Abstract
Background
Red blood cell (RBC) transfusion decreases intermittent hypoxemia (IH) events beyond the first week of life. This benefit may be related to improved perfusion to the respiratory control network. Perfusion index (PI) is a perfusion measure provided by the pulse oximeter. We hypothesized that the benefit in IH after RBC transfusion is associated with a rise in PI. In addition, we assessed the value of PI and clinical measures in predicting the effect of RBC transfusion on IH.
Study Design and Methods
We prospectively enrolled infants less than 30 weeks gestational age. PI and oxygen saturation (SpO2) were monitored with high-resolution pulse oximeters 24 hours pre and post RBC transfusion. Data was analyzed at three postnatal periods, epoch 1: first week of life (1 to 7 days of life), epoch 2: 2 to 4 weeks of life (8 to 28 days of life), and epoch 3: 4 to 8 weeks of life.
Results
One hundred eighteen transfusions were analyzed. IH measures significantly decreased post transfusion in epochs 2 and 3. PI significantly increased after transfusion, but it did not correlate with the decrease in IH measures. Mechanical ventilation, fraction of inspired oxygen (FiO2), and IH measures influenced the effects on oxygenation.
Conclusions
RBC transfusion improved IH after the first week of life. The benefit in IH did not correlate with PI increase after transfusion. Pre transfusion respiratory support and IH measures predicted the effect of transfusion on oxygenation.
Keywords: red blood cell transfusion, preterm infants, perfusion, hypoxemia
INTRODUCTION
Intermittent Hypoxemia (IH), defined as episodic drops in oxygen saturation, is common in preterm infants.1–3 The incidence of IH in extremely low gestational age infants changes during the first 2 months of life.1,2 There is low IH frequency during the first week of life, followed by a progressive increase over weeks 2-3, plateaus around 4 weeks, and decreases at weeks 6-8.1,2 Intermittent hypoxemia is associated with both short and long term morbidities such as retinopathy of prematurity,2 neurodevelopmental impairment, and late death.1,3–5 Red blood cell (RBC) transfusion results in IH improvement, particularly beyond the first week of life.1 Perhaps, the main rationale for RBC transfusion in preterm infants is improvement in oxygenation.6 There are two proposed mechanisms for beneficial effect of RBC transfusion on oxygenation. The first relates to greater cardiovascular stability with increased perfusion to the respiratory control network leading to improved central respiratory drive and subsequent less IH.1,7–9 The second suggests greater stability of oxygenation due to a rise in hematocrit leading to less IH in the presence of apnea.1,10
Perfusion index (PI) is a noninvasive measure of perfusion provided by the bedside pulse oximeter. Perfusion index is calculated from the ratio of the pulsatile to non-pulsatile signal at the monitoring site.11,12 Perfusion index correlates with superior vena cava flow,13 detects critical left heart obstructive disease,14 and patent ductus arteriosus.15,16 Furthermore, Kanmaz et al. noted that RBC transfusion is associated with a significant increase in PI and suggested that PI may be a useful marker for the need of transfusion.17 Therefore, we wanted to assess if the benefit in IH seen after RBC transfusion is associated with a rise in PI in preterm infants at different postnatal ages. In addition, we assessed the predictive value of PI, hematocrit, mechanical ventilation, fraction of inspired oxygen (FiO2) and IH; in order to identify infants who will benefit the most from the RBC transfusion in terms of oxygenation.
MATERIALS AND METHODS
Study Design and Data Collection
This was a prospective cohort study conducted at the University of Kentucky Medical Center Neonatal Intensive Care Unit between November 2014 and October 2015. The study was approved by the University of Kentucky Institutional Review Board. Infants with gestational age (GA) less than 30 weeks were approached in the first week of life and informed consent was obtained from parent(s). Infants were then followed and oxygen saturation was continuously monitored in the first 2 months of life. Infants who received RBC transfusion per the NICU transfusion guidelines were included in the analyses. The following is a summary of the local NICU transfusion guidelines: Hematocrit threshold of <35% for mechanically ventilated neonates or FiO2 requirement >40%, hematocrit <28% for infants on non-invasive respiratory support or FiO2 requirement <40%, and hematocrit <22% for neonates on no respiratory support. RBC transfusion at 15ml/kg was administered over a 3 hour period. Oxygen saturation (SpO2) and PI were monitored using continuous high-resolution (2s averaging time and 1Hz sampling rate) pulse oximeters (Radical 7: Masimo, Irvine, CA, USA). The target oxygen saturation in our unit is 90-95%. Patients were continuously monitored for the first 8 weeks of life and data was stored on serial data recorders. Novel programs were utilized to filter (Matlab, Natick, MA, USA) and analyze (SAS Institute, Cary, NC, USA) data. Variables related to demographics, weight, respiratory measures and medications were collected.
