37.0 | (7.82, 74.2) | 43.4 | (16.8, 75.9) | <0.01 |
| MBzP | 2055 | −4.39 | (−24.9, 21.7) | 12.7 | (−7.40, 37.1) | 0.04 |
| MnBP | 2055 | −0.23 | (−14.7, 16.7) | 17.9 | (3.51, 34.2) | 0.01 |
| MiBP | 2055 | −0.33 | (−16.5, 18.9) | 10.1 | (−6.67, 29.9) | 0.18 |
| MEP | 2055 | −7.62 | (−24.7, 13.4) | 4.22 | (−12.1, 23.6) | 0.34 |
| Adolescents (12–19) | ||||||
| ∑DEHP | 2154 | 8.46 | (−6.28, 25.5) | 4.65 | (−8.91, 20.2) | 0.63 |
| MCNP | 1651 | 6.33 | (−11.6, 27.9) | 5.16 | (−9.39, 22.1) | 0.49 |
| MCOP | 1651 | 15.0 | (−8.20, 44.2) | 21.2 | (−3.69, 52.6) | 0.08 |
| MBzP | 2154 | 5.81 | (−7.92, 21.6) | 7.75 | (−8.36, 26.7) | 0.24 |
| MnBP | 2154 | 5.21 | (−6.54, 18.4) | 5.54 | (−6.24, 18.8) | 0.26 |
| MiBP | 2154 | 0.79 | (−13.1, 16.8) | 2.02 | (−9.51, 15.0) | 0.42 |
| MEP | 2154 | −8.13 | (−24.3, 11.5) | −1.91 | (−16.9, 15.8) | 0.82 |
Models adjusted for urinary creatinine (continuous, ln-transformed), age (continuous), gender (categorical), family PIR (continuous), race/ethnicity (categorical), BMI z-score (continuous) and sampling cycle (categorical).
Abbreviations: %∆, adjusted percent change compared to participants who reported Never consuming school lunch; CI, confidence interval.
No statistically significant associations were observed between school lunch consumption and urinary phthalate metabolite concentrations among adolescents in fully adjusted models. However, the trend for increasing urinary MCOP concentrations with Sometimes and Always school lunch consumption compared to Never was suggestive (p=0.08). Adolescents who Always ate school lunch had 21.2% higher urinary concentrations of MCOP (95% CI: −3.69– 52.6%) compared to those who Never ate school lunch. Models with interaction terms between school lunch consumption category (treated continuously) and age category (children vs. adolescents) confirmed that associations were greater in magnitude for children compared to adolescents for all phthalate metabolites, although interaction terms were not always statistically significant (p for interaction as follows: ∑DEHP=<0.01; MCNP=0.26; MCOP=0.20; MBzP=0.12; MnBP=0.03; MEP=0.04).
When restricting our analyses to participants who were examined between November 1 and April 30, we found the effect estimates to be larger but less precise than those observed in the primary analyses (results not shown). The lack of precision was likely due to the smaller sample sizes; 51% of children and adolescents participated during the November-April period.
Because our sample included data across a wide range of time, we wanted to assess whether any of the associations we observed differed across sampling cycle. Figure 2 shows median concentrations of creatinine-corrected urinary ∑DEHP, MCOP, MnBP, and MEP (for comparison) in Always and Never groups in children alone. The greatest relative differences in median concentrations between Always and Never groups for ∑DEHP were 33% in the most recent cycle, which was comparable to 31% in the first cycle. On the other hand, the relative differences between Always and Never groups in MCOP and MnBP median levels increased between the first cycle (MCOP, 17%, and MnBP, 0%) and the most recent cycle (MCOP, 55%, and MnBP, 33%). These differences were not statistically significant in models with an interaction term between school lunch consumption category and time; however, samples sizes by year may not have been sufficient for this comparison.
Figure 2.
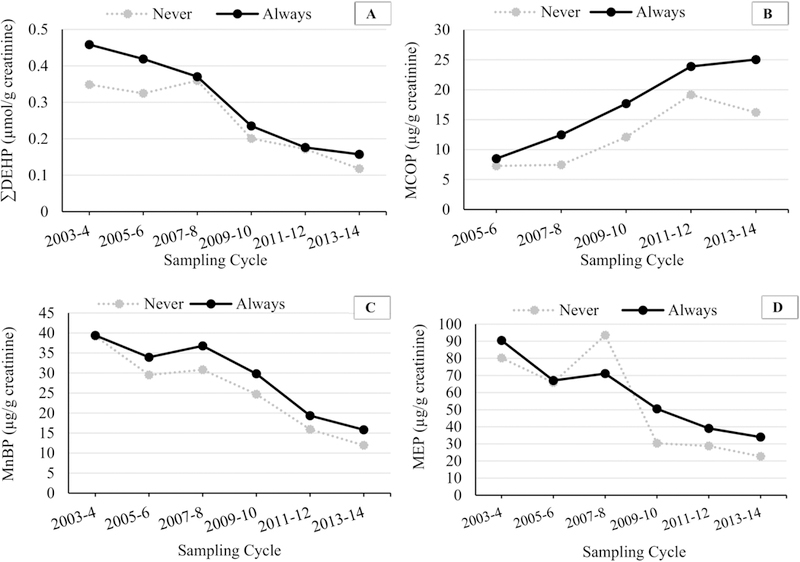
Median urinary phthalate metabolite concentrations (µg/g creatinine) in children (ages 6–11) by school lunch consumption category over time.
Footnote: MCOP was not measured in the 2003–2004 sampling cycle. n (Never) were: 33 (2003–4), 33 (2005–6), 25 (2007–8), 35 (2009–10), 37 (2011–12), 54 (2013–14). n (Always) were: 237 (2003–4), 259 (2005–6), 299 (2007–8), 305 (2009–10), 271 (2011–12), 273 (2013–14)
In our secondary analysis, we examined associations between energy intake from cafeteria food and urinary phthalate metabolites (Tables 3 and Table S3). Percent of total energy intake from cafeteria food in children was categorized as Low (0%, n=1366), Medium (1.5–35%, n=318), and High (35–100%, n=318). In this age group, increased urinary MCOP concentrations were observed in children in the High vs. Low category. For adolescents, categorizations were similar to children: Low (0%, n=1715); Medium (1.9–34%, n=293); and High (34–100%, n=221). Adolescents in the High vs. Low category had higher urinary concentrations of MCOP, MnBP, and MEP. Effect estimates were imprecise and confidence intervals always included the null, which could be attributed to small sample sizes in Medium and High energy intake categories.
Table 3.
Adjusteda percent change in urinary phthalate metabolite concentrations by energy intake categories.b
| Total energy intake from cafeteria food (% of TEI) | ||||||
|---|---|---|---|---|---|---|
| Medium (1.5–35%) | High (35–100%) | |||||
| Children | n | %∆ | 95% CI | %∆ | 95% CI | p trend |
| ∑DEHP | 1883 | −4.11 | (−15.8, 9.13) | 1.11 | (−12.7, 17.9) | 0.93 |
| MCOP | 1593 | −0.40 | (−13.7, 14.9) | 12.5 | (−6.96, 36.1) | 0.29 |
| Medium (1.9–34%) | High (34–100%) | |||||
| Adolescents | n | %∆ | 95% CI | %∆ | 95% CI | p trend |
| ∑DEHP | 2078 | −3.44 | (−14.5, 9.04) | −4.40 | (−24.0, 20.2) | 0.63 |
| MCOP | 1584 | 9.09 | (−11.0, 33.8) | 10.9 | (−10.1, 36.7) | 0.25 |
| Total fat intake from cafeteria food (% of TEI) | ||||||
| Medium (0.04–10%) | High (10–36%) | |||||
| Children | n | %∆ | 95% CI | %∆ | 95% CI | p trend |
| ∑DEHP | 1883 | −3.63 | (−16.6, 11.4) | 1.82 | (−11.9, 17.7) | 0.96 |
| MCOP | 1593 | 4.08 | (−11.2, 22.0) | 5.97 | (−9.05, 23.5) | 0.42 |
| Medium (0.03–10.5) | High (10.6–39.3) | |||||
| Adolescents | n | %∆ | 95% CI | %∆ | 95% CI | p trend |
| ∑DEHP | 2078 | −1.29 | (−14.6, 14.1) | −6.39 | (−22.9, 13.7) | 0.52 |
| MCOP | 1584 | 5.13 | (−13.8, 28.1) | 14.80 | (−10.7, 47.5) | 0.24 |
Models adjusted for urinary creatinine (continuous, ln-transformed), age (continuous), gender (categorical), family PIR (continuous), race/ethnicity (categorical), BMI z-score (continuous) and sampling cycle (categorical).