The primary outcome measures for IH were defined as 1) a drop in SpO2 to less than 80% for ≥4s and ≤3min duration (IH-SpO2<80) and 2) overall percent time spent with SpO2 <80% (%time-SpO2<80). The lower limit of 4s duration was based on the previous data by Abu Jawdeh et al. and the upper limit of 3 min duration was used to differentiate intermittent from sustained hypoxemia.1 Other outcome measures included additional SpO2 thresholds of 85% and 90%.
A RBC transfusion was eligible for analysis if no other RBC transfusion was administered 24 hours pre or post transfusion. We then analyzed changes in IH frequency (IH-SpO2<80, IH-SpO2<85, IH-SpO2<90), percent time spent below threshold (%time-SpO2<80, %time-SpO2<85, %time-SpO2<90), mean PI, and variability of PI during the 24 hours pre and post RBC transfusion. Additionally, we determined the associated changes in hematocrit and respiratory characteristics.
To account for the effect of postnatal age on IH following RBC transfusion2, the 8-week monitoring period was stratified into three epochs and analyzed separately; epoch 1: first week of life (1 to 7 days of life), epoch 2: 2 to 4 weeks of life (8 to 28 days of life), and epoch 3: 4 to 8 weeks of life.1 In order to assess which preterm infants benefit the most from RBC transfusion, we evaluated the predictive value of the following pre RBC transfusion variables: PI, hematocrit, mechanical ventilation, FiO2 requirement, and IH primary measures.
Statistical Analysis
To compare epochs in Table 1, continuous variables were presented as mean ± standard deviation (SD) and categorical variables were expressed as frequencies and percentages. Sample means and SDs were also utilized in Figures 2 and 3 to visually compare pre and post RBC transfusion values for each epoch. Pearson’s correlations were used to quantify associations between changes in different variables. To account for statistical correlation arising from repeated measurements, i.e. multiple observations per subject, generalized estimating equations with robust standard errors were utilized for inference. Finally, linear mixed models with robust standard errors were utilized to obtain results for Table 3, in which change in IH measures (IH-SpO2<80 or %time-SpO2<80) after RBC transfusion was the outcome of interest. The primary predictors were pre RBC transfusion mechanical ventilation, FiO2 requirement, and pre RBC transfusion IH measures. The models also controlled for pre RBC transfusion PI and hematocrit. All tests were two-sided at the 5% significance level. Analyses were conducted in SAS version 9.4 (SAS Institute, Cary, NC, USA).
TABLE 1.
Baseline characteristics of enrolled patients among epochs
| Epoch 1 n=22 |
Epoch 2 n=63 |
Epoch 3 n=33 |
p value | |
|---|---|---|---|---|
| Gestational age in weeks (n) (Mean ± SD) | (22) 25.8 ± 1.3 | (62) 25.6 ± 1.3 | (33) 25.6 ± 1.2 | 0.8 |
| Birth weight in grams (n) (Mean ± SD) | (22) 807 ± 162 | (62) 796 ± 171 | (33) 803 ± 179 | 0.94 |
| Postnatal age in days (n) (Mean ± SD) | (22) 4.6 ± 1.6 | (62) 18.0 ± 6.4 | (33) 43.5 ± 8.9 | <0.001 |
| Weight day of transfusion in grams (n) (Mean ± SD) | (22) 808 ± 153 | (62) 982 ± 247 | (33) 1475 ± 434 | <0.001 |
| Number of Transfusions Per Patient, Median (Interquartile Range) | (22) 2 (1-2) | (63) 5 (3-7) | (33) 8 (6-10) | <0.001 |
| Male, n (%) | 15/22 (68%) | 36/62 (58%) | 21/33 (64%) | 0.7 |
| Caucasian, n (%) | 17/22 (77%) | 53/61 (87%) | 27/33 (82%) | 0.7 |
| Respiratory Support | ||||
| Conventional ventilator, n (%) | 18 (86%) | 50 (86%) | 21 (68%) | 0.22 |
| Non-invasive ventilation, n (%) | 3 (14%) | 8 (14%) | 10 (32%) | |
| NIPPV, n (%) | 2 (10%) | 6 (10%) | 7 (23%) | |
| CPAP, n (%) | 1 (5%) | 2 (3%) | 3 (10%) | |
| Missing data for type of respiratory support, n (%) | 1 (5%) | 5 (8%) | 2 (6%) | |
| Supplemental oxygen, n (%) | 19/21 (90%) | 54/58 (93%) | 29/31 (94%) | 0.92 |
| Pre RBC transfusion FiO2 (n) (Mean ± SD) | (21) 30.0 ± 9.3 | (59) 42.7 ± 21.0 | (31) 44.8 ± 20.8 | <0.001 |
| Caffeine, n (%) | 21/22 (95%) | 62/62 (100%) | 31/33 (94%) | 0.55 |
SD, standard deviation;
NIPPV, nasal intermittent positive pressure ventilation;
CPAP, continuous positive airway pressure; p for mean difference;
FIGURE 2.
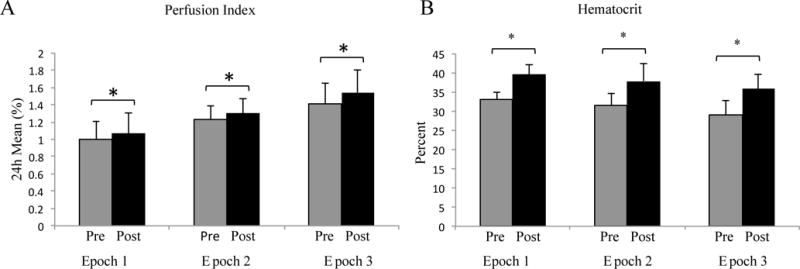
Mean PI and Hematocrit levels for all the 3 epochs pre and post RBC transfusion. There was a statistically significant increase in the PI (A) and hematocrit (B) after RBC transfusion in all the three epochs (*p<0.05). Mean/standard deviation
FIGURE 3.
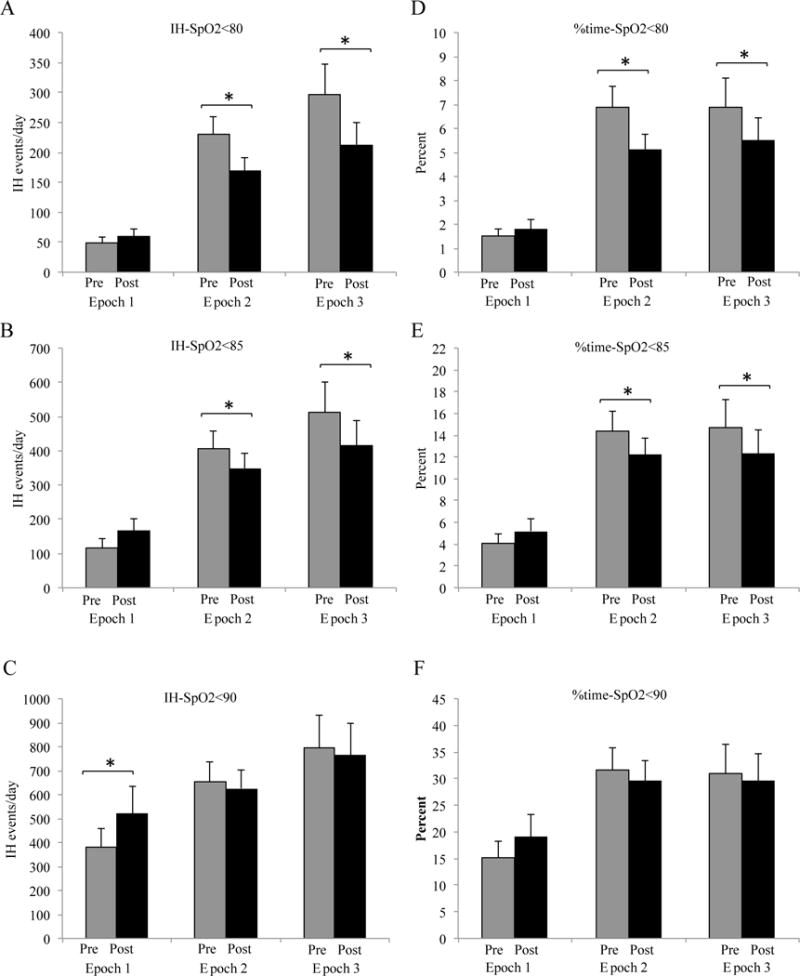
IH events/day and % time below threshold pre and post transfusion. 3A-C: IH-SpO2<80 and IH-SpO2<85 decreased in epochs 2 and 3 (*p<0.04) while IH-SpO2<90 increased in epoch 1 (*p=0.04). 3D-F: % time-SpO2<80 and % time-SpO2<85 decreased in epochs 2 and 3 (*p<0.04). There was a decrease in % time-SpO2<90 in epochs 2 (p=0.2) and 3 (p=0.3) and increase in epoch 1 (p=0.07). Mean/standard deviation
TABLE 3.