Reference categories are Low (0%) total energy intake or total energy intake from fat via cafeteria food, and Medium/High categories represent exposed individuals divided by the median percent.
Abbreviations: %∆, adjusted percent change; CI, confidence interval; TEI, total energy intake.
Associations between percent of total energy intake from fat that came through cafeteria food and urinary phthalate metabolites are presented in Table 3 and Table S4. Children in the High category (10–36% of total energy intake from fat through cafeteria food, n=317) had higher urinary concentrations of MCOP and MnBP compared to children in the Low category (0%, n=1367). Adolescents in the High category (10.6–39.3% of total energy intake from fat through cafeteria food, n=256) had higher urinary concentrations of MCOP, MnBP, and MEP compared to those in the Low category (0%, n=1716). However, these associations were also imprecise and none obtained statistical significance.
Since the findings differed via these two approaches, we calculated means and 95% confidence intervals for total energy intake from cafeteria food and for total energy intake from fat from cafeteria food within our school lunch consumption categories (Table S5). While patterns were as expected (i.e., higher percentages in children and adolescents who Sometimes or Always ate school lunch compared to Never), we did note that among those who reported Never eating school lunch, there was reporting of some energy intake and energy intake from fat from cafeteria food.
4. DISCUSSION
In this cross-sectional analysis of a generalizable US population, we found a significantly positive association between school lunch consumption and urinary phthalate metabolite concentration in children, but not in adolescents. Associations with 24-hour recall of caloric or fat intake from school cafeteria food, however, were null.
These conflicting findings may be interpreted in two ways. First, if we assume that 24-hour recall has greater validity than reporting of Never, Sometimes, or Always eating school lunch, then the associations observed with school lunch consumption categories may be due to residual confounding from exposure to other phthalate-containing dietary sources. Some data suggest that US children in a low income bracket who are enrolled in the school lunch program, and are thus most likely to Always eat school lunch, have poorer diets.33 This could include a greater likelihood of consuming fast foods or foods that are packaged and more likely to contain phthalates. Ideally, we could examine the association between urinary phthalate metabolites and school lunch in an analysis stratified by income level; however, this is impossible in the current dataset given that almost all individuals who are in the lowest PIR category Always eat school lunch (because it is provided to them free-of-charge). A study focusing on a lower income population and collecting more detailed dietary information would better serve to disentangle this issue.
A second explanation for the differences in these results may be that the 24-hour dietary recall variables are subject to greater measurement error than our variable assessing Never, Sometimes, or Always school lunch consumption. For children ages 6–11, parents assist in dietary recall since it can be difficult for children to remember what they ate; however, since parents are not present at school this recall may be particularly poor.34 For adolescents ages 12–19, recall may be better and indeed the trends for associations with urinary phthalate metabolites are more suggestive (though still statistically null) in the 24-hour recall analysis. However, there are also issues with reporting in this age group that may be dependent on BMI.35 Additionally, sample sizes were small for Medium and High exposure categories in our 24-hour recall analysis (~300 per exposed category), which could lead to reduced precision in our results.