Predictors of the effect of RBC transfusions on IH.
| Predictor | Outcome Measure | Epoch 1 | Epoch 2 | Epoch 3 | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Coefficient | (95% CI) | p | Coefficient | 95% CI | p | Coefficient | 95% CI | p | |||||
| Perfusion Index | IH-SpO2<80 | −26.9 | −74.6 | 20.7 | 0.22 | −14.8 | −103.6 | 74.1 | 0.73 | 99.4 | −75 | 273.7 | 0.20 |
| %time-SpO2<80 | −1.20 | −3.00 | 0.58 | 0.16 | −0.50 | −2.96 | 1.99 | 0.69 | 1.86 | −2.95 | 6.67 | 0.40 | |
| Hematocrit | IH-SpO2<80 | −0.33 | −9.1 | 8.4 | 0.93 | 12.7 | −2.5 | 27.9 | 0.10 | −5.56 | −14.39 | 3.27 | 0.20 |
| %time-SpO2<80 | −0.03 | −0.15 | 0.09 | 0.60 | 0.40 | 0.12 | 0.72 | 0.01 | −0.14 | −0.49 | 0.20 | 0.40 | |
| Mechanical ventilation | IH-SpO2<80 | 35 | −4.4 | 74.5 | 0.07 | −60.8 | 140 | 18.5 | 0.13 | −84.1 | −173.7 | 5.4 | 0.06 |
| %time-SpO2<80 | 2.40 | 0.46 | 4.48 | 0.02 | −2.30 | −4.69 | −0.03 | 0.050 | −2.30 | −5.52 | 0.73 | 0.10 | |
| FiO2 | IH-SpO2<80 | −1.52 | −3.01 | −0.03 | 0.047 | −0.82 | −1.7 | 0.05 | 0.06 | 2.01 | −0.23 | 4.24 | 0.07 |
| %time-SpO2<80 | −0.10 | −0.14 | −0.04 | 0.002 | −0.04 | −0.08 | 0.00 | 0.07 | 0.07 | −0.01 | 0.15 | 0.09 | |
| IH-SpO2<80 | IH-SpO2<80 | −0.49 | −1.1 | 0.12 | 0.10 | −0.38 | −0.8 | 0.04 | 0.07 | −0.24 | −0.49 | 0.003 | 0.053 |
| %time-SpO2<80 | %time-SpO2<80 | 0.55 | −0.19 | 1.29 | 0.13 | −0.29 | −0.52 | −0.06 | 0.02 | −0.28 | −0.65 | 0.08 | 0.10 |
RESULTS
Fifty preterm infants met criteria for enrollment. Thirty-nine infants received RBC transfusions that were eligible for analysis for a total of 118 transfusions (22, 63 and 33 RBC transfusions in epochs 1, 2, and 3, respectively). The median (IQR) of eligible transfusions were as follows: 2(1-2), 5(3-7) and 8(6-10) for epochs 1, 2, and 3, respectively (Table 1). Figure 1 shows the flow diagram for patient enrollment, transfusion eligibility, and number of infants who received transfusions during each epoch. There were no significant differences in GA, birth weight, gender, and race across all 3 epochs (Table 1). The majority of infants required respiratory support, supplemental oxygen and caffeine therapy (Table 1). The FiO2 requirement (mean ± standard deviation) increased to 35.2% ±11.6 (p=0.1), 43.7% ± 19.3 (p=0.9) and 47.8% ± 24.7 (p=0.3) in epochs 1, 2 and 3 respectively but was not statistically significant.
FIGURE 1.