Despite these incongruencies, our results identify children who Always consume school lunches as a group experiencing higher levels of exposure to some phthalates. Additional studies are necessary to identify whether this is truly due to dietary intake through school cafeteria food or due to other sources.
A recent study by Varshavsky et al. found that consuming food away from home in children and adolescents was associated with elevated urinary phthalate metabolite levels.36 Furthermore, they found that children and adolescents with Any energy intake from cafeteria food had significantly higher urinary concentrations compared to those with No energy intake from cafeteria or other food outside the home. Their analysis differed from ours in that they: 1) Had greater sample sizes in their exposed groups (~500 for children and ~400 for adolescents); 2) Utilized a ‘cleaner’ comparison group, restricted to individuals who never ate food away from home; and 3) found associations with an androgen-disruptor sum which was based on phthalate intake estimates and their anti-androgenic potency.36 The findings specific to cafeteria food intake are likely attributable to their reference group, which may explain why they observed significant associations with total energy intake from this source whereas we did not.
It has been well described that children in general may be particularly vulnerable to phthalates because of potential impacts on their development as well as higher exposure in this age group.16 Analysis of urinary phthalate metabolite concentrations over time in the nationally representative NHANES population shows that children consistently have higher concentrations of many phthalates compared to adolescents and adults.13 Previous research has pointed to diet as an important source of exposure in this age group, as it is in others, because of the use of phthalates in food packaging materials.14 Trasande et al. observed positive associations between ∑DEHP and caloric intake in a population of children and adolescents combined, and also noted associations with specific food groups.37 Watkins et al. reported that young children in Ohio who recently consumed food from fast food restaurants or that was stored in plastic containers had elevated urinary concentrations of MCOP.38 In other countries as well, researchers have reported associations between dietary patterns and urinary concentrations of phthalates in these age groups. In Sweden, for example, phthalates were associated with intake of fast food, cheese, and ice cream.39 School cafeteria lunches have not been examined previously as a potential dietary source of phthalate exposure in children or adolescents. A recent study in Italy examining phthalates in cooked school meals found increases in concentrations of DEHP and DnBP, the parent phthalate of MnBP, in cooked school meals before and after packaging.15 Our data suggest that school lunch consumption may be a contributor to elevated phthalate levels in young children as well. Additionally, the associations that were most precise and greatest in magnitude were with phthalate metabolites that have been found in food packaging materials or noted as linked to dietary exposures in the above studies (∑DEHP, MCOP, and MnBP). We also did not observe any associations between phthalates that are used more commonly in personal care products and not expected to be found in dietary sources (e.g., MEP16) in adjusted models.
The consequences of elevated urinary phthalate metabolite levels in childhood are not completely clear. DEHP has been studied most commonly, and, while several adverse health effects have been associated with childhood exposure, data are inconclusive. Studies analyzing urinary ∑DEHP concentrations in children found positive associations with reproductive health outcomes, such as reduced testosterone,5 early breast development,40 and later pubic hair development.6,40 Additionally, Weng et al. found a positive association between urinary ∑DEHP and thyroid hormone levels in boys ages 9–10.12 Data assessing the association between DiNP and is lacking, but animal models suggest toxic effects of this compound as well.41 In humans there is also some evidence for an association between asthma and MCOP in children.8,9
We noted some differences in associations by age of the participants as well as calendar year. With regard to age, we noted associations between school lunch consumption categories and urinary phthalate metabolites in children but not adolescents. We speculate that these differences may be due to increases in other dietary and non-dietary sources of exposure among adolescents. Total energy intake overall and from fat is higher among adolescents, and in our dataset a lower percentage of energy intake from cafeteria food (8.69% vs. 12.4%) was observed in adolescents compared to children. Thus, phthalate exposure through other sources could have obscured any associations.
We explored differences in associations by calendar year because phthalate exposure has changed dramatically over time in the US population,13 and types of phthalates used in food packaging has likely changed as well. We observed that ∑DEHP, MBzP, MnBP and MEP concentrations decreased from 2003 to 2014 for both children and adolescents; however, MCOP and MiBP concentrations have increased. This may be a result of replacement of DEHP in consumer products with other phthalate esters such as DiNP.17,42,43 Because of changes in phthalate exposure and use in products, we expected that associations between phthalate metabolites and school lunch consumption would change based on calendar year. We observed some differences in associations, although they were not statistically significant. Urinary concentrations of ∑DEHP in children who Always ate school lunch were elevated in the first survey cycle but were more similar to those who Never ate school lunch in the last survey cycle (Figure 2). On the other hand, MCOP concentrations were very similar in Always vs. Never school lunch consumers in the first cycle, but showed a greater contrast in the last survey cycle.
In addition to our primary research findings, we noted differences in demographic characteristics of children who Always eat school lunch. The National Lunch Assistance program provides free and reduced lunch to children who need financial assistance. In the 2003–2014 NHANES data, 79% of children and adolescents who are eligible for free lunch and 69% who are eligible for reduced lunch reported Always eating lunch provided by the school. We also observed that participants who are Mexican-American or non-Hispanic Black are more likely to Always eat school lunch. Thus, children and adolescents who are lower income or who are Mexican-American or non-Hispanic Black may be disproportionally exposed to higher phthalate concentrations due to their increased school lunch consumption. This may be an important environmental justice issue to address.
It is important to note that school lunches are an important source of nutrition for these age groups. School lunch represents a large portion of daily caloric intake in children particularly44 and a majority of school lunches meet standards for key nutrients.45 Thus, strategies for reducing use of phthalate-containing food packaging and handling materials in the preparation of school lunches should be considered, rather than avoiding eating school lunch.
We acknowledge there are several limitations in this analysis. We used conveniently available data to address a novel research question; however, the NHANES assessment of school lunch consumption was not ideal for this analysis. Because phthalate metabolites are metabolized and excreted rapidly,46 information on whether or not school lunch was consumed on the day (or the day before) the urine sampling would have been preferable. This may have been the case only for individuals who reported Always consuming school lunch in our analysis. Additionally, it was very difficult in this dataset to isolate the phthalate exposure originating from school lunch consumption and not from other dietary sources throughout the day. This could both have obscured some associations, if the proportion of phthalate exposure coming from school lunch was only moderate compared to that from other sources. Alternatively, it could have led to an upward bias in associations if only those individuals who consumed school lunch regularly were also consuming other foods (e.g., fast food) with phthalate contamination. We attempted to utilize 24-hour dietary recall data in order to better characterize the association between urinary phthalate metabolites and calories or fat consumed in the school cafeteria, but 24-hour questionnaires are highly subject to measurement error, especially in children whose parents are primarily providing responses. Thus, these results should be treated as preliminary findings that should be verified in a study with more accurate characterization of school lunch consumption and the ability to isolate that exposure source from others dietary and non-dietary.
Other limitations to our analysis exist as well. The questionnaire to assess school lunch consumption was administered at the time of the urine sample collection, but asked about school lunch consumption over the past year. This could have led to issues with recall. However, because we expect that the individual would be more likely to report consumption over the most recent week or weeks—which is the data most relevant to the present analysis—we would not expect this to have a major influence on our results. Furthermore, as NHANES is a biomonitoring study, all data were cross-sectional. Spot urine measurements of urinary phthalate metabolites have substantial variability and may not capture levels over longer periods of time. Finally, there may be residual confounding due to variation in dietary patterns by socioeconomic status, race, or time that we did not sufficiently account for with our covariate adjustment.
Despite the limitations, this study has major strengths. The NHANES study is representative of the US population making our results generalizable for the age range examined. Our sample size was also very large and diverse, which increased the power of our analysis and allowed us to analyze differences in associations over time and by demographic characteristics. Finally, we are the first, to our knowledge, to investigate the association between self-reported school lunch consumption and phthalate body burden in US children and adolescents.