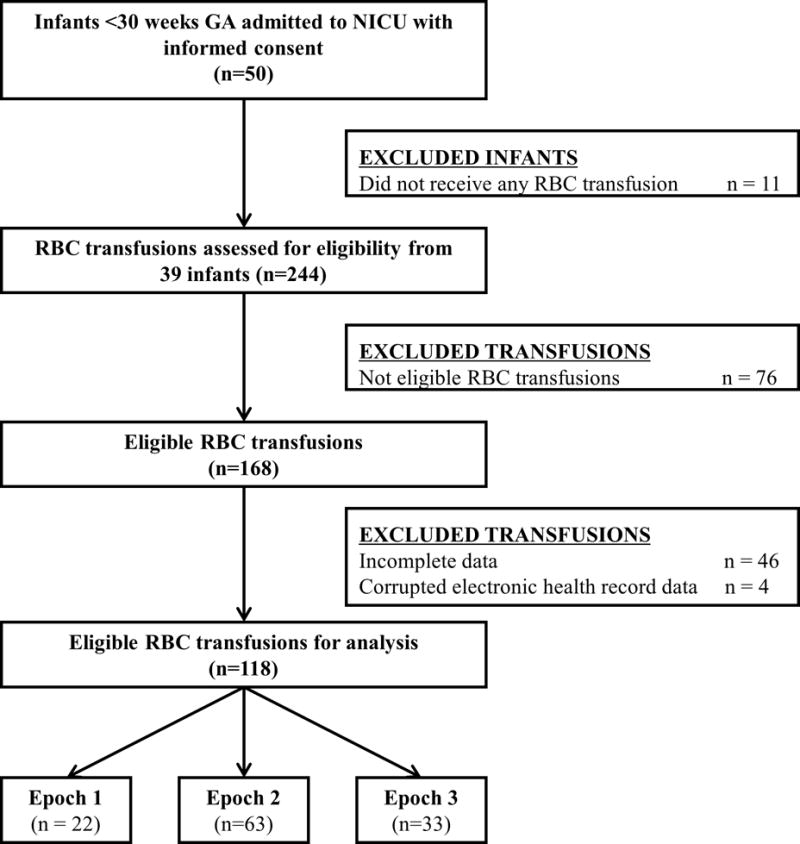
Flow diagram for patient enrollment and transfusion eligibility
Changes in Measures Pre and Post RBC Transfusion
As represented in Figure 2A, there was a statistically significant but minimal increase in mean 24 hour PI after RBC transfusion across all epochs. There was no difference in variability of PI between pre and post RBC transfusion in all 3 epochs (pre-post: −0.07 ± 0.33, p=0.2; −0.01 ± 0.12, p=0.5; −0.05 ± 0.15, p=0.1 in epochs 1, 2 and 3 respectively). In epoch 1, there was no change in IH-SpO2<80 and IH-SpO2<85 post RBC transfusion; interestingly, there was a significant increase in IH-SpO2<90 (Figure 3). Overall, %time-SpO2<80, %time-SpO2<85 and %time-SpO2<90 did not significantly change in epoch 1 (Figure 3). In epochs 2 and 3, we found a significant decrease in IH-SpO2<80 and IH-SpO2<85 and no change in IH-SpO2<90 (Figure 3). Overall %time-SpO2<80% and %time-SpO2<85 improved in epoch 2 and 3 with no changes in %time-SpO2<90. As expected, mean hematocrit significantly increased 24 hours after RBC transfusion across all three epochs (Figure 2B).
Correlations of Changes Pre and Post RBC Transfusion
There was no significant correlation between changes in PI and IH pre and post RBC transfusion in any of the 3 epochs (Table 2). There was no correlation between changes in hematocrit and IH pre and post RBC transfusion in epochs 1 and 2 (Table 2). In epoch 3, there was a positive correlation between the change in hematocrit and IH measures that was statistically significant for %time-SpO2<80 (Table 2).
TABLE 2.