5. CONCLUSIONS
Children ages 6–11 who reported Always eating school lunch had significantly elevated urinary concentrations of ∑DEHP, MCOP, and MnBP compared to those who Never ate school lunch. However, no associations were observed between energy intake or fat intake from cafeteria sources over the previous 24-hours and phthalate metabolites. Our findings highlight a subset of the population with higher phthalate exposure: children who Always eat school lunch. Additional research to verify school lunch as the source of this elevated exposure is warranted.
Supplementary Material
Highlights.
We examined urinary phthalate metabolites by school lunch consumption categories.
Children reporting Always eating school lunch had higher levels of some metabolites.
Calories consumed from school cafeteria food were not associated with metabolites.
No associations were observed between school lunch and phthalates in US adolescents.
Further investigation of elevated phthalate exposure in children who Always eat school lunch is required.
Acknowledgements
Funding support was provided by the Intramural Research Program of the National Institute of Environmental Health Sciences, National Institutes of Health.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Hauser R, Calafat AM. Phthalates and human health. Occupational and environmental medicine 2005;62(11):806–818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gray LE Jr., Wilson VS, Stoker T, et al. Adverse effects of environmental antiandrogens and androgens on reproductive development in mammals. International journal of andrology 2006;29(1):96–104. [DOI] [PubMed] [Google Scholar]
- 3.Huang HB, Kuo PL, Chang JW, Jaakkola JJ, Liao KW, Huang PC. Longitudinal assessment of prenatal phthalate exposure on serum and cord thyroid hormones homeostasis during pregnancy-Tainan birth cohort study (TBCS). Science of The Total Environment 2018;619:1058–1065. [DOI] [PubMed] [Google Scholar]
- 4.Huang PC, Kuo PL, Guo YL, Liao PC, Lee CC. Associations between urinary phthalate monoesters and thyroid hormones in pregnant women. Human reproduction 2007;22(10):2715–2722. [DOI] [PubMed] [Google Scholar]
- 5.Meeker JD, Ferguson KK. Urinary phthalate metabolites are associated with decreased serum testosterone in men, women, and children from NHANES 2011–2012. The Journal of clinical endocrinology and metabolism 2014;99(11):4346–4352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wolff MS, Teitelbaum SL, McGovern K, et al. Phthalate exposure and pubertal development in a longitudinal study of US girls. Human reproduction 2014;29(7):1558–1566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wen HJ, Chen CC, Wu MT, et al. Phthalate exposure and reproductive hormones and sex-hormone binding globulin before puberty - Phthalate contaminated-foodstuff episode in Taiwan. PloS one 2017;12(4):e0175536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hoppin JA, Jaramillo R, London SJ, et al. Phthalate exposure and allergy in the U.S. population: results from NHANES 2005–2006. Environmental health perspectives 2013;121(10):1129–1134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bertelsen RJ, Carlsen KC, Calafat AM, et al. Urinary biomarkers for phthalates associated with asthma in Norwegian children. Environmental health perspectives 2013;121(2):251–256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Trasande L, Attina TM. Association of exposure to di-2-ethylhexylphthalate replacements with increased blood pressure in children and adolescents. Hypertension 2015;66(2):301–308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Trasande L, Sathyanarayana S, Spanier AJ, Trachtman H, Attina TM, Urbina EM. Urinary phthalates are associated with higher blood pressure in childhood. The Journal of pediatrics 2013;163(3):747–753 e741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Weng TI, Chen MH, Lien GW, et al. Effects of Gender on the Association of Urinary Phthalate Metabolites with Thyroid Hormones in Children: A Prospective Cohort Study in Taiwan. International journal of environmental research and public health 2017;14(2). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zota AR, Calafat AM, Woodruff TJ. Temporal trends in phthalate exposures: findings from the National Health and Nutrition Examination Survey, 2001–2010. Environmental health perspectives 2014;122(3):235–241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Serrano SE, Braun J, Trasande L, Dills R, Sathyanarayana S. Phthalates and diet: a review of the food monitoring and epidemiology data. Environmental health : a global access science source 2014;13(1):43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cirillo T, Fasano E, Castaldi E, Montuori P, Amodio Cocchieri R. Children’s exposure to Di(2-ethylhexyl)phthalate and dibutylphthalate plasticizers from school meals. Journal of agricultural and food chemistry 2011;59(19):10532–10538. [DOI] [PubMed] [Google Scholar]
- 16.U.S. Environmental Protection Agency. America’s Children and the Environment, Third Edition. Washington, D.C. 2013. [Google Scholar]
- 17.Department of Health and Human Services. Fourth National Report on Human Exposure to Environmental Chemicals, 2009. Atlanta, GA: Centers for Disease Control and Prevention [Google Scholar]
- 18.Centers for Disease Control and Prevention. NHANES Response Rates and Population Totals 2009; https://wwwn.cdc.gov/nchs/nhanes/ResponseRates.aspx. Accessed 07/20/2018.
- 19.Centers for Disease Control and Prevention. Diet Behavior and Nutrition - DBQ 2013; https://wwwn.cdc.gov/nchs/data/nhanes/2013-2014/questionnaires/DBQ_H.pdf Accessed 09/03/2018.
- 20.Centers for Disease Control and Prevention. National Health and Nutrition Examination Survey 2013–2014 Data Documentation, Codebook, and Frequencies Diet Behavior & Nutrition 2015a; https://wwn.cdc.gov/Nchs/Nhanes/2013-2014/DBQ_H.htm. Accessed 09/03/2018.
- 21.Centers for Disease Control and Prevention. National Health and Nutrition Examination Survey 2013–2014 Data Documentation, Codebook, and Frequencies Diet Behavior & Nutrition 2015b; https://wwn.cdc.gov/Nchs/Nhanes/2013-2014/DBQ_H.htm. Accessed 09/03/2018.
- 22.Centers for Disease Control and Prevention. NHANES MEC Interviewers Procedure Manual 2009; https://www.cdc.gov/nchs/data/nhanes/nhanes_09_10/mecinterviewers.pdf. Accessed 02/24/18.
- 23.Zota AR, Phillips CA, Mitro SD. Recent fast food consumption and bisphenol A and phthalates exposures among the US population in NHANES, 2003–2010. Environmental health perspectives 2016;124(10):1521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Centers for Disease Control and Prevention. Laboratory Procedure Manual:Phthalate Metabolites 2010; https://wwwn.cdc.gov/nchs/data/nhanes/2007-2008/labmethods/phthte_e_met_phthalate_metabolites.pdf. Accessed 09/03/2018.
- 25.Barr DB, Silva MJ, Kato K, et al. Assessing Human Exposure to Phthalates Using Monoesters and Their Oxidized Metabolites as Biomarkers. Environmental health perspectives 2003;111(9):1148–1151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.R Core Team. R: A language and environment for statistical computing Vienna, Austria: R Foundation for Statistical Computing; 2017. [Google Scholar]
- 27.World Health Organization. Growth reference 5–19 years. BMI-for-age (5–19 years) 2017; http://www.who.int/growthref/en/. Accessed 07/28/17.
- 28.Renwick T, Fox L. The Supplemental Poverty Measure: 2015 2016; https://www.census.gov/content/dam/Census/library/publications/2016/demo/p60-258.pdf. Accessed 07/28/17.