Correlations of changes in PI, Hematocrit and IH
| Epoch | ΔIH Events < 80% | Δ%time < 80% | |||
|---|---|---|---|---|---|
|
| |||||
| r | p value | r | p value | ||
| Δ Perfusion Index | 1 | −0.05 | 0.46 | −0.18 | 0.61 |
| 2 | 0.18 | 0.13 | 0.14 | 0.38 | |
| 3 | −0.16 | 0.22 | 0.07 | 0.51 | |
|
| |||||
| Δ Hematocrit | 1 | 0.1 | 0.33 | −0.03 | 0.88 |
| 2 | −0.11 | 0.37 | −0.05 | 0.86 | |
| 3 | 0.271 | 0.08 | 0.322 | 0.02 | |
Δ represents change in value: post RBC transfusion - pre RBC transfusion.
r = correlation coefficient
Factors Associated with the Effect of RBC Transfusion on IH measures
Linear mixed models were utilized to assess factors that influenced the effect of RBC transfusion on IH. The models controlled for pre RBC transfusion PI, hematocrit, mechanical ventilation, FiO2 requirement and IH-SpO2<80 or %time-SpO2<80. The results are presented in Table 3.
DISCUSSION
Our study shows an increase in perfusion (as represented by the rise in PI) after RBC transfusion. However, this increase does not correlate with the improvement in oxygenation. Consistent with Abu Jawdeh et al.,1 our study shows that IH improved post RBC transfusion only beyond the first week of life.2 In addition, our results replicate the lack of benefit in oxygenation after RBC transfusion in the first week of life. This study also demonstrates that pre RBC transfusion mechanical ventilation need, FiO2 requirement and IH measures influence the effect of RBC transfusions on oxygenation.
Similar to a study by Kanmaz et al.,17 our results show a significant increase in PI post RBC transfusion. The increase in PI is minimal and may not be clinically significant. The observed increase in PI did not correlate with a decrease in IH measures following RBC transfusion. The effect of RBC transfusion on PI may be related to volume expansion. In contrast, RBC transfusion effect on IH is likely due to changes in oxygen carrying capacity and stabilization of oxygenation.6,10,18
The effect of RBC transfusion on IH varied based on postnatal age. There was significant improvement in oxygenation after RBC transfusion in epochs 2 and 3. However, there was no significant change in IH measures after RBC transfusion during the first week of life; in fact, an increase in IH frequency occurred for IH-SpO2<90. This increase in IH events in epoch 1 after transfusion for IH-SpO2<90 reflects the increase in milder events (SpO2 ≥85%); although all trended in the same direction. The etiology of this reproducible lack of benefit in oxygenation after RBC transfusion in early postnatal life is unknown, but may be influenced by multiple factors. The lack of benefit may be related to the already low incidence of IH during this period.1,2 Other factors may include inadequate compensatory mechanisms to overcome the changes in blood flow, volume status and blood viscosity associated with RBC transfusion during early postnatal life.6,18–20 Furthermore, the higher proportions of high-affinity fetal hemoglobin in early postnatal life may have an impact on the effect of RBC transfusion on oxygenation.10,20 The lack of benefit in oxygenation after RBC transfusion in the first week of life raises important concerns regarding liberal transfusion thresholds during early postnatal life and the need to further evaluate any adverse respiratory effects in this time period. In addition, studies to further evaluate mechanisms and factors that influence the effect of RBC transfusion on IH in the first week of life are imperative.
Respiratory support (mechanical ventilation and FiO2) and IH measures influenced the effect of RBC transfusions on oxygenation (Table 3). As expected, patients on mechanical ventilation benefited more from RBC transfusion compared to extubated infants in epoch 2 and approached significance in epoch 3. Interestingly, in epoch 1, patients on mechanical ventilation had no improvement or worsening in oxygenation after RBC transfusion. We speculate the findings seen in the first week of life in ventilated infants may relate to patient characteristics including immaturity of compensatory mechanisms, severe lung disease with poor pulmonary reserves and subsequent lung fluid overload from RBC transfusion.6,10,20 Increased FiO2 requirement pre RBC transfusion was associated with a significant decrease in IH measures post transfusion during epoch 1. After the first week of life, higher IH measures pre RBC transfusion were associated with greater benefit in oxygenation that was statistically significant in epoch 2 and approached significance (p=0.053) in epoch 3. Extent of FiO2 requirement and IH measures are closely related as FiO2 adjustment is often based on oxygen desaturations. Our sample size may not have been large enough to reach statistical significance in all epochs; however, FiO2 and IH measures are promising objective tools able to guide transfusion management. Overall, the results of the study show that postnatal age, along with type of respiratory support and IH measures, influence the effect of RBC transfusion on oxygenation. Further studies to evaluate mechanisms as to how these factors influence the effect of RBC transfusion on IH are needed.