- 29.Silva MJ, Barr DB, Reidy JA, et al. Urinary levels of seven phthalate metabolites in the US population from the National Health and Nutrition Examination Survey (NHANES) 1999–2000. Environmental health perspectives 2004;112(3):331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wittassek M, Angerer J. Phthalates: metabolism and exposure. International journal of andrology 2008;31(2):131–138. [DOI] [PubMed] [Google Scholar]
- 31.Colacino JA, Harris TR, Schecter A. Dietary intake is associated with phthalate body burden in a nationally representative sample. Environmental health perspectives 2010;118(7):998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Centers for Disease Control and Prevention. National Health and Nutrition Examination Surve 2013–2014 Data Documentation, Codebook, and Frequencies Demographic Variables and Sample Weights 2015; https://wwwn.cdc.gov/Nchs/Nhanes/2013-2014/DEMO_H.htm. Accessed 09/03/18.
- 33.Gu X, Tucker KL. Dietary quality of the US child and adolescent population: trends from 1999 to 2012 and associations with the use of federal nutrition assistance programs. Am J Clin Nutr 2017;105(1):194–202. [DOI] [PubMed] [Google Scholar]
- 34.Livingstone MB, Robson PJ, Wallace JM. Issues in dietary intake assessment of children and adolescents. Br J Nutr 2004;92 Suppl 2:S213–222. [DOI] [PubMed] [Google Scholar]
- 35.Skinner AC, Steiner MJ, Perrin EM. Self-reported energy intake by age in overweight and healthy-weight children in NHANES, 2001–2008. Pediatrics 2012;130(4):e936–942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Varshavsky JR, Morello-Frosch R, Woodruff TJ, Zota AR. Dietary sources of cumulative phthalates exposure among the US general population in NHANES 2005–2014. Environment International 2018; 115:417–429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Trasande L, Sathyanarayana S, Jo Messito M, R SG, Attina TM, Mendelsohn AL. Phthalates and the diets of U.S. children and adolescents. Environ Res 2013;126:84–90. [DOI] [PubMed] [Google Scholar]
- 38.Watkins DJ, Eliot M, Sathyanarayana S, et al. Variability and predictors of urinary concentrations of phthalate metabolites during early childhood. Environ Sci Technol 2014;48(15):8881–8890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Larsson K, Ljung Bjorklund K, Palm B, et al. Exposure determinants of phthalates, parabens, bisphenol A and triclosan in Swedish mothers and their children. Environ Int 2014;73:323–333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Shi H, Cao Y, Shen Q, Zhao Y, Zhang Z, Zhang Y. Association Between Urinary Phthalates and Pubertal Timing in Chinese Adolescents. Journal of epidemiology 2015;25(9):574–582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.National Toxicology Program. NTP-CERHR Monograph on the Potential Human Reproductive and Developmental Effects of Di-isononyl Phthalate (DINP) 2003; https://ntp.niehs.nih.gov/ntp/ohat/phthalates/dinp/dinp_monograph_final.pdf. Accessed 09/03/18. [PubMed]
- 42.U.S Environmental Protection Agency. Phthalates Action Plan 2012; https://www.epa.gov/sites/production/files/2015-09/documents/phthalates_actionplan_revised_2012-03-14.pdf. Accessed 09/03/18.
- 43.Rodgers KM, Rudel RA, Just AC. Phthalates in food packaging, consumer products, and indoor environments. Toxicants in Food Packaging and Household Plastics Springer, London, 2014. 31–59. [Google Scholar]
- 44.Story M The third School Nutrition Dietary Assessment Study: findings and policy implications for improving the health of US children. Journal of the American Dietetic Association 2009;109(2 Suppl):S7–13. [DOI] [PubMed] [Google Scholar]
- 45.Centers for Disease Control and Prevention. Results from the School Health Policies and Practices Study 2014 2015; https://www.cdc.gov/healthyyouth/data/shpps/pdf/shpps-508-final_101315.pdf. Accessed 09/03/18.
- 46.Preau JL Jr., Wong LY, Silva MJ, Needham LL, Calafat AM. Variability over 1 week in the urinary concentrations of metabolites of diethyl phthalate and di(2-ethylhexyl) phthalate among eight adults: an observational study. Environmental health perspectives 2010;118(12):1748–1754. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