Maintaining hematocrit above a certain consensus threshold is the major indication for RBC transfusion in NICUs worldwide.21,22 Consistent with previous studies, our results suggest that hematocrit alone is a weak predictor of the effect of RBC transfusion on oxygenation.1,5,7,9,23,24 Although hematocrit significantly increased post RBC transfusion, the change in hematocrit did not correlate with improved oxygenation after RBC transfusion except in epoch 3 where a poor correlation was noted (Table 2). We speculate that hematocrits are closely followed in the NICU and the levels in our infants may not have been low enough to result in significant cardiorespiratory instability.
A limitation to this study is not having evaluated other hemodynamic factors such as blood pressure, heart rate, and volume status. We also lack documentation of other neonatal morbidities that may have affected PI and oxygenation such as presence of intraventricular hemorrhage, patent ductus arteriosus, and sepsis. As our model included multiple variables, the current sample size may have lacked sufficient power to reach significance in certain epochs. The possible variation in RBC transfusion indications among providers is a limitation, but likely minimized by our unit consensus transfusion guidelines.
CONCLUSION
Red blood cell transfusion is associated with decreased IH events after the first week of life. The lack of benefit in oxygenation after RBC transfusion in the first week of life is an interesting finding now reported twice from two separate cohorts. This finding requires further investigation especially after possible worsening in oxygenation reported in this study. Our primary aim to assess the value of PI as an indication for RBC transfusion did not yield positive findings. We documented factors, other than hematocrit, that should be considered before RBC transfusion administration; including mechanical ventilation, FiO2 requirement and IH measures. Our study is a stepping stone towards larger studies aimed at finding objective bedside measures to guide RBC transfusion administration.
Acknowledgments
This project described was supported by: (1) the National Center for Research Resources, UL1RR033173, and is now at the National Center for Advancing Translational Sciences. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH and (2) The Gerber Foundation.
The authors would like to thank all the Neonatal Intensive Care Unit nurses, research nurses, and personnel, fellow physicians and attending neonatologists at University of Kentucky Children’s Hospital who all have paved the way in making sure we had good data collection.
Statement of Financial Support: The study was funded by: 1) National Center for Research Resources, UL1RR033173 and is now at the National Center for Advancing Translational Sciences; 2) The Gerber Foundation
Footnotes
Financial Disclosure Statement: The authors have no financial relationships relevant to this article to disclose.
Conflict of Interest: None
References
- 1.Abu Jawdeh EG, Martin RJ, Dick TE, et al. The effect of red blood cell transfusion on intermittent hypoxemia in ELBW infants. J Perinatol. 2014;34:921–5. doi: 10.1038/jp.2014.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Di Fiore JM, Bloom JN, Orge F, et al. A higher incidence of intermittent hypoxemic episodes is associated with severe retinopathy of prematurity. J Pediatr. 2010;157:69–73. doi: 10.1016/j.jpeds.2010.01.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Martin RJ, Wang K, Koroglu O, et al. Intermittent hypoxic episodes in preterm infants: do they matter? Neonatology. 2011;100:303–10. doi: 10.1159/000329922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Di Fiore JM, Poets CF, Gauda E, et al. Cardiorespiratory events in preterm infants: interventions and consequences. J Perinatol. 2016;36:251–8. doi: 10.1038/jp.2015.165. [DOI] [PubMed] [Google Scholar]
- 5.Poets CF, Pauls U, Bohnhorst B. Effect of blood transfusion on apnoea, bradycardia and hypoxaemia in preterm infants. European journal of pediatrics. 1997;156:311–6. doi: 10.1007/s004310050607. [DOI] [PubMed] [Google Scholar]
- 6.Banerjee J, Leung TS, Aladangady N. Cerebral blood flow and oximetry response to blood transfusion in relation to chronological age in preterm infants. Early human development. 2016;97:1–8. doi: 10.1016/j.earlhumdev.2015.10.017. [DOI] [PubMed] [Google Scholar]
- 7.Joshi A, Gerhardt T, Shandloff P, et al. Blood transfusion effect on the respiratory pattern of preterm infants. Pediatrics. 1987;80:79–84. [PubMed] [Google Scholar]
- 8.Zagol K, Lake DE, Vergales B, et al. Anemia, apnea of prematurity, and blood transfusions. J Pediatr. 2012;161:417–21 e1. doi: 10.1016/j.jpeds.2012.02.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Seidel D, Blaser A, Gebauer C, et al. Changes in regional tissue oxygenation saturation and desaturations after red blood cell transfusion in preterm infants. J Perinatol. 2013;33:282–7. doi: 10.1038/jp.2012.108. [DOI] [PubMed] [Google Scholar]
- 10.Sands SA, Edwards BA, Kelly VJ, et al. A model analysis of arterial oxygen desaturation during apnea in preterm infants. PLoS computational biology. 2009;5:e1000588. doi: 10.1371/journal.pcbi.1000588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kroese JK, van Vonderen JJ, Narayen IC, Walther FJ, Hooper S, Te Pas AB. The perfusion index of healthy term infants during transition at birth. European journal of pediatrics. 2015 doi: 10.1007/s00431-015-2650-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Piasek CZ, Van Bel F, Sola A. Perfusion index in newborn infants: a noninvasive tool for neonatal monitoring. Acta Paediatr. 2014;103:468–73. doi: 10.1111/apa.12574. [DOI] [PubMed] [Google Scholar]
- 13.Takahashi S, Kakiuchi S, Nanba Y, et al. The perfusion index derived from a pulse oximeter for predicting low superior vena cava flow in very low birth weight infants. J Perinatol. 2010;30:265–9. doi: 10.1038/jp.2009.159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Granelli A, Ostman-Smith I. Noninvasive peripheral perfusion index as a possible tool for screening for critical left heart obstruction. Acta Paediatr. 2007;96:1455–9. doi: 10.1111/j.1651-2227.2007.00439.x. [DOI] [PubMed] [Google Scholar]
- 15.Gomez-Pomar E, Makhoul M, Westgate PM, et al. Relationship between perfusion index and patent ductus arteriosus in preterm infants. Pediatr Res. 2017 doi: 10.1038/pr.2017.10. [DOI] [PubMed] [Google Scholar]
- 16.Khositseth A, Muangyod N, Nuntnarumit P. Perfusion index as a diagnostic tool for patent ductus arteriosus in preterm infants. Neonatology. 2013;104:250–4. doi: 10.1159/000353862. [DOI] [PubMed] [Google Scholar]
- 17.Kanmaz HG, Sarikabadayi YU, Canpolat E, et al. Effects of red cell transfusion on cardiac output and perfusion index in preterm infants. Early human development. 2013;89:683–6. doi: 10.1016/j.earlhumdev.2013.04.018. [DOI] [PubMed] [Google Scholar]
- 18.Dani C, Pratesi S, Fontanelli G, et al. Blood transfusions increase cerebral, splanchnic, and renal oxygenation in anemic preterm infants. Transfusion. 2010;50:1220–6. doi: 10.1111/j.1537-2995.2009.02575.x. [DOI] [PubMed] [Google Scholar]
- 19.Nelle M, Hocker C, Zilow EP, et al. Effects of red cell transfusion on cardiac output and blood flow velocities in cerebral and gastrointestinal arteries in premature infants. Archives of disease in childhood Fetal and neonatal edition. 1994;71:F45–8. doi: 10.1136/fn.71.1.f45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Orkin SH, Nathan DG. Nathan and Oski’s hematology of infancy and childhood. 7th. Philadelphia: Saunders/Elsevier; 2009. [Google Scholar]
- 21.Kirpalani H, Whyte RK, Andersen C, et al. The Premature Infants in Need of Transfusion (PINT) study: a randomized, controlled trial of a restrictive (low) versus liberal (high) transfusion threshold for extremely low birth weight infants. J Pediatr. 2006;149:301–7. doi: 10.1016/j.jpeds.2006.05.011. [DOI] [PubMed] [Google Scholar]
- 22.Bell EF, Strauss RG, Widness JA, et al. Randomized trial of liberal versus restrictive guidelines for red blood cell transfusion in preterm infants. Pediatrics. 2005;115:1685–91. doi: 10.1542/peds.2004-1884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Westkamp E, Soditt V, Adrian S, et al. Blood transfusion in anemic infants with apnea of prematurity. Biol Neonate. 2002;82:228–32. doi: 10.1159/000065891. [DOI] [PubMed] [Google Scholar]
- 24.Keyes WG, Donohue PK, Spivak JL, et al. Assessing the need for transfusion of premature infants and role of hematocrit, clinical signs, and erythropoietin level. Pediatrics. 1989;84:412–7. [PubMed] [Google Scholar]


